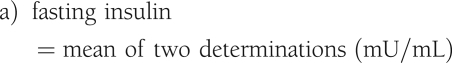Abstract
OBJECTIVE
To examine the relationship of fasting indicators of insulin sensitivity with a more invasive measure of insulin sensitivity (frequently sampled intravenous glucose tolerance test [FSIVGTT]) and the effect of Tanner stage and ethnicity on that relationship.
RESEARCH DESIGN AND METHODS
Data were analyzed from 149 overweight girls (97 Hispanic and 52 African American) who were either in the early stages of maturation defined by Tanner stages 1 or 2 (52 Hispanic and 18 African American) or in the later stages of maturation defined by Tanner stages 4 and 5 (45 Hispanic and 34 African American). Fasting indicators of insulin sensitivity (IS) included fasting insulin and glucose and the homeostasis model assessment of insulin resistance (HOMA-IR). IS was derived from an FSIVGTT with minimal modeling.
RESULTS
In Tanner stages 1 and 2, all fasting indicators were significantly associated with IS: (fasting insulin: r = −0.67, P < 0.01; HOMA: r = −0.66, P < 0.01) with no significant influence of ethnicity on these relationships. In Tanner stages 4 and 5, however, all fasting indicators were associated with IS in African American girls (fasting insulin: r = −0.55, P < 0.01; HOMA: r = −0.47, P < 0.01), but none of the indicators were significantly associated with IS in Hispanic girls.
CONCLUSIONS
Fasting indicators were reflective of IS for girls in Tanner stages 1 and 2, regardless of ethnicity and may provide a clinical measure of future risk for type 2 diabetes. In the latter stages of maturation, however, more invasive measures are warranted to adequately determine IS in clinical practice.
The prevalence of type 2 diabetes has dramatically increased in children and adolescents, particularly within the Hispanic and African American communities (1). The incidence of cases in high-risk ethnic populations accounts for about 50% of all new cases of adolescent diabetes diagnosed in the U.S. (2). Although the pathogenesis of type 2 diabetes in children and adolescents has not been described well, both insulin resistance and the resulting compensatory hyperinsulinemia likely play an important role as antecedents of type 2 diabetes (3). African American and Hispanic children are more insulin resistant than their non-Hispanic white peers (4,5). The increased prevalence of insulin resistance and subsequent related metabolic complications in these children pose an increased risk for future morbidity later in life. Considering the relevance of insulin resistance for the development of type 2 diabetes and other metabolic complications, close monitoring of insulin sensitivity (IS) may be helpful in the prevention of the long-term complications associated with insulin resistance, particularly in at-risk populations.
The euglycemic-hyperinsulinemic clamp (6) and the minimal modeling analysis of the frequently sampled intravenous glucose tolerance test (FSIVGTT) (7) are currently considered the gold standard measures for the assessment of IS. However, those measures are not easily applied in a large number of children and adolescents because of their invasiveness, complexity, and related costs. Therefore, a variety of measures derived from fasting plasma and insulin concentrations have been proposed to simplify the measurement of insulin sensitivity (IS). The measures include the homeostasis model assessment of insulin resistance (HOMA-IR) (8) and simply fasting insulin concentrations. While being less invasive and more feasible, the accuracy of proxy measures has been questioned. Factors such as ethnicity, puberty, and weight status appear to affect the relationship of fasting measures with direct measures of IS (9). The most widely used surrogate measures, their advantages and disadvantages, are summarized in a thorough review by Cutfield and Hofman (10). The aim of the present analysis was to examine the relationship of the FSIVGTT in pre- and postpubertal African American and Hispanic girls.
RESEARCH DESIGN AND METHODS
Baseline data from a total of three studies were included from the University of Southern California (USC) Center for Transdisciplinary Research on Energetics and Cancer (TREC). All studies were performed at USC. Included were girls from the Insulin Resistance and Declining Physical Activity Levels in African American and Latina Girls Study (Transitions) (18 African American and 52 Hispanic girls) in Tanner stages 1 and 2. Transitions is a longitudinal assessment of physical activity in African American and Hispanic girls through adolescence. Tanner stages 4 and 5 included the Strength and Nutrition Outcomes in Latino Adolescents (SANO) (53 Hispanic girls) and the Strength and Nutrition Outcomes in African American Youth (STAND) (39 African American girls) studies (11).
Both studies examined the effect of a randomized control trial, including the combination of nutrition modification and strength training, on metabolic and adiposity parameters in overweight Hispanic and African American teenagers. In total, the analysis included 149 female, overweight children and adolescents of Hispanic (n = 97) and African American (n = 52) descent. Both ethnicities were compared regarding the relationships of fasting measures of IS with a more invasive measure of IS (FSIVGTT) in Tanner stages 1 and 2 as well as in Tanner stages 4 and 5. Tanner stages 1 and 2 were combined for the prepubertal and early puberty group, and Tanner stages 4 and 5 were combined for the adolescent group.
Inclusion criteria for participants were Hispanic or African American ethnicity, defined by all four grandparents being of Hispanic or African American descent, and a BMI that was at or above the 85th percentile for age and sex (12). Participants were excluded from the study if they had diabetes, suffered from a major illness since birth, or had a condition known to affect body composition or IS. Written informed consent was obtained from parents and youth assent from participants.
Anthropometric measures
A pediatric health care provider conducted a medical history and physical examination and determined maturation using the criteria of Tanner at every annual visit (13). Height was measured to the nearest 0.1 cm by a wall-mounted stadiometer, and weight was recorded to the nearest 0.1 kg by a balance beam medical scale. BMI and BMI percentiles for age and sex were determined based upon established Centers for Disease Control normative curves using Epi Info 2000, version 1.1 (14).
Measures of IS.
All girls participated in a FSIVGTT, assessing IS, acute insulin response (AIR), and disposition index (DI). After a 12-h fasting period, intravenous catheters were placed in an anticubital vein in both arms. Two fasting blood samples (−15 and −5 min) were followed by a 0.3 g/kg body weight intravenous glucose administration at time point 0. At 20 min, insulin (0.02 units/kg body weight, Humulin R; Eli Lilly, Indianapolis, IN) was intravenously administered. Blood samples were taken at 2, 4, 8, 19, 22, 30, 40, 50, 70, 100, and 180 min. IS, AIR, and DI as an indicator for β-cell capacity were calculated with MINMOD Millennium 2003 (15). Blood samples from the FSIVGTT were centrifuged for 10 min at 2,500 rpm and plasma aliquots were frozen at −80°C until further analysis. Insulin concentrations were measured with a human insulin ELISA (Linco, St. Charles, MO) (intraassay coefficient of variation: 4.7–7%, interassay coefficient of variation: 9.1–1%). The assay is a double antibody Sandwich ELISA based on the capture of insulin to wells of a microtiter plate coated with monoclonal mouse anti-human insulin antibodies and the binding of a second biotinylated monoclonal mouse anti-human antibody to the captured insulin. Glucose concentrations were measured with the glucose oxidase technique (Yellow Springs Instrument analyzer; YIS Inc., Yellow Springs, OH).
Body composition.
Body composition (total body fat and total lean mass) for all participants was determined with dual-energy X-ray absorptiometry using a Hologic QDR 4500 W (Hologic, Bedford, MA). Abdominal fat (visceral adipose tissue, subcutaneous adipose tissue, and hepatic fat fraction [HFF]) were assessed by magnetic resonance imaging using a General Electric 1.5 Tesla magnet (Waukesha, WI). Multiple-slice axial TR 400/16 view of the abdomen at the level of the umbilicus was analyzed for volume of adipose tissue (liter).
Fasting measures.
Fasting measures were calculated by taking the mean of the two fasting blood samples (−15 and −5) for glucose and insulin of the FSIVGTT. The following fasting indices were evaluated:
Data analysis.
Descriptive statistics for all variables of interest were determined for both ethnic groups. Firstly, linear regression modeling was used to determine whether ethnicity or Tanner stage significantly interact with the fasting measures. Secondly, the relationship between IS and fasting measures was assessed with partial Pearson correlations, adjusted for age, fat mass, and fat-free mass (FFM). P values of <0.05 were considered to be statistically significant. Results are expressed as means ± SD.
RESULTS
Linear regression models testing the relationship of ethnicity and Tanner stage with fasting measures showed a significant interaction of Tanner stage with fasting insulin (β = 1.19; P < 0.01) and HOMA (β = 1.12; P < 0.01) on the relationship with IS. Therefore, Tanner stages were separated into Tanner stages 1 and 2 versus Tanner stages 4 and 5 and were tested separately for further analysis.
No significant interaction of ethnicity with either fasting measure (fasting insulin [β = 0.24; P > 0.05] and HOMA [β = 0.29; P > 0.05]) was observed in Tanner stages 1 and 2. Therefore African American and Hispanic girls were analyzed together in that Tanner stage.
We did find a significant interaction of ethnicity with both fasting insulin (β = 1.59; P < 0.01) and HOMA (β = 1.36; P < 0.05), testing the relationship of fasting measures with IS in Tanner stages 4 and 5. Consequently, this led to a separate analysis of ethnicities in that group of participants.
Characteristics for participants from both ethnicities in Tanner stages 1 and 2 as well as Tanner stages 4 and 5 are summarized in Table 1. In Tanner stages 1 and 2, African American girls had more FFM and less visceral adipose tissue compared with Hispanic girls. They also had significantly lower fasting glucose concentrations. African American girls were not more insulin resistant according to IS and HOMA, but they had a higher DI compared with Hispanic girls. In Tanner stages 4 and 5, African American girls weighed more and had a higher FFM compared with Hispanic girls. Hispanic girls had higher fasting glucose and lower DI compared with African American girls.
Table 1.
Participants’ characteristics separated by Tanner stage
| Tanner stages 1 and 2 |
Tanner stages 4 and 5 |
|||||
|---|---|---|---|---|---|---|
| African American | Hispanic | P | African American | Hispanic | P | |
| n | 18 | 52 | 34 | 45 | ||
| Age (years) | 8.9 ± 0.8 | 9.4 ± 1.6 | 0.13 | 15.4 ± 1 | 15.3 ± 1 | 0.55 |
| Height (cm) | 141.6 ± 5.9 | 137.1 ± 11.1 | 0.08 | 165.4 ± 7.8 | 158.9 ± 15.4 | 0.02 |
| Weight (kg) | 46.0 ± 11.6 | 41.3 ± 12.7 | 0.13 | 98.9.±23.5 | 86.5 ± 18.5 | 0.01 |
| BMI (kg/m2) | 22.9 ± 5.1 | 25.1 ± 4.2 | 0.11 | 36.1 ± 8 | 33.8 ± 6.9 | 0.19 |
| Fat mass (kg) | 14.6 ± 6.6 | 15.6 ± 8.3 | 0.59 | 35.9 ± 12.4 | 35.7 ± 10.9 | 0.93 |
| FFM (kg) | 33.02 ± 6.0 | 28.9 ± 8.0 | 0.05 | 54.6.±7.9 | 48.8 ± 8 | 0.00 |
| SAT (L) | 3.7 ± 3.2 | 4.3 ± 2.8 | 0.45 | 15.5 ± 26.8 | 9.8 ± 3.5 | 0.00 |
| VAT (L) | 0.35 ± 0.33 | 0.73 ± 0.60 | 0.02 | 1.2 ± 0.8 | 1.3 ± 0.8 | 0.35 |
| HFF (%) | 4.1 ± 1.8 | 6.5 ± 6.7 | 0.17 | 3.9 ± 1.9 | 6.5 ± 6.2 | 0.02 |
| Fasting insulin (μU/mL) | 11.9 ± 9.9 | 13.6 ± 10.8 | 0.54 | 20.7 ± 10.1 | 21.3 ± 11.9 | 0.79 |
| Fasting glucose (mg/L) | 87.5 ± 6.7 | 92.6 ± 5.4 | 0.00 | 87.9 ± 6.4 | 91.5 ± 5.5 | 0.01 |
| IS [(× 10−4/min−1)/(μU/mL)] | 3.0 ± 1.8 | 3.3 ± 1.8 | 0.59 | 1.3 ± 0.8 | 1.6 ± 0.8 | 0.10 |
| DI (× min−1) | 3650 ± 2024 | 2678 ± 1148 | 0.01 | 2230 ± 1456 | 1633 ± 745 | 0.02 |
| HOMA-IR | 2.7 ± 2.3 | 3.1 ± 2.6 | 0.50 | 4.5 ± 2.4 | 4.8 ± 2.7 | 0.65 |
SAT, subcutaneous adipose tissue; VAT, visceral adipose tissue.
Tanner stages 1 and 2
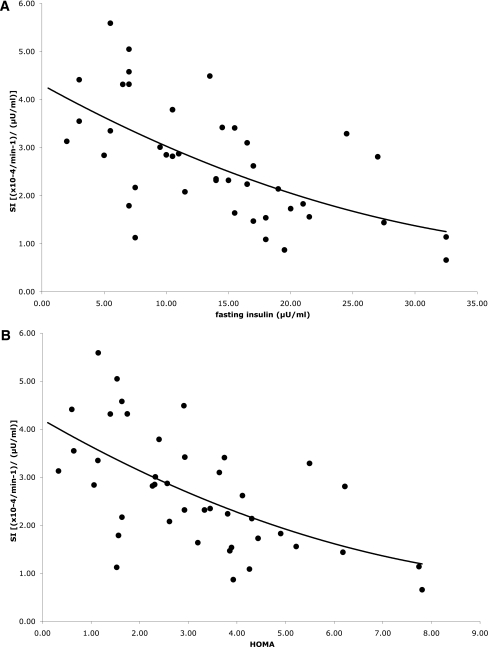
Figure 1 shows the relationship of IS with fasting measures for both ethnicities. In partial correlations adjusting for age, fat, and FFM, IS was significantly correlated with fasting insulin (r = −0.67; P < 0.01) and HOMA (r = −0.66; P < 0.01) for all participants.
Figure 1.
Relationship of fasting insulin (A) and HOMA (B) with IS in Tanner stages 1 and 2.
Tanner stages 4 and 5
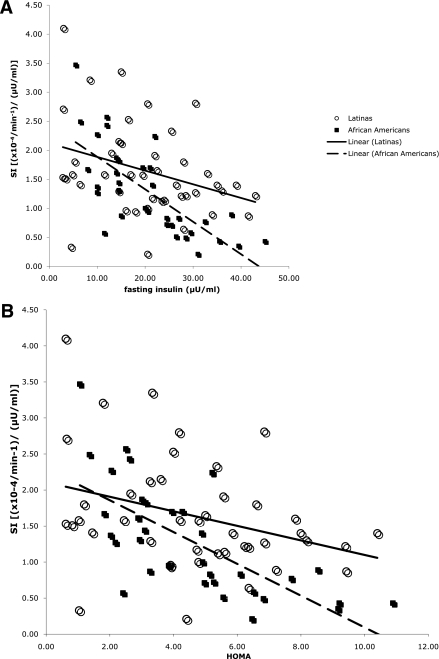
Figure 2 shows the relationship of IS and fasting measures for both ethnicities, adjusted for age, fat mass, and FFM. For African American girls, fasting insulin (r = −0.55; P < 0.01) and HOMA (r = −0.47; P < 0.01) were significantly associated with IS. However, in Hispanic girls, none of the fasting measures were associated with IS (fasting insulin: r = −0.09, P = 0.55; HOMA: r = 0.12, P = 0.45).
Figure 2.
Relationship of fasting insulin (A) and HOMA (B) with IS in Tanner stages 4 and 5.
For African American girls, fasting insulin was associated with total fat mass (r = 0.68; P < 0.01), total lean mass (r = 0.74; P < 0.01), and fasting glucose (r = 0.53; P < 0.01). In Hispanic girls, fasting insulin was associated with total fat mass (r = 0.35; P < 0.01), total lean mass (r = 0.42; P < 0.01), but was unrelated to fasting glucose. Fasting insulin was associated with HFF in both African American (r = 0.58; P < 0.01) and Hispanic girls (r = 0.45; P < 0.01).
CONCLUSIONS
The aim of this study was to examine the relationship of fasting plasma markers of IS with IS derived from a more invasive measure (FSIVGTT) in ethnic minority girls. Our results show that fasting indicators of IS were modestly associated with IS derived from the FSIVGTT in prepubertal and early puberty girls, independent of ethnicity. In postpubertal adolescents however, fasting indicators were associated with IS in African American girls, but not in Hispanic girls.
Insulin sensitive tissues include muscle, liver, adipose tissue, and the brain (16). Because fasting measures primarily reflect insulin suppression of hepatic glucose production, they are mostly thought to represent hepatic IS, whereas more invasive measures are thought to be more indicative of whole-body IS (17). The observation that HFF is related to both fasting measures and IS in early Tanner stages suggests a significant contribution of hepatic IS to overall IS in prepurbertal and early puberty. In the adolescent group, HFF was related to both fasting insulin and IS in the African American girls, but only to fasting insulin in the Hispanic girls. The difference in the relative contribution of hepatic IS to whole-body IS in more advanced Tanner stages may explain the missing association of fasting measures with IS in Hispanic girls. The ethnic specific difference in the relationship of fasting measures with whole-body IS is in accordance with other studies showing a better reflection of body IS by fasting measures in African American women compared with non-Hispanic white women (9). Our results suggest that ethnicity is an important component in determining the clinical utility of fasting measures as less invasive surrogates for IS. The current study extends the comparison between fasting measures of IS and IS published by Alvarez et al. (9) between non-Hispanic white and African Americans to the Hispanic ethnicity.
In addition to ethnicity, our results do demonstrate the importance of pubertal stage in the association of fasting measures with IS. Even though other publications find the relationship between fasting insulin and IS to be independent of Tanner stage (18), the frequent finding of a significant effect of puberty on IS (19) may contribute to the findings in this article. All participants examined in the current study were overweight. Other work has discussed how the relationship of SI and fasting measures may depend on specific characterizations of the group investigated, including ethnicity (9), weight status (20), or health status (21). Our results underline that not only weight status, but also ethnicity and pubertal status play an important role for the relationship of IS and fasting measures of IS.
The fasting measures examined in this article are only a selection of possible surrogates. Several measures based on fasting insulin and glucose in children and adolescents have been investigated regarding their pros and cons as surrogate measures for IS (10). Fasting insulin concentrations can be highly variable because of serum half-life, cyclicity, and rapid responsiveness to hormonal and metabolic milieu (21). To account for variability, we used the mean of two fasted samples taken separated by 5 min. Although this is one less sample than recommended by Matthews et al. (22), we found reliable correlations and consider the results acceptable.
Fasting measures including HOMA-IR are based on the assumption of a linear relationship between glucose and insulin concentrations. A linear relationship can be observed with normal glucose tolerance, but not in participants with impaired fasting glucose (IFG) or impaired glucose tolerance (IGT) (17). Fasting insulin and fasting glucose were correlated for both ethnicities in Tanner stages 1 and 2, but only in African American girls in Tanner stages 4 and 5. The lack of relationship between fasting insulin and fasting glucose in late pubertal Hispanic girls may be an explanation for our discrepancy in the relationship of IS and fasting measures. The mismatch between fasting insulin and glucose may well be because of the higher HFF in the Hispanic group. With a χ2 test, we examined whether the Hispanic girls in Tanner stages 4 and 5 presented with higher frequency of IFG or IGT. IFG and IGT were classified according to the revised criteria of the World Health Organization (23). The frequency of both IFG and IGT was not different between ethnicities and thus cannot explain our findings. The current study only included girls because the Transitions study only enrolled girls. It remains to be tested, if our results hold, if both sexes are taken into consideration.
In our adolescent participants, HFF was significantly higher in Hispanic compared with African American girls, which may possibly contribute to the disruption of a relationship between fasting measures and IS in that ethnicity. Hepatic fat is associated with visceral fat and insulin resistance, especially in Hispanic adolescents (24,25). The individual fat depots in Hispanics are only related to fasting measures, but not IS, suggesting that the relative contribution of hepatic insulin resistance to overall insulin resistance in Hispanic girls is regulated differently compared with African American girls. The results suggest that IS and fasting measures equally reflect overall IS in African American girls independent of Tanner stage. Different compensatory mechanisms for overall insulin resistance between the ethnicities may also play a role in the explanation for the different relationship of fasting measures and IS in late puberty between ethnicities (5).
In association with the significant difference in HFF between the ethnicities, one would expect differences in fasting insulin and glucose concentrations as well. While fasting insulin concentrations were not different between the ethnicities, fasting glucose was significantly higher in Hispanic girls. The limited power of fasting insulin to suppress hepatic glucose output also supports the idea of more hepatic insulin resistance in the Hispanic girls. The progressive failure of the β-cell to adjust to the increased insulin demands is evident in the significantly lower β-cell capacity (DI) in Hispanic girls.
A limitation of this study is that we have no data on IS derived from a glucose clamp in our group of participants. However, the FSIVGTT, especially the current protocol including insulin administration, was shown to produce results equivalent to the euglycemic glucose clamp in nondiabetic normal weight and obese adults (7). Furthermore, the FSIVGTT was shown to be a reliable measure in children, even in a modified shortened form (18).
In summary, the current study delivers evidence for the clinical value of fasting measures of IS in prepubertal or early puberty African American and Hispanic girls. More invasive measures are necessary postpuberty to obtain an accurate estimation of IS—at least in Hispanic girls. Our results do emphasize the importance of ethnicity and Tanner stage as factors to consider when determining the clinical usefulness of fasting measures as an indicator for IS.
Acknowledgments
This work was supported by the University of Southern California Transdisciplinary Research on Energetics and Cancer (U54-CA-116848), the National Institute of Child Health and Human Development (R01-HD/HL-33064), the Dr. Robert C. and Veronica Atkins Foundation, the National Cancer Institute (T32-CA-09492), and a 2009–2011 Fellowship in Health Disparities from Pfizer’s Medical and Academic Partnership program awarded to T.C.A. No other potential conflicts of interest relevant to this article were reported.
T.C.A. researched data and wrote the manuscript. R.E.H. researched data and helped to write the manuscript. C.J.L. is the statistician on the manuscript, did parts of the statistical analysis, and contributed to discussion. J.N.D. helped with data analysis and reviewed and edited the manuscript. M.J.W. (research pediatrician for study participants) reviewed and edited the manuscript. D.S.-M. (principal investigator for the Transitions study) reviewed and edited the manuscript. M.I.G. (principal investigator for the SANO/STAND studies) edited and reviewed the manuscript.
Parts of this study were presented in abstract form at the Annual Meeting of the Obesity Society, San Diego, California, 8–12 October 2010.
References
- 1.Goran MI, Lane C, Toledo-Corral C, Weigensberg MJ. Persistence of pre-diabetes in overweight and obese Hispanic children: association with progressive insulin resistance, poor beta-cell function, and increasing visceral fat. Diabetes 2008;57:3007–3012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dabelea D, Bell RA, D’Agostino RB, Jr, et al. Writing Group for the SEARCH for Diabetes in Youth Study Group Incidence of diabetes in youth in the United States. JAMA 2007;297:2716–2724 [DOI] [PubMed] [Google Scholar]
- 3.Haffner SM, Stern MP, Hazuda HP, Pugh JA, Patterson JK. Hyperinsulinemia in a population at high risk for non-insulin-dependent diabetes mellitus. N Engl J Med 1986;315:220–224 [DOI] [PubMed] [Google Scholar]
- 4.Freedman DS, Khan LK, Dietz WH, Srinivasan SR, Berenson GS. Relationship of childhood obesity to coronary heart disease risk factors in adulthood: the Bogalusa Heart Study. Pediatrics 2001;108:712–718 [DOI] [PubMed] [Google Scholar]
- 5.Goran MI, Bergman RN, Cruz ML, Watanabe R. Insulin resistance and associated compensatory responses in African-American and Hispanic children. Diabetes Care 2002;25:2184–2190 [DOI] [PubMed] [Google Scholar]
- 6.DeFronzo RA, Tobin JD, Andres R. Glucose clamp technique: a method for quantifying insulin secretion and resistance. Am J Physiol 1979;237:E214–E223 [DOI] [PubMed] [Google Scholar]
- 7.Bergman RN, Prager R, Volund A, Olefsky JM. Equivalence of the insulin sensitivity index in man derived by the minimal model method and the euglycemic glucose clamp. J Clin Invest 1987;79:790–800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985;28:412–419 [DOI] [PubMed] [Google Scholar]
- 9.Alvarez JA, Bush NC, Hunter GR, Brock DW, Gower BA. Ethnicity and weight status affect the accuracy of proxy indices of insulin sensitivity. Obesity (Silver Spring) 2008;16:2739–2744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cutfield WS, Hofman PL. Simple fasting methods to assess insulin sensitivity in childhood. Horm Res 2005;64(Suppl. 3):25–31 [DOI] [PubMed] [Google Scholar]
- 11.Hasson RE, Adam TC, Davis JN, et al. Ethnic differences in insulin action in obese African-American and Latino adolescents. J Clin Endocrinol Metab 2010;95:4048–4051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ogden CL, Flegal KM, Carroll MD, Johnson CL. Prevalence and trends in overweight among US children and adolescents, 1999-2000. JAMA 2002;288:1728–1732 [DOI] [PubMed] [Google Scholar]
- 13.Tanner JM. Growth and maturation during adolescence. Nutr Rev 1981;39:43–55 [DOI] [PubMed] [Google Scholar]
- 14.Flegal KM, Ogden CL, Carroll MD. Prevalence and trends in overweight in Mexican-American adults and children. Nutr Rev 2004;62:S144–S148 [DOI] [PubMed] [Google Scholar]
- 15.Bergman RN, Phillips LS, Cobelli C. Physiologic evaluation of factors controlling glucose tolerance in man: measurement of insulin sensitivity and beta-cell glucose sensitivity from the response to intravenous glucose. J Clin Invest 1981;68:1456–1467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Catalano KJ, Stefanovski D, Bergman RN. Critical role of the mesenteric depot versus other intra-abdominal adipose depots in the development of insulin resistance in young rats. Diabetes 2010;59:1416–1423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tripathy D, Almgren P, Tuomi T, Groop L. Contribution of insulin-stimulated glucose uptake and basal hepatic insulin sensitivity to surrogate measures of insulin sensitivity. Diabetes Care 2004;27:2204–2210 [DOI] [PubMed] [Google Scholar]
- 18.Cutfield WS, Bergman RN, Menon RK, Sperling MA. The modified minimal model: application to measurement of insulin sensitivity in children. J Clin Endocrinol Metab 1990;70:1644–1650 [DOI] [PubMed] [Google Scholar]
- 19.Goran MI, Gower BA. Longitudinal study on pubertal insulin resistance. Diabetes 2001;50:2444–2450 [DOI] [PubMed] [Google Scholar]
- 20.Kim SH, Abbasi F, Reaven GM. Impact of degree of obesity on surrogate estimates of insulin resistance. Diabetes Care 2004;27:1998–2002 [DOI] [PubMed] [Google Scholar]
- 21.Mather KJ, Hunt AE, Steinberg HO, et al. Repeatability characteristics of simple indices of insulin resistance: implications for research applications. J Clin Endocrinol Metab 2001;86:5457–5464 [DOI] [PubMed] [Google Scholar]
- 22.Matthews DR, Lang DA, Burnett MA, Turner RC. Control of pulsatile insulin secretion in man. Diabetologia 1983;24:231–237 [DOI] [PubMed] [Google Scholar]
- 23.Alberti KG, Zimmet PZ. Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: diagnosis and classification of diabetes mellitus provisional report of a WHO consultation. Diabet Med 1998;15:539–553 [DOI] [PubMed] [Google Scholar]
- 24.Cruz ML, Shaibi GQ, Weigensberg MJ, Spruijt-Metz D, Ball GD, Goran MI. Pediatric obesity and insulin resistance: chronic disease risk and implications for treatment and prevention beyond body weight modification. Annu Rev Nutr 2005;25:435–468 [DOI] [PubMed] [Google Scholar]
- 25.Kelley DE, McKolanis TM, Hegazi RA, Kuller LH, Kalhan SC. Fatty liver in type 2 diabetes mellitus: relation to regional adiposity, fatty acids, and insulin resistance. Am J Physiol Endocrinol Metab 2003;285:E906–E916 [DOI] [PubMed] [Google Scholar]