Abstract
OBJECTIVE
Receptor activity–modifying proteins (RAMPs) 1, 2, and 3 are unusual accessory proteins that dictate the binding specificity of two G protein–coupled receptors involved in energy homeostasis: calcitonin gene–related peptide (CGRP) and amylin receptors. These proteins are expressed throughout the central nervous system (CNS), including in the brain regions involved in the regulation of energy homeostasis, but the significance of CNS RAMPs in the control of energy balance remains unknown.
RESEARCH DESIGN AND METHODS
To examine the functional significance of modulating neuronal RAMP1, we assessed the effect of overexpressing human RAMP1 (hRAMP1) in the CNS on body energy balance.
RESULTS
Nestin/hRAMP1 transgenic mice have a remarkably decreased body weight associated with reduced fat mass and circulating leptin levels. The transgenic mice exhibited higher energy expenditure as indicated by increased oxygen consumption, body temperature, and sympathetic tone subserving brown adipose tissue (BAT). Consistent with this, the nestin/hRAMP1 transgenic mice had elevated BAT mRNA levels of peroxisome proliferator–activated receptor γ coactivator 1α and uncoupling protein 1 and 3, and these changes can be reversed by chronic blockade of sympathetic nervous system signaling. Furthermore, metabolic response to amylin was enhanced in the nestin/hRAMP1 mice whereas the response to CGRP was blunted, possibly the result of higher expression of CGRP in the CNS.
CONCLUSIONS
These data demonstrate that CNS RAMP1 plays a pivotal role in the regulation of energy homeostasis by promoting energy expenditure.
The discovery of receptor activity–modifying proteins (RAMPs) was a hallmark in the conceptual evolution of the G protein–coupled receptors. Involvement of RAMPs extended the pharmacology and mechanism of function of these receptors from a single seven-transmembrane protein to a receptor complex with a specific chaperone (1,2). RAMPs (RAMP1, RAMP2, and RAMP3) are small, single-pass integral membrane proteins with a large extracellular N-terminal domain and a small intracellular COOH-terminal domain (3,4). RAMP1 interacts with calcitonin-like receptor (CLR) to yield calcitonin gene–related peptide (CGRP) receptor binding specificity (1,5). Association of CLR with RAMPs 2 or 3 yields receptors for adrenomedullin (1,5). On the other hand, interaction of RAMP1, 2, or 3 with calcitonin receptor (CTR) changes the ligand specificity from calcitonin to generate distinct receptors with high affinity for amylin (6,7). Of note, the expression level of RAMPs is subject to regulation during physiological challenge, such as pregnancy and hypoxia, and is altered in pathological states, including cardiovascular disease, diabetes, and sepsis (2,4).
A number of studies have shown that both amylin and CGRP are involved in the control of food intake and metabolism. Amylin and CGRP administered systemically, as a single bolus or chronically, decreased food intake and body weight in rodents (8–10). Amylin and CGRP also decreased food intake and caused weight loss following single or chronic intracerebroventricular (ICV) administration (8,11,12). Conversely, treatment with selective antagonists of the amylin receptor or CGRP receptor increased food intake, body weight, and adiposity in rats (13,14). The potency of ICV administration of amylin and CGRP as compared with systemic administrations indicates that the effects of amylin and CGRP on food intake and body weight are mediated by the central nervous system (CNS). Thus, amylin and CGRP, which are released by peripheral tissues (pancreas and gastrointestinal tract, respectively) in response to nutrient stimulus following ingestion of food, may act as afferent signals to inform the CNS about the status of nutrient availability and energy reserves, thereby regulating energy balance.
Consistent with the effects of amylin and CGRP on food intake and energy balance, RAMPs are expressed in several brain regions including the hypothalamus and brainstem nuclei involved in the regulation of energy homeostasis (2,15,16). The distribution of CTR and CLR overlaps with RAMP proteins, particularly RAMP1 and RAMP2 in several brain nuclei (2). Of note, in the brain, amylin-induced c-fos activation was found to codistribute with distinct RAMP1, 2, and 3 mRNA (17). However, the importance of CNS RAMPs for energy balance is unknown.
Here, we used a mouse model that overexpresses the human RAMP1 gene in the CNS to demonstrate that neuronal RAMP1 is critically involved in the regulation of energy homeostasis. We show that elevation of RAMP1 in the CNS increases energy expenditure and modulates the brain actions of amylin and CGRP.
RESEARCH DESIGN AND METHODS
All procedures were approved by the University of Iowa Animal Care and Use Committee. Nestin-hRAMP1 transgenic mice were produced by mating Nestin-cre mice with green fluorescence protein (GFP)flox-hRAMP1 mice. Unless otherwise indicated, we used 12- to 24-week-old Tg(RAMP1) line 28412/3 (18) crossed with nestin-cre mice having a B6;129 mixed genetic background, yielding mice with contributions from the B6, 129, and SJL genetic backgrounds. For some studies, we confirmed the phenotype using Tg(RAMP1) line 28245/3, and where indicated, nestin-cre was introduced by an intercross with mice obtained from The Jackson Laboratory (stock 003771) in only the B6 genetic background, yielding mice with contributions from only the B6 and SJL genetic backgrounds. Genotype of the mice was determined by PCR as described previously (18). Mice were housed in groups of 3–5 per cage and maintained on 12-h light-dark cycle with lights on at 6:00 a.m.. Room temperature was maintained at 22°C. Food and water were available ad libitum except when the mice were fasted or had their food restricted as described below.
Body weight and food intake studies.
Mice were weighed once a week for several weeks. To measure food intake, mice were housed separately in regular cages and allowed to acclimate for at least 3 days before beginning the measurements of 24-h food intake. To assess the effect of fasting on the feeding behavior, individually housed mice (12–16 weeks old) were fasted for 24 h. Food was returned at the onset of the dark cycle. Food intake and body weight were recorded 24 h after the food was returned.
A group of wild-type mice had their body weights paired to that of transgenic mice by adjusting the daily food intake, beginning at weaning (4 weeks of age) until the mice reached 12 weeks of age. Parallel groups of control and transgenic mice were fed ad libitum.
To test the effect of ICV injection of amylin and CGRP, a cannula was implanted into the lateral cerebral ventricle as described previously (19). Mice were fasted for 24 h before ICV injection of vehicle (saline, 2 μl) or 0.2 nmol of amylin (Bachem, Torrance, CA) or CGRP (Sigma-Aldrich, St. Louis, MO). The dosage of amylin and CGRP was chosen based on preliminary studies in wild-type mice. Food was returned 1 h after the ICV injections, corresponding to the onset of the dark cycle. Body weight and food intake were then recorded after 24 h.
Fat pad analysis.
Magnetic resonance imaging was performed in anesthetized mice as described previously (20). In addition, different fat depots including gonadal fat, mesenteric fat, retroperitoneal fat, and BAT were dissected at death and weighed.
To examine the adipocytes, mice were perfused with fresh saline, followed by 4% paraformaldehyde (Electron Microscopy Sciences, Hatfield, PA), and retroperitoneal fat was dissected, postfixed in 4% paraformaldehyde at 4°C overnight. Tissue blocks were sectioned (7 μm) on a sliding microtome, mounted onto gelatin-coated slides, and stained with standard hematoxylin and eosin method. Slides were visualized and pictures were obtained using Olympus BX-51 light microscope (ESICO, Davenport, IA). Cell sizes were analyzed using National Institutes of Health ImageJ software (http://rsbweb.nih.gov). At least three slides from each animal were analyzed.
Measurements of O2 consumption, body temperature, and locomotor activity.
O2 consumption was measured in mice housed individually in regular cages with free access to food and water. Regular filter lids were replaced with well-sealed filter lids. Room air drawn through each cage was dried by passage through two successive columns of CaSO4 (Drierite, Arcos, Newark, NJ) desiccant, then analyzed for O2 (model S-3A/II, AEI Technologies, Pittsburgh, PA) content.
Radiotelemetric measurement was used to record body temperature and locomotor activity in conscious mice using TA10TA-F20 transmitter (Data Sciences, St.Paul, MN) which was implanted in the peritoneal cavity under anesthesia. After 7 days recovery, body temperature and locomotor activity were collected continuously for 6 days and analyzed using Data Science Dataquest software.
Sympathetic nervous system activity.
We used direct multifiber recording to measure baseline BAT sympathetic nerve activity as described previously (19). Briefly, mice were anesthetized by intraperitoneal injection of ketamine/xylaxine cocktail and the nerve subserving interscapular BAT was isolated, suspended to bipolar electrodes (Cooner Wire, Chatsworth, CA) and secured with silicone gel (Kwik-Cast; World Precision Instruments, Sarasota, FL). An average of three separate measurements during a 30-min recording period was taken as baseline value for each animal.
Α β-adrenergic blocker, propranolol, was used to interrupt the sympathetic nervous system signaling. For this, osmotic minipumps containing either vehicle (saline) or propranolol (10 mg/kg/day) were implanted subcutaneously in anesthetized mice. After 2 weeks, body weight was determined and mice killed by CO2 asphyxiation. BAT was collected from each mouse, freeze-clamped, and stored at −80°C.
RNA isolation and quantitative RT-PCR.
Total RNA from various tissues was extracted using the Trizol method (Invitrogen, Carlsbad, CA) and reverse transcribed with oligo-dT and Multiscribe reverse transcriptase (Applied Biosystems, NJ) (18,20). RNA levels were measured by quantitative RT-PCR using IQ SYBR Green Supermix kit (Bio-Rad Laboratories, Hercules, CA), and β-actin was used as internal control. Primers are provided in Supplementary Table 1.
Western blotting.
BAT proteins were extracted using lysis buffer, resolved by 10% SDS-PAGE, and transferred to polyvinylidene fluoride membranes, which were incubated with UCP1 antibody (#sc-6528; Santa Cruz Biotechnology, Santa Cruz, CA) or glyceraldehyde-3-phosphate dehydrogenase antibody (#sc-20357; Santa Cruz Biotechnology) followed by horseradish peroxidase–conjugated secondary antibodies. Blots were detected with enhanced chemiluminescence and measured using National Institutes of Health ImageJ and reported relative to the signal from control mice.
Analytical procedures.
The cerebrospinal fluid was collected as described previously (21). Plasma was obtained by centrifuging (5,000 rpm for 8 min) the blood. Leptin was measured by an ELISA kit (Crystal Chem Inc., Downers Grove, IL), and CGRP was measured using an EIA kit (Cayman Chemical Company, Ann Arbor, MI).
Statistical analysis.
Data are presented as means ± SEM. Analysis of difference was performed using Student t test, one- or two-way ANOVA. When ANOVA reached significance, the Fisher test was used to compare the mean values among the different levels of mice groups and treatments. A value of P < 0.05 was considered to be significant.
RESULTS
Genetic overexpression of RAMP1 in the CNS.
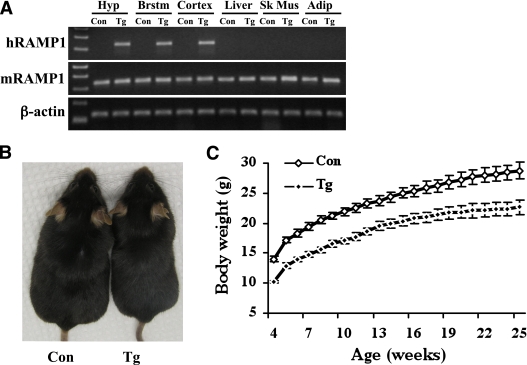
To examine the functional significance of modulating neuronal RAMP1, we assessed the effect of overexpressing human RAMP1 (hRAMP1) in the CNS on body energy balance. The strategy we used was to breed mice expressing GFPflox-hRAMP1, in which an upstream GFP cDNA includes a translational stop sequence and a polyadenylation signal that prevents expression of hRAMP1, with mice expressing cre under the control of the CNS-specific nestin promoter (nestin/hRAMP1 mice) (18). In this way, hRAMP1 expression is dependent on Cre recombinase excision of GFP at flanking loxP sites. The success of this strategy is demonstrated by the expression of hRAMP1 in the brain (hypothalamus, brainstem, and cortex) but not in the peripheral tissues such as the liver, skeletal muscle, and adipose tissue (Fig. 1A). The levels of hRAMP1 and endogenous mouse RAMP1 were measured by quantitative RT-PCR. The transgenic mice have about 1.5-fold overall elevation of total RAMP1 mRNA in the brain. A similar elevation was measured in the hypothalamus and brainstem (Supplementary Fig. 1). The elevation was due to the expression of hRAMP1, as the levels of mouse RAMP1 mRNA in those tissues was comparable between nestin/hRAMP1 mice and littermate controls. Likewise, the hRAMP1 transgene was not detectable in peripheral tissues (liver, skeletal muscle, and white adipose tissue) by quantitative RT-PCR, and the mouse RAMP1 mRNA in these peripheral tissues was not altered.
FIG. 1.
Nestin/hRAMP1 transgenic mice have reduced body weight. A: A representative PCR comparing the expression of human and mouse RAMP1 in various tissues between nestin/hRAMP1 transgenic (Tg) mice and wild-type controls (Con). Hyp, hypothalamus; Brstm, brainstem; Sk Mus, skeletal muscle; Adip, white adipose tissue. B: A photograph of a nestin/hRAMP1 transgenic mouse and a wild-type control littermate. C: Growth curve of nestin/hRAMP1 and control mice (n = 12–19 per group). (A high-quality color representation of this figure is available in the online issue.)
Transgenic mice overexpressing RAMP1 in the CNS have reduced body weight.
To investigate the importance of neuronal RAMP1 for body weight regulation, we monitored the weight of nestin/hRAMP1 mice and littermate controls. Body weight was comparable between nontransgenic mice and single-transgenic mice containing only the hRAMP1 or nestin-cre transgenes (data not shown). Strikingly, nestin/hRAMP1 mice exhibited much lower body weight compared with littermate controls (Fig. 1B–C). This difference in body weight can be detected at birth with nestin/hRAMP1 mice weighing about 17% less than the controls (1.77 ± 0.06 vs. 2.13 ± 0.04 g, P < 0.05) and was sustained at weaning (with transgenic mice weighing about 18% less than controls) (Fig. 1C). After 25 weeks, transgenic mice tended to gain less weight (12 ± 1 g) than controls (14 ± 1 g), but this was not statistically different. The difference in body weight was observed in both male and female mice (Supplementary Fig. 2). The nose-anus length of nestin/hRAMP1 mice (9.27 ± 0.10 cm) is slightly, but significantly (P < 0.05), reduced as compared with littermate controls (9.84 ± 0.08 cm), supporting a role for CNS RAMP1 in growth regulation.
The reduced body weight was maintained throughout the life span. Compared with control littermates between 2 and 12 months, both male and female nestin/hRAMP1 mice had 25% and 16% reduction in body weight, respectively (n = 78–122). The lower body weight was still present in several 3-year-old nestin/hRAMP1 mice (data not shown). To ascertain that the lean phenotype is not the result of a nonspecific effect of the transgene, we analyzed the body weight of a second GFPflox-hRAMP1 transgenic line. The body weight of nestin/hRAMP1 line 28245/3 was 19% lower compared with littermates (P < 0.05, n = 8–14). Finally, the lower body weight phenotype was reproduced in transgenic mice produced by crossing GFPflox-hRAMP1 line 28412/3 with two lines of nestin-cre mice (see research design and methods). Together, these data indicate that overexpression of hRAMP1 in the CNS leads to body weight reduction irrespective of the genetic background and the phenotype persists throughout the life span.
Reduced adiposity in mice overexpressing RAMP1 in the CNS.
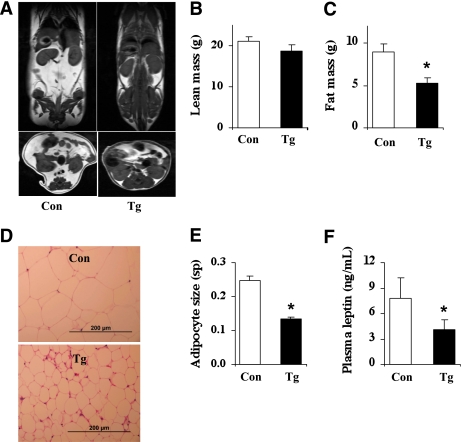
Using MRI, we found that nestin/hRAMP1 mice had significant reduction in total fat mass, but not in total lean mass (Fig. 2A–C). Expressed as percent of body weight, total fat mass was significantly (P = 0.02) lower in transgenic mice (22.2 ± 2.6%) as compared with controls (29.8 ± 2.3%). The reduction in fat mass was confirmed by weighing the fat pads (Supplementary Fig. 3). In addition, adipocytes (obtained from retroperitoneal fat) of nestin/hRAMP1 mice were approximately half the size of adipocytes from control animals (Fig. 2D–E). Consistent with the reduced fat mass, the plasma level of the adipocyte-derived hormone leptin was significantly reduced in the nestin/hRAMP1 mice (Fig. 2F). These results indicate that reduced adiposity accounts for the decreased body weight of nestin/hRAMP1 mice.
FIG. 2.
Reduced adiposity of nestin/hRAMP1 transgenic mice. A: Representative T1-weighted MRI showing a decrease in fat mass in nestin/hRAMP1 mice as compared with controls. Coronal (top panels) and axial abdominal (bottom panels) sections of wild-type and nestin/hRAMP1 mice are shown. B and C: Comparison of total lean mass (B) and total fat mass (C) between wild-type and nestin/hRAMP1 mice. D and E: Smaller white adipocyte (obtained from retroperitoneal fat) stained with hematoxylin and eosin (D) and size measured using square pixels (sp) (E). F: Lower circulating levels of the adipocyte-derived hormone, leptin, in nestin/hRAMP1 mice. *P < 0.05 vs. controls; n = 5–18. (A high-quality color representation of this figure is available in the online issue.)
To determine whether the decreased adiposity in the transgenic mice was driven mainly by CNS hRAMP1 or just the consequence of reduced body weight, we measured fat mass in control mice that have their body weight matched to that of transgenic mice (Supplementary Fig. 4). We found that the decreased fat mass cannot be explained entirely by the reduction in body weight, therefore, implicating the overexpression of the transgene in the CNS.
Orexigenic profile of hypothalamic neuropeptides in mice overexpressing RAMP1 in the CNS.
To test whether alterations in the hypothalamic mechanisms that regulate energy balance may account for the lower adiposity and subsequent leanness of nestin/hRAMP1 mice, we analyzed the RNA levels of key hypothalamic neuropeptides involved in the control of energy homeostasis (22,23). This analysis revealed unanticipated alterations in the neuropeptide expression levels. Unexpectedly, the expression level of the anorexigenic proopiomelanocortin (pomc) gene was significantly reduced in the nestin/hRAMP1 mice (Supplementary Fig. 5A). The expression of another anorexigenic gene, cocaine- and amphetamine-related transcript (cart), was lower in the nestin/hRAMP1 mice, but the difference did not reach statistical significance.
Similarly, the mRNA level of orexin, an orexigenic peptide, was decreased in nestin/hRAMP1 mice. There was no significant alteration in the mRNA levels of melanin-concentrating hormone (MCH). On the other hand, the expression of neuropeptide y (NPY) gene npy was increased in the nestin/hRAMP1 mice as compared with the controls (Supplementary Figure 5B). Of note, the mRNA level of agouti-related protein (agrp), another orexigenic peptide expressed in NPY neurons, was not altered.
We also examined whether these changes in hypothalamic gene expression were due to the transgene (hRAMP1) or a consequence of reduced body weight. We found that control mice with body weights matched to that of transgenic animals had similar alterations in the expression of pomc, orexin, and npy than the transgenic mice (Supplementary Figure 5C and D).
Overexpression of RAMP1 in the CNS does not alter feeding behavior.
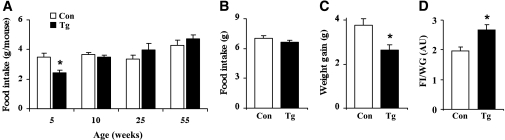
At 5 weeks of age, nestin/hRAMP1 mice had reduced food intake (Fig. 3A). However, at later ages, 10 weeks and beyond, food intake was not different in nestin/hRAMP1 mice as compared with littermate controls (Fig. 3A). Thus, although reduced food consumption in young nestin/hRAMP1 mice may contribute to the lower body weight and adiposity, the similar food intake at later ages indicates that food intake does not contribute to maintaining the lean phenotype of the nestin/hRAMP1 mice.
FIG. 3.
Food intake of nestin/hRAMP1 transgenic mice. A: Twenty-four-hour food intake of nestin/hRAMP1 transgenic (Tg) mice and littermate controls (Con) at different ages. B–D: Effect of refeeding after 24-h fasting period in 12- to 16-week-old nestin/hRAMP1 transgenic and control mice: food intake (B), body weight gain (C), and the ratio of food intake (FI) (g) to weight gain (WG) (g) (D). *P < 0.05 vs. controls; n = 8–20.
To test whether challenging the mice may reveal difference in appetite control and feeding behavior, we fasted 12- to 16-week-old transgenic and littermate control mice for 24 h and measured their food intake 24 h after the food was returned. Overnight fasting caused similar weight loss in transgenic mice (2.3 ± 1.1 g) relative to controls (2.4 ± 0.5 g, P = 0.4). Food intake after fasting was not different between the transgenic and control mice (Fig. 3B), but the transgenic mice gained significantly less weight (Fig. 3C), suggesting that in the transgenic animals ingested fuels are not stored, but rather metabolized. This was confirmed by calculating the food intake relative to weight gain ratio (indirect indicator of calories dissipated as heat) which was significantly increased in the nestin/hRAMP1 mice (Fig. 3D) supporting the assumption that these mice have increased metabolism.
Increase energy expenditure in mice overexpressing RAMP1 in the CNS.
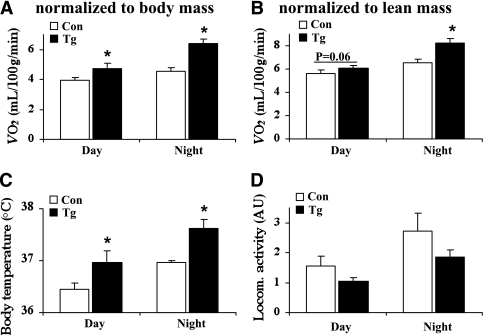
Consistent with the increased metabolism, oxygen consumption, expressed as a function of either body mass (Fig. 4A) or lean mass (Fig. 4B), was significantly higher during daytime and nighttime in the transgenic mice. Furthermore, nestin/hRAMP1 mice had significantly higher body temperatures than control mice (Fig. 4B). Locomotor activity tends to be lower in nestin/hRAMP1 mice, but it was not significantly different (P = 0.12 and P = 0.24 during the day and the night, respectively) (Fig. 4C).
FIG. 4.
Increased energy expenditure in nestin/hRAMP1 transgenic mice. Compared with littermate controls (Con), nestin/hRAMP1 transgenic (Tg) mice have increased oxygen consumption (VO2, normalized to body mass [A] or total lean mass [B]) and body temperature (C) and a slight, but not significant, reduction in locomotor activity (D). *P < 0.05 vs. controls; n = 8–12.
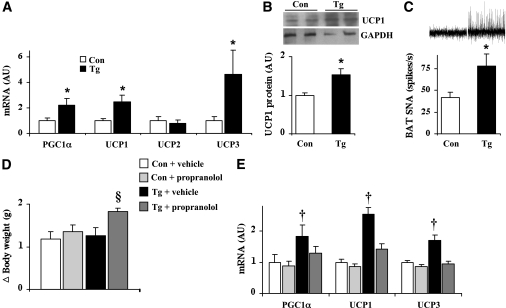
In line with the elevated energy expenditure, nestin/hRAMP1 mice exhibited significant increase in the mRNA levels of peroxisome proliferator–activated receptor γ coactivator 1α (PGC1α), uncoupling protein (UCP) 1 and UCP3, but not UCP2, in BAT (Fig. 5A). Consistent with the increased mRNA, BAT UCP1 protein was increased in the nestin/hRAMP1 mice (Fig. 5B). In addition, nestin/hRAMP1 mice exhibited ∼90% higher BAT sympathetic nerve activity (Fig. 5C).
FIG. 5.
Energy expenditure–promoting pathways are enhanced in nestin/hRAMP1 transgenic mice. A: Compared with controls, nestin/hRAMP1 mice have elevated BAT mRNA levels, by quantitative RT-PCR, of PGC1α, UCP1, and UCP3, but not UCP2. B: Consistent with the mRNA levels, UCP1 protein measured by Western blot was increased in the BAT of nestin/hRAMP1 mice. C: Increased sympathetic nerve activity (SNA) recorded from the nerve subserving interscapular BAT. D and E: Continuous subcutaneous infusion of β-adrenergic receptor antagonist, propranolol (10 mg/kg/day for 2 weeks), accelerated the weight gain (D) and normalized the elevated expression levels of PGC1α, UCP1, and UCP3 in BAT (E) of transgenic mice. *P < 0.05 vs. controls, §P < 0.05 vs. Tg + vehicle, †P < 0.05 vs. Con + vehicle and Tg + propranolol; n = 4–12.
To determine whether the increase in sympathetic outflow could explain the changes in BAT gene expression in transgenic mice, we tested the effect of blocking the sympathetic nervous system signaling by subcutaneous infusion of propranolol, a β-adrenergic receptor antagonist, for 2 weeks. Transgenic mice treated with propranolol gained about 45% more weight than those treated with vehicle (Fig. 5D). In addition, chronic blockade of β-adrenergic receptors reversed the increase in mRNA levels of BAT PGC1α, UCP1, and UCP3 (Fig. 5E). These data indicate that overexpression of hRAMP1 in the CNS triggers an increase in sympathetic nerve outflow to thermogenic brown adipocytes leading to an increase in energy expenditure through changes in the expression of key BAT genes.
Brain actions of amylin and CGRP are differentially altered in mice overexpressing RAMP1 in the CNS.
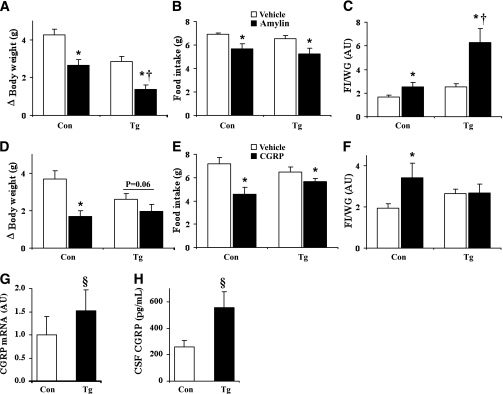
Since RAMP1 is a common subunit for both amylin and CGRP receptors (1,5,24), we tested whether neuronal overexpression of hRAMP1 alters the physiological responses to both of these peptides. In control mice, ICV injection of amylin and CGRP decreased food intake and body weight and promoted the burning of calories as indicated by the increased food intake to weight gain ratio (Fig. 6A–C). Surprisingly, the transgenic mice exhibited contrasting responses to amylin and CGRP. The amylin-induced weight loss and increase in food intake to weight gain ratio was significantly enhanced in the transgenic animals (Fig. 6A–C). In contrast, exogenous CGRP had a blunted effect on food intake and body weight and did not further increase food intake to weight gain ratio in the nestin/hRAMP1 mice (Fig. 6D–F). The increased sensitivity to amylin demonstrates that RAMP1 is critical for neuronal action of this hormone and may contribute to the increased energy expenditure of the nestin/hRAMP1 mice.
FIG. 6.
Effects of amylin and CGRP in nestin/hRAMP1 mice. A–C: Effect of ICV administration of amylin (0.2 nmol) on body weight, food intake, and food intake (FI, g) to weight gain (WG, g) ratio in nestin/hRAMP1 mice (Tg) as compared with controls (Con). D–F: Effect of ICV administration of CGRP (0.2 nmol) on body weight, food intake, and food intake to weight gain ratio in nestin/hRAMP1 transgenic mice as compared with littermate controls. G and H: Increased hypothalamic CGRP mRNA levels (G), measured by quantitative RT-PCR, and CGRP levels in the cerebrospinal fluid (H) in nestin/hRAMP1 mice. *P < 0.05 vs. vehicle, †P < 0.05 vs. controls treated with amylin, §P < 0.05 vs. controls; n = 4–10.
Given that chronic activation of the CGRP receptor can generate a self-sustaining feedback loop leading to increased expression of CGRP (encoded by the calca gene) in cultured neurons (18), we hypothesized that the blunted response to exogenous CGRP in the nestin/hRAMP1 mice may be the result of increased endogenous CGRP expression in these animals. In line with our hypothesis, the nestin/hRAMP1 mice exhibited significantly higher levels of hypothalamic CGRP mRNA (Fig. 6G) and cerebrospinal fluid CGRP protein (Fig. 6H). In contrast, there was no significant difference (P = 0.87) in the plasma CGRP between the transgenic and control mice. Together with the increased sensitivity to amylin, this increase in brain CGRP may play a role in the increased metabolism and energy expenditure of the nestin/hRAMP1 animals.
DISCUSSION
The present investigation revealed a novel and critical role for CNS RAMPs in the control of energy homeostasis. We show that genetic elevation of RAMP1 in the CNS lead to a marked lean phenotype in mice owing to reduced adiposity. Food intake was reduced only in 5-week-old transgenic mice. In contrast, the transgenic mice exhibited marked increase in energy expenditure associated with elevated expression profile of BAT genes that promote thermogenesis. Importantly, even when normalized to lean body mass, energy expenditure is still elevated in the transgenic animals. Furthermore, we describe a key role for the sympathetic nervous system in mediating the reduction in adiposity and the increase in BAT gene expression profile. Finally, in the transgenic mice we demonstrated enhanced potency of central action of amylin whereas the action of CGRP was blunted, possibly as the result of higher expression of CGRP in the CNS. To our knowledge, this is the first report to document a role for CNS RAMP1 in the regulation of energy homeostasis.
Our results indicate that one functional role of the CNS RAMP1 is to modulate activity in peripheral organs that are crucial for the maintenance of body energy balance. Indeed, CNS RAMP1 appears to activate the pathways that promote energy expenditure as indicated by the increased PGC1α, UCP1, and UCP3 mRNA levels in BAT of nestin/hRAMP1 transgenic mice. PGC-1α is thought to play a pivotal role in the control of brown fat development and function by regulating the mitochondrial and thermogenic genes, including ucp1 (25). The critical role for UCP1 has been revealed using a mouse model that lacks the ucp1 gene. These mice were found to be unable to maintain body temperature during cold exposure (26). In addition, at thermoneutrality the ucp1 null mice became obese and have increased sensitivity to diet-induced obesity (27).
Importantly, we showed that chronic infusion of β-adrenergic receptor antagonist reversed the elevated gene expression level of these genes in BAT of nestin/hRAMP1 mice, which was associated with increased weight gain in these transgenic animals. These finding demonstrate the key role of the sympathetic nervous system in mediating the increased thermogenesis in mice overexpressing RAMP1 in the CNS. The sympathetic nervous system is an important regulatory mechanism of metabolism and thermogenesis, and an alteration in this system can play a significant role in disease states such as obesity (28,29). The autonomic center in the brain receives afferent signals about the status of body energy reserves leading to autonomic and behavioral adaptations aimed at maintaining body weight homeostasis (22,30). Brown adipocytes receive dedicated noradrenergic innervations, and a centrally triggered increase in sympathetic traffic lead to alterations in thermogenic activity in BAT resulting in an increase in energy expenditure (29,31). The elevated sympathetic nerve traffic to BAT in transgenic mice confirms the importance of the sympathetic nervous system in the lean phenotype of these animals.
It is striking that nestin/hRAMP1 mice exhibited higher energy expenditure and leanness in spite of the orexigenic expression profile of hypothalamic neuropeptides. However, such gene expression profile is consistent with the decreased circulating levels of leptin and may be the consequence of counter-regulatory mechanisms triggered by the reduced fat stores in the transgenic mice. Alternatively, the increased expression of neuropeptides such as NPY may contribute to the alterations in sympathetic nerve traffic and energy expenditure in the transgenic mice as NPY has been shown to amplify norepinephrine action and elicit sympathetic excitation (32,33).
The enhanced CNS amylin action in terms of weight reducing effect and increase in food intake to weight gain ratio combined with the increased CGRP levels are in line with the reduced body weight and fat mass and increased energy expenditure of the nestin/hRAMP1 transgenic mice. Several reports have shown that systemic or central amylin administration increase energy expenditure as assessed by indirect calorimetry and body temperature (34–36). This is corroborated by the finding that the reduction in body weight and fat mass induced by amylin treatment was not entirely explained by the reduction in caloric intake (37).
Our findings are also consistent with the essential role of RAMPs as chaperones for both amylin and CGRP receptors and the importance of central action of both amylin and CGRP in the regulation of energy balance (1,5,24). CTR and CLR appear to associate with RAMPs in the endoplasmic reticulum and are cotrafficked to the cell surface where they form stable complexes acting as receptors for amylin and CGRP, respectively (2). In COS-7 cells, only RAMP1 or RAMP3 can generate an amylin receptor phenotype (6). However, there is still little known about the formation of amylin and CGRP receptor complexes in tissues and the contribution of the resulting heterodimers to physiological actions of amylin and CGRP. Our findings demonstrate that in the CNS, RAMP1 is important for amylin action. Our data also indicate the relevance of RAMP1 for the expression and action of brain CGRP.
The use of a mouse model in which hRAMP1 was overexpressed throughout the CNS using a cre-inducible transgenic approach may be viewed as a limitation to the current study because this strategy does not allow the identification of a specific brain site that drives the phenotype. However, in mice, RAMP1 mRNA was found to be widely distributed throughout the brain (38). In addition, an increase in RAMP1 mRNA levels under various conditions including hypertension, heart failure, and pregnancy has been reported (2). Another limitation of our study relates to the fact that the nestin-cre strategy yields transgene expression not only in the CNS but also in peripheral nervous system, which may have contributed to the phenotype we describe in the transgenic mice.
Our study indicates that RAMP1 is a potential therapeutic target to modulate body energy balance. The feasibility of modulating RAMP1 as a therapeutic target has been established by the efficacy of RAMP1 antagonism for treating migraine (39,40). Amylin signaling in the brain has recently emerged as a potential target for the treatment of obesity (41,42). Therefore, a better understanding of the complex mechanism of amylin and CGRP receptor signaling and the precise role of each component in controlling energy balance is critical to enhance the design of novel and effective pharmacological therapies to reduce metabolic disorders.
ACKNOWLEDGMENTS
This work is supported by National Institutes of Health grants DE-016511, DE-018149, and HL-084207.
No potential conflicts of interest relevant to this article were reported.
Z.Z., A.F.R., and K.R. designed the research and wrote the manuscript. Z.Z. and A.K. performed the metabolic studies. Z.Z. and X.L. performed gene and protein expression analysis. D.A.M. performed the sympathetic nerve activity studies. D.R.T. performed the MRI studies. Z.Z., X.L., D.A.M., A.K., D.R.T., A.F.R., and K.R. analyzed data. All authors read and commented on the manuscript.
We are grateful to Dr. Curt D. Sigmund, University of Iowa, for providing access to equipments and reagents used in these studies.
Footnotes
This article contains Supplementary Data online at http://diabetes.diabetesjournals.org/lookup/suppl/doi:10.2337/db10-0692/-/DC1.
REFERENCES
- 1.McLatchie LM, Fraser NJ, Main MJ, et al. RAMPs regulate the transport and ligand specificity of the calcitonin-receptor-like receptor. Nature 1998;393:333–339 [DOI] [PubMed] [Google Scholar]
- 2.Hay DL, Poyner DR, Sexton PM. GPCR modulation by RAMPs. Pharmacol Ther 2006;109:173–197 [DOI] [PubMed] [Google Scholar]
- 3.Hay DL, Christopoulos G, Christopoulos A, Sexton PM. Amylin receptors: molecular composition and pharmacology. Biochem Soc Trans 2004;32:865–867 [DOI] [PubMed] [Google Scholar]
- 4.Udawela M, Hay DL, Sexton PM. The receptor activity modifying protein family of G protein coupled receptor accessory proteins. Semin Cell Dev Biol 2004;15:299–308 [DOI] [PubMed] [Google Scholar]
- 5.Poyner DR, Sexton PM, Marshall I, et al. International Union of Pharmacology. XXXII. The mammalian calcitonin gene-related peptides, adrenomedullin, amylin, and calcitonin receptors. Pharmacol Rev 2002;54:233–246 [DOI] [PubMed] [Google Scholar]
- 6.Christopoulos G, Perry KJ, Morfis M, et al. Multiple amylin receptors arise from receptor activity-modifying protein interaction with the calcitonin receptor gene product. Mol Pharmacol 1999;56:235–242 [DOI] [PubMed] [Google Scholar]
- 7.Leuthäuser K, Gujer R, Aldecoa A, et al. Receptor-activity-modifying protein 1 forms heterodimers with two G-protein-coupled receptors to define ligand recognition. Biochem J 2000;351:347–351 [PMC free article] [PubMed] [Google Scholar]
- 8.Krahn DD, Gosnell BA, Levine AS, Morley JE. Effects of calcitonin gene-related peptide on food intake. Peptides 1984;5:861–864 [DOI] [PubMed] [Google Scholar]
- 9.Arnelo U, Blevins JE, Larsson J, et al. Effects of acute and chronic infusion of islet amyloid polypeptide on food intake in rats. Scand J Gastroenterol 1996;31:83–89 [DOI] [PubMed] [Google Scholar]
- 10.Lutz TA. Amylinergic control of food intake. Physiol Behav 2006;89:465–471 [DOI] [PubMed] [Google Scholar]
- 11.Lutz TA, Rossi R, Althaus J, Del Prete E, Scharrer E. Amylin reduces food intake more potently than calcitonin gene-related peptide (CGRP) when injected into the lateral brain ventricle in rats. Peptides 1998;19:1533–1540 [DOI] [PubMed] [Google Scholar]
- 12.Rushing PA, Hagan MM, Seeley RJ, Lutz TA, Woods SC. Amylin: a novel action in the brain to reduce body weight. Endocrinology 2000;141:850–853 [DOI] [PubMed] [Google Scholar]
- 13.Lutz TA, Rossi R, Althaus J, Del Prete E, Scharrer E. Evidence for a physiological role of central calcitonin gene-related peptide (CGRP) receptors in the control of food intake in rats. Neurosci Lett 1997;230:159–162 [DOI] [PubMed] [Google Scholar]
- 14.Rushing PA, Hagan MM, Seeley RJ, et al. Inhibition of central amylin signaling increases food intake and body adiposity in rats. Endocrinology 2001;142:5035–5038 [DOI] [PubMed] [Google Scholar]
- 15.Husmann K, Sexton PM, Fischer JA, Born W. Mouse receptor-activity-modifying proteins 1, -2 and -3: amino acid sequence, expression and function. Mol Cell Endocrinol 2000;162:35–43 [DOI] [PubMed] [Google Scholar]
- 16.Oliver KR, Kane SA, Salvatore CA, et al. Cloning, characterization and distribution of receptor activity central nervous system modifying proteins in the rat. Eur J Neurosci 2001;14:618–628 [DOI] [PubMed] [Google Scholar]
- 17.Barth SW, Riediger T, Lutz TA, Rechkemmer G. Peripheral amylin activates circumventricular organs expressing calcitonin receptor a/b subtypes and receptor-activity modifying proteins in the rat. Brain Res 2004;997:97–102 [DOI] [PubMed] [Google Scholar]
- 18.Zhang ZM, Winborn CS, Marquez de Prado B, Russo AF. Sensitization of calcitonin gene-related peptide receptors by receptor activity-modifying protein-1 in the trigeminal ganglion. J Neurosci 2007;27:2693–2703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rahmouni K, Morgan DA, Morgan GM, Mark AL, Haynes WG. Role of selective leptin resistance in diet-induced obesity hypertension. Diabetes 2005;54:2012–2018 [DOI] [PubMed] [Google Scholar]
- 20.Rahmouni K, Fath MA, Seo S, et al. Leptin resistance contributes to obesity and hypertension in mouse models of Bardet-Biedl syndrome. J Clin Invest 2008;118:1458–1467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Morgan DA, Thedens DR, Weiss R, Rahmouni K. Mechanisms mediating renal sympathetic activation to leptin in obesity. Am J Physiol Regul Integr Comp Physiol 2008;295:R1730–R1736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Morton GJ, Cummings DE, Baskin DG, Barsh GS, Schwartz MW. Central nervous system control of food intake and body weight. Nature 2006;443:289–295 [DOI] [PubMed] [Google Scholar]
- 23.Coll AP, Farooqi IS, O’Rahilly S. The hormonal control of food intake. Cell 2007;129:251–262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Evans BN, Rosenblatt MI, Mnayer LO, Oliver KR, Dickerson IM. CGRP-RCP, a novel protein required for signal transduction at calcitonin gene-related peptide and adrenomedullin receptors. J Biol Chem 2000;275:31438–31443 [DOI] [PubMed] [Google Scholar]
- 25.Seale P, Kajimura S, Spiegelman BM. Transcriptional control of brown adipocyte development and physiological function—of mice and men. Genes Dev 2009;23:788–797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Enerbäck S, Jacobsson A, Simpson EM, et al. Mice lacking mitochondrial uncoupling protein are cold-sensitive but not obese. Nature 1997;387:90–94 [DOI] [PubMed] [Google Scholar]
- 27.Feldmann HM, Golozoubova V, Cannon B, Nedergaard J. UCP1 ablation induces obesity and abolishes diet-induced thermogenesis in mice exempt from thermal stress by living at thermoneutrality. Cell Metab 2009;9:203–209 [DOI] [PubMed] [Google Scholar]
- 28.Dulloo AG. Biomedicine. A sympathetic defense against obesity. Science 2002;297:780–781 [DOI] [PubMed] [Google Scholar]
- 29.Lowell BB, Bachman ES. Beta-Adrenergic receptors, diet-induced thermogenesis, and obesity. J Biol Chem 2003;278:29385–29388 [DOI] [PubMed] [Google Scholar]
- 30.Elmquist JK, Elias CF, Saper CB. From lesions to leptin: hypothalamic control of food intake and body weight. Neuron 1999;22:221–232 [DOI] [PubMed] [Google Scholar]
- 31.Morrison SF, Nakamura K, Madden CJ. Central control of thermogenesis in mammals. Exp Physiol 2008;93:773–797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Martin JR, Knuepfer MM, Westfall TC. Hemodynamic effects of posterior hypothalamic injection of neuropeptide Y in awake rats. Am J Physiol 1991;261:H814–H824 [DOI] [PubMed] [Google Scholar]
- 33.Rasmusson AM, Schnurr PP, Zukowska Z, Scioli E, Forman DE. Adaptation to extreme stress: post-traumatic stress disorder, neuropeptide Y and metabolic syndrome. Exp Biol Med (Maywood) 2010;235:1150–1162 [DOI] [PubMed] [Google Scholar]
- 34.Mack C, Wilson J, Athanacio J, et al. Pharmacological actions of the peptide hormone amylin in the long-term regulation of food intake, food preference, and body weight. Am J Physiol Regul Integr Comp Physiol 2007;293:R1855–R1863 [DOI] [PubMed] [Google Scholar]
- 35.Wielinga PY, Löwenstein C, Muff S, Munz M, Woods SC, Lutz TA. Central amylin acts as an adiposity signal to control body weight and energy expenditure. Physiol Behav 2010;101:45–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lutz TA. The role of amylin in the control of energy homeostasis. Am J Physiol Regul Integr Comp Physiol 2010;298:R1475–R1484 [DOI] [PubMed] [Google Scholar]
- 37.Roth JD, Hughes H, Kendall E, Baron AD, Anderson CM. Antiobesity effects of the beta-cell hormone amylin in diet-induced obese rats: effects on food intake, body weight, composition, energy expenditure, and gene expression. Endocrinology 2006;147:5855–5864 [DOI] [PubMed] [Google Scholar]
- 38.Ueda T, Ugawa S, Saishin Y, Shimada S. Expression of receptor-activity modifying protein (RAMP) mRNAs in the mouse brain. Brain Res Mol Brain Res 2001;93:36–45 [DOI] [PubMed] [Google Scholar]
- 39.Olesen J, Diener HC, Husstedt IW, et al. BIBN 4096 BS Clinical Proof of Concept Study Group Calcitonin gene-related peptide receptor antagonist BIBN 4096 BS for the acute treatment of migraine. N Engl J Med 2004;350:1104–1110 [DOI] [PubMed] [Google Scholar]
- 40.Ho TW, Ferrari MD, Dodick DW, et al. Efficacy and tolerability of MK-0974 (telcagepant), a new oral antagonist of calcitonin gene-related peptide receptor, compared with zolmitriptan for acute migraine: a randomised, placebo-controlled, parallel-treatment trial. Lancet 2008;372:2115–2123 [DOI] [PubMed] [Google Scholar]
- 41.Roth JD, Roland BL, Cole RL, et al. Leptin responsiveness restored by amylin agonism in diet-induced obesity: Evidence from nonclinical and clinical studies. Proc Natl Acad Sci U S A 2008;105:7257–7262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Trevaskis JL, Coffey T, Cole R, et al. Amylin-mediated restoration of leptin responsiveness in diet-induced obesity: magnitude and mechanisms. Endocrinology 2008;149:5679–5687 [DOI] [PubMed] [Google Scholar]