Abstract
OBJECTIVE
To assess complication prevalence and identify protective factors in patients with diabetes duration of ≥50 years. Characterization of a complication-free subgroup in this cohort would suggest that some individuals are protected from diabetes complications and allow identification of endogenous protective factors.
RESEARCH DESIGN AND METHODS
Cross-sectional, observational study of 351 U.S. residents who have survived with type 1 diabetes for ≥50 years (Medalists). Retinopathy, nephropathy, neuropathy, and cardiovascular disease were assessed in relation to HbA1c, lipids, and advanced glycation end products (AGEs). Retrospective chart review provided longitudinal ophthalmic data for a subgroup.
RESULTS
A high proportion of Medalists remain free from proliferative diabetic retinopathy (PDR) (42.6%), nephropathy (86.9%), neuropathy (39.4%), or cardiovascular disease (51.5%). Current and longitudinal (the past 15 years) glycemic control were unrelated to complications. Subjects with high plasma carboxyethyl-lysine and pentosidine were 7.2-fold more likely to have any complication. Of Medalists without PDR, 96% with no retinopathy progression over the first 17 years of follow-up did not experience retinopathy worsening thereafter.
CONCLUSIONS
The Medalist population is likely enriched for protective factors against complications. These factors might prove useful to the general population with diabetes if they can be used to induce protection against long-term complications. Specific AGE combinations were strongly associated with complications, indicating a link between AGE formation or processing with development of diabetic vasculopathy.
The rising prevalence of diabetes and its vascular complications is a global public health issue (1). Studies evaluating diabetic patients over 20–30 years have identified complication risk factors including worse glycemic control, longer diabetes duration, hypertension, and hyperlipidemia (2–10). However, other than glycemic and systemic control, no clinical or biochemical factors have conclusively been shown to protect against long-term complications.
The Golden Year Study provided a description of U.K. patients with diabetes for ≥50 years (11); however this study described nephropathy but did not characterize other complications or their relationship to glycemic control. A more recent survey of U.S. patients with type 1 diabetes for ≥50 years (Medalists) suggested that over 40% remain free from multiple complications; however, this was self-reported data only (12).
This study performed a cross-sectional characterization of all four vascular complications in a large Medalist cohort. In addition, we evaluated longitudinal ophthalmic outcomes in relation to clinical and biochemical markers including glycemic control, oxidative stress, inflammatory markers, lipoprotein subpopulations, and advanced glycation end products (AGEs). Our goal was to establish whether or not a substantial proportion of Medalists is protected from advanced diabetic vascular complications and, if so, to explore potential correlates of protection within this unique group.
RESEARCH DESIGN AND METHODS
Design overview
We performed a cross-sectional, observational study of U.S. residents with at least 50 years of insulin-dependent diabetes. Longitudinal data on retinopathy progression was obtained via chart review in patients followed at the Joslin Diabetes Center eye clinic (Boston, MA). The Joslin Institutional Review Board approved the study.
Participants and data sources/measurement
By 31 December 2007, 443 patients who received Joslin 50-year medals from 1997 to 2007 had been contacted, and 351 Medalists had completed the single study visit. Reasons for nonparticipation were death (n = 32), inability to travel/poor health (n = 48), lack of interest (n = 27), and/or visit duration (n = 4). Most Medalists (77.8%, n = 277) received routine care outside of the Joslin clinic. Medalists came from 42 states (Supplementary Table 1), most commonly Massachusetts (16.0%), New York (8.6%), Florida (7.1%), and California (6.8%). All subjects were evaluated at the Joslin clinic with medical history, clinical and ophthalmic exam, and blood and urine collection.
HbA1c was determined by high-performance liquid chromatography (Tosoh G7 and 2.2, Tokyo, Japan); C-reactive protein measured by particle-enhanced immunonephelometry (BN ProSpec Analyzer; Dade Behring, Newark, DE); lipid profiles determined by standard methods (kits from Roche Diagnostics, Indianapolis, IN; Denka Seiken, Tokyo, Japan; AsahiKasei, Tokyo, Japan); total plasma apolipoprotein A-I concentrations measured by turbidimetric immunoassay (Wako Diagnostics, Richmond, VA); and apolipoprotein A-I containing HDL subpopulations determined as previously described (13). C-peptide was measured by radioimmunoassay (Diagnostic Systems Laboratory, Webster, TX). Total RNA was isolated from peripheral blood mononuclear cells using TRIReagent (Molecular Research Center, Inc., Cincinnati, OH). Standard quantitative real-time PCR (qPCR) was performed (TaqMan Universal PCR Master Mix kits, Applied Biosystems) to assess oxidative stress marker mRNA levels.
Fructose-lysine (measured as furosine), carboxymethyl-lysine (CML), and carboxyethyl-lysine (CEL) were determined by gas chromatography mass spectrometry using isotope dilution technique and an adaptation of Sell’s protocol (14). Upon hydrolysis, the samples were dried, spiked with standards of CML-d4 and CEL-d3, cleaned over LC-18 sep-pack column, and derivatized into trifluoroacetic anhydrides. Measured masses were CML (m/z = 392), CML-d4 (m/z = 396), CEL (m/z = 79), CEL d-3 (m/z = 382), and furosine (m/z = 110) quantified using CML-d4 standard. Pentosidine was determined by high-performance liquid chromatography (15).
Retinopathy
Early Treatment Diabetic Retinopathy Study (ETDRS)-protocol seven standard field stereoscopic fundus photographs were graded for clinical severity of diabetic retinopathy with adjudication of discrepancies. Patients with no-mild nonproliferative diabetic retinopathy in the more severely affected eye were compared with patients with retinal neovascularization or scatter laser photocoagulation scars indicative of proliferative diabetic retinopathy (PDR) in either eye. Dates of diabetic retinopathy progression were obtained for 97 Medalists followed at the Joslin clinic.
Nephropathy
Renal status was based on the average of urinary albumin-to-creatinine ratios (ACRs) from two spot urine samples. Subjects without microalbuminuria (ACR <70 μg/mg creatinine) were compared with those with microalbuminuria (ACR ≥70 μg/mg creatinine). Cystatin C levels were assessed via nephelometry on a BN ProSpec Analyzer (Dade Behring, Newark, DE).
Neuropathy
The Michigan Neuropathy Screening Instrument physical assessment portion (16) was used to evaluate foot appearance, vibration sensation, muscle stretch reflexes, and monofilament testing. Scores >2 on the Michigan Neuropathy Screening Instrument were considered positive for neuropathy.
Cardiovascular
Medalists reporting a history of coronary artery disease, angina, heart attack, or with a prior cardiac/leg angioplasty or bypass graft surgery were deemed positive for cardiovascular disease.
Statistical analysis
SAS (v. 9.1) was used to perform Wilcoxon rank sum analysis for bivariate analyses involving continuous variables and χ2 analysis to examine categorical variables. Multivariable logistic regression was performed to adjust for possible confounding. Cox proportional hazards analysis was used to examine the relationship of variables to diabetic retinopathy worsening over time. Log-rank tests confirmed significant differences in survival distributions between groups. P values <0.05 were considered statistically significant.
RESULTS
Clinical characteristics
Medalists (n = 351) had a mean ± SD age of 67.5 ± 7.5 years and diabetes duration of 56.5 ± 5.7 years. Characteristics (Table 1) were consistent with type 1 diabetes: mean age at diagnosis 11.0 ± 6.3 years, BMI 26.0 ± 5.1 kg/m2, and HLA DR3 or DR4 risk alleles present in 90.8% of subjects. HbA1c levels were 7.3 ± 1.0% with HDL levels of 1.62 ± 0.51 mmol/L and LDL levels of 2.22 ± 0.63 mmol/L. Six percent of subjects demonstrated random C-peptide >0.13 nmol/L.
Table 1.
Baseline characteristics of 50-year Medalist cohort
| 50-year Medalist subjects: baseline characteristics (N = 351) | |
|---|---|
| Characteristic | |
| Age (years) | 67.5 ± 7.5 [51.0–89.3] (351) |
| Age at diagnosis (years) | 11.0 ± 6.3 [0–31] (351) |
| Duration of diabetes (years) | 56.5 ± 5.7 [50–80] (351) |
| Sex: male | 47.0 (165) |
| Smoking history | 41.1 (138) |
| History of hypertension | 56.1 (185) |
| ACE inhibitor use | 40.1 (140) |
| Lipid-lowering medication | 64.5 (220) |
| Weight (kg) | 72.8 ± 15.0 [41.3–131.5] (347) |
| Height (m) | 1.67 ± 0.10 [1.20–1.93] (347) |
| BMI (kg/m2) | 26.0 ± 5.1 [15.9–58.0] (347) |
| Insulin dose (units/kg) | 0.46 ± 0.17 [0.01–1.13] (334) |
| sBP (mmHg) | 134.5 ± 19.7 [90–190] (271) |
| dBP (mmHg) | 60.9 ± 8.3 [37–83] (271) |
| Mean arterial pressure (mmHg) | 85.4 ± 10.2 [59.3–111.3] (271) |
| Current HbA1c (%) | 7.3 ± 1.0 [5.0–14.0] (342) |
| Longitudinal HbA1c (%) | 7.7 ± 1.0 [5.7–10.6] (73) |
| Total cholesterol (mg/dL) | 163.9 ± 33.9 [76–299] (313) |
| HDL (mg/dL) | 62.4 ± 19.5 [28–142] (313) |
| LDL (mg/dL) | 85.9 ± 24.2 [14–187] (313) |
| Triglycerides (mg/dL) | 78.3 ± 46.1 [17–391] (313) |
| C-peptide >0.4 ng/mL | 6.0 (19) |
Data are mean ± SD [range] (N) or % (N).
Complications
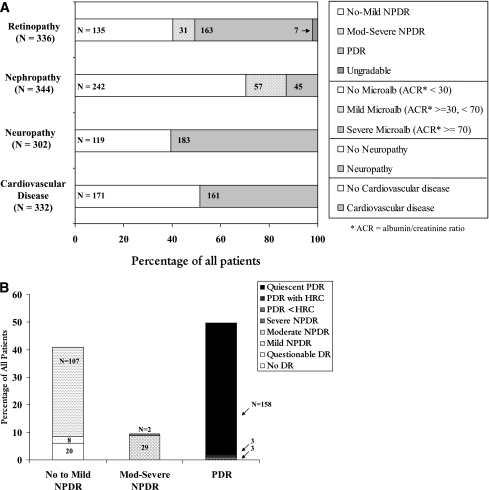
High proportions of Medalists remained free from PDR (49.4%), nephropathy (86.9%), neuropathy (39.4%), or cardiovascular disease (51.5%) (Fig. 1A). Of 255 subjects for whom all three microvascular complications were characterized, 21.2% were free of PDR, nephropathy, and neuropathy. A bimodal diabetic retinopathy distribution was present with 40.2% having no-mild nonproliferative diabetic retinopathy and 48.5% having PDR (Fig. 1B). In contrast, 86.9% of Medalists had no nephropathy (ACR <70 μg/mg creatinine) with only 4.7% exhibiting proteinuria (ACR ≥300 μg/mg creatinine).
Figure 1.
A: Prevalence of micro- and macrovascular diabetes complications in the 50-year Medalist cohort. mod, moderate; microalb, microalbuminuria; NPDR, nonproliferative diabetic retinopathy. B: Bimodal distribution of diabetic retinopathy severity in the Medalist cohort. DR, diabetic retinopathy; HRC, high-risk characteristics; Mod, moderate; NPDR, nonproliferative diabetic retinopathy.
Factors associated with complications after 50 years of diabetes differ from those in shorter duration diabetes
Factors associated with individual complications were not necessarily consistent with those established for shorter duration diabetes (Supplementary Table 2). No significant relationship was found between glycemic control and any complication in the Medalist cohort.
Longitudinal glycemic control (HbA1c from 1993 onward) was assessed for 73 Medalists (20.1%) followed at the Joslin clinic. The average number of HbA1c measurements was 20.4. Mean HbA1c was 7.7%. This subgroup did not differ from the overall cohort on age, age of diagnosis, duration of diabetes, blood pressure, lipid profile, or complication status. Current HbA1c was highly correlated with longitudinal HbA1c (r = 0.82, P < 0.001). Consistent with the lack of association between current HbA1c and complications, no significant relationship was found between longitudinal HbA1c and complications.
Systolic (sBP) and diastolic (dBP) blood pressure, also typically associated with complications in subjects with shorter duration disease, did not correlate with microvascular complications in the Medalists. Longer duration of diabetes was associated with increased nephropathy (P = 0.009), neuropathy (P = 0.009), and cardiovascular disease (P = 0.03), but not retinopathy. Cardiovascular disease was more prevalent in subjects with lower sBP (P = 0.01) and mean arterial pressure (P = 0.004), lower heart rate (P = 0.002), and lower total cholesterol (P < 0.001) or LDL (P < 0.001), likely because of high percentages of these subjects on hypertensive and/or lipid-lowering medications.
Subjects with nephropathy were more likely to report a history of smoking (P < 0.001). Neuropathy was related to older current age (P < 0.001), increased height (P = 0.003), and heavier weight (P = 0.03). Macrovascular complications were related to older current age (P = 0.02).
Lipoprotein subpopulations were assessed (Supplementary Table 2). Higher rates of cardiovascular disease were related to elevations in lipoprotein(a) (P = 0.04) and decreases in HDL (P < 0.001). Higher levels of pre-β2 HDL were associated with each complication, but these elevations were statistically significant only for nephropathy (P = 0.02) and cardiovascular disease (P = 0.006).
Inflammatory marker evaluation revealed that higher levels of C-reactive protein were observed for cardiovascular disease (P < 0.001), and significant elevations of soluble vascular cell adhesion molecule-1 were present in those with neuropathy (P = 0.02) and nephropathy (P = 0.05) but not retinopathy. Other inflammatory markers did not differ between subjects with and without complications. Similarly, oxidative stress biomarkers including urinary 8-isoprostane and mRNA levels of superoxide dismutase, heme oxygenase 1, catalase, and glutamate-cysteine ligase catalytic subunit were not associated with complication status.
Long-term data on retinopathy progression and stabilization
Factors associated with prolonged protection from complications were evaluated in 97 Medalists with median ophthalmic follow-up of 23 years (Q1, Q3: 8, 31 years), number of visits 33 (4, 63 visits), and diabetes duration 56 years (53, 61 years). This group was not significantly different from the cross-sectional cohort in age, age at diagnosis, current or longitudinal HbA1c, sBP or dBP, or heart rate, but it did have higher total cholesterol levels (4.46 vs. 4.25 mmol/L, P = 0.03).
Over time, 46 patients (47.4%) without PDR at baseline progressed to PDR (median time to PDR: 38.4 years). Subjects who developed PDR had higher sBP than those who did not develop PDR (144 vs. 125 mmHg, P = 0.02), but there was no relationship between PDR development and current or longitudinal HbA1c, age, age at diagnosis, diabetes duration, sex, BMI, dBP, heart rate, or lipid parameters.
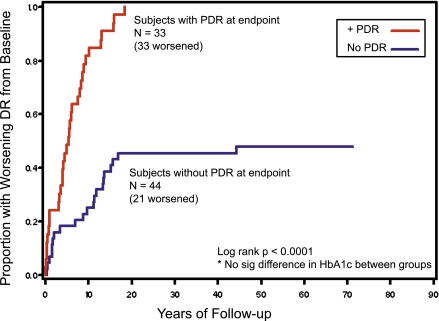
Of 25 subjects with no baseline diabetic retinopathy in either eye, 11 (44%) remained free of PDR in both eyes at the last visit. The retinopathy progression rate was slower in Medalists who did not progress to PDR in either eye as compared with those that did (P < 0.001) (Fig. 2). Of the 24 Medalists (52%) whose retinopathy did not worsen over the first 17 years of follow-up, 23 (96%) did not worsen thereafter with follow-up out to a minimum duration of diabetes of 50.4 years and median follow-up of 13 years (Q1, Q3: 6, 27 years).
Figure 2.
Worsening retinopathy in Medalists who do and do not progress to PDR. DR, diabetic retinopathy; sig, significant. (A high-quality color representation of this figure is available in the online issue.)
Complication associations with AGEs
Given the lack of correlation between current HbA1c and complications, we assessed markers of long-term glycemic control in the Medalists by evaluating the early glycation product fructose-lysine/fructosamine and AGE concentrations including CEL, an AGE derived from methylglyoxal; pentosidine, a glycoxidation product; and CML, a glycoxidation and advanced lipoxidation product. CEL and fructose-lysine CML were significantly elevated in the Medalists as compared with nondiabetic, age-matched control subjects (n = 23, mean age 67.7 years) (Supplementary Table 3).
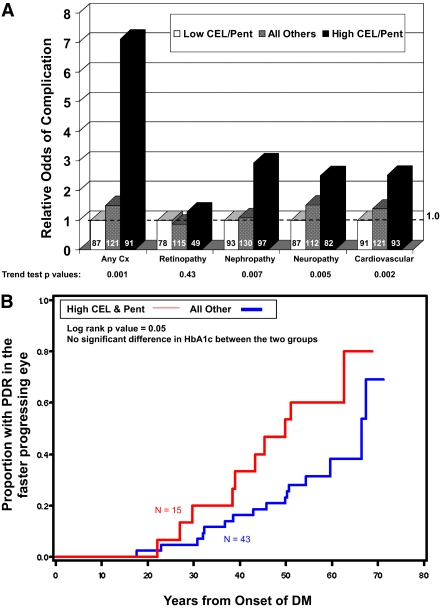
A combined biomarker of CEL and pentosidine was highly associated with complication status. Subjects with both CEL and pentosidine ≥median levels (“high CEL and pentosidine”: CEL ≥5.3 μmol/mol lysine and pentosidine ≥1.0 pmol/mg protein) were the most likely to have any complication (P = 0.001) or suffer from nephropathy (P = 0.007), neuropathy (P = 0.005), or cardiovascular disease (P = 0.002). Subjects with either CEL or pentosidine (but not both) at or above the median had an intermediate risk of severe complications, and subjects with both CEL and pentosidine below the median had the lowest complication risk. The odds of complications in Medalists with high CEL and pentosidine as compared with those with low CEL and pentosidine were 7.2-fold for any complication, 1.3-fold for retinopathy, 3.1-fold for nephropathy, 2.5-fold for neuropathy, and 2.3-fold for cardiovascular disease (Fig. 3A).
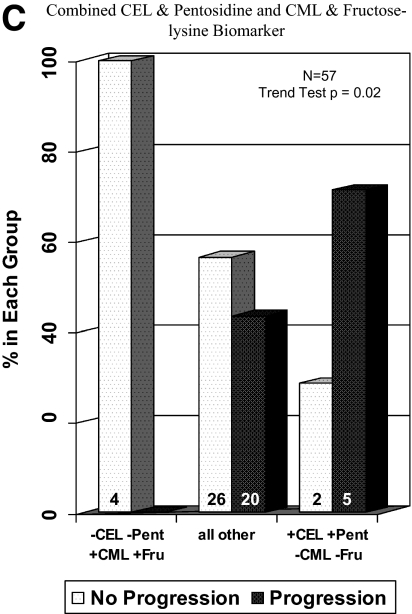
Figure 3.
A: Relative odds of complications associated with high vs. low CEL and pentosidine levels. Cx, complication; Pent, pentosidine. B: Risk of PDR development by CEL and pentosidine levels. DM, diabetes; Pent, pentosidine. C: Progression to PDR in patients grouped by combined CEL and pentosidine and CML and fructose-lysine biomarker. Fru, fructose; Pent, pentosidine. (A high-quality color representation of this figure is available in the online issue.)
The relationship between current AGE concentrations and risk of progression to PDR was examined in Medalists with longitudinal follow-up. Increased risk of PDR was seen in subjects with high CEL and pentosidine as compared with the rest of the cohort (P = 0.05) (Fig. 3B). A combination biomarker that also included CML and fructose-lysine segmented by median (“low CML and fructose-lysine”: CML <59.8 and fructose-lysine <1,004 μmol/mol lysine) was even more strongly associated with PDR outcome (P = 0.02) (Fig. 3C). None of the four subjects with low CEL and pentosidine (CEL and pentosidine levels below the median) and high CML and fructose-lysine (CML and fructose-lysine levels above the median) progressed to PDR over the course of follow-up. Conversely, five of seven subjects with high CEL and pentosidine and low CML and fructose-lysine progressed to PDR.
CONCLUSIONS
Given survival with unexpectedly few complications despite extremely long duration diabetes, the Medalist group appears to be enriched for factors protective against morbidity and mortality. The survival bias inherent in studying the Medalist cohort thereby permits evaluation of complication-protective factors in addition to a conventional assessment of risk factors. The clinical results from these cross-sectional and longitudinal studies provide strong support for the existence of endogenous factors in some individuals that can reduce and even prevent diabetes complications. We have also demonstrated that individuals who do not develop advanced retinopathy over long durations of diabetes are unlikely to experience further worsening of retinopathy once they have had 17 or more years of follow-up. This finding indicates that protective mechanisms against complication development are activated early and chronically in the course of the disease.
Behavioral skills leading to improved control of blood glucose, blood pressure, and cholesterol are clearly crucial in preventing complications in diabetic patients in general. Indeed, the mean Medalist HbA1c of 7.3% indicates good glycemic control in many subjects, and it is possible that Medalists without complications had better control during early diabetes than those who developed complications. However, the existence of additional unique protective factors in some Medalists is supported by the fact that glycemic control was not related to complications in this cohort despite a wide range of HbA1c (5–14%). It is also important to note that most Medalists developed diabetes when strict glycemic control was neither possible nor the standard of medical care. By 1993, when the results of the Diabetes Control and Complications Trial (DCCT) were first reported, the most recently diagnosed Medalist had already had diabetes for 36 years. Thus, it is unlikely that most Medalists instituted current standard strict glycemic control of HbA1c within the first few decades of diagnosis. The lack of retinopathy progression in the subset of Medalists who have been followed chronically at the Joslin clinic suggests that protective factors may have several roles including neutralization of initiating toxic effects of hyperglycemia, combating mechanisms responsible for complication progression, and even facilitation of glycemic or metabolic memory. Future longitudinal study of glycemic control in the Medalist cohort will provide important information on the effect of endogenous protective factors on metabolic memory.
The AGE results confirm a robust association with complications and suggest AGE specificity in associations with both protection from and risk for development of complications. The association of complications with CEL implicates increased methyglyoxal production, which itself is associated with various cellular and matrix dysfunctions (17), while increased pentosidine likely reflects increased ascorbic acid degradation because of oxidant production (18). These data are the first to suggest that specific AGEs may decrease the risk for diabetes complications because high current CML and fructose-lysine concentrations were negatively correlated with PDR development. Previous reports indicated that AGEs and their precursors may be important in the pathogenesis of diabetes complications (19–25); however it is unexpected that lower current levels of CML and fructose-lysine are inversely related to PDR development in light of reports indicating an association between elevations of these AGEs measured in other tissues and retinopathy (24). Previous studies finding positive correlations between AGEs and diabetes complications have measured AGEs in skin samples or other target tissues (24). In contrast, this study examined plasma AGE concentrations. Given the lack of relationship between HbA1c and complications in this group, it is possible that an adaptive mechanism, which can alter the processing of CML and fructose-lysine, may provide protection against development of PDR and possibly other complications of diabetes.
Several limitations are present in this study. The presence of retinopathy and nephropathy were studied in detail, whereas neuropathy and cardiovascular complications were only estimated clinically. Despite these limitations, known risk factors for neuropathy (height and male sex) and cardiovascular disease (dyslipidemia) were confirmed, indicating a reasonable assessment of these complications. Another limitation derives from the study’s cross-sectional nature, which prevents conclusions concerning the predictive value of AGEs for complication development. Finally, because of the nature of this unique cohort, there is difficulty in establishing an adequate control group for external comparison. The Medalist group as a whole must have survival characteristics that allowed them to outlive many peers with diabetes. It is difficult, however, to characterize a comparison cohort in the same rigorous manner as has been done for the Medalists—including obtaining blood samples and fundus photographs—of age- and sex-matched patients diagnosed during the same timeframe who are already deceased. Thus, we have chosen as a first step in this study to focus on subjects with and without complications within the Medalist cohort in order to identify factors associated with complications or protection. Future studies, already underway, may focus on further comparisons of the Medalist cohort as a whole to pre-established groups of shorter-lived patients with diabetes in order to establish factors allowing survival of this group over a time period in which systemic control of diabetes was not emphasized as heavily as it is in the post-DCCT era. Given the unique nature of the Medalist cohort, it will be important to investigate and validate these findings in groups of patients with shorter duration diabetes and faster progression of microvascular complications in order to assess generalizability to the overall population of diabetic patients. However, even if protection factors are unique to the select Medalist group, future mechanistic understanding and target identification arising from these results may provide novel preventative interventions pertinent to all patients with diabetes.
Supplementary Material
Acknowledgments
The following organizations supported this research: the Juvenile Diabetes Research Foundation (JDRF 8-2005-358, 8-2008-363, 25-2008-383); the National Institutes of Health (NIH K12 EY16335, T32DK007260, R24 DK083957-01, P30DK036836-23); the Brehm Foundation; the Beatson Foundation; and Eli Lilly (F3Z-US-X024). No other potential conflicts of interest relevant to this article were reported.
J.K.S. researched data and wrote the manuscript. H.A.K. researched data and reviewed and edited the manuscript. J.D.C. researched data. B.F.A. researched data. E.J.S. researched data. D.R.S. researched data. C.M.S. researched data. V.M.M. researched data and contributed to discussion. A.D. contributed to discussion. L.P.A. reviewed and edited the manuscript and contributed to discussion. G.L.K. reviewed and edited the manuscript and contributed to discussion.
Parts of this study were presented at the 67th Scientific Sessions of the American Diabetes Association, Chicago, Illinois, 22–26 June 2007, and at the 70th Scientific Sessions of the American Diabetes Association, Orlando, Florida, 25–29 June 2010.
The authors thank Alysha Berger, Leah Whelan, Joshua Geltman, Margaret Stockman, and Ann Kopple of the Joslin Diabetes Center for their assistance in clinical research and the Beetham Eye Institute (Joslin Diabetes Center) staff for ocular evaluation of the Medalist patients.
Footnotes
This article contains Supplementary Data online at http://care.diabetesjournals.org/lookup/suppl/doi:10.2337/dc10-1675/-/DC1.
See accompanying editorial, p. 1060.
References
- 1.Shaw JE, Sicree RA, Zimmet PZ. Global estimates of the prevalence of diabetes for 2010 and 2030. Diabetes Res Clin Pract 2010;87:4–14 [DOI] [PubMed] [Google Scholar]
- 2.The Diabetes Control and Complications Trial Research Group The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med 1993;329:977–986 [DOI] [PubMed] [Google Scholar]
- 3.UK Prospective Diabetes Study Group Tight blood pressure control and risk of macrovascular and microvascular complications in type 2 diabetes: UKPDS 38. BMJ 1998;317:703–713 [PMC free article] [PubMed] [Google Scholar]
- 4.Cruickshanks KJ, Ritter LL, Klein R, Moss SE, The Wisconsin Epidemiologic Study of Diabetic Retinopathy The association of microalbuminuria with diabetic retinopathy. Ophthalmology 1993;100:862–867 [DOI] [PubMed] [Google Scholar]
- 5.Chew EY, Klein ML, Ferris FL, 3rd, et al. Association of elevated serum lipid levels with retinal hard exudate in diabetic retinopathy: Early Treatment Diabetic Retinopathy Study (ETDRS) Report 22. Arch Ophthalmol 1996;114:1079–1084 [DOI] [PubMed] [Google Scholar]
- 6.Sjølie AK, Stephenson J, Aldington S, et al. Retinopathy and vision loss in insulin-dependent diabetes in Europe: the EURODIAB IDDM Complications Study. Ophthalmology 1997;104:252–260 [DOI] [PubMed] [Google Scholar]
- 7.Gale EA. Glucose control in the UKPDS: what did we learn? Diabet Med 2008;25(Suppl. 2):9–12 [Review. PubMed PMID: 18717972.] [DOI] [PubMed] [Google Scholar]
- 8.Klein R, Klein BE, Moss SE, Davis MD, DeMets DL. The Wisconsin Epidemiologic Study of Diabetic Retinopathy. II. Prevalence and risk of diabetic retinopathy when age at diagnosis is less than 30 years. Arch Ophthalmol 1984;102:520–526 [DOI] [PubMed] [Google Scholar]
- 9.Mitchell P, Smith W, Wang JJ, Attebo K. Prevalence of diabetic retinopathy in an older community: the Blue Mountains Eye Study. Ophthalmology 1998;105:406–411 [DOI] [PubMed] [Google Scholar]
- 10.Henricsson M, Nilsson A, Groop L, Heijl A, Janzon L. Prevalence of diabetic retinopathy in relation to age at onset of the diabetes, treatment, duration and glycemic control. Acta Ophthalmol Scand 1996;74:523–527 [DOI] [PubMed] [Google Scholar]
- 11.Bain SC, Gill GV, Dyer PH, et al. Characteristics of type 1 diabetes of over 50 years duration (the Golden Years Cohort). Diabet Med 2003;20:808–811 [DOI] [PubMed] [Google Scholar]
- 12.Keenan HA, Costacou T, Sun JK, et al. Clinical factors associated with resistance to microvascular complications in diabetic patients of extreme disease duration: the 50-year Medalist Study. Diabetes Care 2007;30:1995–1997 [DOI] [PubMed] [Google Scholar]
- 13.Asztalos BF, Roheim PS, Milani RL, et al. Distribution of ApoA-I-containing HDL subpopulations in patients with coronary heart disease. Arterioscler Thromb Vasc Biol 2000;20:2670–2676 [DOI] [PubMed] [Google Scholar]
- 14.Sell DR, Strauch CM, Shen W, Monnier VM. 2-aminoadipic acid is a marker of protein carbonyl oxidation in the aging human skin: effects of diabetes, renal failure and sepsis. Biochem J 2007;404:269–277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Odetti P, Fogarty J, Sell DR, Monnier VM. Chromatographic quantitation of plasma and erythrocyte pentosidine in diabetic and uremic subjects. Diabetes 1992;41:153–159 [DOI] [PubMed] [Google Scholar]
- 16.Feldman EL, Stevens MJ, Thomas PK, Brown MB, Canal N, Greene DA. A practical two-step quantitative clinical and electrophysiological assessment for the diagnosis and staging of diabetic neuropathy. Diabetes Care 1994;17:1281–1289 [DOI] [PubMed] [Google Scholar]
- 17.Ahmed N, Thornalley PJ. Advanced glycation endproducts: what is their relevance to diabetic complications? Diabetes Obes Metab 2007;9:233–245 [DOI] [PubMed] [Google Scholar]
- 18.Fan X, Monnier VM. Vitamin C-mediated Maillard reaction in the lens probed in a transgenic-mouse model. Ann N Y Acad Sci 2008;1126:194–200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Beisswenger PJ, Drummond KS, Nelson RG, Howell SK, Szwergold BS, Mauer M. Susceptibility to diabetic nephropathy is related to dicarbonyl and oxidative stress. Diabetes 2005;54:3274–3281 [DOI] [PubMed] [Google Scholar]
- 20.Brownlee M. The pathological implications of protein glycation. Clin Invest Med 1995;18:275–281 [PubMed] [Google Scholar]
- 21.Vlassara H, Palace MR. Diabetes and advanced glycation endproducts. J Intern Med 2002;251:87–101 [DOI] [PubMed] [Google Scholar]
- 22.Stern DM, Yan SD, Yan SF, Schmidt AM. Receptor for advanced glycation endproducts (RAGE) and the complications of diabetes. Ageing Res Rev 2002;1:1–15 [DOI] [PubMed] [Google Scholar]
- 23.Fosmark DS, Torjesen PA, Kilhovd BK, et al. Increased serum levels of the specific advanced glycation end product methylglyoxal-derived hydroimidazolone are associated with retinopathy in patients with type 2 diabetes mellitus. Metabolism 2006;55:232–236 [DOI] [PubMed] [Google Scholar]
- 24.Genuth S, Sun W, Cleary P, et al. DCCT Skin Collagen Ancillary Study Group Glycation and carboxymethyllysine levels in skin collagen predict the risk of future 10-year progression of diabetic retinopathy and nephropathy in the Diabetes Control and Complications Trial and Epidemiology of Diabetes Interventions and Complications participants with type 1 diabetes. Diabetes 2005;54:3103–3111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Amore A, Cirina P, Conti G, et al. Amadori-configurated albumin induces nitric oxide-dependent apoptosis of endothelial cells: a possible mechanism of diabetic vasculopathy. Nephrol Dial Transplant 2004;19:53–60 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.