Abstract
OBJECTIVE
Many guidelines recommend reduced consumption of salt in patients with type 1 diabetes, but it is unclear whether dietary sodium intake is associated with mortality and end-stage renal disease (ESRD).
RESEARCH DESIGN AND METHODS
In a nationwide multicenter study (the FinnDiane Study) between 1998 and 2002, 2,807 enrolled adults with type 1 diabetes without ESRD were prospectively followed. Baseline urinary sodium excretion was estimated on a 24-h urine collection. The predictors of all-cause mortality and ESRD were determined by Cox regression and competing risk modeling, respectively.
RESULTS
The median follow-up for survival analyses was 10 years, during which 217 deaths were recorded (7.7%). Urinary sodium excretion was nonlinearly associated with all-cause mortality, such that individuals with the highest daily urinary sodium excretion, as well as the lowest excretion, had reduced survival. This association was independent age, sex, duration of diabetes, the presence and severity of chronic kidney disease (CKD) (estimated glomerular filtration rate [eGFR] and log albumin excretion rate), the presence of established cardiovascular disease, and systolic blood pressure. During follow-up, 126 patients developed ESRD (4.5%). Urinary sodium excretion was inversely associated with the cumulative incidence of ESRD, such that individuals with the lowest sodium excretion had the highest cumulative incidence of ESRD.
CONCLUSIONS
In patients with type 1 diabetes, sodium was independently associated with all-cause mortality and ESRD. Although we have not demonstrated causality, these findings support the calls for caution before applying salt restriction universally. Clinical trials must be performed in diabetic patients to formally test the utility/risk of sodium restriction in this setting.
Blood pressure control is a key target for the prevention and management of the complications of type 1 diabetes. To this end, many treatment guidelines have promoted the potential utility of dietary salt restriction in patients with diabetes (1,2), as a means to reduce blood pressure levels and, with it, potentially modify the risk and severity of complications. However, any restriction in dietary sodium intake is also associated with activation of the sympathetic nervous system (3) and the renin-angiotensin-aldosterone system (4) and increased LDL cholesterol (4). Some studies have also suggested that salt restriction reduces insulin sensitivity in patients with type 2 diabetes (5) and healthy human volunteers (6). In the context of type 1 diabetes, and especially in patients with chronic kidney disease (CKD) who have the greatest risk of adverse outcomes (7,8), activation of these pathways may offset or overshadow any gains achieved from modest and transient blood pressure lowering. Hence, in this study we aimed to determine the association between dietary salt intake and 1) all-cause mortality and 2) end-stage renal disease (ESRD) in 2,807 patients with type 1 diabetes from the large nationwide multicenter cohort of Finnish adults with type 1 diabetes (the Finnish Diabetic Nephropathy [FinnDiane] Study) (7,9).
RESEARCH DESIGN AND METHODS
Study sample
This study is part of the ongoing FinnDiane Study, with the aim to identify genetic, clinical, and environmental risk factors for diabetic nephropathy in patients with type 1 diabetes (7,9). In FinnDiane, type 1 diabetes was defined as an onset of diabetes before the age of 35 years and permanent insulin treatment initiated within 1 year of diagnosis. For the current study, outcomes were ascertained in all patients from the FinnDiane prospective cohort without ESRD at baseline and who were enrolled between January 1998 and December 2002 (n = 2,807). The ethics committees of all participating centers approved the study protocol. Written informed consent was obtained from each patient, and the study was performed in accordance with the Declaration of Helsinki.
Cohort characteristics
At baseline, data on medication and diabetes complications were registered with the use of a standardized questionnaire, which was completed by the physician based upon medical files. Blood pressure was measured twice in the sitting position after a 10-min rest, and the average of these two measurements was used in the analysis. Height, weight, and waist-to-hip ratio were recorded, and blood was drawn for the measurements of HbA1c, lipids, and creatinine. The glomerular filtration rate (GFR) was estimated using the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) formula. Macrovascular disease was defined as a history of myocardial infarction, a coronary artery procedure (bypass surgery or angioplasty), stroke, limb amputation, or peripheral artery procedure, which was verified from the medical files. HbA1c and creatinine were determined by standardized assays at each center. Serum lipid and lipoprotein concentrations were analyzed centrally by automated enzymatic methods (Hoffmann-LaRoche, Basel, Switzerland). In addition, the urinary sodium concentration was measured in a single 24-h urine collection with an ion-selective electrode (Roche Diagnostics, Basel, Switzerland) performed centrally at the University of Helsinki. During follow-up all patients were managed by their own practitioner and diabetes team, without any attempt to standardize care. In particular, there were no limitations or requests to modify the diet or sodium intake by study organizers.
Ascertainment of outcomes
Deaths from any cause through 17 March 2010 were identified via a search of the Finnish National Death Registry and center databases. All deaths were confirmed with death certificate data. In each case, vital status was verified from the Finnish National Death Registry. ESRD was defined as the requirement for dialysis or kidney transplantation and identified via a search of the renal registries and center databases and verified from medical files.
Statistical analysis
In this article, we aimed to identify the predictors of the all-cause mortality and the cumulative incidence of ESRD. To evaluate the independent predictors of all-cause mortality in individuals with type 1 diabetes, we used Cox proportional hazards models. Model selection from candidate variables was accomplished by minimization of the Akaike and Bayesian information criteria. Overall, Cox model fit was assessed by 1) approximation of cumulative Cox-Snell residuals to (−log) Kaplan-Meier estimates, residual plots, and specific testing of the proportional hazards assumption and 2) Harrell C statistic and added-variable goodness-of-fit tests. Cox model performance was adjudged by the explained variation using 5,000 bootstrap repetitions of the whole dataset, adjusting for covariates (10).
The predictors of the cumulative incidence of ESRD, accounting for the competing event of pre-ESRD death, were ascertained using a Fine and Gray model (11), which extends the Cox proportional hazards model to competing risk data by considering the subdistribution hazard. The strength of the association between each predictor variable and the outcome was assessed using the subhazard ratio (12), which is the ratio of hazards associated with the cumulative incidence function (CIF) in the presence of and in the absence of a prognostic factor. Because standard errors of the Fine-Gray model are robust (Huber-White type), model selection was guided by information criteria (Akaike information criterion [AIC] and Bayesian information criterion [BIC]). The model specification was established by residual analysis, DFBETA measures of influence across each model covariate, and formal check of proportionality by the use of time-varying covariate effect. The Fine-Gray model was implemented in Stata statistical software (V11, 2009; College Station, TX) using the stcrreg module. In both models, covariate functional form (including assessment of nonlinear effect) was adjudged by residual-by-time analysis, fractional polynomials, and (cubic) regression splines, where appropriate. The potential for multiple colinearity was tested using the variance inflation factor and condition number, where variance inflation factor <10 and condition number <30 are desirable.
RESULTS
Cohort characteristics
The cohort in which 24-h urinary sodium excretion was estimated comprised 2,807 adult patients with type 1 diabetes. The cohort characteristics at baseline are summarized in Table 1. Briefly, approximately half of the participants were men (51%). The mean age of the participants was 39 years, with a median duration of diabetes of 20 years. Forty-seven percent of patients had hypertension (defined by the use of antihypertensive agents and/or a blood pressure >140/90). At baseline, 15% had a urinary albumin excretion rate (AER) in the microalbuminuric range and 15% had a urinary AER in the macroalbuminuric range. Sixty-five percent had a urinary AER in the normoalbuminuric range. A further 5% of study participants were unclassified because of an inadequate number of urine collections. Twelve percent of patients had an estimated GFR (eGFR) <60 mL/min/1.73 m2, most of whom also had macroalbuminuria.
Table 1.
Baseline clinical characteristics of patients with type 1 diabetes from the FinnDiane Study, stratified according to 24-h urinary sodium excretion
| Quartile |
|||
|---|---|---|---|
| Low, <102 mmol/day | Middle, 102–187 mmol/day | High, >187 mmol/day | |
| Age (years) | 38 ± 13 | 39 ± 12 | 39 ± 12 |
| Male (%) | 32.6* | 49.2 | 71.5* |
| Duration of diabetes (years) | 22 ± 12 | 22 ± 12 | 20 ± 11* |
| Insulin dose (units/kg) | 0.7 ± 0.2 | 0.7 ± 0.2 | 0.7 ± 0.2 |
| HbA1c (%) | 8.4 ± 1.5 | 8.4 ± 1.5 | 8.4 ± 1.4 |
| BMI (kg/m2) | 24.7 ± 3.4* | 25.0 ± 3.5 | 26.1 ± 3.5* |
| Hypertension (%) | 44.5* | 50.2 | 53.6* |
| Blood pressure (mmHg) | |||
| Systolic | 132 ± 18 | 133 ± 18 | 135 ± 18* |
| Diastolic | 78 ± 9* | 79 ± 9 | 81 ± 10* |
| Antihypertensive medication use (%) | 33.6 | 37.1 | 39.3* |
| ACE inhibition | 28.9 | 27.4 | 24.4 |
| Angiotensin receptor blocker | 6.2 | 5.1 | 3.8 |
| Calcium channel blocker | 7.3 | 8.5 | 10.0 |
| β-blocker | 9.7 | 10.3 | 9.0 |
| Diuretic | 9.4 | 10.0 | 8.1 |
| Lipid-lowering therapy (%) | 10.8 | 9.5 | 10.8 |
| Cholesterol (mmol/L) | |||
| Total§ | 5.0 ± 0.9 | 5.0 ± 0.9 | 5.0 ± 1.0 |
| LDL§ | 3.1 ± 0.8 | 3.1 ± 0.8 | 3.2 ± 0.8* |
| HDL§ | 1.3 ± 0.4 | 1.3 ± 0.4 | 1.3 ± 0.4* |
| Triglycerides (mmol/L) | 1.3 ± 0.9 | 1.2 ± 0.9 | 1.3 ± 0.9 |
| Any retinopathy (%) | 52 | 50 | 52 |
| Retinopathy requiring laser therapy (%) | 29 | 31 | 28 |
| Current smoker (%) | 23 | 24 | 27 |
| Established macrovascular disease (%) | 9.2 | 7.5 | 6.4 |
| Normoalbuminuria (%) | 68.7 | 69.9 | 71.1 |
| Microalbuminuria (%) | 16.4 | 14.5 | 13.8 |
| Macroalbuminuria (%) | 14.9 | 15.6 | 15.2 |
| eGFR (mL/min/1.73 m2) | 84 ± 23 | 84 ± 22 | 87 ± 21* |
*Versus middle quartiles, univariate P < 0.05 (for independent predictors of baseline urinary sodium excretion in a multivariate regression model see Supplementary Table 1).
§To convert values for cholesterol to milligrams per deciliter, divide by 0.02586.
Urinary sodium excretion
The mean urinary sodium excretion in Finnish patients with type 1 diabetes was 150 ± 20 mmol/day. As previously described in the Finnish population (13,14), the mean daily sodium excretion was significantly lower in women (131 mmol/day) than in men (160 mmol/day; P < 0.001). In addition, sodium excretion was independently associated with age, duration of diabetes, body mass, HDL cholesterol levels, the presence of established cardiovascular disease, and renal function (both eGFR and albuminuria) on multivariate regression (Supplementary Table 1). Blood pressure levels were higher in individuals with high sodium intake (Table 1), but after adjusting for age and sex there was no association between urinary sodium excretion and the achieved level of blood pressure or the modality of antihypertensive therapy, including the chronic use of diuretics, as previously shown (15). Although insulin may directly modify renal sodium excretion (16), the insulin dose was not associated with urinary sodium excretion.
Mortality and urinary sodium excretion
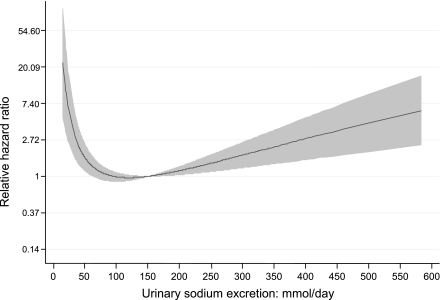
The median follow-up for survival analyses was 10 years during which 217 deaths were recorded (7.7%). Urinary sodium excretion was significantly associated with all-cause mortality (P < 0.001). This association was nonlinear, such that individuals with the highest daily urinary sodium excretion, as well as the lowest excretion, had reduced cumulative survival (Fig. 1). After adjusting for parameters associated with daily urinary sodium excretion, as well as other factors independently associated with all-cause mortality (including age, sex, duration of diabetes, the presence and severity of CKD [eGFR and log AER]), the presence of established cardiovascular disease and systolic blood pressure urinary sodium excretion remained significantly associated with all-cause mortality on multivariate Cox regression analysis (P < 0.001; full regression model is presented as Supplementary Table 2). There were no significant interactions identified. Notably, the relationship between sodium intake and mortality was independent of the severity of CKD at baseline, the presence or absence of hypertension, its severity, or its means of treatment.
Figure 1.
The association between 24-h urinary sodium excretion and all-cause mortality modeled within the conventional Cox model as a cubic regression spline presented as Supplementary Table 2.
ESRD and urinary sodium excretion
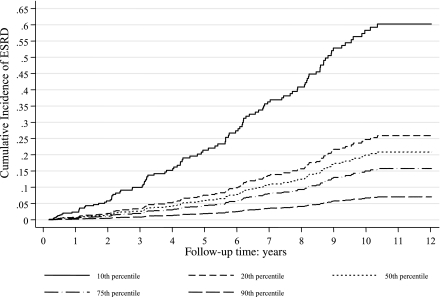
During a median of 10 years of follow-up 126 patients developed ESRD (4.5%). All but four of these patients had a urinary albumin excretion in the macroalbuminuric range at baseline. Consequently, to examine the association between urinary sodium excretion and ESRD, we considered only this subgroup of macroalbuminuric patients (n = 424) with their 122 ESRD events. As with mortality, urinary sodium excretion was also significantly associated with ESRD (Fig. 2). After adjusting for parameters associated with daily urinary sodium excretion, as well as other factors independently associated with ESRD and taking into account the competing risk of pre-ESRD death, urinary sodium excretion remained significantly associated with the cumulative incidence of ESRD (P < 0.001). Individuals with the lowest sodium excretion had the highest cumulative incidence of ESRD. The full Fine-Gray competing risk model is presented as Supplementary Table 3. Notably, this model incorporates an interaction between sodium excretion and estimated GFR modeled as a cubic regression spline. The relationship between sodium intake and the cumulative incidence of ESRD was not demonstrated to be associated with the presence or absence of hypertension, its severity, or its means of treatment.
Figure 2.
The cumulative incidence of ESRD over the 10th, 25th, 50th, 75th, and 90th percentiles of 24-h urinary sodium excretion, adjusted for other covariate predictors and accounting for pre-ESRD mortality as the competing risk (full Fine-Gray proportional hazards competing risk regression model is presented as Supplementary Table 3).
CONCLUSIONS
Many guidelines recommend and advocate that patients with type 1 diabetes should aim to restrict their dietary intake of salt (1,2). Such recommendations have been inferred from data showing that a reduced sodium intake can result in a modest short-term fall in blood pressure in some (but not all) patients with type 1 diabetes (17). However, the epidemiological associations between sodium intake and long-term outcomes have not been previously explored specifically in patients with type 1 diabetes. Small studies have suggested an association between dietary sodium intake with proliferative retinopathy (18) and albuminuria in type 1 diabetes (19). However, this is the first study to show that dietary sodium intake is associated with all-cause mortality in patients with type 1 diabetes and correlated with the development of ESRD in patients with macroalbuminuria, after adjusting for baseline risk factors. Importantly, this association appears to be nonlinear, with an increased risk of death and ESRD in individuals with low sodium excretion, as well as reduced survival in those with high sodium intakes.
That both high and low sodium intake may be associated with adverse mortality outcomes is consistent with epidemiological data from nondiabetic individuals. For example, in the Finnish general population, a high sodium intake has been associated with an increased risk of mortality (14). In so far as two-thirds of our patients with type 1 diabetes are free of CKD and hypertension, and these complications-free individuals have an age-sex standardized mortality rate not significantly different from that of the general population (7,8), similar predictors of mortality may also be anticipated. At the same time, a negative association between sodium intake and mortality has been also observed in hypertensive men (20) and in aggressively treated patients with type 2 diabetes (21). It is possible to speculate that the balance of diabetic patients enrolled in our study, which included both healthy individuals and others with established complications, reflects one or other pole, thus cumulatively generating a U-shaped association between salt excretion and mortality. However, the shape of this association was consistent across all subgroups, including those individuals with or without established renal or cardiovascular complications (Supplementary Table 2), suggesting that other factors are also involved.
Strengths of our study include its very large cohort of individuals with type 1 diabetes, high participation rate, access to subsidized care (75–100% of costs), and contemporary treatment regimens, including a range of insulin regimens, statins, blockers of the RAS, and self-monitoring technologies. We used validated methods to identify deaths, and all deaths in our cohort were confirmed through death records. Surveillance bias is unlikely given the uniform vital status follow-up procedures used by our staff masked to participants’ CKD status levels. In our questionnaire, we had broad data on tobacco or alcohol use, diet, education, socioeconomic status, other possible confounders (e.g., insulin resistance), or the severity of disease. Few changes in diabetes treatment and health care over the short study period will have affected mortality results. Our study also specifically examined ESRD within the paradigm of a formal competing risks (Fine-Gray) model, which looked at the cumulative incidence of ESRD while taking into consideration (in an estimation sense) the competing risk of death. This strategy is especially important in patients with type 1 diabetes, since cause-specific analysis may be confounded when individuals at highest risk of ESRD (e.g., macroalbuminuric or hypertensive patients) are also at increased risk of pre-ESRD mortality.
Another strength of this study is the fact that the dietary intake of sodium in our cohort is very similar to that documented in the general population, both in Finland (13,14) and globally (22). We have also recently published dietary surveys on a subset of 817 FinnDiane patients, collected with a 3-day food record completed twice with a 2- to 3-month interval, describing a mean daily sodium consumption of 7.2 ± 2.0 g/day (23). Twenty-seven percent of patients achieved target levels of less than 6 g of NaCl (23), a figure no different to the general Finnish population. Such data suggest that advice to restrict dietary salt intake in our cohort of diabetic patients is not promulgated beyond universal recommendations for the general population or is equally ineffective. Although it is possible that more aggressive recommendations for salt restriction or their uptake may be more likely in high-risk patients (confounding by indication), the association between salt and adverse outcomes appeared to be independent of conventional factors that physicians may normally use to stratify risk.
It is fundamental to state with respect to the association of sodium and adverse outcomes that statistical independence of effect does not imply (strict) causality (24) and additional trials should be performed to formally test our findings and the potential utility/risk of sodium restriction in the context of diabetes. Any pathophysiological mechanisms that may underlie our findings also remain to be established. Certainly, sodium restriction results in increased atherosclerosis in experimental models (25). In humans, salt restriction increased activity of the renin-angiotensin-aldosterone system (4) and increased sympathetic activity (3) and insulin resistance (5,6), each of which may contribute to the development and progression of diabetes complications. We were unable to observe any association between sodium intake and blood pressure indexes in our cohort. Consistent with these findings, short-term studies of salt restriction in patients with type 1 diabetes have also revealed variable outcomes, with both increases and falls in blood pressure observed in different individuals, and no net effect overall (17). Notably, in this study, the clinical characteristics of salt-sensitive patients were not different from those in whom blood pressure levels rose with salt restriction, particularly with regard to the presence and severity of CKD. This suggests that even in the setting of diabetes and CKD, universal recommendations regarding salt intake may be problematic.
Several study limitations need to be considered. First, these studies were also limited by reliance on sodium excretion data obtained from a single urine collection. Because of dietary variability, there may be substantial variation in salt excretion on a day-to-day basis, meaning that a single value may not accurately reflect habitual sodium intake. Nonetheless, this method is regarded as the best way to estimate dietary sodium intake and substantially more effective than dietary recall. There appears to be only a modest coefficient of variation (21), comparable with other commonly used parameters like lipid levels. Even in the setting of renal impairment, the urinary excretion of sodium is largely maintained along with volume homeostasis, as a result of adaptations in residual nephrons. This has been suggested to be because sodium intake may be physiologically set (22). Second, our data are observational. Although observational studies have a number of potential advantages, it is also possible that associations demonstrated in this study may be because of confounding by unmeasured factors or ones that are difficult to quantify. For example, variability in dietary sodium intake may be passively associated with a range of differences in diet composition, processing, and preparation that may impact adverse outcomes in diabetic individuals. In addition, the appetite for salt may be associated with differences in neuroendocrine effectors that may themselves impact on the development and progression of diabetes complications.
In summary, the association between sodium intake and adverse outcomes in type 1 diabetes is complex. In the setting of type 1 diabetes, individuals with the lowest sodium excretion (the best available marker for their low salt intake) have an increased risk of all-cause mortality and ESRD. Individuals with a high salt intake also have an increased risk of mortality. Although we have not demonstrated causality, these findings further support the calls for caution before applying salt restriction universally (26), since clinical outcomes may be different or paradoxical in certain settings. In particular, the specific requirements for diabetic individuals should not be inferred from general population data but must be validated in studies performed specifically in diabetic patients.
Supplementary Material
Acknowledgments
The FinnDiane Study is supported by grants from the Folkhälsan Research Foundation, the Wilhelm and Else Stockmann Foundation, the Liv och Hälsa Foundation, and the Finnish Medical Society (Finska Läkaresällskapet). M.C.T. is supported by the Bootle bequest and Kidney Health Australia.
No potential conflicts of interest relevant to this article were reported.
M.C.T. and J.M. wrote and edited the article and performed statistical analyses. C.F. collected data and wrote and edited the article. V.H., L.T., A.A., J.W., N.T., M.S., and D.G. collected data. P.-H.G. collected data and wrote and edited the article. C.F. and P.-H.G. had full access to all data in the study and take responsibility for the integrity of data and the accuracy of data analysis.
The authors also acknowledge the generous support of many physicians and nurses at each center, previously presented in detail (7,9).
Footnotes
This article contains Supplementary Data online at http://care.diabetesjournals.org/lookup/suppl/doi:10.2337/dc10-1722/-/DC1.
References
- 1.Bantle JP, Wylie-Rosett J, Albright AL, et al. American Diabetes Association Nutrition recommendations and interventions for diabetes: a position statement of the American Diabetes Association. Diabetes Care 2008;31(Suppl. 1):S61–S78 10.2337/dc08-S061 [DOI] [PubMed] [Google Scholar]
- 2.Graham I, Atar D, Borch-Johnsen K, et al. European Society of Cardiology (ESC) European Association for Cardiovascular Prevention and Rehabilitation (EACPR) Council on Cardiovascular Nursing. European Association for Study of Diabetes (EASD) International Diabetes Federation Europe (IDF-Europe) European Stroke Initiative (EUSI) Society of Behavioural Medicine (ISBM) European Society of Hypertension (ESH) WONCA Europe (European Society of General Practice/Family Medicine) European Heart Network (EHN) European Atherosclerosis Society (EAS) European guidelines on cardiovascular disease prevention in clinical practice: full text. Fourth Joint Task Force of the European Society of Cardiology and other societies on cardiovascular disease prevention in clinical practice (constituted by representatives of nine societies and by invited experts). Eur J Cardiovasc Prev Rehabil 2007;14(Suppl. 2):S1–S113 10.1097/01.hjr.0000277983.23934.c9 [DOI] [PubMed] [Google Scholar]
- 3.Grassi G, Dell’Oro R, Seravalle G, Foglia G, Trevano FQ, Mancia G. Short- and long-term neuroadrenergic effects of moderate dietary sodium restriction in essential hypertension. Circulation 2002;106:1957–1961 10.1161/01.CIR.0000033519.45615.C7 [DOI] [PubMed] [Google Scholar]
- 4.Graudal NA, Galløe AM, Garred P. Effects of sodium restriction on blood pressure, renin, aldosterone, catecholamines, cholesterols, and triglyceride: a meta-analysis. JAMA 1998;279:1383–1391 10.1001/jama.279.17.1383 [DOI] [PubMed] [Google Scholar]
- 5.Petrie JR, Morris AD, Minamisawa K, et al. Dietary sodium restriction impairs insulin sensitivity in noninsulin-dependent diabetes mellitus. J Clin Endocrinol Metab 1998;83:1552–1557 10.1210/jc.83.5.1552 [DOI] [PubMed] [Google Scholar]
- 6.Garg R, Williams GH, Hurwitz S, Brown NJ, Hopkins PN, Adler GK. Low-salt diet increases insulin resistance in healthy subjects. Metabolism. 29 Oct 2010 [Epub ahead of print] [DOI] [PMC free article] [PubMed]
- 7.Groop PH, Thomas MC, Moran JL, et al. FinnDiane Study Group The presence and severity of chronic kidney disease predicts all-cause mortality in type 1 diabetes. Diabetes 2009;58:1651–1658 10.2337/db08-1543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Orchard TJ, Secrest AM, Miller RG, Costacou T. In the absence of renal disease, 20 year mortality risk in type 1 diabetes is comparable to that of the general population: a report from the Pittsburgh Epidemiology of Diabetes Complications Study. Diabetologia 2010;53:2312–2319 10.1007/s00125-010-1860-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mäkinen VP, Forsblom C, Thorn LM, et al. FinnDiane Study Group Metabolic phenotypes, vascular complications, and premature deaths in a population of 4,197 patients with type 1 diabetes. Diabetes 2008;57:2480–2487 10.2337/db08-0332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Royston P. Explained variation for survival models. Stata J 2006;6:83–96 [Google Scholar]
- 11.Fine J, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc 1999;94:496–509 10.2307/2670170 [DOI] [Google Scholar]
- 12.Grunkemeier GL, Jin R, Eijkemans MJ, Takkenberg JJ. Actual and actuarial probabilities of competing risks: apples and lemons. Ann Thorac Surg 2007;83:1586–1592 10.1016/j.athoracsur.2006.11.044 [DOI] [PubMed] [Google Scholar]
- 13.Laatikainen T, Pietinen P, Valsta L, Sundvall J, Reinivuo H, Tuomilehto J. Sodium in the Finnish diet: 20-year trends in urinary sodium excretion among the adult population. Eur J Clin Nutr 2006;60:965–970 10.1038/sj.ejcn.1602406 [DOI] [PubMed] [Google Scholar]
- 14.Tuomilehto J, Jousilahti P, Rastenyte D, et al. Urinary sodium excretion and cardiovascular mortality in Finland: a prospective study. Lancet 2001;357:848–851 10.1016/S0140-6736(00)04199-4 [DOI] [PubMed] [Google Scholar]
- 15.Wilcox CS, Mitch WE, Kelly RA, et al. Response of the kidney to furosemide. I. Effects of salt intake and renal compensation. J Lab Clin Med 1983;102:450–458 [PubMed] [Google Scholar]
- 16.Lee IH, Dinudom A, Sanchez-Perez A, Kumar S, Cook DI. Akt mediates the effect of insulin on epithelial sodium channels by inhibiting Nedd4-2. J Biol Chem 2007;282:29866–29873 10.1074/jbc.M701923200 [DOI] [PubMed] [Google Scholar]
- 17.Gerdts E, Svarstad E, Myking OL, Lund-Johansen P, Omvik P. Salt sensitivity in hypertensive type-1 diabetes mellitus. Blood Press 1996;5:78–85 10.3109/08037059609062112 [DOI] [PubMed] [Google Scholar]
- 18.Roy MS, Janal MN. High caloric and sodium intakes as risk factors for progression of retinopathy in type 1 diabetes mellitus. Arch Ophthalmol 2010;128:33–39 10.1001/archophthalmol.2009.358 [DOI] [PubMed] [Google Scholar]
- 19.Holler C, Abrahamian H, Auinger M. Effect of nutrition on microalbuminuria in patients with type 1 diabetes: prospective data evaluation over 5 years. Acta Med Austriaca 1999;26:168–172 [in German] [PubMed] [Google Scholar]
- 20.Alderman MH, Madhavan S, Cohen H, Sealey JE, Laragh JH. Low urinary sodium is associated with greater risk of myocardial infarction among treated hypertensive men. Hypertension 1995;25:1144–1152 [DOI] [PubMed] [Google Scholar]
- 21.Ekinci EI, Clarke S, Thomas MC, et al. Dietary salt intake and mortality in patients with type 2 diabetes. Diabetes Care 2011;34:703–709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McCarron DA, Stern JS, Graudal N. Public policy and dietary sodium restriction. JAMA 2010;303:1916–1918 [DOI] [PubMed] [Google Scholar]
- 23.Ahola AJ, Mikkila V, Makimattila S, Forsblom C, Freese R, Groop PH. Energy and nutrient intakes and adherence to dietary guidelines among Finnish adults with type 1 diabetes. Ann Med. 3 Nov 2010 [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 24.Schafer J. Marginal modeling of intensive longitudinal data by generalized estimating equations. In Models For Intensive Longitudinal Data. Walls T, Schafer J, Eds. New York, Oxford University Press, 2006, p. 38–62 [Google Scholar]
- 25.Ivanovski O, Szumilak D, Nguyen-Khoa T, et al. Dietary salt restriction accelerates atherosclerosis in apolipoprotein E-deficient mice. Atherosclerosis 2005;180:271–276 10.1016/j.atherosclerosis.2004.12.020 [DOI] [PubMed] [Google Scholar]
- 26.Alderman MH. Reducing dietary sodium: the case for caution. JAMA 2010;303:448–449 10.1001/jama.2010.69 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.