Abstract
OBJECTIVE
To assess the effect of intraday glucose variability (GV) on cardiovascular outcomes in a reanalysis of Hyperglycemia and Its Effect After Acute Myocardial Infarction on Cardiovascular Outcomes in Patients With Type 2 Diabetes Mellitus (HEART2D) study data.
RESEARCH DESIGN AND METHODS
Type 2 diabetic patients after acute myocardial infarction were randomized to an insulin treatment strategy targeting postprandial (PRANDIAL; n = 557) or fasting/interprandial (BASAL; n = 558) hyperglycemia. GV was calculated as mean amplitude of glycemic excursions (MAGE), mean absolute glucose (MAG) change, and SD.
RESULTS
The PRANDIAL strategy resulted in an 18% lower MAG than BASAL (mean [SEM] difference 0.09 [0.04] mmol/L/h, P = 0.02). In addition, MAGE and SD were lower in the PRANDIAL group, however, not significantly. HbA1c levels and cardiovascular event rates were comparable between groups.
CONCLUSIONS
A PRANDIAL strategy demonstrated lower intraday GV vs. a BASAL strategy with similar overall glycemic control but did not result in a reduction in cardiovascular outcomes. This does not support the hypothesis that targeting GV would be beneficial in reducing subsequent secondary cardiovascular events.
Short-term variation in blood glucose (BG) levels is a daily challenge for patients with diabetes. It confers a possible increased risk for hypoglycemia, and it has been suggested that glucose variability (GV) is related to cardiovascular risk (1–3). However, reanalysis of the Diabetes Control and Complications Trial (DCCT) and DCCT/Epidemiology of Diabetes Interventions and Complications (EDIC) dataset examining the predictive value of GV on microvascular and neurologic complications did not show an effect of GV independent from mean glucose and HbA1c (4–6), and randomized controlled trials (RCTs) specifically targeting GV are lacking (7,8).
To assess the effect of intraday GV on cardiovascular outcomes we re-examined data from Hyperglycemia and Its Effect After Acute Myocardial Infarction on Cardiovascular Outcomes in Patients With Type 2 Diabetes Mellitus study (HEART2D; clinical trial reg. no. NCT00191282, clinicaltrials.gov) (9).
RESEARCH DESIGN AND METHODS
The HEART2D study included 1,115 type 2 diabetic patients who had had a recent myocardial infarction; patients well-controlled with diet or treated with intensive insulin therapy were excluded. It was designed to investigate possible differences between two insulin treatment strategies on time until first combined cardiovascular event (a composite of cardiovascular death, nonfatal myocardial infarction, nonfatal stroke, coronary revascularization, or hospitalization for acute coronary syndrome) (9). Within 21 days after admission for acute myocardial infarction, patients were randomized to one of two insulin treatment strategies: one targeted postprandial hyperglycemia with thrice-daily premeal insulin lispro (PRANDIAL; n = 557), and the other targeted fasting/interprandial hyperglycemia with once-daily insulin glargine or twice-daily NPH (BASAL; n = 558). The study succeeded in achieving similar HbA1c levels in both strategies, which allowed the authors to look at effects of targeting postprandial glucose values independent from glycemic control. There was no significant difference between groups in time to first combined cardiovascular event (hazard ratio 0.98 [95% CI 0.8–1.21]). Though the PRANDIAL group achieved significantly lower mean postprandial blood glucose values, the between-group difference was less than expected. The trial was halted early due to futility with a lower than expected overall number of cardiovascular events. We evaluated the effect of glycemic variability to help further the interpretation of HEART2D results.
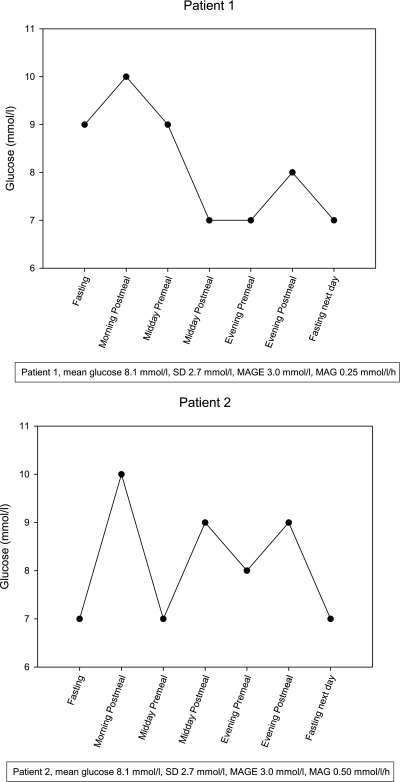
In the present analysis we calculated mean GV in both strategies from 7-point self-measured BG profiles collected prior to study visits during the study period, obtained over 24 h from breakfast to breakfast the next morning. This adds to the original analysis since postprandial excursions contribute to GV, but GV encompasses more than postprandial excursions alone. Because no gold standard for quantifying GV exists (7), we calculated mean absolute glucose (MAG) change, mean amplitude of glycemic excursions (MAGE), and SD. MAG is the summated change in glucose per unit of time (MAG = |ΔGlucose|/ΔTime) (Fig. 1), which showed in the intensive care unit a stronger association with mortality than SD (10). This is the first time MAG is used in a diabetic population. MAGE is the average of all BG increases or decreases that are >1 SD of all BG measures (11). Differences between regimens and those experiencing versus those not experiencing a cardiovascular event were assessed using a pattern mixed-model repeated-measurement analysis. The model included strategy, baseline GV, randomization factors, and an additional factor for study duration (defined as ≤30, >30 and ≤42, and >42 months) (9).
Figure 1.
Two fictitious patients with identical mean glucose, SD, and MAGE, but different patterns of variability expressed by MAG change.
RESULTS
The original HEART2D study analysis showed that HbA1c did not differ between groups during the study (mean [SEM] PRANDIAL 7.7 [0.1] %, BASAL 7.8 [0.1] %, P = 0.4). We found that the PRANDIAL strategy resulted in a significantly lower MAG compared with the BASAL strategy (mean [SEM] PRANDIAL 0.40 [0.03] mmol/L/h, BASAL 0.49 [0.02] mmol/L/h, difference 0.09 [0.04] mmol/L/h, P = 0.02). Also MAGE and SD were lower in the PRANDIAL group, however, not significantly (MAGE PRANDIAL 3.14 [0.22] mmol/L, BASAL 3.32 [0.17] mmol/L, difference 0.18 [0.27] mmol/L, P = 0.50; SD PRANDIAL 1.42 [0.09] mmol/L, BASAL 1.58 [0.07] mmol/L, difference 0.16 [0.11] mmol/L, P = 0.15). Additionally, taking the two randomization groups together, there was no difference in GV between those experiencing versus those not experiencing a cardiovascular event (mean [SEM] MAG 0.47 [0.03] mmol/L/h vs. 0.44 [0.02] mmol/L/h, P = 0.57; MAGE 3.35 [0.23] mmol/L vs. 3.16 [0.16] mmol/L, P = 0.49; SD 1.56 [0.10] mmol/L vs. 1.48 [0.07] mmol/L, P = 0.52).
CONCLUSIONS
We found that in type 2 diabetic patients after acute myocardial infarction an insulin regimen targeting postprandial hyperglycemia lowered intraday GV compared with a basal insulin strategy, with similar overall glycemic control. However, GV decreases shown in the PRANDIAL strategy did not translate into a reduction in cardiovascular outcomes compared with treatment with BASAL strategy.
It might be possible that these negative results are explained by the patient group studied, i.e., type 2 diabetic patients with advanced atherosclerosis. Another possibility is that patients with diabetes, in contrast with critically ill patients without previously diagnosed diabetes (10), are not affected by GV because of the ability of cells to adapt to the harmful effects of changing ambient glucose.
A strong point of the current study is the assessment of GV by MAG. MAG takes GV into account to its fullest extent as opposed to MAGE, which neglects glycemic swings smaller than 1 SD and assesses only the increases or decreases of the glucose profile (11). Furthermore, MAG calculates glucose change over time, whereas SD does not take time into account (Fig. 1).
In conclusion, the results of the present analysis showed that targeting postprandial glucose decreased intraday GV by 18% without a corresponding reduction in subsequent secondary cardiovascular events at least in this population. Further studies looking at different groups of patients are needed to investigate whether reducing GV will reduce cardiovascular risk independently from HbA1c.
Acknowledgments
L.K. and S.J.J. are employed by Eli Lilly and Company, sponsors of the HEART2D study, and own stock in Eli Lilly and Company. No other potential conflicts of interest relevant to this article were reported.
S.E.S. contributed to discussion and wrote the manuscript. L.K. and S.J.J. researched data, contributed to discussion, and reviewed and edited the manuscript. J.H.D. contributed to discussion and reviewed and edited the manuscript.
Footnotes
Clinical trial reg. no. NCT00191282, clinicaltrials.gov.
See accompanying editorial, p. 1058.
References
- 1.Borg R, Kuenen JC, Carstensen B, et al. ADAG Study Group HbA1(c) and mean blood glucose show stronger associations with cardiovascular disease risk factors than do postprandial glycaemia or glucose variability in persons with diabetes: the A1C-Derived Average Glucose (ADAG) study. Diabetologia 2011;54:69–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nalysnyk L, Hernandez-Medina M, Krishnarajah G. Glycaemic variability and complications in patients with diabetes mellitus: evidence from a systematic review of the literature. Diabetes Obes Metab 2010;12:288–298 [DOI] [PubMed] [Google Scholar]
- 3.Monnier L, Colette C, Mas E, et al. Regulation of oxidative stress by glycaemic control: evidence for an independent inhibitory effect of insulin therapy. Diabetologia 2010;53:562–571 [DOI] [PubMed] [Google Scholar]
- 4.Kilpatrick ES, Rigby AS, Atkin SL. The effect of glucose variability on the risk of microvascular complications in type 1 diabetes. Diabetes Care 2006;29:1486–1490 [DOI] [PubMed] [Google Scholar]
- 5.Siegelaar SE, Kilpatrick ES, Rigby AS, Atkin SL, Hoekstra JB, Devries JH. Glucose variability does not contribute to the development of peripheral and autonomic neuropathy in type 1 diabetes: data from the DCCT. Diabetologia 2009;52:2229–2232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kilpatrick ES, Rigby AS, Atkin SL. Effect of glucose variability on the long-term risk of microvascular complications in type 1 diabetes. Diabetes Care 2009;32:1901–1903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Siegelaar SE, Holleman F, Hoekstra JB, DeVries JH. Glucose variability: does it matter? Endocr Rev 2010;31:171–182 [DOI] [PubMed] [Google Scholar]
- 8.Siegelaar SE, Kulik W, van Lenthe H, Mukherjee R, Hoekstra JB, Devries JH. A randomized clinical trial comparing the effect of basal insulin and inhaled mealtime insulin on glucose variability and oxidative stress. Diabetes Obes Metab 2009;11:709–714 [DOI] [PubMed] [Google Scholar]
- 9.Raz I, Wilson PW, Strojek K, et al. Effects of prandial versus fasting glycemia on cardiovascular outcomes in type 2 diabetes: the HEART2D trial. Diabetes Care 2009;32:381–386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hermanides J, Vriesendorp TM, Bosman RJ, Zandstra DF, Hoekstra JB, Devries JH. Glucose variability is associated with intensive care unit mortality. Crit Care Med 2010;38:838–842 [DOI] [PubMed] [Google Scholar]
- 11.Service FJ, Molnar GD, Rosevear JW, Ackerman E, Gatewood LC, Taylor WF. Mean amplitude of glycemic excursions, a measure of diabetic instability. Diabetes 1970;19:644–655 [DOI] [PubMed] [Google Scholar]