Abstract
OBJECTIVE
We investigated in a cross-sectional study the levels of serum and urinary damage markers in diabetic patients (n = 94) and nondiabetic control subjects (n = 45) to study the association of glomerular (IgG), proximal tubular (kidney injury molecule [KIM]-1, N-acetyl-β-d-glucosaminidase [NAG], neutrophil gelatinase–associated lipocalin [NGAL], and cystatin C), and distal tubular (heart fatty acid–binding protein [H-FABP]) damage markers with kidney disease severity, as assessed by albuminuria and estimated glomerular filtration rate (eGFR).
RESEARCH DESIGN AND METHODS
Damage markers were measured in triplicate in fresh morning urine samples and in plasma.
RESULTS
Of the diabetic patients, 41 were normoalbuminuric, 41 were microalbuminuric, and 12 were macroalbuminuric. Urinary NAG (ninefold), NGAL (1.5-fold), and H-FABP (3.5-fold) were significantly elevated in normoalbuminuric diabetic patients compared with nondiabetic control subjects. Urinary concentrations of all markers increased per albuminuria stratum, except KIM-1. All urinary damage markers, except KIM-1, were significantly associated with albuminuria, independent of age, sex, and plasma concentrations of the corresponding biomarker (standard βs between 0.35 and 0.87; all P ≤ 0.001). All urinary damage markers, except KIM-1, were significantly associated with the eGFR in univariate models (standard βs between −0.38 and −0.21; all P < 0.04). After adjusting for age, sex, plasma concentration of the corresponding damage marker, and albuminuria, only the association of H-FABP with eGFR remained significant (standard β −0.26; P = 0.037).
CONCLUSIONS
Glomerular and tubular markers are associated with albuminuria, independently of eGFR, suggesting that albuminuria reflects both glomerular and tubulointerstitial damage. Only urinary H-FABP is associated with eGFR independently of albuminuria and, therefore, may be a promising urinary damage marker to assess diabetic kidney disease.
Diabetic nephropathy occurs in 20–40% of patients with diabetes and is the leading cause of chronic kidney disease and end-stage renal disease in the U.S. and many other Western countries (1,2). The onset of elevated levels of urinary albumin excretion is an early sign of diabetic nephropathy. Various studies have shown that in subjects with diabetes, microalbuminuria predicts the occurrence of macroalbuminuria and renal function decline (3). As a result, high albuminuria has become an established renal risk marker in these patients (4).
Classically, albuminuria is regarded as the consequence of diabetes-induced glomerular damage. More recently, it is increasingly appreciated that the renal tubulointerstitium plays a role in the pathogenesis of diabetic nephropathy, with prolonged exposure to a variety of metabolic and hemodynamic injuring factors associated with sustained diabetes disease as contributing factors (5,6). Furthermore, persistent albuminuria secondary to glomerular lesions may be directly harmful to renal tubular cells, leading to tubular inflammation and tubulointerstitial fibrosis (7,8).
Several tubular damage markers recently have been discovered. Increased levels of these markers are supposed to indicate proximal tubular damage in the case of kidney injury molecule (KIM)-1, neutrophil gelatinase–associated lipocalin (NGAL), N-acetyl-β-d-glucosaminidase (NAG), and cystatin C and distal tubular damage in the case of heart fatty acid–binding protein (H-FABP). These tubular damage markers have been extensively investigated in the field of predicting the occurrence of acute kidney injury after various nephrotoxic insults, such as ischemia during cardiac surgery, sepsis, and administration of contrast medium (9–11). Little research has been done in patients with chronic kidney disease. In this study, we investigated the serum and urinary levels of the aforementioned damage markers in diabetic patients and nondiabetic control subjects in order to investigate the relation of these markers to the severity of kidney disease as assessed by albuminuria and the estimated glomerular filtration rate (eGFR). As a secondary aim, we investigated whether these damage markers are related to eGFR independent of albuminuria.
RESEARCH DESIGN AND METHODS
Type 1 and type 2 diabetic patients who were visiting a diabetes specialty clinic were recruited. Inclusion was between April 2009 and September 2009. They were stratified by the amount of albuminuria, based on a first morning urine void. We included 94 diabetic patients, of whom 41 had normoalbuminuria, 41 had microalbuminuria, and 12 had macroalbuminuria. To ensure that macroalbuminuria was the consequence of diabetic nephropathy, patients with macroalbuminuria were required to have diabetic retinopathy. Patients who had cancer, infections or inflammatory conditions, and renal disease other than diabetic nephropathy; those who used nephrotoxic drugs; those who had a renal transplant; or those who were pregnant were excluded. Patients aged <18 years also were excluded. For a control group, we included 45 nondiabetic subjects without chronic kidney disease (1:2 vs. diabetic patients). Subjects were excluded from the control group if they had a fasting glucose >7.0 mmol/L, used glucose-lowering medication, had an eGFR <60 mL/min per 1.73 m2, or had an albumin-to-creatinine ratio (ACR) >30 mg/g. The study protocol was approved by the local ethics committee.
Measurements
We instructed patients to collect their first morning urine void on the day of their clinic visit. Blood pressure was assessed with a single measurement. Urinary albumin concentration was determined by nephelometry (BNII; Dade Behring Diagnostics, Marburg, Germany). Serum and urine creatinine were measured with an enzymatic creatinine assay (Roche, Mannheim, Germany). Glucose, HbA1c, and cholesterol levels were measured with standard laboratory testing.
Definitions
We defined history of cardiovascular disease as having had a myocardial infarction, a stroke, surgery, or endovascular treatment for coronary carotid or peripheral (legs, abdominal, and aorta) artery disease. Hypertension was defined as systolic blood pressure >140 mmHg, diastolic blood pressure >90 mmHg, or use of blood pressure–lowering medication. Normoalbuminuria was defined as an ACR <3 mg/mmol, microalbuminuria as an ACR 3–30 mg/mmol, and macroalbuminuria as an ACR >30 mg/mmol (12). Serum creatinine values were used to calculate an eGFR, using the abbreviated Modification of Diet in Renal Disease formula.
Damage markers
We measured IgG as a glomerular damage marker; KIM-1, NGAL, NAG, and cystatin CAs as markers of proximal tubular damage; and H-FABP as a marker of distal tubular damage. Urinary concentrations of KIM-1, NGAL, H-FABP, and IgG were measured by enzyme-linked immunosorbent assay. For KIM-1 and NGAL, antibodies were obtained from R&D Systems (Minneapolis, MN). H-FABP and IgG antibodies were obtained from Hytest (Turku, Finland). We measured urinary concentration of NAG using a modified enzyme assay according to Lockwood and corrected for nonspecific conversion (HaemoScan, Groningen, the Netherlands). Cystatin C was measured by nephelometry (reagents obtained from Siemens [Marburg, Germany]). We measured all samples in triplicate in fresh samples of both urine and plasma. Urinary damage-marker concentrations are expressed per millimole creatinine.
Statistical analyses
Analyses were performed with SPSS version 16.0 (SPSS, Chicago, IL). Characteristics were calculated per albuminuria stratum. Parametric variables were expressed as means ± SD, whereas nonparametric variables were given as median (interquartile range). We tested P values for trend over albuminuria strata in diabetic patients using ANOVA for normal distributed variables. For nonnormal distributed variables, we used a Kruskall-Wallis test. Logarithmic transformation of damage-marker excretion was applied. To investigate the association between the individual damage marker and eGFR or albuminuria, we performed a linear regression analysis by using eGFR or albuminuria as independent variables and the various damage markers as dependent variables. Various models were gradually built to adjust for possible confounding. First, we investigated the crude association between the damage marker and eGFR or albuminuria. Second, we performed multivariate analysis, adjusting for age and sex. Third, we tested whether plasma levels of the corresponding damage marker influenced the association between the damage marker and eGFR or albuminuria. Finally, to investigate whether knowledge about urinary damage-marker concentrations may be of value to assess the severity of diabetic kidney disease in addition to knowledge about albuminuria status, we performed multivariable regression analysis with eGFR as dependent variable and the damage markers as independent variables, adjusting for albuminuria. We added the use of diuretics, the use of ACE inhibitors/angiotensin receptor blockers (ARBs), and the type of diabetes to the final multivariable models (model 3 for albuminuria and model 4 for eGFR) as a sensitivity analysis. If any of these covariates showed a significant association with either albuminuria or eGFR, we tested for interaction by entering the urinary biomarker, the investigated characteristic, and their product term in the multivariable regression model. For all analyses, a two-sided P < 0.05 was considered statistically significant.
RESULTS
A total of 94 patients with diabetes and 45 control subjects participated in this study. Characteristics of patients and control subjects are given in Table 1. Patients in the microalbuminuric and macroalbuminuric groups were more often male and smokers, more often had a history of cardiovascular disease, used more antihypertensive medications, and had a lower eGFR when compared with normoalbuminuric diabetic subjects.
Table 1.
Characteristics of nondiabetic control subjects (n = 45) and diabetic patients (n = 94) according to albuminuria stratum
| Nondiabetic subjects | Subjects with diabetes | P value in diabetes | |||
|---|---|---|---|---|---|
| Normoalbuminuria | Microalbuminuria | Macroalbuminuria | |||
| n | 45 | 41 | 41 | 12 | |
| Age (years) | 53 ± 13 | 59 ± 13 | 64 ± 12* | 63 ± 13† | 0.12 |
| Male sex (%) | 56 | 73 | 66 | 83 | 0.48 |
| Diabetes duration (years) | — | 24 ± 11 | 20 ± 9 | 27 ± 8 | 0.064 |
| Type diabetes (% type 2) | — | 49 | 78 | 83 | 0.007 |
| History of cardiovascular disease (%) | 0 | 22 | 45 | 64 | 0.018 |
| Smoking (%) | 22 | 10 | 32 | 33 | 0.035 |
| BMI (kg/m2) | 27 ± 6 | 30 ± 5† | 32 ± 6‡ | 32 ± 5† | 0.28 |
| Systolic blood pressure (mmHg) | 132 ± 16 | 139 ± 15† | 141 ± 17‡ | 152 ± 14* | 0.052 |
| Diastolic blood pressure (mmHg) | 74 ± 9 | 78 ± 10 | 77 ± 77 | 77 ± 13 | 0.90 |
| Antihypertensive medication (%) | 18 | 72* | 95* | 100* | 0.006 |
| ACE inhibitors/ARBs (%) | 4 | 65* | 80* | 83* | 0.24 |
| Diuretics | 11 | 32* | 63* | 75* | 0.015 |
| Hypertension | 36 | 85* | 97* | 100* | 0.071 |
| HbA1c (%) | 5.4 ± 0.3 | 7.7 ± 1.0* | 7.6 ± 1.3* | 7.8 ± 0.7* | 0.87 |
| eGFR Modification of Diet in Renal Disease (mL/min per 1.73 m2) | 86 ± 14 | 85 ± 21 | 72 ± 22‡ | 55 ± 24‡ | <0.001 |
| ACR (mg/mmol) | 0.56 (0.44–1.0) | 0.70 (0.36–1.21) | 8.7 (5.6–13.7)* | 115 (71–130)* | <0.001 |
Parametric variables are expressed as means ± SD and nonparametric variables are median (interquartile range).
*P < 0.001;
†P < 0.05;
‡P < 0.01 vs. nondiabetic control subjects, calculated using the independent-samples t test for normal distributed variables and the Mann-Whitney U test for nonnormal distributed variables. Hypertension was defined as systolic blood pressure >140 mmHg, diastolic blood pressure >90 mmHg, or use of antihypertensive medication.
Damage-marker concentrations
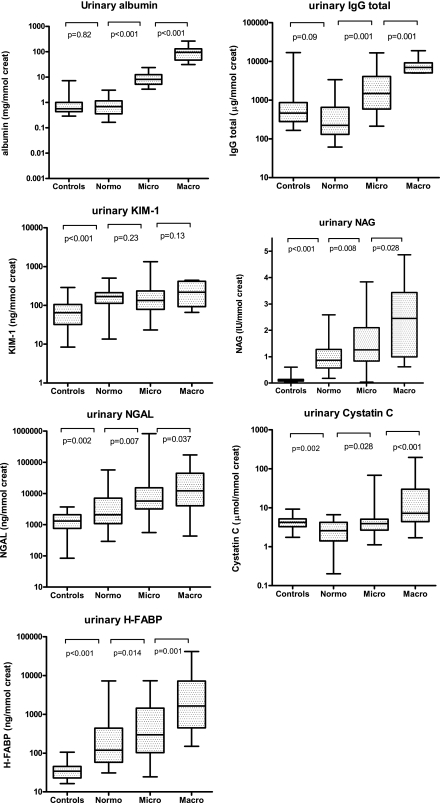
Damage-marker concentrations in nondiabetic control subjects and in diabetic patients are shown in Table 2 and Fig. 1. Urinary damage-marker concentrations of NAG, NGAL, and H-FABP were higher in normoalbuminuric patients with diabetes than in control subjects and increased with increasing categories of albuminuria in patients with diabetes. In contrast, cystatin C was lower in normoalbuminuric diabetic patients than in nondiabetic control subjects but again increased with increasing categories of albuminuria in patients with diabetes. KIM-1 was higher in normoalbuminuric patients with diabetes than in control subjects but was not different between the categories of albuminuria in patients with diabetes (Fig. 1 and Table 2). Differences between the categories of albuminuria were most pronounced for the glomerular marker IgG (>30-fold increase from normoalbuminuria to macroalbuminuria) and the distal tubular marker H-FABP (>21-fold). Differences were less pronounced for the proximal tubular markers (from no increase to an 8.5-fold increase for NGAL). Plasma concentrations of the various markers are given in Supplementary Table 1.
Table 2.
Damage-marker concentrations in nondiabetic control subjects and in diabetic patients according to albuminuria stratum
| Nondiabetic subjects | Subjects with diabetes | P value in diabetes | |||
|---|---|---|---|---|---|
| Normoalbuminuria | Microalbuminuria | Macroalbuminuria | |||
| Albumin (mg/mmol) | 0.56 (0.44–1.0) | 0.70 (0.36–1.21) | 8.7 (5.6–13.7)* | 115 (71–130)* | <0.001 |
| Glomerular | |||||
| IgG (μg/mmol) | 465 (279–867) | 242 (151–751) | 1,320 (590–3,780)‡ | 7,379 (5,079–9,202)* | <0.001 |
| Proximal tubular | |||||
| KIM-1 (ng/mmol) | 65 (33–104) | 168 (116–216)* | 122 (73–221)* | 305 (112–417)* | 0.53 |
| NAG (units/mmol) | 0.10 (0.07–0.14) | 0.9 (0.6–1.4)* | 1.2 (0.9–2.1)* | 2.5 (1.4–3.4)* | <0.001 |
| NGAL (μg/mmol) | 1.3 (0.8–2.0) | 2.1 (1.1–7.2)‡ | 5.5 (2.9–14.0)* | 18.0 (6.9–45.1)* | 0.001 |
| Cystatin C (μg/mmol) | 4.2 (3.3–5.1) | 2.6 (1.8–4.4)‡ | 3.9 (2.7–5.0) | 10.3 (6.7–33.3)* | <0.001 |
| Distal tubular | |||||
| H-FABP (ng/mmol) | 34 (24–44) | 130 (59–413)* | 300 (104–1,215)* | 2,742 (712–7,199)* | <0.001 |
Data are per mmol urinary creatinine concentration and given as medians (25th–75th percentile).
*P < 0.001;
‡P < 0.01 vs. nondiabetic control subjects, calculated using the Mann-Whitney U test.
Figure 1.
Biomarker concentrations in nondiabetic control subjects and in diabetic patients, according to albuminuria stratum (normoalbuminuria, microalbuminuria, and macroalbuminuria). Box plots show medians (25th–75th percentile). Significance was tested using the Mann-Whitney U test. Control subjects were included when subjects had a fasting glucose <7.0 mmol/L or did not use glucose-lowering medication.
Associations of damage markers with albuminuria in diabetic patients
Associations between the various urinary damage markers and albuminuria were analyzed in all diabetic patients. All damage markers were found to be significantly associated with albuminuria in crude models, except for KIM-1 (Table 3, model 1). Also, the results did not materially change after adjusting for age and sex (model 2) and, additionally, for the plasma concentration of the corresponding damage marker (model 3) and for eGFR (model 4). All investigated damage markers, except KIM-1, remained associated with albuminuria. When information on use of diuretics, the use of ACE inhibitors/ARBs, or the type of diabetes was added to the multivariable regression analyses, only the type of diabetes was a significant covariate for the association between urinary KIM-1, NAG, NGAL, cystatin C, and H-FABP with albuminuria. Sex was a significant covariate in only the association between NGAL and albuminuria. We subsequently tested for an interaction between these covariates and these damage markers in their association with albuminuria. Of all tested interaction terms, only the interaction between sex and NGAL reached statistical significance.
Table 3.
Multivariable regression analysis of various damage markers vs. albuminuria or eGFR
| Damage markers vs. albuminuria | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| IgG | KIM-1 | NGAL | Cystatin C | NAG | H-FABP | |||||||||
| Model | Standard β | P | Standard β | P | Standard β | P | Standard β | P | Standard β | P | Standard β | P | ||
| 1 | 0.78 | <0.001 | 0.15 | 0.195 | 0.34 | 0.001 | 0.49 | <0.001 | 0.37 | <0.001 | 0.48 | <0.001 | ||
| 2 | 0.80 | <0.001 | 0.13 | 0.252 | 0.42 | <0.001 | 0.51 | <0.001 | 0.37 | <0.001 | 0.49 | <0.001 | ||
| 3 |
0.87 |
<0.001 |
0.12 |
0.311 |
0.45 |
<0.001 |
0.35 |
0.001 |
0.60 |
<0.001 |
0.50 |
<0.001 |
||
| Damage markers vs. eGFR |
||||||||||||||
| Albumin |
IgG |
KIM-1 |
NGAL |
Cystatin C |
NAG |
H-FABP |
||||||||
| Model | Standard β |
P |
Standard β |
P |
Standard β |
P |
Standard β |
P |
Standard β |
P |
Standard β |
P |
Standard β |
P |
| 1 | −0.37 | <0.001 | −0.35 | 0.034 | −0.13 | 0.24 | −0.38 | <0.001 | −0.26 | 0.012 | −0.32 | 0.002 | −0.34 | 0.001 |
| 2 | −0.32 | <0.001 | −0.34 | 0.026 | 0.03 | 0.74 | −0.29 | 0.004 | −0.28 | 0.002 | −0.19 | 0.053 | −0.35 | <0.001 |
| 3 | −0.32 | 0.001 | −0.39 | 0.018 | −0.08 | 0.42 | −0.26 | 0.014 | −0.08 | 0.21 | −0.23 | 0.074 | −0.36 | 0.001 |
| 4 | — | — | 0.03 | 0.91 | 0.11 | 0.27 | −0.11 | 0.311 | 0.07 | 0.30 | 0.02 | 0.91 | −0.26 | 0.037 |
Bold print indicates associations between damage markers and albuminuria or eGFR that reach statistical significance. Model 1: crude; model 2: adjustment for age and sex; model 3: adjustment for age, sex, and plasma concentration of the corresponding damage marker; model 4: adjustment for age, sex, plasma concentration of the corresponding damage marker, and albuminuria.
Associations of damage markers with eGFR in diabetic patients
Table 3 also shows the associations between the various urinary damage markers and eGFR. Again, all damage markers, except KIM-1, were significantly associated with eGFR in crude models (model 1). However, after adjusting for age and sex, only albumin, the glomerular marker IgG, the proximal tubular markers NGAL and cystatin C, and the distal tubular marker H-FABP remained significantly associated with eGFR (model 2). When additionally adjusted for plasma concentration of the corresponding damage marker, IgG, albumin, NGAL, and H-FABP remained significant, whereas cystatin C lost significance. The association between the distal tubular marker H-FABP and eGFR was the only association that remained significant, after additional adjustment for albuminuria (standard β −0.26; P = 0.037). When information on sex, the use of diuretics, the use of ACE inhibitors/ARBs, or the type of diabetes was added to the multivariable regression analyses, only sex was a significant covariate for the association between urinary cystatin C with eGFR, but no significant interaction was found between sex and urinary cystatin C.
CONCLUSIONS
In the current study, we measured markers of glomerular, proximal tubular, and distal tubular damage to study the relationship between these damage markers with the severity of diabetic kidney disease as assessed by albuminuria and eGFR. We found that urinary concentrations of glomerular, proximal tubular, and distal tubular damage markers were higher when patients had more albuminuria. Interestingly, even in normoalbuminuric diabetic patients some of these markers already were elevated compared with nondiabetic subjects. In regression analyses, all damage markers that were significantly associated with albuminuria also were significantly associated with eGFR, except cystatin C and NAG. H-FABP was the only damage marker significantly associated with eGFR after adjustment for albuminuria.
In diabetic patients, the urinary concentration of the investigated glomerular damage marker, IgG, increased over the three albuminuria groups and is strongly associated with albuminuria. In healthy subjects, only trace amounts of these large–molecular weight proteins are leaked by the glomerulus. These data suggest, therefore, that in albuminuric diabetic patients, the amount of albuminuria reflects glomerular damage, at least to a certain extent. Similar observations have been previously described (13,14).
In general, all investigated tubular markers, except KIM-1, increased in higher albuminuria strata. Only a limited number of studies have, to date, investigated the association of tubular markers with the severity of chronic kidney disease in diabetic nephropathy. Our results, with respect to the proximal tubular markers, are similar to these studies insofar that they also showed that the urinary level of tubular damage markers parallels the degree of urinary albumin excretion (15–18). It can be argued that this may be a result of the tubulotoxic effect of albumin and other proteins that are leaked into the tubular lumen (7). However, such tubulotoxic effects cannot fully explain our findings because some of the markers under investigation already were increased in the normoalbuminuric diabetic patients compared with nondiabetic control subjects, whereas albuminuria (and eGFR) in these normoalbuminuric diabetic patients was comparable with nondiabetic control subjects. A limited number of other studies (17–19) have previously described elevated levels of tubular markers in normoalbuminuric diabetic subjects when compared with healthy control subjects. Urinary excretion of NGAL and lysosomal enzymes, such as NAG and cathepsin, have, for instance, been found to be increased. These data suggest a role of the tubulointerstitium in the pathogenesis and progression of renal damage in patients with diabetes. This assumption is strengthened by two other observations in our study. First, we found that some of these markers were associated with diabetes-related factors in normoalbuminuric diabetic subjects, such as BMI, duration of diabetes, and metabolic regulation. Second, to our knowledge, we are the first to show that a marker of distal tubular damage, H-FABP, also is associated with albuminuria, despite the fact that the distal tubule is assumed to be less sensitive to the toxic effects of urinary proteins.
Data in literature also support the finding that in diabetic kidney disease, the severity of chronic kidney disease depends not only on the severity of glomerular lesions but also on tubulointerstitial damage. A histological study (20) showed that proximal tubular basement membrane width already is thickened in normoalbuminuric diabetic patients compared with healthy control subjects. Another study (21) in microalbuminuric diabetic patients with similar GFR showed that only 29% had typical histological glomerular features of diabetic nephropathy, whereas 42% had severe tubulointerstitial lesions disproportional to the mild glomerular involvement.
All markers that were associated with albuminuria also were correlated with eGFR in crude models. However, the association of eGFR with the urinary NAG concentration lost significance after adjusting for age and sex, and the association with urinary cystatin C lost significance after additional adjustment for its plasma concentration. In healthy subjects, cystatin C is a marker for eGFR, with impaired GFR being associated with higher levels of cystatin C. Our data imply that the urinary concentration of cystatin C is largely determined by the amount that is filtrated through the glomerulus in subjects with diabetic nephropathy.
As secondary aim in our study, we investigated which damage markers are associated with eGFR independent of albuminuria. Albuminuria is widely acknowledged to be associated with eGFR in subjects with diabetic nephropathy (3,22). We found that the associations of all markers under investigation with eGFR lost statistical significance after adjusting for albuminuria, except for H-FABP. In addition, we found that the urinary H-FABP concentration already was significantly elevated in normoalbuminuric patients with diabetes compared with nondiabetic control patients who had a similar eGFR. These data suggest that urinary H-FABP concentration is a promising marker to predict the clinical outcome of diabetic nephropathy in addition to albuminuria.
We acknowledge that the current study has limitations. First, the current study is a single-center study, and the association of the measured damage markers with albuminuria and eGFR needs to be confirmed. Second, this study has a cross-sectional design. Whether the damage markers, as assessed in our study, predict the progression of diabetic nephropathy has to be investigated in long-term, prospective, observational studies. Finally, having a larger number of patients would have allowed us to perform the analyses in specific subgroups. However, the results of our multivariable regression analyses in which interactions were tested showed that there were almost no significant interactions between these covariates and the various urinary damage markers in their association with albuminuria or eGFR. This suggests that the associations between these damage markers and albuminuria or eGFR are not different in men versus women, in subjects using or not using diuretics, in subjects using or not using ACE inhibitors/ARBs, or in subjects with type 1 versus type 2 diabetes. The strengths of this study are that we measured a wide range of kidney damage markers, representing different parts of the nephron, ranging from the glomerulus, proximal, to distal tubules. Furthermore, we measured all our markers in fresh urine samples to ensure optimal sample quality. It has previously been shown for several markers that frozen storage and freeze-thaw cycles lead to a systematic decrease and increase in variability (9,23,24), which may negatively influence association studies such as the present one. Finally, most authors investigating urinary excretion of renal damage markers did not measure plasma concentrations (9,25). We also measured the corresponding plasma concentration of all urinary markers to rule out that a high plasma concentration instead of renal damage caused a high urinary concentration. This appeared to be the case for cystatin C.
In conclusion, urinary concentrations of most investigated damage markers are elevated in patients with diabetes when compared with nondiabetic control subjects. These damage markers are associated with the severity of diabetic nephropathy as assessed by albuminuria and eGFR. Interestingly, some of these markers already are elevated in normoalbuminuric diabetic patients with normal eGFR. This renders these proteins as potential sensitive markers of early diabetic kidney damage. Only the distal tubular marker H-FABP was found to be associated with eGFR independent of albuminuria, suggesting that especially measuring urinary H-FABP concentrations may be useful to assess the severity of diabetic kidney damage in addition to measuring albuminuria.
Supplementary Material
Acknowledgments
No potential conflicts of interest relevant to this article were reported.
F.L.N. and W.E.B. held the outpatient clinic, researched data, and wrote the manuscript. S.J.L.B. researched data and contributed to discussion. H.v.G. contributed to discussion and reviewed the manuscript. W.v.O. conducted measurements and contributed to discussion. P.E.d.J. reviewed the manuscript and contributed to discussion. H.B. helped in the outpatients clinic and contributed to the manuscript. R.T.G. researched data and edited the manuscript.
The authors thank Katinka Mulder, University Medical Center Groningen, Groningen, the Netherlands, for help with reviewing the manuscript.
Footnotes
This article contains Supplementary Data online at http://care.diabetesjournals.org/lookup/suppl/doi:10.2337/dc10-1545/-/DC1.
References
- 1.KDOQI KDOQI clinical practice guidelines and clinical practice recommendations for diabetes and chronic kidney disease. Am J Kidney Dis 2007;49:S12–S154 10.1053/j.ajkd.2006.12.005 [DOI] [PubMed] [Google Scholar]
- 2.American Diabetes Association Standards of medical care in diabetes: 2007. Diabetes Care 2007;30(Suppl. 1):S4–S41 10.2337/dc07-S004 [DOI] [PubMed] [Google Scholar]
- 3.O’Hare AM, Hailpern SM, Pavkov ME, et al. Prognostic implications of the urinary albumin to creatinine ratio in veterans of different ages with diabetes. Arch Intern Med 2010;170:930–936 10.1001/archinternmed.2010.129 [DOI] [PubMed] [Google Scholar]
- 4.Mogensen CE, Christensen CK. Predicting diabetic nephropathy in insulin-dependent patients. N Engl J Med 1984;311:89–93 10.1056/NEJM198407123110204 [DOI] [PubMed] [Google Scholar]
- 5.Thomas MC, Burns WC, Cooper ME. Tubular changes in early diabetic nephropathy. Adv Chronic Kidney Dis 2005;12:177–186 10.1053/j.ackd.2005.01.008 [DOI] [PubMed] [Google Scholar]
- 6.Comper WD, Hilliard LM, Nikolic-Paterson DJ, Russo LM. Disease-dependent mechanisms of albuminuria. Am J Physiol Renal Physiol 2008;295:F1589–F1600 10.1152/ajprenal.00142.2008 [DOI] [PubMed] [Google Scholar]
- 7.Abbate M, Zoja C, Remuzzi G. How does proteinuria cause progressive renal damage? J Am Soc Nephrol 2006;17:2974–2984 10.1681/ASN.2006040377 [DOI] [PubMed] [Google Scholar]
- 8.Zoja C, Garcia PB, Remuzzi G. The role of chemokines in progressive renal disease. Front Biosci 2009;14:1815–1822 10.2741/3343 [DOI] [PubMed] [Google Scholar]
- 9.Han WK, Wagener G, Zhu Y, Wang S, Lee HT. Urinary biomarkers in the early detection of acute kidney injury after cardiac surgery. Clin J Am Soc Nephrol 2009;4:873–882 10.2215/CJN.04810908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Haase M, Bellomo R, Devarajan P, Schlattmann P, Haase-Fielitz A, NGAL Meta-analysis Investigator Group Accuracy of neutrophil gelatinase-associated lipocalin (NGAL) in diagnosis and prognosis in acute kidney injury: a systematic review and meta-analysis. Am J Kidney Dis 2009;54:1012–1024 10.1053/j.ajkd.2009.07.020 [DOI] [PubMed] [Google Scholar]
- 11.Bagshaw SM, Bellomo R. Early diagnosis of acute kidney injury. Curr Opin Crit Care 2007;13:638–644 10.1097/MCC.0b013e3282f07570 [DOI] [PubMed] [Google Scholar]
- 12.Levey AS, Eckardt KU, Tsukamoto Y, et al. Definition and classification of chronic kidney disease: a position statement from Kidney Disease: Improving Global Outcomes (KDIGO). Kidney Int 2005;67:2089–2100 10.1111/j.1523-1755.2005.00365.x [DOI] [PubMed] [Google Scholar]
- 13.Satchell SC, Tooke JE. What is the mechanism of microalbuminuria in diabetes: a role for the glomerular endothelium? Diabetologia 2008;51:714–725 10.1007/s00125-008-0961-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jefferson JA, Shankland SJ, Pichler RH. Proteinuria in diabetic kidney disease: a mechanistic viewpoint. Kidney Int 2008;74:22–36 10.1038/ki.2008.128 [DOI] [PubMed] [Google Scholar]
- 15.Bolignano D, Coppolino G, Campo S, et al. Urinary neutrophil gelatinase-associated lipocalin (NGAL) is associated with severity of renal disease in proteinuric patients. Nephrol Dial Transplant 2008;23:414–416 10.1093/ndt/gfm541 [DOI] [PubMed] [Google Scholar]
- 16.Piwowar A, Knapik-Kordecka M, Fus I, Warwas M. Urinary activities of cathepsin B, N-acetyl-beta-D-glucosaminidase, and albuminuria in patients with type 2 diabetes mellitus. Med Sci Monit 2006;12:CR210–CR214 [PubMed] [Google Scholar]
- 17.Mohammadi-Karakani A, Asgharzadeh-Haghighi S, Ghazi-Khansari M, Hosseini R. Determination of urinary enzymes as a marker of early renal damage in diabetic patients. J Clin Lab Anal 2007;21:413–417 10.1002/jcla.20212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bolignano D, Lacquaniti A, Coppolino G, et al. Neutrophil gelatinase-associated lipocalin as an early biomarker of nephropathy in diabetic patients. Kidney Blood Press Res 2009;32:91–98 10.1159/000209379 [DOI] [PubMed] [Google Scholar]
- 19.Uslu S, Efe B, Alataş O, et al. Serum cystatin C and urinary enzymes as screening markers of renal dysfunction in diabetic patients. J Nephrol 2005;18:559–567 [PubMed] [Google Scholar]
- 20.Brito PL, Fioretto P, Drummond K, et al. Proximal tubular basement membrane width in insulin-dependent diabetes mellitus. Kidney Int 1998;53:754–761 10.1046/j.1523-1755.1998.00809.x [DOI] [PubMed] [Google Scholar]
- 21.Fioretto P, Mauer M, Brocco E, et al. Patterns of renal injury in NIDDM patients with microalbuminuria. Diabetologia 1996;39:1569–1576 10.1007/s001250050616 [DOI] [PubMed] [Google Scholar]
- 22.Keane WF, Zhang Z, Lyle PA, et al. RENAAL Study Investigators Risk scores for predicting outcomes in patients with type 2 diabetes and nephropathy: the RENAAL study. Clin J Am Soc Nephrol 2006;1:761–767 10.2215/CJN.01381005 [DOI] [PubMed] [Google Scholar]
- 23.Lambers Heerspink HJ, Nauta FL, van der Zee CP, et al. Alkalinization of urine samples preserves albumin concentrations during prolonged frozen storage in patients with diabetes mellitus. Diabet Med 2009;26:556–559 10.1111/j.1464-5491.2009.02721.x [DOI] [PubMed] [Google Scholar]
- 24.Haase-Fielitz A, Haase M, Bellomo R. Instability of urinary NGAL during long-term storage. Am J Kidney Dis 2009;53:564–565; author reply 566 10.1053/j.ajkd.2009.01.009 [DOI] [PubMed] [Google Scholar]
- 25.Hofstra JM, Deegens JK, Willems HL, Wetzels JF. Beta-2-microglobulin is superior to N-acetyl-beta-glucosaminidase in predicting prognosis in idiopathic membranous nephropathy. Nephrol Dial Transplant 2008;23:2546–2551 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.