Abstract
OBJECTIVE
To compare the efficacy and safety of duloxetine and amitriptyline in painful diabetic neuropathy (PDN).
RESEARCH DESIGN AND METHODS
In this randomized, double-blind, cross-over, active-control trial, 58 patients received amitriptyline and duloxetine orally once daily at bedtime, each for 6 weeks with optional dose uptitration fortnightly. Single-blinded placebo washout was given for 2 weeks between the two treatments and a single-blinded placebo run-out phase of 4 weeks was given at the end of the treatment period. Pain relief was measured by the patient’s global assessment of efficacy, using a visual analog scale (0–100) as a primary end point, and overall improvement and adverse events were assessed as secondary outcome measures. Median pain score reductions of >50%, 25–50%, and <25% were considered good, moderate, and mild responses, respectively.
RESULTS
There was a significant improvement in pain with both treatments compared with their baseline values (P < 0.001 for both). Good, moderate, and mild pain relief was achieved in 55, 24, and 15% of patients, respectively, on amitriptyline and 59, 21, and 9% of patients, respectively, on duloxetine. There were no significant differences in various other outcome measures between the groups. Of the reported adverse events, dry mouth was significantly more common with amitriptyline than duloxetine (55 vs. 24%; P < 0.01). Although, numerically, more patients preferred duloxetine, overall this was not statistically significant (48 vs. 36%; P = 0.18).
CONCLUSIONS
Both duloxetine and amitriptyline demonstrated similar efficacy in PDN. A large, multicentric clinical trial in other populations could possibly demonstrate the superiority of either drug.
The management of painful diabetic neuropathy (PDN) includes intensive glycemic control and drugs for pain relief. The American Diabetes Association recommends amitriptyline, a tricyclic antidepressant, as the first choice; however, titration to higher doses is limited by its anticholinergic adverse effects (1). The selective serotonin norepinephrine reuptake inhibitor, duloxetine, is approved by the Food and Drug Administration for the treatment of PDN. It attenuates persistent pain mechanisms, including the central sensitization and hyperexcitability of the spinal and supraspinal pain-transmitting pathways. Duloxetine has been evaluated in placebo-controlled trials for a variety of chronic pain, including PDN (2–6). It not only relieves pain but also improves functionality and quality of life in patients with PDN. An extensive search of literature did not reveal any head-to-head comparison of duloxetine with amitriptyline, an established first-line therapy for PDN (1). Thus, the current study was planned to compare the efficacy and safety of duloxetine with amitriptyline in PDN.
RESEARCH DESIGN AND METHODS
Patients of either sex with type 2 diabetes, aged between 18 and 75 years, who were on stable glucose-lowering medications during the preceding month and who had PDN for at least 1 month were considered for the study. Patients who had a pain score of >50%, as assessed by visual analog scale (VAS), were enrolled in the study. Those previously exposed to medications for PDN, regardless of dose and duration, were considered after 2 weeks of placebo washout in a single-blind manner. The study was initiated after approval by the institutional ethics committee and was conducted following the principles of the Declaration of Helsinki. Written informed consent was obtained from each subject prior to enrollment.
PDN was confirmed by 1) the patient’s medical history, 2) a diabetic neuropathy symptom (DNS) score of >1 point (7), 3) a diabetic neuropathy examination (DNE) score of >3 points (8), 4) a modified neuropathy symptom score (NSS) (9,10), and 5) increased thresholds on the vibration perception test and monofilament test. Patients were excluded if they had any clinically significant or unstable medical or psychiatric illnesses. Patients with other causes of neuropathy; renal dysfunction (serum creatinine >132 μmol/L); liver disease (alanine aminotransferase and aspartate aminotransferase more than three time times the normal level); epilepsy; psychiatric illness; uncontrolled hypertension; malignancy; substance abuse; those taking anticonvulsants, antidepressants, local anesthetics, or opioids; those who were pregnant; lactating women; or those being treated with any investigational drug within the last 30 days were excluded from the study.
A 14-week, randomized (computer-generated randomization of blocks of four), double-blind, cross-over, active-control with optional dose titration, equivalence clinical trial was conducted. All patients underwent an initial 2-week run-in period in order to achieve a baseline state (during which the patients were withdrawn from any existing medication for PDN), followed by 6 weeks of treatment with each drug and a washout period of 2 weeks between the two therapies. At the end of 14 weeks, patients entered a 4-week run-out phase, during which placebo response was evaluated in a single-blind manner.
Three doses each of amitriptyline (10, 25, or 50 mg once daily at bed time) and duloxetine (20, 40, or 60 mg once daily at bed time) were used in the study. Wockhardt Limited (Mumbai, India) and Sun pharmaceutical Industries Limited (Mumbai, India) provided amitriptyline and duloxetine, respectively, as free samples. Treatment was started with the lowest dose of either drug, with fortnightly assessments with optional uptitration.
The primary end point of the study was the reduction in the median pain score from baseline, as assessed by the patient’s global assessment of efficacy by the VAS (0–100 points). Secondary end points included the assessment of pain by the short-form McGill Pain Questionnaire (11); an 11-point Likert scale for pain (0 = no pain and 10 = excruciating pain); overall improvement by DNE score, DNS score, modified NSS, and the 24-point Hamilton Rating Scale for Depression (12); change in sleep pattern (increased, unchanged, or decreased); and patient self-evaluation of overall change on the basis of a 7-point patient global impression of change (PGIC) scale. Treatment-emergent adverse events (TEAEs) were assessed by clinical and laboratory evaluation of fasting and postprandial plasma glucose, A1C levels, lipid profile, urea, creatinine, aspartate aminotransferase/alanine aminotransferase, and alkaline phosphatase. The patient’s preference of treatment was assessed by direct questionnaire. Demographic characteristics were noted and all the parameters were measured before and after treatment with both drugs and compared.
Uptitration was done after assessment of the therapeutic response and tolerability by the above-mentioned scores. Blinding and randomization were carried out by an independent person unrelated to the study. The drug packets were administered to patients serially according to the patient’s reporting sequence. A median pain score reduction on the basis of the VAS of >50%, between 25 and 50%, and <25% were considered as good, moderate, and mild responses, respectively. Overall improvement in pain intensity of ≥30 and ≥50% also was assessed. Assessments for depression and change in sleep pattern were performed at the beginning and end of each of the treatments, PGIC was assessed at the end of each treatment period, and patient preference of treatment was carried out fortnightly after beginning the second treatment, whereas all other assessments were performed at each of the 2-weekly follow-up visits. The investigator administered the drug and performed assessment for the efficacy and safety at each visit and was accessible by telephone to all patients throughout the study. No additional dose escalation was carried out in the case of appearance of adverse events, lack of benefit with the initial two doses (no benefit on patient VAS), patient’s satisfaction with pain relief, the highest dose was reached, or patient’s noncompliance. Patients were reminded by telephone calls for the due visits and also were traced for missed visits. In case of a missed visit, the patient was given the scheduled dose if he/she turned up during the stipulated time of 2 weeks or else the placebo washout was given and the next scheduled drug was started. Patients were not allowed any pain medication other than up to 3 g per day paracetamol as a rescue medication during the trial period, except 24 h prior to assessment. Compliance was assessed by direct questioning and pill counting. Success of blinding was assessed by the accuracy of the physician’s prediction at the end of the study.
Statistical analysis
The sample size calculation was based on the means and SDs observed for pain scores in previous trials of duloxetine versus placebo and amitriptyline versus placebo in PDN. The efficacy of both drugs was estimated to be 70%. To prove that there is no difference in efficacy between the two treatments, with two-sided significance levels of 2.5% and power of 80%, a within-subject SD of 20%, and a maximum-allowable difference of 10%, the total number of patients to be included was 44. Because of its cross-over design, with 40% noncompliance and loss to follow-up, we decided to include a total of 60 patients in the study. The primary and secondary efficacy analyses were performed on the intention-to-treat population, defined as patients who received at least one dose of randomized study medication and had at least one postbaseline efficacy assessment. Values are expressed as means ± SD, median with interquartile range (IQR), and numbers and percentages.
The median pain scores, the patient VAS, the Likert pain scale, and the McGill Pain Questionnaire were compared using the Wilcoxon matched-pair test. In addition, data from all visits were compared with baseline scores using the Friedman test for intragroup comparison. PGIC was compared by the McNemar-Bowker test. The Hamilton Depression Rating Scale, altered sleep pattern, percentage of patients showing improvement, treatment preference, and incidence of adverse events were compared using the χ2 test or Fisher exact test. Baseline parameters were compared by the Student paired or unpaired t test, χ2 test, or Fisher exact test. A P value <0.05 was considered significant. SPSS (version 12.0) was used to perform analysis.
RESULTS
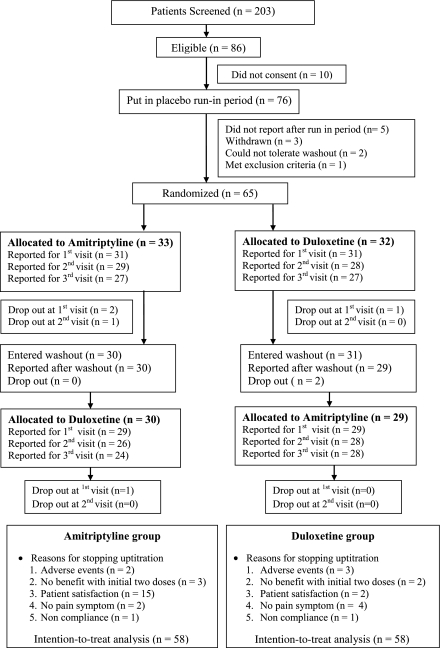
The study was conducted between March 2009 and April 2010. Patient demography, clinical characteristics, and disposition through the study period are summarized in Table 1 and Fig. 1, respectively. Of 203 patients screened, 86 were found to be eligible and 65 patients reported for randomization following the run-in period. Of a total of 58 patients, 53 (91%) completed the study with >80% compliance. The reasons for noncompliance included frequent follow-up visits and long distance from home to the hospital. Scores at baseline both before and after the crossover were similar in the two groups, and, thus, the data were pooled. No patient suffered from depression as per the Hamilton Depression Rating Scale. Previously tried treatments for neuropathic pain included the use of pregabalin in 20%, amitriptyline in 8%, and duloxetine and gabapentin in 2% of patients each.
Table 1.
Baseline demographic parameters of patients who completed the study
| Characteristics | Values |
|---|---|
| Age (years) | 52.5 (48.2–62) |
| Sex | |
| Male | 27 (47) |
| Female | 31 (53) |
| Height (cm) | 158.8 (153.6–166.7) |
| Weight (kg) | 69 (64.0–77.7) |
| Waist circumference (cm) | 93.1 (88.2–99.1) |
| BMI (kg/m2) | 26.8 (24.7–29.3) |
| Duration of diabetes (years) | 7 (2–12) |
| Duration of pain (months) | 18 (6–36) |
| Site of pain | |
| Foot | 45 (78) |
| Foot and hand | 13 (22) |
| Hypertension | 43 (74) |
| Vibration perception threshold score | 25 (14–33.8) |
| DNS (>1 point abnormal) | 58 (100) |
| DNE (>3 points abnormal) | 58 (100) |
| NSS (1–9) | 7 ± 1.8 |
| A1C (%) | 8.2 ± 1.7 |
Data are median (IQR), means ± SD, or n (%). n = 58 patients.
Figure 1.
CONSORT statement.
With duloxetine, 59% (n = 34) of patients showed good improvement, 22% (n = 13) showed moderate improvement, and 9% (n = 5) showed mild improvement. With amitriptyline, 55% (n = 32) of patients showed good improvement, 24% (n = 14) showed moderate improvement, and 16% (n = 9) showed mild improvement (Fig. 2).
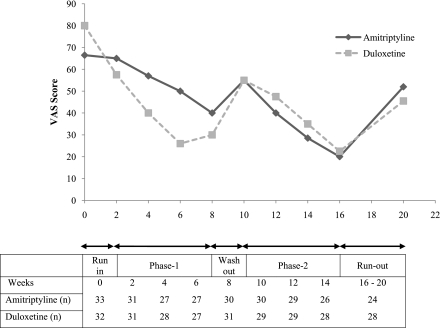
Figure 2.
Median VAS pain scores of patients.
Overall pain relief of >30% was observed in 64 and 62% of patients with duloxetine and amitriptyline, respectively, and >50% improvement was seen in 59 and 55% of patients with duloxetine and amitriptyline, respectively. No significant difference in the efficacy between the two treatments was observed on other scales as well (McGill Pain Questionnaire and Likert scale), and both drugs significantly relieved pain at 6 weeks (P < 0.001 for both). Significant improvement in sleep and overall well being was observed with both drugs (P < 0.001 for both).
Of the total study population 5, 14, and 30% preferred 20, 40, and 60 mg duloxetine, respectively, whereas 5, 22, and 9% preferred 10, 25, and 50 mg of amitriptyline, respectively, based on pain relief and tolerability. In the whole cohort, 48% (n = 28) of patients preferred duloxetine and 36% (n = 21) of patients preferred amitriptyline, but it was not statistically significant (P = 0.18). In addition, 16% of patients had no preference, out of which 9% were equally satisfied and 7% were equally dissatisfied with either treatment options. Forty-eight percent (n = 28) of patients with amitriptyline and 65% (n = 38) with duloxetine were uptitrated to their highest doses of 50 and 60 mg, respectively. Significantly more patients preferred a higher dose of duloxetine (n = 17) compared with amitriptyline (n = 5; P < 0.02).
Table 2 depicts TEAEs with the two drugs. The events were similar between amitriptyline (n = 111) and duloxetine (n = 112). The number of mild TEAEs with duloxetine was higher compared with amitriptyline (P < 0.02), whereas the number of moderate to severe TEAEs, limiting dose uptitration and requiring intervention or discontinuation of the drug, was higher with amitriptyline (P < 0.01). Moderate to severe TEAEs were more common with amitriptyline compared with duloxetine (51 vs. 24% of patients; P < 0.01). Of all the events, dry mouth was significantly more common with amitriptyline (P < 0.01). The more common adverse events reported with duloxetine were constipation and somnolence. No significant change in weight was noticed with either therapy. The mean A1C levels improved from 8.2 to 7.8% (P < 0.01). The fasting and postprandial plasma glucose levels, lipid profile, and renal and liver function tests did not change significantly with either of the drugs over the treatment period. The investigator was able to identify the prescribed drug correctly in 34% of the patients at the run-out phase. The median VAS (IQR) score increased from 26 (10–40) to 40 (20–54) at the end of run-out phase (P < 0.001), and the average run-out duration was 21 ± 12 days.
Table 2.
Adverse events observed
| Adverse events | Duloxetine | Amitriptyline | P |
|---|---|---|---|
| Somnolence | 18 (31) | 21 (36) | 0.89 |
| Dry mouth | 14 (24) | 32 (55) | <0.001 |
| Constipation | 22 (37) | 10 (17) | 0.06 |
| Lethargy | 14 (24) | 19 (33) | 0.30 |
| Insomnia | 9 (15) | 6 (10) | 0.71 |
| Uneasiness | 11 (19) | 6 (10) | 0.40 |
| Dizziness | 7 (12) | 8 (14) | 0.86 |
| Nausea | 6 (10) | 3 (5) | 0.58 |
| Anorexia | 5 (8) | 1 (2) | 0.25 |
| Headache | 4 (7) | 3 (5) | 0.78 |
| Pain abdomen | 1 (2) | 1 (2) | 0.95 |
| Itching | 1 (2) | 1 (2) | 0.95 |
| Total adverse events (n) | 112 | 111 | |
| Mild events* | 98 | 82 | <0.02 |
| Moderate to severe events† | 14 | 29 | <0.01 |
Data are n (%). n = 58 in each group.
*Mild: not requiring any intervention or discontinuation.
†Moderate to severe: limiting dose uptitration, requiring intervention or discontinuation of the drug.
CONCLUSIONS
The current study compared the efficacy and safety of duloxetine with amitriptyline head to head in patients with PDN. Both drugs demonstrated comparable efficacy and safety as per the established pain-rating scales for PDN. Although, numerically, more patients preferred duloxetine over amitriptyline, this did not reach the level of significance.
In the present trial, both duloxetine and amitriptyline demonstrated similar efficacy (>30% pain relief). More than 50% improvement in pain score was observed in 59% of patients with duloxetine, compared with 49–52% in various placebo-controlled trials (4,5,13), and 55% with amitriptyline, compared with 51–58% in other placebo-controlled and active-control trials (14–16). Improvement in pain was significant with both drugs as per patient assessment by the VAS, the 11-point Likert scale, and the McGill Pain Questionnaire. These findings are in concordance with previous studies (17,18) where pain evaluation was confirmed by similar well-established pain-evaluation scores. In addition, neuropathy status was evaluated using well-established scales like the DNS score, DNE score, and modified NSS, which also demonstrated significant improvement. The pain scores demonstrated pain resurgence during the washout and run-out phase (Fig. 2). This suggests that the drugs do not affect the basic pathophysiology of PDN; however, it also is too short a duration of therapy to comment on the same. There was comparable improvement in sleep with both drugs, as per the sleep score. There was a significant improvement in A1C over the trial period, and thus the reduction in cumulative glycemic burden can be expected to contribute to the pain relief and may have a confounding effect on the efficacy assessment (13,19). However, this is negated because of the cross-over study design and demonstration of pain resurgence during the washout and run-out phases.
The various published placebo-controlled studies on duloxetine for treating PDN have parallel designs for efficacy and safety evaluation (3,20). The current study has a cross-over design and dose titration was gradual, from a low 20–60 mg daily compared with trials evaluating 20–120 mg fixed daily doses. It was considered appropriate to plan two weekly evaluations because previous trials have demonstrated efficacy within 2 weeks of the onset of therapy. Therefore, the study was planned to be of shorter duration (i.e., 6 weeks of active drug therapy compared with a duration of 12–52 weeks, as in other trials [5,21]). The most preferred effective doses were 60 mg duloxetine and 25 mg amitriptyline, which are lower than the recommended dose range of 60–120 mg and 25–150 mg for duloxetine and amitriptyline, respectively (1,22,23). Even at these doses, moderate pain relief was achieved, and as conferred in the previous studies the lower doses of amitriptyline are effective because of the possible lower body size and weight of the Indian population (17,18,24).
The overall safety assessment was comparable for both duloxetine and amitriptyline, except for the intensity of adverse events. Dose escalation with amitriptyline was limited by its TEAEs (especially dry mouth). There were no significant differences in the laboratory parameters before and after the treatment, suggesting the safety of both drugs. Adverse events with both drugs were comparable with those reported in the previous studies (5,13,14,17,20,21,25).
The strengths of the study include the randomized, active-control, cross-over, double-blind, and adequately powered design with a good compliance rate. In addition, use of multiple established rating scales for evaluating neuropathy as well as efficacy and gradual dose uptitration enhanced the outcome evaluation. The study was limited by the lack of a placebo arm, which might have enhanced the sensitivity in pain relief with the test drug in PDN. However, the placebo used during the run-out phase in a single-blind manner led to the reappearance of pain in most of the patients necessitating intervention.
In conclusion, both amitriptyline and duloxetine demonstrated comparable efficacy, safety, and tolerability in the management of PDN. A similar head-to-head comparison in a multicentric clinical trial using a larger sample size could possibly demonstrate the superiority of either drug.
Acknowledgments
The authors are thankful to Wockhardt Limited, Mumbai, India, and Sun Pharmaceutical Industries Limited, Mumbai, India, for providing amitriptyline and duloxetine, respectively, as free samples.
No potential conflicts of interest relevant to this study were reported.
H.K. helped conduct the study, researched and analyzed data, and drafted the manuscript. D.H. contributed to the study concept and design and discussion of the study results and reviewed the final manuscript. A.B., P.D., D.B., and A.C. helped conduct the study and reviewed and edited the manuscript. D.B. was working as a resident during the study in Pgimer, Chandigarh.
Parts of this study were presented in abstract form at the 13th World Congress of Pain, Montreal, Canada, 29 August–2 September 2010.
The authors thank R. Walia, Assistant Professor, Department of Endocrinology, Postgraduate Institute of Medical Education and Research, Chandigarh, for revising the final draft of the manuscript.
References
- 1.American Diabetes Association Standards of medical care in diabetes: 2009. Diabetes Care 2009;32(Suppl. 1):S13–S61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sultan A, Gaskell H, Derry S, Moore RA. Duloxetine for painful diabetic neuropathy and fibromyalgia pain: systematic review of randomised trials. BMC Neurol 2008;8:29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lunn MP, Hughes RA, Wiffen PJ. Duloxetine for treating painful neuropathy or chronic pain. Cochrane Database Syst Rev 2009;7:CD007115. [DOI] [PubMed] [Google Scholar]
- 4.Raskin J, Pritchett YL, Wang F, et al. A double-blind, randomized multicenter trial comparing duloxetine with placebo in the management of diabetic peripheral neuropathic pain. Pain Med 2005;6:346–356 [DOI] [PubMed] [Google Scholar]
- 5.Goldstein DJ, Lu Y, Detke MJ, Lee TC, Iyengar S. Duloxetine vs. placebo in patients with painful diabetic neuropathy. Pain 2005;116:109–118 [DOI] [PubMed] [Google Scholar]
- 6.Kajdasz DK, Iyengar S, Desaiah D, et al. Duloxetine for the management of diabetic peripheral neuropathic pain: evidence-based findings from post hoc analysis of three multicenter, randomized, double-blind, placebo-controlled, parallel-group studies. Clin Ther 2007;29(Suppl.):2536–2546 [DOI] [PubMed] [Google Scholar]
- 7.Meijer JW, van Sonderen E, Blaauwwiekel EE, et al. Diabetic neuropathy examination: a hierarchical scoring system to diagnose distal polyneuropathy in diabetes. Diabetes Care 2000;23:750–753 [DOI] [PubMed] [Google Scholar]
- 8.Meijer JW, Smit AJ, Sonderen EV, Groothoff JW, Eisma WH, Links TP. Symptom scoring systems to diagnose distal polyneuropathy in diabetes: the Diabetic Neuropathy Symptom Score. Diabet Med 2002;19:962–965 [DOI] [PubMed] [Google Scholar]
- 9.Dyck PJ. Detection, characterization, and staging of polyneuropathy: assessed in diabetics. Muscle Nerve 1988;11:21–32 [DOI] [PubMed] [Google Scholar]
- 10.Dyck PJ, Sherman WR, Hallcher LM, et al. Human diabetic endoneurial sorbitol, fructose, and myo-inositol related to sural nerve morphometry. Ann Neurol 1980;8:590–596 [DOI] [PubMed] [Google Scholar]
- 11.Melzack R. The short-form McGill Pain Questionnaire. Pain 1987;30:191–197 [DOI] [PubMed] [Google Scholar]
- 12.Snaith RP. Hamilton rating scale for depression. Br J Psychiatry 1977;131:431–432 [DOI] [PubMed] [Google Scholar]
- 13.Ziegler D. Treatment of diabetic neuropathy and neuropathic pain: how far have we come? Diabetes Care 2008;31(Suppl. 2):S255–S261 [DOI] [PubMed] [Google Scholar]
- 14.Morello CM, Leckband SG, Stoner CP, Moorhouse DF, Sahagian GA. Randomized double-blind study comparing the efficacy of gabapentin with amitriptyline on diabetic peripheral neuropathy pain. Arch Intern Med 1999;159:1931–1937 [DOI] [PubMed] [Google Scholar]
- 15.Zin CS, Nissen LM, Smith MT, O’Callaghan JP, Moore BJ. An update on the pharmacological management of post-herpetic neuralgia and painful diabetic neuropathy. CNS Drugs 2008;22:417–442 [DOI] [PubMed] [Google Scholar]
- 16.Max MB, Lynch SA, Muir J, Shoaf SE, Smoller B, Dubner R. Effects of desipramine, amitriptyline, and fluoxetine on pain in diabetic neuropathy. N Engl J Med 1992;326:1250–1256 [DOI] [PubMed] [Google Scholar]
- 17.Jose VM, Bhansali A, Hota D, Pandhi P. Randomized double-blind study comparing the efficacy and safety of lamotrigine and amitriptyline in painful diabetic neuropathy. Diabet Med 2007;24:377–383 [DOI] [PubMed] [Google Scholar]
- 18.Bansal D, Bhansali A, Hota D, Chakrabarti A, Dutta P. Amitriptyline vs. pregabalin in painful diabetic neuropathy: a randomized double blind clinical trial. Diabet Med 2009;26:1019–1026 [DOI] [PubMed] [Google Scholar]
- 19.Chong MS, Hester J. Diabetic painful neuropathy: current and future treatment options. Drugs 2007;67:569–585 [DOI] [PubMed] [Google Scholar]
- 20.Wernicke JF, Wang F, Pritchett YL, et al. An open-label 52-week clinical extension comparing duloxetine with routine care in patients with diabetic peripheral neuropathic pain. Pain Med 2007;8:503–513 [DOI] [PubMed] [Google Scholar]
- 21.Raskin J, Wang F, Pritchett YL, Goldstein DJ. Duloxetine for patients with diabetic peripheral neuropathic pain: a 6-month open-label safety study. Pain Med 2006;7:373–385 [DOI] [PubMed] [Google Scholar]
- 22.Dworkin RH, O’Connor AB, Backonja M, et al. Pharmacologic management of neuropathic pain: evidence-based recommendations. Pain 2007;132:237–251 [DOI] [PubMed] [Google Scholar]
- 23.Ziegler D. Painful diabetic neuropathy: advantage of novel drugs over old drugs? Diabetes Care 2009;32(Suppl. 2):S414–S419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.World Health Organization. BMI/WHO global infobase country comparison [Internet]. Available from https://apps.who.int/infobase/report.aspx?rid=118 Accessed 9 December 2010
- 25.Wernicke JF, Gahimer J, Yalcin I, Wulster-Radcliffe M, Viktrup L. Safety and adverse event profile of duloxetine. Expert Opin Drug Saf 2005;4:987–993 [DOI] [PubMed] [Google Scholar]