Abstract
OBJECTIVE
Pulse pressure (PP), an estimate of arterial stiffness, has been shown to be associated with incident cardiovascular disease (CVD) in patients with type 1 diabetes (T1D). However, diabetic kidney disease, a strong predictor of CVD, was not previously taken into account. Furthermore, the role of PP as a predictor of diabetic nephropathy is not known. Therefore, we prospectively investigated the associations between PP and these diabetes complications in patients with T1D.
RESEARCH DESIGN AND METHODS
A total of 4,509 patients from the FinnDiane Study participated. Follow-up data on incident CVD events and renal status (median 5.3 years) were available in 69 and 76% of the patients, respectively. Altogether, 269 patients (8.6%) had an incident CVD event and 370 patients (10.8%) progressed to a higher level of albuminuria or to end-stage renal disease.
RESULTS
PP was higher at baseline in patients who experienced a CVD event (66 ± 18 vs. 52 ± 14 mmHg; P < 0.001) or progressed in their renal status (58 ± 18 vs. 54 ± 15 mmHg; P < 0.01) during follow-up. In a Cox regression model, PP was independently associated with a first ever CVD event (hazard ratio per 10 mmHg 1.22 [95% CI 1.10–1.34]) but not progression of renal disease (1.00 [0.89–1.12]) after adjustments for traditional risk factors.
CONCLUSIONS
PP, a marker of arterial stiffness, is a risk factor for cardiovascular complications but not for diabetic nephropathy in patients with T1D.
Whereas systolic blood pressure (SBP) increases with age in the western population, diastolic blood pressure (DBP) generally increases during adulthood, peaks at 55–60 years of age, and thereafter starts to decrease as the result of arterial stiffening (1). This results in an increased pulse pressure (PP) that is associated with cardiovascular disease (CVD) (2). We have shown a premature increase in PP in patients with type 1 diabetes (T1D) compared with nondiabetic subjects (3). The results indicated an accelerated arterial aging in patients with T1D that may contribute to a higher risk of CVD.
PP has been shown to be a strong predictor of CVD in the general population, especially in elderly subjects aged >65 years (4,5). The EURODIAB Prospective Complications Study extended this finding to patients with T1D because PP was shown to be associated with incident CVD (6). However, diabetic nephropathy, a strong CVD risk factor (7), was not taken into account. Furthermore, Soedamah-Muthu et al. (8) reported that PP predicted all-cause but not cardiovascular mortality in the same cohort.
Although PP was shown to be associated with diabetic nephropathy in a cross-sectional study (3), it is not known whether PP could be used as a predictor of progression of diabetic nephropathy. However, blood pressure per se has been shown to predict incipient and overt diabetic nephropathy, although the results have been conflicting (9–11).
Therefore, the aim was to explore the role of PP in the development of CVD and progression of diabetic nephropathy in a large, homogeneous and nationwide cohort of patients with T1D.
RESEARCH DESIGN AND METHODS
The Finnish Diabetic Nephropathy (FinnDiane) Study is an ongoing, nationwide, prospective multicenter study seeking clinical, genetic, biochemical, and environmental risk factors for diabetes complications, with an emphasis on diabetic nephropathy. Participating study centers comprise diabetes and renal outpatient clinics at all five university central hospitals, all 16 central hospitals, the majority (N = 27) of regional hospitals, and 31 major primary health care centers in Finland. Patients with T1D (International Classification of Diseases, 10th Revision, code E10) were recruited at routine outpatient visits. On the basis of medical records, the attending physician completed a standardized check-list regarding diabetes complications and medication. Follow-up data have been collected since 2004 (7). The study protocol is in accordance with the Declaration of Helsinki as revised in 2000 and approved by the local ethics committee in each study center. Written informed consent was obtained from each patient.
At baseline, patients underwent a thorough clinical investigation that took place in conjunction with a regular visit to the attending physician. Patients with complete information on clinical data were selected. Follow-up data on the occurrence of CVD and verified renal status were collected by reexamination of the patients or review of the medical files. The median (range) follow-up time for the study population was 5.3 (0.0–14.0) years.
Blood pressure was measured by standard methods. After the patient had rested for at least 10 min, blood pressure was measured twice in a sitting position by a trained nurse. The mean of the two recordings was used in the analyses. PP was calculated as (SBP − DBP in mmHg), and mean arterial pressure was calculated as (1/3 SBP + 2/3 DBP in mmHg).
Diabetes complications
CVD was defined as any of the following events: myocardial infarction, coronary artery procedure (bypass surgery or angioplasty), stroke (ischemic or hemorrhagic), limb amputation because of ischemia, or a peripheral artery procedure. Renal status was based on the urinary albumin excretion rate (AER) in two of three consecutive overnight or 24-h urine collections. Normal AER was defined as <20 μg/min or <30 mg/24 h, microalbuminuria was defined as 20 μg/min ≤ AER <200 μg/min or 30 mg/24 h ≤ AER <300 mg/24 h, and macroalbuminuria was defined as AER ≥200 μg/min or AER ≥300 mg/24 h. End-stage renal disease (ESRD) was defined as patients undergoing dialysis or having received a kidney transplant. Progression was defined as a change from one level to a higher level of AER or the development of ESRD. Diabetic nephropathy was defined as macroalbuminuria or ESRD. Patients with a kidney transplant were excluded from the CVD analysis. Data on CVD events and progression of albuminuria were available in 3,110 patients (69% of all patients with data on PP) versus 3,424 patients (76%), respectively.
Arterial stiffness by pulse-wave analysis
In a subpopulation of 408 patients, both arterial stiffness measured by pulse-wave analysis (PWA) and follow-up data were available. The median (range) follow-up time was 6.1 (0.0–11.0) years. Applanation tonometry (SphygmoCor, Atcor Medical, Sydney, Australia) is a widely used noninvasive method to estimate arterial stiffness by analyzing arterial pressure waveforms (12). The pulse wave was recorded from the radial artery of the right arm with a high-fidelity micromanometer (SPT-301, Millar Instruments, Houston, TX). A model of the central pressure waveform was synthesized by the SphygmoCor software using a validated generalized mathematic transformation to calculate the augmentation index (AIx) and aortic PP as indicators of arterial stiffness (13).
Assays
Fasting blood samples were analyzed for HbA1c, serum lipids, and lipoproteins. HbA1c was determined locally by standardized assays, and serum lipid and lipoprotein concentrations were determined centrally by automated enzymatic methods (Hoffman-LaRoche, Basel, Switzerland). Estimated glomerular filtration rate was calculated using the Modification of Diet in Renal Disease four-variable equation (14,15). AER was assessed from an overnight or a 24-h urine collection by immunoturbidimetry.
Statistical analyses
All analyses were performed with SPSS 17.0.1 (SPSS Inc., Chicago, IL). Baseline characteristics are presented as means ± SD for normally distributed values and as median with interquartile range for non-normally distributed values and percentages. Differences between groups for normally distributed variables were tested using ANOVA, and for nonparametric data the Mann–Whitney or Kruskal–Wallis test was used. When P values were adjusted for age, ANCOVA was used for continuous variables and logistic regression for categoric variables. For categoric variables, the χ2 test was used when appropriate. Risk factors for the progression of diabetes complications were assessed using Cox proportional hazard survival regression showing results as hazard ratios with 95% CIs. Age, sex, HbA1c, total cholesterol, estimated glomerular filtration rate, history of smoking, use of antihypertensive medication, and PP were used as covariates. To account for a potential multicolinearity, we chose to use age but not duration of diabetes in the models, because the associations were stronger between age at baseline and incident events at follow-up. Similarly, total cholesterol was chosen of the cholesterol indices. SBP was not included in the analysis because of the strong association with PP (r = 0.83; P < 0.001.)
RESULTS
Altogether, 4,509 patients were studied, 52% of whom were male. At baseline their mean age was 39 ± 12 years, SBP was 134 ± 19 mmHg, DBP was 80 ± 10 mmHg, PP was 55 ± 16 mmHg, and HbA1c was 8.5 ± 1.5%. A total of 2,763 patients had normal AER, 561 patients had microalbuminuria, 611 patients had macroalbuminuria, 292 patients had ESRD, and 282 patients could not be classified with respect to their renal status because of insufficient number of urine collections or disease duration <15 years. At baseline, 9% of patients had experienced a CVD event. Table 1 shows patient characteristics according to baseline quartiles of PP.
Table 1.
Patient characteristics according to baseline quartiles of pulse pressure
| 1st | 2nd | 3rd | 4th | P value | Age-corrected P value | |
|---|---|---|---|---|---|---|
| Patients (N) | 1,145 | 1,080 | 1,137 | 1,147 | — | — |
| Sex (% women) | 42 | 50 | 58 | 56 | <0.001 | <0.001 |
| Age (years) | 33 ± 9 | 36 ± 10 | 39 ± 12 | 47 ± 12 | <0.001 | — |
| Duration (years) | 17 ± 9 | 19 ± 11 | 22 ± 12 | 30 ± 12 | <0.001 | <0.001 |
| Age at onset (years) | 16 ± 11 | 17 ± 11 | 17 ± 11 | 17 ± 11 | 0.25 | <0.001 |
| BMI (kg/m2) | 24.6 ± 3.7 | 24.8 ± 3.8 | 25.2 ± 3.5 | 25.5 ± 3.7 | <0.001 | 0.025 |
| HbA1c (%) | 8.5 ± 1.7 | 8.4 ± 1.5 | 8.4 ± 1.4 | 8.5 ± 1.5 | 0.16 | 0.022 |
| Total cholesterol (mmol/L) | 4.9 ± 1.0 | 4.9 ± 1.0 | 4.9 ± 1.0 | 5.2 ± 1.1 | <0.001 | <0.001 |
| HDL cholesterol (mmol/L) | 1.6 ± 0.5 | 1.6 ± 0.5 | 1.6 ± 0.6 | 1.6 ± 0.5 | 0.49 | 0.073 |
| LDL cholesterol (mmol/L) | 3.0 ± 0.8 | 3.0 ± 1.1 | 3.0 ± 0.9 | 3.2 ± 0.9 | <0.001 | 0.159 |
| Triacylglycerol (mmol/L) | 1.02 (0.77–1.43) | 1.00 (0.75–1.41) | 1.04 (0.77–1.46) | 1.10 (0.81–1.58) | 0.03 | <0.001 |
| Insulin dose (IU/kg) | 0.74 ± 0.26 | 0.72 ± 0.26 | 0.70 ± 0.25 | 0.66 ± 0.24 | <0.001 | 0.005 |
| SBP (mmHg) | 118 ± 10 | 127 ± 10 | 136 ± 11 | 156 ± 16 | <0.001 | <0.001 |
| DBP (mmHg) | 80 ± 9 | 80 ± 10 | 79 ± 10 | 79 ± 11 | 0.39 | 0.022 |
| PP (mmHg) | 38 ± 5 | 48 ± 2 | 57 ± 3 | 76 ± 12 | <0.001 | <0.001 |
| Mean arterial pressure (mmHg) | 80 ± 9 | 82 ± 10 | 85 ± 10 | 92 ± 11 | <0.001 | <0.001 |
| History of CVD (%) | 4 | 6 | 7 | 19 | <0.001 | <0.001 |
| History of smoking (%) | 47 | 45 | 47 | 50 | 0.109 | 0.241 |
| Antihypertensive medication (%) | 24 | 28 | 38 | 62 | <0.001 | <0.001 |
| eGFR (mL/min/1.73 m2) | 92 ± 30 | 93 ± 31 | 90 ± 34 | 87 ± 34 | <0.001 | <0.001 |
| Diabetic nephropathy (%) | 13 | 15 | 20 | 40 | <0.001 | <0.001 |
Values are expressed as means ± SD, percent, or median (interquartile range).
eGFR, estimated glomerular filtration rate.
A total of 269 patients (8.6%) had an incident CVD event during follow-up. Of those, 178 had their first ever CVD event. Altogether, 370 patients (10.8%) progressed to a higher level of albuminuria or to ESRD during follow-up (148 to microalbuminuria, 75 to macroalbuminuria, and 147 to ESRD). Table 2 demonstrates baseline characteristics according to outcomes during follow-up.
Table 2.
Baseline characteristics according to occurrence of an incident CVD event (coronary event, stroke, peripheral vascular event) and progression in renal status (defined as any increase in albuminuria level or progression to ESRD) during follow-up
| Incident CVD event |
Progression in renal status |
|||
|---|---|---|---|---|
| Event | No event | Progressors | Nonprogressors | |
|
N |
269 |
2,698 |
373 |
2,830 |
| Age (years) |
49 ± 10* |
37 ± 12 |
39 ± 12 |
38 ± 13 |
| Age at onset (years) |
17 ± 11 |
17 ± 11 |
15 ± 11† |
17 ± 11 |
| HbA1c (%) |
8.8 ± 1.6* |
8.4 ± 1.5 |
9.4 ± 1.7† |
8.3 ± 1.4 |
| Total cholesterol (mmol/L) |
5.3 ± 1.2* |
4.9 ± 0.9 |
5.4 ± 1.3† |
4.9 ± 0.9 |
| SBP (mmHg) |
148 ± 20* |
132 ± 17 |
141 ± 21† |
132 ± 17 |
| DBP (mmHg) |
81 ± 10* |
79 ± 10 |
83 ± 10† |
79 ± 10 |
| PP (mmHg) |
66 ± 18* |
52 ± 14 |
58 ± 18† |
54 ± 15 |
| Mean arterial pressure (mmHg) |
90 ± 10* |
83 ± 9 |
89 ± 10† |
83 ± 9 |
| History of CVD (%) |
34* |
5 |
12† |
7 |
| History of smoking (%) |
60* |
40 |
61† |
39 |
| Antihypertensive medication (%) |
77* |
31 |
60† |
35 |
| eGFR (mL/min/1.73 m2) |
57 ± 33* |
90 ± 31 |
68 ± 41† |
88 ± 32 |
| Diabetic nephropathy | 59* | 15 | NA | NA |
Values are expressed as number, means ± SD, or percentage.
Patients with a kidney transplant are excluded from the CVD analysis.
eGFR, estimated glomerular filtration rate; NA, not applicable.
*P < 0.05 vs. no event.
†P < 0.05 vs. nonprogressors.
Survival plots
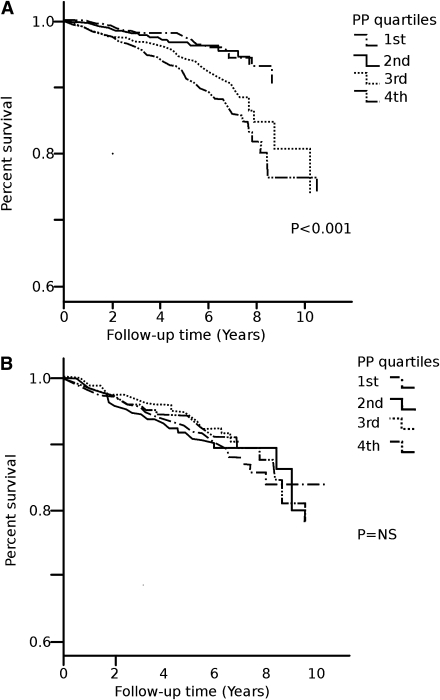
Age-adjusted survival curves demonstrate a positive association between PP and the occurrence of an incident hard CVD event during follow-up (Fig. 1A). Thus, patients in the highest PP quartiles (third and fourth) more often had a CVD event than those in the first and second quartiles. No association was observed between PP and the progression in renal status (Fig. 1B).
Figure 1.
A: Age-adjusted survival curves for an incident CVD event (coronary event, stroke, peripheral vascular event) by quartiles of PP. B: Age-adjusted survival curves for any progression in renal status (defined as any increase in albuminuria level or progression to ESRD) by quartiles of PP.
Multivariate models
Because of the high proportion of patients with a previous CVD event at baseline, only patients who had their first ever CVD event (N = 178) during follow-up (Table 3) were included in the Cox regression analysis. PP independently predicted a first ever CVD event. Inclusion of AER or diabetic nephropathy into the model did not change the results.
Table 3.
Cox regression analysis for an incident CVD event (coronary event, stroke, peripheral vascular event) and progression in renal status (defined as any increase in albuminuria level or progression to ESRD)
| Incident CVD event |
Progression in renal status |
|||
|---|---|---|---|---|
| HR (95% CI) | P value | HR (95% CI) | P value | |
| Age (years) |
1.06 (1.04–1.08) |
<0.001 |
1.00 (0.99–1.02) |
0.81 |
| Male sex |
1.11 (0.79–1.54) |
0.55 |
2.01 (1.47–2.76) |
<0.001 |
| HbA1c (%) |
1.06 (0.95–1.18) |
0.32 |
1.41 (1.30–1.53) |
<0.001 |
| Total cholesterol (mmol/L) |
1.26 (1.10–1.45) |
0.001 |
1.19 (1.04–1.37) |
0.01 |
| eGFR (mL/min/1.73 m2) |
0.99 (0.98–0.99) |
<0.001 |
1.00 (0.99–1.01) |
0.88 |
| History of smoking |
1.45 (1.20–1.59) |
0.001 |
1.20 (0.92–1.41) |
0.15 |
| Antihypertensive medication |
0.43 (0.31–0.71) |
<0.001 |
0.58 (0.42–0.81) |
0.001 |
| PP (per 10 mmHg) | 1.22 (1.10–1.34) | <0.001 | 1.00 (0.89–1.12) | 0.96 |
Variables are from baseline.
eGFR, estimated glomerular filtration rate; HR, hazard ratio.
PP did not predict progression of renal disease. Furthermore, no associations were observed between PP and progression to microalbuminuria, macroalbuminuria, or ESRD when analyzed separately.
After dividing the patients by albuminuria at baseline, a similar Cox regression analysis was performed (Table 4). The number of first ever CVD events at follow-up was 51 in patients with normal AER, 27 in patients with microalbuminuria, and 77 in patients with macroalbuminuria. PP and age independently predicted a first ever CVD event at follow-up in all groups.
Table 4.
Cox regression analysis for an incident CVD event (coronary event, stroke, peripheral vascular event) in patients by albuminuria status at baseline
| CVD event | ||||||
|---|---|---|---|---|---|---|
| Normoalbuminuria | Microalbuminuria | Macroalbuminuria | ||||
| HR (95% CI) | P value | HR (95% CI) | P value | HR (95% CI) | P value | |
| Age (years) | 1.08 (1.04–1.11) | <0.001 | 1.09 (1.05–1.14) | <0.001 | 1.04 (1.01–1.07) | 0.009 |
| Male sex | 1.24 (0.67–2.28) | 0.49 | 1.12 (0.43–2.95) | 0.82 | 1.15 (0.67–1.98) | 0.68 |
| HbA1c (%) | 1.14 (0.88–1.47) | 0.33 | 1.29 (0.95–1.76) | 0.11 | 1.04 (0.89–1.22) | 0.61 |
| Total cholesterol (mmol/L) | 1.44 (1.05–1.98) | 0.02 | 0.92 (0.55–1.52) | 0.73 | 1.24 (1.02–1.52) | 0.03 |
| eGFR (mL/min/1.73 m2) | 0.99 (0.97–1.01) | 0.23 | 1.01 (0.99–1.03) | 0.31 | 0.99 (0.98–1.00) | 0.046 |
| History of smoking | 1.52 (1.10–1.74) | 0.02 | 1.26 (0.15–1.70) | 0.52 | 1.31 (0.84–1.59) | 0.17 |
| Antihypertensive medication | 0.50 (0.27–0.95) | 0.03 | 0.69 (0.26–1.82) | 0.46 | 0.96 (0.23–4.10) | 0.96 |
| PP (per 10 mmHg) | 1.31 (1.07–1.61) | 0.009 | 1.41 (1.04–1.93) | 0.03 | 1.18 (1.03–1.38) | 0.02 |
Variables are from baseline.
eGFR, estimated glomerular filtration rate; HR, hazard ratio.
Arterial stiffness by PWA and incident CVD
Of the 408 patients with PWA data available, 35 had an incident CVD event during follow-up. Both aortic PP (61 ± 21 vs. 46 ± 18 mmHg, P < 0.001) and AIx (24.3 ± 9.0 vs. 19.5 ± 12.1%, P < 0.05) were increased in the patients with an incident CVD event. Similarly, age (61 ± 21 vs. 46 ± 18 years, P < 0.001), brachial SBP (151 ± 23 vs. 137 ± 22 mmHg, P < 0.001), and brachial PP (75 ± 21 vs. 63 ± 17 mmHg, P < 0.001) were higher in the patients with a CVD event. A larger proportion of the patients with an incident CVD event had diabetic nephropathy (57 vs. 37%, P < 0.001) and previous CVD events (80 vs. 7%, P < 0.001) at baseline.
Aortic PP was independently associated with incident CVD events during follow-up (1.29 [1.02–1.62] per 10 mmHg, P = 0.03) after adjusting for age, sex, total cholesterol, HbA1c, diabetic nephropathy, and history of smoking in the Cox model. However, after inclusion of previous CVD in the model, aortic PP was no longer a predictor. Finally, AIx was not independently associated with an incident CVD event in a similar model.
CONCLUSIONS
The main finding of this study was that PP predicted a first ever CVD event even after adjustments for renal disease in patients with T1D. Another novel finding was that PP also predicted incident CVD in diabetic patients irrespectively of the degree of diabetic nephropathy. Notably, PP did not influence the progression of diabetic nephropathy.
We previously showed a premature increase in PP in patients with T1D compared with nondiabetic control subjects (3). However, because of the cross-sectional design of that study, we could not answer the question whether an early increase in PP also increased the risk for CVD in these patients. The current study, on the other hand, shows that PP indeed increases the risk for a first ever CVD event in these patients. This risk increased by 18–41% (per 10 mmHg PP) depending on renal status.
Our results are in line with earlier data showing PP to be a predictor of CVD in patients with diabetes. In the Hoorn Study, PP correlated positively with cardiovascular mortality (16). However, despite the large study sample (N = 2,484), it included no more than 208 patients with type 2 diabetes. Cockcroft et al. (17) reported PP to predict coronary heart disease in a large-scale study of patients with type 2 diabetes. In T1D, results from the EURODIAB cohort showed PP to associate with incident CVD after correcting for age and sex in a multivariate model (6). However, the association was not independent of further adjustments. Notably, diabetic kidney disease was not included in the prospective analysis. This is important because the presence and severity of diabetic nephropathy are shown to be strongly linked to the excess mortality associated with T1D (7). Moreover, we included only patients with hard CVD events (coronary event, stroke, peripheral vascular event) in the analysis, whereas patients with electrocardiogram abnormalities and angina pectoris were included in the EURODIAB study.
We did not observe an association between PP and progression in renal status. To our knowledge, there are no other prospective studies on PP and diabetic nephropathy in T1D in the literature, but blood pressure per se has been demonstrated to play a role in the development of microvascular complications (18). Mean arterial pressure predicted the development of microalbuminuria and macroalbuminuria in patients with T1D from the Steno Diabetes Center (10). Similar results have been reported by the Microalbuminuria Collaborative Study Group (19). Notably, there are also data available demonstrating no difference in blood pressure in patients who progress in renal status and those who do not (9,11,20).
The data in the small subset of patients in whom PWA was measured support the findings. We recently showed that central PP is more strongly associated with CVD than AIx in patients with T1D in the FinnDiane cohort (21). Therefore, it was not surprising that central PP was associated with incident CVD in this prospective analysis. The data are further supported by Roman et al. (22), who reported central PP to be a strong predictor of incident CVD in a large-scale study.
This study cannot provide the mechanisms behind the relationship between arterial stiffness and CVD events. However, as the arteries stiffen, less energy is absorbed during the systolic cardiac phase and released during the diastolic phase (23). The phenomenon results in increased aortic stiffness, increased left ventricular afterload, increased LV mass and oxygen demand, and decreased stroke volume, and consequently potentiates the development of arterial disease (24). The increase in brachial PP can be considered a robust measure of arterial stiffness, especially in older subjects (5,25). Because the increase in PP occurs 15 to 20 years earlier in patients with T1D and is indicative of accelerated vascular aging, PP may be used as a predictor of future CVD events in these patients (3).
The strengths of this study are the large number of well-characterized patients and the prospective study design. The FinnDiane represents a homogenous, nationwide cohort of adults with T1D studied between 1997 and 2010. A limitation is that the blood pressure recordings were obtained on a single occasion. However, if anything, this only would dilute our findings.
In view of the combined results, PP, a marker of arterial stiffness, is a risk factor for cardiovascular complications but not for diabetic nephropathy in patients with T1D.
Supplementary Material
Acknowledgments
The study was supported by grants from the Folkhälsan Research Foundation, Helsinki University Central Hospital Research Funds (EVO), the Wilhelm and Else Stockmann Foundation, the Waldemar von Frenckell Foundation, the Liv och Hälsa Foundation, the Finnish Medical Society (Finska Läkaresällskapet), the Diabetes Research Foundation, the Paavo Nurmi Foundation, the Biomedicum Helsinki Foundation, the Nylands Nation Foundation, and the Paulo Foundation.
No potential conflicts of interest relevant to this article were reported.
D.G. researched data and wrote the article. J.W., C.F., L.T., M.R.-B., N.T., M.S., V.H., and P.-H.G. edited the article.
The skilled assistance of Anna Sandelin, Jaana Tuomikangas, Sinikka Lindh, Jessica Thorn, and Susanne Ström of the FinnDiane Study is gratefully acknowledged. The authors also thank Joakim Janér, MD, PhD, of the Department of Pediatrics, Helsinki University Central Hospital, for the graphic contribution in the figures. The authors acknowledge all the physicians and nurses at each participating study center.
Footnotes
This article contains Supplementary Data online at http://care.diabetesjournals.org/lookup/suppl/doi:10.2337/dc10-2013/-/DC1.
*A complete list of the members of the FinnDiane Study Group can be found in the Supplementary Data.
References
- 1.Franklin SS, Gustin W, 4th, Wong ND, et al. Hemodynamic patterns of age-related changes in blood pressure. The Framingham Heart Study. Circulation 1997;96:308–315 [DOI] [PubMed] [Google Scholar]
- 2.Boutouyrie P, Tropeano AI, Asmar R, et al. Aortic stiffness is an independent predictor of primary coronary events in hypertensive patients: a longitudinal study. Hypertension 2002;39:10–15 10.1161/hy0102.099031 [DOI] [PubMed] [Google Scholar]
- 3.Rönnback M, Fagerudd J, Forsblom C, Pettersson-Fernholm K, Reunanen A, Groop PH. Finnish Diabetic Nephropathy (FinnDiane) Study Group. Altered age-related blood pressure pattern in type 1 diabetes. Circulation 2004;110:1076–1082 10.1161/01.CIR.0000139903.29522.8D [DOI] [PubMed] [Google Scholar]
- 4.Franklin SS, Khan SA, Wong ND, Larson MG, Levy D. Is pulse pressure useful in predicting risk for coronary heart Disease? The Framingham heart study. Circulation 1999;100:354–360 [DOI] [PubMed] [Google Scholar]
- 5.Glynn RJ, Chae CU, Guralnik JM, Taylor JO, Hennekens CH. Pulse pressure and mortality in older people. Arch Intern Med 2000;160:2765–2772 10.1001/archinte.160.18.2765 [DOI] [PubMed] [Google Scholar]
- 6.Schram MT, Chaturvedi N, Fuller JH, Stehouwer CD, EURODIAB Prospective Complications Study Group Pulse pressure is associated with age and cardiovascular disease in type 1 diabetes: the Eurodiab Prospective Complications Study. J Hypertens 2003;21:2035–2044 10.1097/00004872-200311000-00012 [DOI] [PubMed] [Google Scholar]
- 7.Groop PH, Thomas MC, Moran JL, et al. FinnDiane Study Group. The presence and severity of chronic kidney disease predicts all-cause mortality in type 1 diabetes. Diabetes 2009;58:1651–1658 10.2337/db08-1543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Soedamah-Muthu SS, Chaturvedi N, Witte DR, Stevens LK, Porta M, Fuller JH, EURODIAB Prospective Complications Study Group Relationship between risk factors and mortality in type 1 diabetic patients in Europe: the EURODIAB Prospective Complications Study (PCS). Diabetes Care 2008;31:1360–1366 10.2337/dc08-0107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mathiesen ER, Rønn B, Jensen T, Storm B, Deckert T. Relationship between blood pressure and urinary albumin excretion in development of microalbuminuria. Diabetes 1990;39:245–249 10.2337/diabetes.39.2.245 [DOI] [PubMed] [Google Scholar]
- 10.Hovind P, Tarnow L, Rossing P, et al. Predictors for the development of microalbuminuria and macroalbuminuria in patients with type 1 diabetes: inception cohort study. BMJ 2004;328:1105 10.1136/bmj.38070.450891.FE [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Giorgino F, Laviola L, Cavallo Perin P, Solnica B, Fuller J, Chaturvedi N. Factors associated with progression to macroalbuminuria in microalbuminuric type 1 diabetic patients: the EURODIAB Prospective Complications Study. Diabetologia 2004;47:1020–1028 10.1007/s00125-004-1413-8 [DOI] [PubMed] [Google Scholar]
- 12.Wilkinson IB, Fuchs SA, Jansen IM, et al. Reproducibility of pulse wave velocity and augmentation index measured by pulse wave analysis. J Hypertens 1998;16:2079–2084 10.1097/00004872-199816121-00033 [DOI] [PubMed] [Google Scholar]
- 13.Chen CH, Nevo E, Fetics B, et al. Estimation of central aortic pressure waveform by mathematical transformation of radial tonometry pressure. Validation of generalized transfer function. Circulation 1997;95:1827–1836 [DOI] [PubMed] [Google Scholar]
- 14.National Kidney Foundation K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Am J Kidney Dis 2002;39(Suppl. 1):S1–S266 10.1016/S0272-6386(02)70081-4 [DOI] [PubMed] [Google Scholar]
- 15.Levey AS, Coresh J, Greene T, et al. Chronic Kidney Disease Epidemiology Collaboration Using standardized serum creatinine values in the modification of diet in renal disease study equation for estimating glomerular filtration rate. Ann Intern Med 2006;145:247–254 [DOI] [PubMed] [Google Scholar]
- 16.Schram MT, Kostense PJ, Van Dijk RA, et al. Diabetes, pulse pressure and cardiovascular mortality: the Hoorn Study. J Hypertens 2002;20:1743–1751 10.1097/00004872-200209000-00017 [DOI] [PubMed] [Google Scholar]
- 17.Cockcroft JR, Wilkinson IB, Evans M, et al. Pulse pressure predicts cardiovascular risk in patients with type 2 diabetes mellitus. Am J Hypertens 2005;18:1463–1467; discussion 1468–1469 10.1016/j.amjhyper.2005.05.009 [DOI] [PubMed] [Google Scholar]
- 18.Fagerudd JA, Tarnow L, Jacobsen P, et al. Predisposition to essential hypertension and development of diabetic nephropathy in IDDM patients. Diabetes 1998;47:439–444 10.2337/diabetes.47.3.439 [DOI] [PubMed] [Google Scholar]
- 19.Predictors of the development of microalbuminuria in patients with T1D mellitus: a seven-year prospective study. The Microalbuminuria Collaborative Study Group. Diabet Med 1999;16:918–925 10.1046/j.1464-5491.1999.00182.x [DOI] [PubMed] [Google Scholar]
- 20.Rossing P, Hougaard P, Parving HH. Risk factors for development of incipient and overt diabetic nephropathy in type 1 diabetic patients: a 10-year prospective observational study. Diabetes Care 2002;25:859–864 10.2337/diacare.25.5.859 [DOI] [PubMed] [Google Scholar]
- 21.Gordin D, Wadén J, Forsblom C, et al. for the FinnDiane Study Group. Arterial stiffness and vascular complications in patients with type 1 diabetes: the Finnish Diabetic Nephropathy (FinnDiane) Study. Ann Med. 3 November 2010 [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 22.Roman MJ, Devereux RB, Kizer JR, et al. High central pulse pressure is independently associated with adverse cardiovascular outcome the strong heart study. J Am Coll Cardiol 2009;54:1730–1734 10.1016/j.jacc.2009.05.070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chemla D, Hébert JL, Coirault C, et al. Total arterial compliance estimated by stroke volume-to-aortic pulse pressure ratio in humans. Am J Physiol 1998;274:H500–H505 [DOI] [PubMed] [Google Scholar]
- 24.Marchais SJ, Guerin AP, Pannier BM, Levy BI, Safar ME, London GM. Wave reflections and cardiac hypertrophy in chronic uremia. Influence of body size. Hypertension 1993;22:876–883 [DOI] [PubMed] [Google Scholar]
- 25.Nichols WW, O’Rourke MF. McDonald’s Blood Flow in Arteries: Theoretic, Experimental and Clinical Principles. 5th ed. London, Edward Arnold, 2005 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.