Abstract
OBJECTIVE
To investigate the relationship between diabetes and future risk of Parkinson’s disease (PD) among older U.S. adults.
RESEARCH DESIGN AND METHODS
A prospective study of self-reported diabetes in 1995 and 1996 in relation to PD diagnosed after 1995 among 288,662 participants of the National Institutes of Health-AARP Diet and Health Study. Multivariate odds ratio (OR) and 95% CI were derived from logistic regression models.
RESULTS
A total of 1,565 participants with PD diagnosed after 1995 were included in the analysis. After adjustment for potential confounders, PD risk was ∼40% higher (OR = 1.41 [95% CI 1.20–1.66]) among diabetic patients than among participants without diabetes. Further analysis showed that the risk elevation was largely limited to individuals who had diabetes for more than 10 years at the time of baseline survey (1.75 [1.36–2.25]). The association with diabetes was seen for both participants with PD diagnosed between 1995 and 1999 and participants with PD diagnosed after 2000. In addition, similar results were obtained after excluding participants with stroke, heart disease, cancers, or poor or fair health status and in subgroup analyses by age, sex, smoking status, and coffee consumption.
CONCLUSIONS
This large study showed that diabetes was associated with a higher future risk of PD and the nature of this association warrants further investigation.
Over the past several decades, type 2 diabetes has become a significant public health problem worldwide. Around ∼10% of middle-aged adults and 20% of the elderly in the U.S. have diabetes (1), in part because of high prevalence of inactive lifestyle and obesity. Diabetic patients often suffer from a variety of neurological consequences, ranging from peripheral neuropathy (2) to higher risk of dementia (3) or Alzheimer’s disease (4). A link between diabetes and Parkinson’s disease (PD) has also been explored in several epidemiological studies, but the results are inconsistent (5–11), ranging from a significant inverse association to a significant positive association. These studies often included few exposed cases (PD participants with diabetes) and did not have information on the duration of diabetes. We therefore examined the history of diabetes in relation to PD risk in the large prospective cohort of the National Institutes of Health (NIH)-AARP Diet and Health Study.
RESEARCH DESIGN AND METHODS
Study participants
The prospective NIH-AARP Diet and Health study was established in 1995 and 1996 by the National Cancer Institute to investigate etiologies of cancer and other age-related chronic diseases (12). AARP, formerly known as American Association of Retired Persons, is a nonprofit and membership-based interest organization for U.S. adults aged 50 years or older. AARP currently has over 40 million members across the U.S. and thus represents a wide range of older U.S. adults. The NIH-AARP Diet and Health cohort is composed of 566,401 AARP members (ages 50–71 years) from six U.S. states (California, Florida, Pennsylvania, New Jersey, North Carolina, and Louisiana) and two metropolitan areas (Atlanta, GA and Detroit, MI), and all participants completed a comprehensive survey on diet and lifestyle at baseline (12). Between 2004 and 2006, a follow-up questionnaire was mailed to surviving participants of the cohort to update lifestyle information and to ascertain the occurrence of major chronic diseases, including PD. A total of 187,499 (55.2%) men and 130,761 (57.6%) women of the baseline participants answered the follow-up survey and were thus eligible for the current study. Of those not in the follow-up survey (n = 248,141), 58,192 (23.5%) were deceased and 189,949 (76.6%) were nonresponders. On the follow-up questionnaire, participants were asked to report whether they had been diagnosed by a doctor as having PD during the following periods: before 1985, 1985–1994, 1995–1999, or 2000 to present. Of the 2,432 self-reported PD patients, we excluded 459 who were diagnosed before 1995 to eliminate cases prevalent at the time of cohort enrollment. Furthermore, we excluded 293 patients whose diagnosis was denied either by the patients themselves or by their treating physicians in the diagnostic validation process as described below and 115 patients with missing information on baseline diabetes status. Of those who did not report a PD diagnosis, we excluded 28,731 participants with missing data on diabetes (n = 20,607) or PD status (n = 8,124). Therefore the final analyses included 1,565 self-reported PD participants diagnosed in or after 1995 and 287,097 participants without PD.
We conducted diagnostic validations for surviving self-reported PD patients, described in detail previously (13). Briefly, with permission from patients, we asked their treating physicians (mostly neurologists) to complete a diagnostic questionnaire and to send us a copy of relevant medical records that were subsequently reviewed by a movement disorder specialist (X.H.). A case was confirmed if the diagnosis was considered clinically definitive or probable by the treating neurologist or if the medical record included a final PD diagnosis or evidence of two or more cardinal signs with one being rest tremor or bradykinesia, a progressive course, responsiveness to dopaminergic treatments, and absence of features that suggest an alternative diagnosis. To date, we have received a total of 1,069 responses from the treating physicians and 940 (87.9%) confirmed the diagnosis. The average age at diagnosis of confirmed PD patients was 66.7 ± 7.3 years.
Exposure assessment
At baseline in 1995 and 1996, participants were asked whether they had ever been told by a doctor that they had diabetes. We did not differentiate type 2 from type 1 diabetes. However, because ∼90–95% of all diagnosed diabetes in adults is type 2 diabetes (14), we believe most patients in our cohort have type 2 diabetes. A similar question was also asked in the follow-up questionnaire along with the calendar year of diabetes diagnosis: before 1985, 1985–1994, 1995–1999, and 2000 to present. With these two questions, we defined the analytic variables for the presence of diabetes and the duration of diabetes at baseline. Because the baseline survey was conducted in 1995 and 1996, diabetic patients who reported diagnoses before 1985 were considered as having ≥10 years of diabetes before baseline and those diagnosed between 1985 and 1994 as having <10 years of diabetes at baseline. The analysis on diabetes duration included fewer participants because 922 eligible diabetic patients did not report the year of diagnosis on the follow-up questionnaire. In addition to diabetes status, the baseline survey also asked participants to report their age, sex, race, education, cigarette smoking, body weight and height, physical activity, general health status, diagnosis of stroke or heart disease, and cancers (12). BMI was calculated as weight in kilograms divided by height squared in meters. For physical activity, we asked how often they did activities at work or home that lasted at least 20 min or increased breath or heart rate or caused sweat, and six categorical answers were allowed (never, rarely, times/week: <1, 1–2, 3–4, and ≥5).
Statistical analysis
We derived multivariate odds ratios (OR) and 95% CI from unconditional logistic regression models, adjusting for baseline age (in 5-year groups), sex, race (white vs. nonwhite), education (<8 years, 8–11 years, 12 years or completed high school, post-high school or some college, and college and post graduate), smoking status (never, past smokers [years since last smoking: ≥35, 30–34, 20–29, 10–19, 1–9], and current smokers [number of cigarettes per day: 1–10, 11–20, >20]), cups of coffee per day (none, <1, 1, 2–3, >3), BMI (kg/m2 <22.5, 22.5–24.9, 25.0–27.4, 27.5–29.9, and ≥30.0), and physical activity (never or rarely, times/week: <1, 1–2, 3–4, 5). Analysis was first conducted with all PD participants diagnosed since 1995 and then separately for PD participants diagnosed between 1995 and 1999 and diagnosed after 2000. For the later group, PD diagnoses were made at least 4 or 5 years after baseline ascertainment of diabetes and therefore reverse causality was less likely.
Certain types of cancers have been linked to both PD and diabetes; furthermore, because diabetic patients often have vascular changes that might complicate PD diagnosis (e.g., vascular parkinsonism) and participants with these chronic diseases or poorer health at baseline might have frequent clinical visits and thus introduce identification bias, we conducted sensitivity analyses by excluding participants with stroke, heart disease, cancers, or poor or fair health from the analysis. Finally, we conducted subgroup analyses according to selected key factors that are known to be associated with PD risk, including baseline age (<65 years and ≥65 years), sex (men and women), smoking status (never and ever [current and past smokers]), and coffee drinking (<2 cups per day and ≥2 cups per day). The statistical significance for linear trend of PD with diabetes duration was tested by assigning a value to each duration category (5 for <10 years and 12 for ≥10 years) and including it as a continuous variable in the regression model. All statistical analyses were conducted using SAS software (Version 9.1, Cary, NC), and the significance tests were two-tailed with α = 0.05.
RESULTS
Table 1 shows population characteristics according to baseline diabetes status. When compared with participants without diabetes, diabetic patients were older and were more likely to be men, nonwhite, and past smokers. They were also more likely to report lower education; higher BMI; less exercise; poorer health status; and a history of heart disease, stroke, or cancers.
Table 1.
Population characteristics according to diabetes status at baseline*
| Variable | Nondiabetes | Diabetes |
|---|---|---|
| N | 267,051 | 21,611 |
| Age (years) | 61.4 ± 5.4 | 62.1 ± 5.1 |
| Male (%) | 57.5 | 66.6 |
| Race (%) | ||
| White | 93.2 | 87.3 |
| Nonwhite | 5.9 | 11.3 |
| Education (%) | ||
| <8 years | 4.0 | 6.5 |
| 8–11 years | 17.1 | 20.4 |
| 12 years or completed high school | 9.4 | 10.2 |
| Post-high school or some college | 22.7 | 23.9 |
| College and postgraduate | 44.6 | 36.2 |
| Smokers (%) | ||
| Never | 38.6 | 34.1 |
| Past | 50.5 | 56.7 |
| Current | 10.0 | 7.9 |
| Coffee consumption (%) | ||
| None | 10.3 | 10.6 |
| <1 cup/day | 16.2 | 17.6 |
| 1 cup/day | 16.1 | 17.4 |
| 2 to 3 cups/day | 41.4 | 39.2 |
| >3 cups/day | 15.5 | 14.6 |
| BMI (kg/m2) | 26.7 ± 4.8 | 29.8 ± 5.8 |
| Physical activity (%) | ||
| Never or rarely | 15.1 | 22.1 |
| <1 time/week | 13.5 | 13.6 |
| 1 to 2 times/week | 22.1 | 20.8 |
| 3 to 4 times/week | 28.3 | 25.0 |
| ≥5 times/week | 20.3 | 17.4 |
| Health status (%) | ||
| Excellent/very good | 59.0 | 22.8 |
| Good | 32.5 | 47.4 |
| Fair or poor | 7.8 | 28.6 |
| Heart disease (%) | ||
| No | 88.1 | 75.6 |
| Yes | 11.6 | 24.0 |
| Stroke (%) | ||
| No | 98.2 | 96.1 |
| Yes | 1.4 | 3.4 |
| Cancers | ||
| No | 88.6 | 86.2 |
| Yes | 7.9 | 9.1 |
*Data are means ± SD for continuous variables and proportions for categorical variables. Participants without diabetes (4,923 or 1.8%) and participants with diabetes (476 or 2.2%) had missing data on BMI; for categorical variables, percentage may not add up to 100% because of missing values.
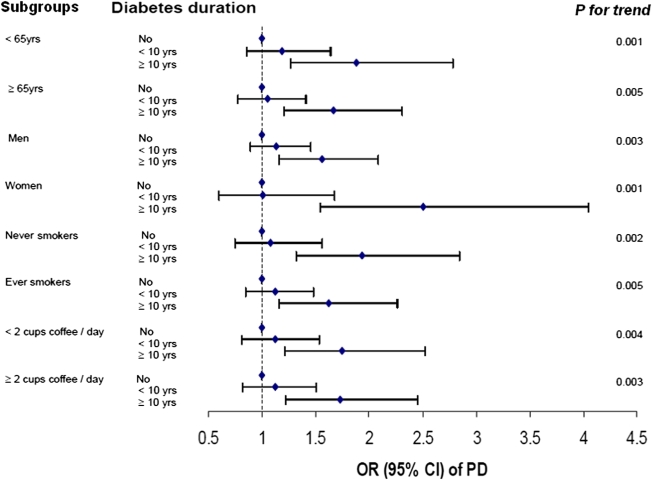
The primary analysis included a total of 1,565 PD patients diagnosed in or after 1995. After adjustment for potential confounders, baseline diabetes was associated with a 41% (OR 1.41 [95% CI 1.20–1.66]) higher risk of PD (Table 2). The risk elevation was higher among individuals who had diabetes for more than 10 years at baseline (1.75 [1.36–2.25]) than among diabetic patients with <10 years of the disease at baseline (1.11 [0.89–1.38]). Excluding participants with heart disease, stroke, cancers or fair/poor health status attenuated the slightly higher risk for patients with <10 years of diabetes but made little difference to patients with more than 10 years of diabetes at baseline. Generally, similar results were obtained when we further analyzed the data according to year of PD diagnosis (1995–1999 vs. after 2000). Finally, the association between diabetes and PD was observed in each subgroup analysis according to age, sex, smoking status, and coffee consumption (Fig. 1).
Table 2.
ORs and 95% CI of PD according to baseline diabetes status and disease duration*
| Diabetes |
Diabetes duration (years) |
|||||
|---|---|---|---|---|---|---|
| No | Yes | None | <10 | ≥10 | P for trend | |
| All Parkinson diagnoses since 1995 | ||||||
| All participants | ||||||
| Number of PD patients | 1,393 | 172 | 1,393 | 88 | 66 | |
| OR (95% CI) | 1.0 | 1.41 (1.20–1.66) | 1.0 | 1.11 (0.89–1.38) | 1.75 (1.36–2.25) | <0.0001 |
| Excluding participants with stroke, heart disease, cancers, and poor/fair health | ||||||
| Number of PD patients | 952 | 79 | 952 | 40 | 30 | |
| OR (95% CI) | 1.0 | 1.34 (1.06–1.69) | 1.0 | 1.00 (0.73–1.38) | 1.80 (1.25–2.60) | 0.008 |
| Parkinson diagnoses between 1995 and 1999 | ||||||
| All participants | ||||||
| Number of PD patients | 465 | 52 | 465 | 25 | 23 | |
| OR (95% CI) | 1.0 | 1.31 (0.97–1.75) | 1.0 | 0.97 (0.64–1.45) | 1.85 (1.21–2.83) | 0.02 |
| Excluding participants with stroke, heart disease, cancers, and poor/fair health | ||||||
| Number of PD patients | 328 | 27 | 328 | 12 | 12 | |
| OR (95% CI) | 1.0 | 1.34 (0.90–2.00) | 1.0 | 0.87 (0.49–1.56) | 2.12 (1.18–3.78) | 0.05 |
| Parkinson diagnosis after 2000 | ||||||
| All participants | ||||||
| Number of PD patients | 928 | 120 | 928 | 63 | 43 | |
| OR (95% CI) | 1.0 | 1.46 (1.20–1.77) | 1.0 | 1.18 (0.91–1.53) | 1.70 (1.25–2.31) | 0.001 |
| Excluding participants with stroke, heart disease, cancers, and poor/fair health | ||||||
| Number of PD patients | 624 | 52 | 624 | 28 | 18 | |
| OR (95% CI) | 1.0 | 1.33 (1.00–1.78) | 1.0 | 1.07 (0.73–1.56) | 1.64 (1.02–2.63) | 0.06 |
*Adjusting for age, sex, race, education, smoking, coffee, BMI, and physical activity. The numbers of participants without PD for the overall analyses were 265,658 (no diabetes), 21,439 (diabetes), 14,280 (diabetes <10 years), and 6,255 (diabetes duration ≥10 years). For the analysis excluding stroke, heart disease, cancers, and poor/fair health, the numbers were 203,299, 11,393, 7,878, and 3,030, respectively.
Figure 1.
ORs and 95% CIs of PD according to diabetes duration in subgroup analyses. Potential confounders include age, sex, race, education, smoking, coffee, BMI, and physical activity; detailed categories of the stratifying variable were adjusted when appropriate. (A high-quality color representation of this figure is available in the online issue.)
CONCLUSIONS
In this large population of older adults in the U.S., we found that diabetes was associated with a modest increase of PD risk. Further analysis suggested that the higher risk was primarily limited to study participants who had had diabetes for more than 10 years before the baseline survey. Multivariate and stratified analyses suggest that these results were not confounded or modified by known or suspected PD risk factors, nor could they be accounted for by poorer baseline health status or the presence of vascular diseases or cancers as shown in the sensitivity analyses.
Over the past decade, diabetes has been reported to be associated with increased risk of dementia (3) and Alzheimer’s disease (4), suggesting a potential role of diabetes or insulin dysregulation in neurodegeneration. Several lines of evidence also indicate a link between diabetes and PD. Insulin receptors are expressed in the substantia nigra (15). The dopamine agonist bromocriptine improves glycemic control (16) and was recently approved for adjunctive treatment of diabetes. Conversely, the insulin sensitizer rosiglitazone protects dopaminergic neurons in animal models of PD (17). From an etiologic perspective, it is also important to point out that both diabetes and PD are age-related chronic diseases and some pathogenic processes may underlie both conditions. For example, systemic chronic inflammation, which increases the risk of diabetes, was also found to be associated with higher risk of PD (18,19). Furthermore, oxidative stress and mitochondria abnormalities have been implicated in both diseases (20–22).
A few epidemiological studies have investigated the association between diabetes and PD. Several case-control studies (6–8,23), but not all (5,24), reported that PD patients were less likely to report diabetes. A closer examination of these studies revealed further uncertainties. For example, the diabetes-PD association did not persist in the multivariate analysis of one study (7) and another study showed different results across subgroups (6). Despite these uncertainties, it has been hypothesized that the lower prevalence of diabetes among PD patients is a result of impaired autonomic functions and fewer cardiovascular risk factors among PD patients (7,8). However, alternative explanations such as chance or bias could not be excluded since these studies were often based on small sample sizes, prevalent cases of patients with PD, and retrospective exposure assessment. For example, a spurious inverse association between diabetes and PD could arise in a case-control study with prevalent cases if PD adversely affects the survival of diabetic patients or vice versa. Finally, case-control studies were not ideal to address the temporal relationships between diabetes and PD because of the use of prevalent cases and sometimes unspecified time window of exposure assessment.
Unlike case-control studies, prospective studies collect exposure data before outcome identification and therefore are less prone to recall and selection biases. To the best of our knowledge, three prospective studies to date have examined diabetes in relation to future risk of PD (9–11). In contrast with case-control studies, none of the prospective studies found lower PD risk among diabetic patients. The Health Professionals Follow-up and Nurses Health Studies reported similar risk of PD between individuals with and without diabetes (RR = 1.04) (11). On the other hand, the Finnish study reported an 83% higher risk of PD among diabetic patients than participants without PD (10). Cases in this study were identified from the Finnish nationwide drug register without further diagnostic validation and therefore some caution is needed in interpreting the result. The third study was conducted in the Physicians Health Study and was the only one that took into account the duration of diabetes in the analyses (9). This study also reported a higher PD risk (RR = 1.34) among diabetic patients, but further analysis showed that the increment was limited to diabetic patients with <10 years of the disease. Therefore, the authors attributed this finding to potential ascertainment bias, reverse causality, or common mechanisms that underlie both diabetes and PD (9).
In addition to some design differences, all previous studies had fewer than 60 exposed cases (PD participants with diabetes), which may have contributed to the heterogeneous results. In comparison, the current study was substantially larger with 172 PD participants with diabetes and 1,393 PD participants without diabetes. Furthermore, in data analysis, we controlled for or stratified by known or suspected PD risk factors to examine the robustness of our result, and similar results were found across subgroups. Like the Finnish and the Physicians Health studies, we observed a modestly higher risk of PD among individuals with baseline diabetes. In contrast with the Physicians Health Study, we found the higher risk was largely limited to diabetic patients who had been diagnosed more than 10 years before baseline. This association was evident for both PD participants diagnosed between 1995 and 1999 and participants diagnosed after 2000; PD diagnoses in the latter group were at least 4 to 5 years after the report of baseline diabetes status. Finally, neither vascular conditions or cancers nor poorer health status at baseline accounted for the association of diabetes with PD risk. Taken together, our results are consistent with the hypothesis that diabetic patients had higher risk of PD, which could not be explained by reverse causality.
The higher PD risk among diabetic patients could potentially be explained by two intertwined possibilities. It is possible that common pathogenic processes such as chronic inflammation or oxidative stress may first lead to diabetes and then years later to a higher risk of PD. Alternatively, one can speculate that diabetes and insulin dysregulation or other aspects of diabetes may themselves increase the risk of PD. In either case, the evidence to date is indirect and the relevance of these speculations to our observed diabetes-PD association needs to be evaluated in future studies.
Our study has several limitations. In such a large population, we had to rely on self-reports to identify PD participants cost effectively. This might have led to diagnostic and reporting errors, particularly underreports since some PD patients might not report their diagnoses on the follow-up questionnaire. However, we were able to confirm ∼88% of diagnoses among self-reported patients whose medical information was available. Furthermore, we excluded from analyses patients in the case group with erroneous reports identified in the validation study. Diabetes was also self-identified; however, previous studies showed satisfactory agreement (κ = 0.76) between self-reports of diabetes and medical records despite a modest sensitivity (66%) (25). The lack of annual glucose tolerance screening in the cohort also resulted in underreports and under-diagnosis of diabetes. Nevertheless, because diabetes status was assessed before PD case ascertainment, inaccurate reporting of diabetes should be nondifferential and thus might have underestimated the true association between diabetes and PD. Another limitation on diabetes assessment was the lack of information on diabetes complications and treatments. We therefore could not evaluate the possibility that diabetes medications and treatments or diabetes complications might have contributed to higher risk of PD, particularly among long-term diabetic patients. It was also possible that diabetic patients might have more frequent physician visits than individuals without diabetes. Although we conducted sensitivity analysis by excluding participants with poor/fair health or several chronic diseases, we could not exclude the possibility of identification bias from this source. Finally, the current analyses were limited to participants of the follow-up survey. A spurious association might be induced if more diabetic patients with PD survived and participated in the follow-up survey than diabetic patients without PD.
In conclusion, this study showed higher PD risk among diabetic patients in a large cohort of older adults. The nature of this association should be evaluated in future studies.
Acknowledgments
This study was supported by the intramural research program of the NIH, the National Institute of Environmental Health Sciences (Z01-ES-101986), and the National Cancer Institute (Z01-CP-010196-02).
No potential conflicts of interest relevant to this article were reported.
Q.X. researched data and wrote the manuscript. Y.P., X.H., A.H., A.B., and A.S. contributed to data collection and discussion and reviewed and edited the manuscript. H.C. developed the study concept, researched data, wrote the manuscript, contributed to discussion, and reviewed and edited the manuscript.
The authors thank the participants of the NIH-AARP Diet and Health study for their important contributions and Dr. Xuguang Guo (Westat) for analytical help.
Footnotes
Q.X. is currently affiliated with the Department of Epidemiology and Health Statistics, Institute of Basic Medical Sciences, Chinese Academy of Medical Sciences, Peking Union Medical College, Beijing, China.
References
- 1.Cowie CC, Rust KF, Byrd-Holt DD, et al. Prevalence of diabetes and impaired fasting glucose in adults in the U.S. population: National Health And Nutrition Examination Survey 1999-2002. Diabetes Care 2006;29:1263–1268 [DOI] [PubMed] [Google Scholar]
- 2.American Diabetes Association American Academy of Neurology Consensus statement: report and recommendations of the San Antonio conference on diabetic neuropathy. Diabetes Care 1988;11:592–597 [DOI] [PubMed] [Google Scholar]
- 3.Peila R, Rodriguez BL, Launer LJ, Honolulu-Asia Aging Study Type 2 diabetes, APOE gene, and the risk for dementia and related pathologies: The Honolulu-Asia Aging Study. Diabetes 2002;51:1256–1262 [DOI] [PubMed] [Google Scholar]
- 4.Arvanitakis Z, Wilson RS, Bienias JL, Evans DA, Bennett DA. Diabetes mellitus and risk of Alzheimer disease and decline in cognitive function. Arch Neurol 2004;61:661–666 [DOI] [PubMed] [Google Scholar]
- 5.Becker C, Brobert GP, Johansson S, Jick SS, Meier CR. Diabetes in patients with idiopathic Parkinson’s disease. Diabetes Care 2008;31:1808–1812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Powers KM, Smith-Weller T, Franklin GM, Longstreth WT, Jr, Swanson PD, Checkoway H. Diabetes, smoking, and other medical conditions in relation to Parkinson’s disease risk. Parkinsonism Relat Disord 2006;12:185–189 [DOI] [PubMed] [Google Scholar]
- 7.Scigliano G, Musicco M, Soliveri P, Piccolo I, Ronchetti G, Girotti F. Reduced risk factors for vascular disorders in Parkinson disease patients: a case-control study. Stroke 2006;37:1184–1188 [DOI] [PubMed] [Google Scholar]
- 8.D’Amelio M, Ragonese P, Callari G, et al. Diabetes preceding Parkinson’s disease onset. A case-control study. Parkinsonism Relat Disord 2009;15:660–664 [DOI] [PubMed] [Google Scholar]
- 9.Driver JA, Smith A, Buring JE, Gaziano JM, Kurth T, Logroscino G. Prospective cohort study of type 2 diabetes and the risk of Parkinson’s disease. Diabetes Care 2008;31:2003–2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hu G, Jousilahti P, Bidel S, Antikainen R, Tuomilehto J. Type 2 diabetes and the risk of Parkinson’s disease. Diabetes Care 2007;30:842–847 [DOI] [PubMed] [Google Scholar]
- 11.Simon KC, Chen H, Schwarzschild M, Ascherio A. Hypertension, hypercholesterolemia, diabetes, and risk of Parkinson disease. Neurology 2007;69:1688–1695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schatzkin A, Subar AF, Thompson FE, et al. Design and serendipity in establishing a large cohort with wide dietary intake distributions: the National Institutes of Health-American Association of Retired Persons Diet and Health Study. Am J Epidemiol 2001;154:1119–1125 [DOI] [PubMed] [Google Scholar]
- 13.Chen H, Huang X, Guo X, et al. Smoking duration, intensity, and risk of Parkinson disease. Neurology 2010;74:878–884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Centers for Disease Control and Prevention National Diabetes Fact Sheet: General Information and National Estimates on Diabetes in the United States, 2007. Atlanta, GA, U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, 2008 [Google Scholar]
- 15.Unger JW, Livingston JN, Moss AM. Insulin receptors in the central nervous system: localization, signalling mechanisms and functional aspects. Prog Neurobiol 1991;36:343–362 [DOI] [PubMed] [Google Scholar]
- 16.Scranton R, Cincotta A. Bromocriptine—unique formulation of a dopamine agonist for the treatment of type 2 diabetes. Expert Opin Pharmacother 2010;11:269–279 [DOI] [PubMed] [Google Scholar]
- 17.Schintu N, Frau L, Ibba M, et al. PPAR-gamma-mediated neuroprotection in a chronic mouse model of Parkinson’s disease. Eur J Neurosci 2009;29:954–963 [DOI] [PubMed] [Google Scholar]
- 18.Pradhan A. Obesity, metabolic syndrome, and type 2 diabetes: inflammatory basis of glucose metabolic disorders. Nutr Rev 2007;65:S152–S156 [DOI] [PubMed] [Google Scholar]
- 19.Chen H, O’Reilly EJ, Schwarzschild MA, Ascherio A. Peripheral inflammatory biomarkers and risk of Parkinson’s disease. Am J Epidemiol 2008;167:90–95 [DOI] [PubMed] [Google Scholar]
- 20.Sandyk R. The relationship between diabetes mellitus and Parkinson’s disease. Int J Neurosci 1993;69:125–130 [DOI] [PubMed] [Google Scholar]
- 21.Friederich M, Hansell P, Palm F. Diabetes, oxidative stress, nitric oxide and mitochondria function. Curr Diabetes Rev 2009;5:120–144 [DOI] [PubMed] [Google Scholar]
- 22.Henchcliffe C, Beal MF. Mitochondrial biology and oxidative stress in Parkinson disease pathogenesis. Nat Clin Pract Neurol 2008;4:600–609 [DOI] [PubMed] [Google Scholar]
- 23.Miyake Y, Tanaka K, Fukushima W, et al. Fukuoka Kinki Parkinson’s Disease Study Group Case-control study of risk of Parkinson’s disease in relation to hypertension, hypercholesterolemia, and diabetes in Japan. J Neurol Sci 2010;293:82–86 [DOI] [PubMed] [Google Scholar]
- 24.Nataraj A, Rajput AH. Parkinson’s disease, stroke, and related epidemiology. Mov Disord 2005;20:1476–1480 [DOI] [PubMed] [Google Scholar]
- 25.Okura Y, Urban LH, Mahoney DW, Jacobsen SJ, Rodeheffer RJ. Agreement between selfreport questionnaires and medical record data was substantial for diabetes, hypertension, myocardial infarction and stroke but not for heart failure. J Clin Epidemiol 2004;57:1096–1103 [DOI] [PubMed] [Google Scholar]