Abstract
OBJECTIVE
An association between insulin resistance and microalbuminuria in type 2 diabetes has often been found in cross-sectional studies. We aimed to reassess this relationship in a prospective Taiwanese cohort of type 2 diabetic subjects.
RESEARCH DESIGN AND METHODS
We enrolled 738 normoalbuminuric type 2 diabetic subjects, aged 56.6 ± 9.0 years, between 2003 and 2005 and followed them through the end of 2009. Average follow-up time was 5.2 ± 0.8 years. We used urine albumin-to-creatinine ratio to define microalbuminuria and the homeostasis model assessment of insulin resistance (HOMA-IR) to assess insulin resistance. The incidence rate ratio and Cox proportional hazards model were used to evaluate the association between HOMA-IR and development of microalbuminuria.
RESULTS
We found incidences of microalbuminuria of 64.8, 83.5, 93.3, and 99.0 per 1,000 person-years for the lowest to highest quartiles of HOMA-IR. Compared with those in the lowest quartile of HOMA-IR, the incidence rate ratios for those in the 2nd, 3rd, and highest quartiles were 1.28 (95% CI 0.88–1.87), 1.44 (0.99–2.08), and 1.52 (1.06–2.20), respectively (trend test: P < 0.001). By comparison with those in the lowest quartile, the adjusted hazard ratios were 1.37 (0.93–2.02), 1.66 (1.12–2.47), and 1.76 (1.20–2.59) for those in the 2nd, 3rd, and highest HOMA-IR quartiles, respectively.
CONCLUSIONS
According to the dose-response effects of HOMA-IR shown in this prospective study, we conclude that insulin resistance could significantly predict development of microalbuminuria in type 2 diabetic patients.
Microalbuminuria has long been recognized as an important biomarker to predict micro- and macrovascular complications and mortality for patients with type 2 diabetes (1). Of many established risk factors linked to the development of microalbuminuria, insulin resistance—one of the fundamental pathogenic features of type 2 diabetes—deserves our special attention. The significant association between insulin resistance and microalbuminuria in type 2 diabetes has frequently been demonstrated across different ethnic populations. In Hong Kong, Chan et al. (2) found that diabetic patients with albuminuria had a higher homeostasis model assessment (HOMA) score than those without albuminuria. In Japan, a case-control study identified the HOMA score as an independent variable for urinary albumin excretion in diabetic patients (3). In Italy, Parvanova et al. (4) showed a significant association between glucose disposal rate and microalbuminuria, and De Cosmo et al. (5) revealed that diabetic males in the highest quartile of the HOMA were more likely than those in the lowest to be associated with microalbuminuria. In Iran (6), diabetic patients in the highest quartile of HOMA were also found more likely to have increased urinary albumin excretion. However, to our best knowledge, research designated to depict the relationship between insulin resistance and microalbuminuria has been conducted primarily with a cross-sectional design. None of the aforementioned results have been reconfirmed by a prospective cohort research. The purpose of this study was to explore the effects of insulin resistance on microalbuminuria development in a prospective Taiwanese cohort of type 2 diabetic subjects.
RESEARCH DESIGN AND METHODS
The study subjects were type 2 diabetic patients enrolled in the diabetes management through an integrated delivery system (DMIDS) project (NCT00288678, ClinicalTrials.gov) (6). The patient recruitment period was between August 2003 and December 2005, with the project carried out through December 2009. All type 2 diabetic subjects were eligible to be recruited unless they met any of the following exclusion criteria: 1) those aged <30 or >70 years; 2) type 1 diabetic patients; 3) pregnant women; and 4) those who had major diabetes complications including leg amputation, uremia, or hospitalization in the previous year as the result of acute myocardial infarction, heart failure, or stroke. The number of original enrollees having at least two interpretable laboratory tests was 1,209. Of these enrollees, 22 subjects receiving insulin injection, 323 with a urine albumin-to-creatinine ratio (ACR) ≥30 mg/g in at least one of the first two urine tests, and 126 subjects with missing fasting insulin data at baseline were excluded. The remaining 738 subjects, who had normoalbuminuria (ACR <30 mg/g in the first two consecutive urine tests), were selected for further investigation. Written informed consent was obtained from all enrollees. The institutional review board at the National Health Research Institutes reviewed and approved this study.
Laboratory tests
Fasting (overnight for at least 8 h) venous blood and spot urine specimens were collected every 6 months. Glycated hemoglobin (HbA1c) was measured by high-performance liquid chromatography (Variant II; Bio-Rad Laboratories, Hercules, CA). Fasting glucose, triglycerides, and HDL cholesterol were measured by an automatic analyzer (Hitachi 7060; Hitachi High Technologies, Tokyo, Japan). Insulin was measured by a chemiluminescent immunometric assay (Immulite 1000; Diagnostic Products, Los Angeles, CA). Urinary albumin was measured by the immunoturbidimetric method (Hitachi 7060; Hitachi High Technologies, Tokyo, Japan). Dipstick urinalysis was performed by an automated chemical analyzer (Clinitek 500; Bayer, Elkhart, IN). All blood and urine samples were kept in temperature-proof containers at 2°C to 8°C, transported by express delivery to a central laboratory, and measured within 8 h.
Variable definition and anthropometric measurement
Waist circumference was measured at the level of the midpoint between the lowest rib and the iliac crest. A mercury sphygmomanometer was used to measure blood pressure after subjects sat for 5 min. Systolic blood pressure was recorded as the first perception of successive sounds. Diastolic blood pressure was marked at the complete disappearance of sound. Blood pressure was measured three times separated by 1 min; the mean of these three measurements was recorded. We used the HOMA of insulin resistance (HOMA-IR) to assess insulin resistance (HOMA-IR = {insulin [mU/L] × fasting glucose [mmol/L]}/22.5) (7).
The primary end point of this study was development of microalbuminuria. Those who had ACR ≥30 mg/g in two consecutive urine tests were defined as having microalbuminuria. The urine samples were excluded from analysis if microscopic urinalysis showed erythrocytes >5/high-power field (HPF), white blood cells >5/HPF, epithelial cells >5/HPF, and appearance of casts or bacteria.
Those who had smoked fewer than 100 cigarettes in their lifetime were defined as nonsmokers. For those who had smoked >100 cigarettes, ex-smokers were defined as having stopped smoking completely at least 1 month prior to recruitment and current smokers as having a daily or occasional smoking habit at the time of recruitment.
Statistical analysis
Data were expressed as mean ± SD for continuous variables, or as counts and proportions for categorical variables. Student t tests and χ2 analyses were used for continuous and categorical variables, respectively. The incidence rate of microalbuminuria in the follow-up period was estimated by the number of observed new microalbuminuria cases per 1,000 person-years. The person-years were calculated as the time elapsed from the date of recruitment until the date of death, microalbuminuria development, or the end of follow-up, whichever came first. The calculation of a 95% CI for the incidence rate was based on the assumption that the observed incident cases followed a Poisson distribution. We estimated the incidence rate of microalbuminuria in different HOMA-IR quartiles not only for all study subjects but also for different subgroups according to their glycemic control (HbA1c ≤8 vs. >8%) and blood pressure control (≤130/80 vs. >130/80 mmHg).
Kaplan-Meier analyses and univariate Cox proportional hazards models were used to explore the association between HOMA-IR and microalbuminuria development. The predictors used in Cox proportional hazards models—including baseline demographic and metabolic profiles (age at diabetes onset, sex, education, diabetes duration, smoking status, BMI, waist circumference, triglycerides and HDL cholesterol, urine ACR, HbA1c, and hypertension status). Multivariable Cox proportional hazards modeling was used to determine the independent effects of HOMA-IR on microalbuminuria development. Covariates that were significant at the 0.25 level were entered into a stepwise model. Study entry was defined as the date of enrollment. Observations were censored at the end of the study or the date that patients died or dropped out of the study, whichever occurred first. Results were expressed as hazard ratios (HRs) and 95% CIs compared with the reference group. To eliminate residual confounding effects, for the survival analysis we also selected subjects who had good metabolic profiles. They were those who met the following criteria at baseline: no ACEI (angiotensin-converting enzyme inhibitor) and ARB (angiotensin II receptor blocker) use, BMI <24 kg/m2, triglycerides <150 mg/dL, waist circumference <80 cm (female) or <90 cm (male), HDL cholesterol >40 mg/dL (male) or >50 mg/dL (female), and blood pressure <130/80 mmHg.
The proportional hazards assumption, the constant HR over time, was evaluated by comparing estimated log-log survival curves for all covariates. All assessed log-log survival plots graphically showed two parallel lines, indicating no violation of the assumption. A test for trend was conducted by treating quartiles of HOMA-IR as a continuous variable.
Analyses were performed with SAS software, version 9.1 (SAS Institute, Cary, NC). A two-sided P value < 0.05 was considered statistically significant.
RESULTS
Table 1 displays demographic and biochemical characteristics of study subjects. Compared with those who retained a status of normoalbuminuria, patients who developed microalbuminuria were more likely to have lower education, higher HOMA-IR, and higher baseline ACR.
Table 1.
Baseline demographic and biochemical characteristics of type 2 diabetic patients with and without progression to microalbuminuria
| Overall | Normoalbuminuria | Microalbuminuria | P value | |
|---|---|---|---|---|
| n | 738 | 491 | 247 | |
| Male, n (%) | 337 (45.6) | 228 (46.4) | 109 (44.3) | 0.116 |
| Education (≤6 years), n (%) | 376 (50.9) | 230 (46.8) | 146 (59.1) | 0.034 |
| Age at diabetes onset (years) | 53.6 ± 8.6 | 53.7 ± 8.1 | 53.4 ± 8.7 | 0.697 |
| Diabetes duration at recruitment (years) | 2.9 ± 2.6 | 2.9 ± 2.7 | 3.0 ± 3.0 | 0.453 |
| Smoking status, n (%) | 0.095 | |||
| Nonsmoker | 545 (73.8) | 365 (74.3) | 180 (72.9) | |
| Ex-smoker | 53 (7.2) | 41 (8.4) | 12 (4.5) | |
| Current smoker | 140 (19.0) | 85 (17.3) | 55 (22.6) | |
| BMI (kg/m2) | 25.1 ± 3.6 | 25.0 ± 3.8 | 25.0 ± 3.9 | 0.871 |
| Waist circumference (cm) | 86.6 ± 10.4 | 88.5 ± 11.1 | 86.5 ± 10.3 | 0.076 |
| HOMA-IR | 3.7 ± 3.1 | 3.5 ± 3.2 | 4.0 ± 3.2 | 0.026 |
| Urine ACR (mg/g) | 8.9 ± 8.0 | 7.4 ± 7.0 | 12.7 ± 8.2 | <0.001 |
| Triglycerides (mg/dL) | 198.8 ± 239.0 | 187.5 ± 145.7 | 192.1 ± 130.2 | 0.623 |
| HDL cholesterol (mg/dL) | 47.3 ± 13.2 | 48.4 ± 13.3 | 49.4 ± 12.9 | 0.327 |
| HbA1c (%) | 8.15 ± 1.86 | 8.01 ± 1.82 | 8.42 ± 1.94 | 0.010 |
| Hypertension, n (%) | 407 (58.3) | 254 (51.7) | 153 (61.9) | 0.141 |
| sBP (mmHg) | 128.7 ± 15.8 | 127 ± 15.0 | 130 ± 17.3 | 0.094 |
| dBP (mmHg) | 80.0 ± 9.7 | 79.5 ± 9.3 | 80.9 ± 10.3 | 0.083 |
| Medication use at baseline, n (%) | ||||
| Statins | 19 | 15 (3.0) | 4 (1.4) | 0.188 |
| Sulfonylurea | 627 | 406 (82.6) | 221 (89.4) | 0.878 |
| Biguanide | 572 | 369 (75.1) | 203 (82.1) | 0.863 |
| Thiazolidinedione | 78 | 51 (10.3) | 27 (10.9) | 0.923 |
| α-Glucosidase inhibitor | 36 | 25 (5.0) | 11 (4.4) | 0.853 |
| Meglitinide | 16 | 11 (2.2) | 5 (2.0) | 0.896 |
| ACEI/ARB | 245 | 155 (31.5) | 90 (36.4) | 0.485 |
| CCB | 196 | 126 (25.6) | 70 (28.3) | 0.426 |
| β-Blocker | 142 | 88 (17.9) | 54 (21.8) | 0.708 |
| Diuretic | 85 | 55 (11.2) | 30 (12.1) | 0.473 |
Data are n (%) or mean ± SD unless otherwise indicated. sBP, systolic blood pressure; dBP, diastolic blood pressure; CCB, calcium channel blocker.
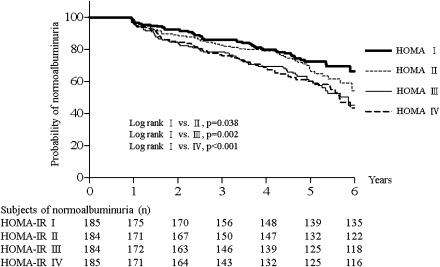
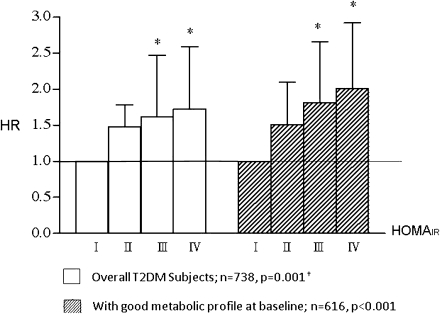
The Kaplan-Meier survival curves in Fig. 1 indicate that the probability of remaining normoalbuminuric for those in the 2nd to 4th HOMA-IR quartiles was significantly lower than for subjects in the lowest quartile. The significant adjusted HRs for those in 3rd and 4th quartiles in the multivariable Cox proportional hazards model, shown in Table 2, confirm the independent association between insulin resistance and microalbuminuria development. Figure 2 shows the adjusted HR and trend test for those with good metabolic profiles were similar to what was observed for overall subjects.
Figure 1.
Kaplan-Meier estimates of probability of normoalbuminuria according to 4 quartiles of HOMA-IR at baseline.
Table 2.
Cox proportional hazards models for progression to microalbuminuria*
| Univariate HR | P value | Multivariable HR† | P value | |
|---|---|---|---|---|
| Sex (male/female) | 0.80 (0.62–1.03) | 0.090 | 0.96 (0.69–1.34) | 0.826 |
| Education (≤6 years/>6 years) | 1.32 (1.02–1.70) | 0.031 | 1.36 (1.00–1.83) | 0.045 |
| Age at diabetes onset (years) | 1.01 (1.00–1.02) | 0.048 | 0.99 (0.97–1.01) | 0.448 |
| Diabetes duration at recruitment (years) | 1.02 (1.01–1.03) | 0.033 | 1.01 (0.96–1.06) | 0.591 |
| Smoking status | ||||
| Ex-smoker/nonsmoker | 0.52 (0.29–0.94) | 0.030 | 0.56 (0.30–1.05) | 0.073 |
| Current smoker/nonsmoker | 1.11 (0.82–1.50) | 0.482 | 1.09 (0.76–1.56) | 0.634 |
| BMI (kg/m2) | 1.00 (0.96–1.03) | 0.966 | — | — |
| Waist circumference (cm) | 0.99 (0.98–1.00) | 0.246 | 0.98 (0.97–1.00) | 0.070 |
| Urine ACR (mg/g) | 1.06 (1.04–1.07) | <0.001 | 1.06 (1.04–1.06) | <0.001 |
| Triglycerides (mg/dL) | 1.00 (0.99–1.00) | 0.417 | — | — |
| HDL cholesterol (mg/dL) | 1.00 (0.99–1.04) | 0.334 | — | — |
| Hypertension (yes/no) | 1.27 (0.98–1.65) | 0.064 | 1.39 (1.05–1.83) | 0.020 |
| HbA1c (%) | 1.57 (1.30–1.89) | <0.001 | 1.26 (1.09–1.43) | 0.026 |
| HOMA-IR | ||||
| Quartile 2/Quartile 1 | 1.41 (0.97–2.06) | 0.068 | 1.37 (0.93–2.02) | 0.110 |
| Quartile 3/Quartile 1 | 1.68 (1.16–2.44) | 0.005 | 1.66 (1.12–2.47) | 0.011 |
| Quartile 4/Quartile 1 | 1.73 (1.21–2.47) | 0.002 | 1.76 (1.20–2.59) | 0.003 |
Data are HR (95% CI).
*The covariates used in Cox proportional hazards models were baseline demographics and biomedical markers.
†The multivariate HRs were derived from a stepwise proportional hazards regression model.
Figure 2.
Adjusted HR of microalbuminuria development in type 2 diabetic patients for overall subjects and those with good metabolic profiles at baseline. Type 2 diabetic patients with good metabolic profiles were those who had no ACEI/ARB use, BMI <24 kg/m2, triglycerides <150 mg/dL, waist circumference <80 cm (female) or <90 cm (male), HDL cholesterol >40 mg/dL (male) or >50 mg/dL (female), and blood pressure <130/80 mmHg. The controlled covariates in the survival analyses included demographics (baseline age, sex, education, smoking status, and diabetes duration) and baseline biomedical profiles (waist circumference, BMI, triglycerides, ACR, HDL cholesterol, HbA1c, and mean arterial pressure). The reference group for each model was those who were in the lowest quartile of the corresponding HOMA-IR index. The mean arterial pressure (MAP) was calculated by the formula: mean arterial pressure = diastolic blood pressure + 1/3 (systolic blood pressure – diastolic blood pressure). T2DM, type 2 diabetes. †Test for trend. *P < 0.05 in multivariable Cox proportional hazards model.
Table 3 shows that the incidence of microalbuminuria generally increased with HOMA-IR. In addition to overall subjects, this dose-response relationship remained true for type 2 diabetic patients with poor glycemic or blood pressure control. Furthermore, we classified the HOMA-IR score based on its tertile instead of quartile. The incidence rate ratios of tertile 3, in comparison with tertile 1, were 1.60 (95% CI 1.00–2.55), 2.71 (1.80–4.15), 1.92 (1.16–3.27), and 1.70 (1.14–2.54) for subgroups with HbA1c ≤8%, HbA1c >8%, blood pressure ≤130/80 mmHg and blood pressure >130/80 mmHg, respectively. Their P values (0.041, 0.022, 0.013, and 0.007, respectively) for dose-response trend tests were also significant (data not shown).
Table 3.
Incidence of microalbuminuria in subjects in different HOMA-IR quartiles, according to their glycemic and blood pressure control
| HOMA-IR quartile at baseline | Development of microalbuminuria (n) | Person-years observed | Microalbuminuria incidence per 1,000 | Incident RR (95% CI) | P value* |
|---|---|---|---|---|---|
| Overall subjects (n = 738) | |||||
| Quartile 1 | 50 | 771.6 | 64.8 | 1.00 | <0.001 |
| Quartile 2 | 62 | 742.0 | 83.5 | 1.28 (0.88–1.87) | |
| Quartile 3 | 66 | 716.2 | 92.1 | 1.42 (0.98–2.06) | |
| Quartile 4 | 69 | 696.9 | 99.0 | 1.52 (1.06–2.20) | |
| Cumulative average HbA1c ≤ 8% (n = 403) | |||||
| Quartile 1 | 27 | 501.2 | 53.8 | 1.00 | 0.657 |
| Quartile 2 | 28 | 452.3 | 61.9 | 1.14 (0.67–1.96) | |
| Quartile 3 | 35 | 371.6 | 94.1 | 1.74 (1.05–2.91) | |
| Quartile 4 | 24 | 336.9 | 71.2 | 1.32 (0.75–2.30) | |
| Cumulative average HbA1c >8% (n = 335) | |||||
| Quartile 1 | 23 | 270.4 | 85.0 | 1.00 | 0.043 |
| Quartile 2 | 34 | 289.7 | 117.4 | 1.38 (0.81–2.37) | |
| Quartile 3 | 38 | 344.6 | 110.3 | 1.29 (0.77–2.20) | |
| Quartile 4 | 45 | 360.0 | 125.0 | 1.47 (0.89–2.46) | |
| Cumulative average BP ≤130/80 mmHg (n = 290) | |||||
| Quartile 1 | 23 | 461.9 | 49.7 | 1.00 | 0.021 |
| Quartile 2 | 22 | 283.6 | 77.5 | 1.55 (0.86–2.81) | |
| Quartile 3 | 25 | 284.6 | 87.8 | 1.76 (0.99–3.13) | |
| Quartile 4 | 28 | 293.2 | 95.5 | 1.91 (1.10–3.36) | |
| Cumulative average BP >130/80 mmHg (n = 448) | |||||
| Quartile 1 | 27 | 309.7 | 87.1 | 1.00 | 0.049 |
| Quartile 2 | 40 | 458.4 | 87.2 | 1.00 (0.61–1.64) | |
| Quartile 3 | 41 | 431.6 | 95.0 | 1.09 (0.67–1.79) | |
| Quartile 4 | 41 | 403.7 | 101.6 | 1.16 (0.71–1.91) |
BP, blood pressure; RR, rate ratio.
*Test for trend for incidence rate ratio from HOMA-IR Q1 to Q4.
CONCLUSIONS
Insulin resistance is shown in this longitudinal study to be a strong predictor in determining the development of microalbuminuria within 5 years for the type 2 diabetic patients with ACR <30 mg/g at enrollment. Compared with those with a HOMA-IR score in the lowest quartile, those in the highest quartile had increased incidences of microalbuminuria by about 17% (for those with poor blood pressure control) to 92% (for those with good blood pressure control). Furthermore, the adjusted dose-response effect of insulin resistance on development of microalbuminuria was significant in overall subjects and the subgroup with good metabolic profiles. Overall, this prospective study provides new epidemiological evidence linking elevated risk of diabetic nephropathy with insulin resistance.
A growing body of studies has demonstrated that insulin resistance is associated with hemodynamic alteration in the kidney. Hyperinsulinemia has been reported to be able to raise glomerular hydrostatic pressure, increase renal vascular permeability, aggravate glomerular hyperfiltration, and enhance renal sodium reabsorption (8–10). Altogether, the steady state of renal endothelial functions and hemodynamic harmonization may be changed because of cascading reactions caused by insulin resistance. This could explain underlying mechanisms for the significant association between the HOMA-IR score and microalbuminuria development observed in this study.
Similar to previous studies (3,11,12), our study found that those with a higher HOMA-IR were more likely to have metabolic syndrome (data not shown) and higher urine ACR at baseline. Metabolic syndrome and urine ACR have been well recognized as risk factors associated with microalbuminuria (2,4,5,13–15). Because of this intertwining feature in the cluster of insulin resistance, metabolic syndrome, and microalbuminuria, we could not completely exclude the possibility that insulin resistance is a biochemical marker rather than a causal factor in the process of microalbuminuria development. In order to ensure the effects of insulin resistance, we calculated the incidence rate ratio and performed multivariable survival analyses for subjects stratified by different baseline characteristics. The causal association is considered to be truly independent because the significant dose-response effect of HOMA-IR on microalbuminuria development was demonstrated (Fig. 2) not only in overall subjects but also in type 2 diabetic subjects with no ACEI/ARB use and good metabolic profiles.
The inverse association between educational attainment and microalbuminuria development is another noteworthy finding. We found that the less educated were also more likely to have higher HbA1c and to be current smokers (data not shown), both of which are risk factors for the development of microalbuminuria. These findings are in accordance with previous studies in both Western and oriental countries (16,17). This universal evidence implies that those with lower educational attainment are at higher risk of developing chronic kidney disease.
Study findings may have to be interpreted cautiously for the following reasons. First, we used the HOMA-IR index instead of the hyperinsulinemic euglycemic glucose clamp (18) to measure insulin resistance. Although some previous papers argued that the HOMA-IR index might have limitations to assess insulin resistance in certain circumstances (19), it is the most frequently used index to evaluate insulin resistance in the general population and diabetic patients (20). The UK Prospective Diabetes Study also used the HOMA-IR index to monitor insulin sensitivity in its type 2 diabetic cohort (21). In line with other epidemiologic studies, we believe the classification of insulin resistance by quartiles in this study is reliable.
Second, we didn’t measure fat deposition by using standardized dual-energy X-ray absorptiometry or computed tomography to evaluate association between abdominal adiposity and nephropathy (22). Instead, abdominal adiposity was estimated by waist circumference, which is suggested as a predictor of insulin resistance and albuminuria (23,24). Although we adjusted for waist circumference in all of our multivariable survival analyses, the HOMA-IR score remained a significant predictor of microalbuminuria development, either in the entire cohort (Table 2) or in a subgroup of individuals with good metabolic profiles (Fig. 2).
Moreover, small sample size in subgroups in quartiles 1–4 of glycemic control or blood pressure control in Table 3 could affect statistical significance. If we classified HOMA-IR based on its tertile, the incidence rate ratios of tertile 3 for subgroups with HbA1c ≤8%, HbA1c >8%, blood pressure ≤130/80 mmHg, and blood pressure >130/80 mmHg were all significant as compared with those in tertile 1. These results indicate the dose-response effects of HOMA-IR on microalbuminuria were robust.
In conclusion, this is the first prospective study investigating an association between insulin resistance and development of microalbuminuria in type 2 diabetic patients. We reconfirm that type 2 diabetic patients predisposed to higher insulin resistance are more likely to develop microalbuminuria. Future research should aim at stopping renal function deterioration by improving insulin sensitivity for type 2 diabetes with high insulin resistance.
Acknowledgments
No potential conflicts of interest relevant to this article were reported.
C.-C.H., S.-J.S., and T.-Y.T. designed the study. C.-C.H., H.-Y.C., Y.-S.L., and S.-J.S. analyzed and interpreted data. C.-C.H. and S.-J.S. wrote the manuscript. S.-J.H., M.-C.H., Y.-C.Y., H.-J.Y., C.-T.C., C.-J.C., T.-Y.T., and K.N.K. reviewed and revised the manuscript. C.-C.H., M.-C.H., Y.-C.Y., H.-J.Y., and C.-T.C. coordinated the data collection. All authors read and approved the final manuscript.
The authors thank the National Health Research Institutes for supporting and funding this study.
References
- 1.de Zeeuw D. Albuminuria: a target for treatment of type 2 diabetic nephropathy. Semin Nephrol 2007;27:172–181 [DOI] [PubMed] [Google Scholar]
- 2.Chan JC, Tomlinson B, Nicholls MG, et al. Albuminuria, insulin resistance and dyslipidaemia in Chinese patients with non-insulin-dependent diabetes (NIDDM). Diabet Med 1996;13:150–155 [DOI] [PubMed] [Google Scholar]
- 3.Takahashi N, Anan F, Nakagawa M, et al. Microalbuminuria, cardiovascular autonomic dysfunction, and insulin resistance in patients with type 2 diabetes mellitus. Metabolism 2004;53:1359–1364 [DOI] [PubMed] [Google Scholar]
- 4.Parvanova AI, Trevisan R, Iliev IP, et al. Insulin resistance and microalbuminuria: a cross-sectional, case-control study of 158 patients with type 2 diabetes and different degrees of urinary albumin excretion. Diabetes 2006;55:1456–1462 [DOI] [PubMed] [Google Scholar]
- 5.De Cosmo S, Minenna A, Ludovico O, et al. Increased urinary albumin excretion, insulin resistance, and related cardiovascular risk factors in patients with type 2 diabetes: evidence of a sex-specific association. Diabetes Care 2005;28:910–915 [DOI] [PubMed] [Google Scholar]
- 6.Huang MC, Hsu CC, Wang HS, Shin SJ. Prospective randomized controlled trial to evaluate effectiveness of registered dietitian-led diabetes management on glycemic and diet control in a primary care setting in Taiwan. Diabetes Care 2010;33:233–239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985;28:412–419 [DOI] [PubMed] [Google Scholar]
- 8.Catalano C, Muscelli E, Quiñones Galvan A, et al. Effect of insulin on systemic and renal handling of albumin in nondiabetic and NIDDM subjects. Diabetes 1997;46:868–875 [DOI] [PubMed] [Google Scholar]
- 9.Tucker BJ, Anderson CM, Thies RS, Collins RC, Blantz RC. Glomerular hemodynamic alterations during acute hyperinsulinemia in normal and diabetic rats. Kidney Int 1992;42:1160–1168 [DOI] [PubMed] [Google Scholar]
- 10.Cohen AJ, McCarthy DM, Stoff JS. Direct hemodynamic effect of insulin in the isolated perfused kidney. Am J Physiol 1989;257:F580–F585 [DOI] [PubMed] [Google Scholar]
- 11.Esteghamati A, Ashraf H, Nakhjavani M, Najafian B, Hamidi S, Abbasi M. Insulin resistance is an independent correlate of increased urine albumin excretion: a cross-sectional study in Iranian Type 2 diabetic patients. Diabet Med 2009;26:177–181 [DOI] [PubMed] [Google Scholar]
- 12.Gallagher EJ, LeRoith D, Karnieli E. The metabolic syndrome–from insulin resistance to obesity and diabetes. Endocrinol Metab Clin North Am 2008;37:559–579 [DOI] [PubMed] [Google Scholar]
- 13.Rachmani R, Levi Z, Lidar M, Slavachevski I, Half-Onn E, Ravid M. Considerations about the threshold value of microalbuminuria in patients with diabetes mellitus: lessons from an 8-year follow-up study of 599 patients. Diabetes Res Clin Pract 2000;49:187–194 [DOI] [PubMed] [Google Scholar]
- 14.Hao Z, Konta T, Takasaki S, et al. The association between microalbuminuria and metabolic syndrome in the general population in Japan: the Takahata study. Intern Med 2007;46:341–346 [DOI] [PubMed] [Google Scholar]
- 15.Lin CC, Liu CS, Li TC, Chen CC, Li CI, Lin WY. Microalbuminuria and the metabolic syndrome and its components in the Chinese population. Eur J Clin Invest 2007;37:783–790 [DOI] [PubMed] [Google Scholar]
- 16.Shoham DA, Vupputuri S, Diez Roux AV, et al. Kidney disease in life-course socioeconomic context: the Atherosclerosis Risk in Communities (ARIC) Study. Am J Kidney Dis 2007;49:217–226 [DOI] [PubMed] [Google Scholar]
- 17.Sabanayagam C, Shankar A, Saw SM, Lim SC, Tai ES, Wong TY. Socioeconomic status and microalbuminuria in an Asian population. Nephrol Dial Transplant 2009;24:123–129 [DOI] [PubMed] [Google Scholar]
- 18.DeFronzo RA, Tobin JD, Andres R. Glucose clamp technique: a method for quantifying insulin secretion and resistance. Am J Physiol 1979;237:E214–E223 [DOI] [PubMed] [Google Scholar]
- 19.Muniyappa R, Lee S, Chen H, Quon MJ. Current approaches for assessing insulin sensitivity and resistance in vivo: advantages, limitations, and appropriate usage. Am J Physiol Endocrinol Metab 2008;294:E15–E26 [DOI] [PubMed] [Google Scholar]
- 20.Wallace TM, Levy JC, Matthews DR. Use and abuse of HOMA modeling. Diabetes Care 2004;27:1487–1495 [DOI] [PubMed] [Google Scholar]
- 21.U.K. Prospective Diabetes Study Group U.K. prospective diabetes study 16. Overview of 6 years’ therapy of type II diabetes: a progressive disease. Diabetes 1995;44:1249–1258 [PubMed] [Google Scholar]
- 22.Hanai K, Babazono T, Nyumura I, et al. Involvement of visceral fat in the pathogenesis of albuminuria in patients with type 2 diabetes with early stage of nephropathy. Clin Exp Nephrol 2010;14:132–136 [DOI] [PubMed] [Google Scholar]
- 23.Racette SB, Evans EM, Weiss EP, Hagberg JM, Holloszy JO. Abdominal adiposity is a stronger predictor of insulin resistance than fitness among 50-95 year olds. Diabetes Care 2006;29:673–678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kramer H, Reboussin D, Bertoni AG, et al. Look Ahead Research Group Obesity and albuminuria among adults with type 2 diabetes: the Look AHEAD (Action for Health in Diabetes) Study. Diabetes Care 2009;32:851–853 [DOI] [PMC free article] [PubMed] [Google Scholar]