Abstract
OBJECTIVE
Because of confounding factors, the effects of dietary n-3 polyunsaturated fatty acids (PUFA) on type 1 diabetes remain to be clarified. We therefore evaluated whether fat-1 transgenic mice, a well-controlled experimental model endogenously synthesizing n-3 PUFA, were protected against streptozotocin (STZ)-induced diabetes. We then aimed to elucidate the in vivo response at the pancreatic level.
RESEARCH DESIGN AND METHODS
β-Cell destruction was produced by multiple low-doses STZ (MLD-STZ). Blood glucose level, plasma insulin level, and plasma lipid analysis were then performed. Pancreatic mRNA expression of cytokines, the monocyte chemoattractant protein, and GLUT2 were evaluated as well as pancreas nuclear factor (NF)-κB p65 and inhibitor of κB (IκB) protein expression. Insulin and cleaved caspase-3 immunostaining and lipidomic analysis were performed in the pancreas.
RESULTS
STZ-induced fat-1 mice did not develop hyperglycemia compared with wild-type mice, and β-cell destruction was prevented as evidenced by lack of histological pancreatic damage or reduced insulin level. The prevention of β-cell destruction was associated with no proinflammatory cytokine induction (tumor necrosis factor-α, interleukin-1β, inducible nitric oxide synthase) in the pancreas, a decreased NF-κB, and increased IκB pancreatic protein expression. In the fat-1–treated mice, proinflammatory arachidonic-derived mediators as prostaglandin E2 and 12-hydroxyeicosatetraenoic acid were decreased and the anti-inflammatory lipoxin A4 was detected. Moreover, the 18-hydroxyeicosapentaenoic acid, precursor of the anti-inflammatory resolvin E1, was highly increased.
CONCLUSIONS
Collectively, these findings indicate that fat-1 mice were protected against MLD-STZ–induced diabetes and pointed out for the first time in vivo the beneficial effects of n-3 PUFA at the pancreatic level, on each step of the development of the pathology—inflammation, β-cell damage—through cytokine response and lipid mediator production.
β-Cells, the principal constituents of islets of Langerhans, control whole body metabolic fuel homeostasis by secreting insulin in response to elevations in plasma glucose concentration. Experimental multiple low-doses streptozotocin (MLD-STZ)–induced diabetes is characterized by extreme insulin deficiency as a result of a decrease in the number of functional β-cells (1,2) by a direct toxic effect of STZ on β-cells and inflammatory reaction against damaged β-cells. Reactive oxygen species (ROS) and nitrogen species such as nitric oxide (NO) specifically toxic to β-cells (3,4) are then produced, leading to β-cell destruction and reduced insulin secretion. Transcription factors, such as nuclear factor-κB (NF-κB), induce the expression of proinflammatory cytokines and enzymes that are critically involved in the pathogenesis of chronic inflammatory diseases including type 1 diabetes (5).
Both genetic and environmental factors are involved in the etiology of type 1 diabetes and dietary factors, and among them polyunsaturated fatty acids (PUFA) are prime candidates for environmental modulators of type 1 diabetes (6). Currently, n-6 PUFA comprise a major part of the fatty acid intake in Western-style diets (7) leading to a relative deficiency in n-3 PUFA, which may predispose to increased risk of inflammatory diseases, such as type 1 diabetes. Indeed, the n-6 PUFA arachidonic acid (AA) is metabolized in activated cells into diverse proinflammatory eicosanoids. Among them, 12-hydroxyeicosatetraenoic acid (12-HETE), generated upon 12-lipoxygenase (LO) activation, is directly toxic to β-cells leading to decreasing insulin secretory function and β-cell death (8). Resistance to type 1 diabetes induction in 12/15-LO knockout mice was recently observed (9). Conversely, lipoxins (LX) are endogenous eicosanoids synthesized locally from AA at sites of inflammation and exhibit proresolving activities. Among them, LXA4 can counteract inflammation in different cell and animal models. LX are considered as endogenous stop signals for inflammation (10–12).
There is growing evidence that dietary n-3 PUFA can be involved in diabetes prevention (13) in reducing the activity of proinflammatory processes (14) in both animals and humans (15–17). Among them, eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) are potent immunomodulators and are equipotent in inhibiting interleukin (IL)-2 production in mice (18). Suresh and Das (19) showed that several n-3 and n-6 PUFA and their eicosanoid metabolites alter the susceptibility of alloxan-induced diabetes in rat. These observations suggest that n-3 PUFA may lower inflammation susceptibility and dampen the inflammatory response in pancreatic tissue by suppressing cytokine production. Lipidomic approaches have demonstrated that potent anti-inflammatory mediators are generated from EPA and DHA (20–23). These newly discovered mediators, termed resolvins and protectins, are involved in the resolution of inflammation and have been shown to inhibit NF-κB activity (20).
Very recently, an in vitro study using two cellular models evaluated the direct impact of n-3 PUFA on the function and viability of pancreatic β-cells (23). The authors showed a strong resistance to the destruction of the cells treated by cytokines by stable cellular production of n-3 PUFAs in mfat-1–transfected β-cells. The in vivo relevance of such conclusions remains to be explored as well as the efficiency of high pancreatic n-3 PUFA in alleviating insulin-dependent diabetes.
Transgenic fat-1 mice carry a C. Elegans gene, fat-1, encoding an n-3 fatty acid desaturase catalyzing the conversion of n-6 to n-3 PUFA (24). There is a remarkable difference in the n-6–to–n-3 PUFA ratio in tissues from wild-type (WT) versus fat-1 transgenic mice (20 to 50 and close to 1, respectively) fed diets high in n-6 and low in n-3 PUFA (24). A single diet can therefore be used to generate mice with different fatty acid profiles (high and low n-6–to–n-3 ratios) eliminating potential confounding dietary factors and allowing in vivo investigation on the role of n-6–to–n-3 ratio in the destruction of pancreatic β-cells.
Thereby, we used fat-1 transgenic mice to determine whether endogenously synthesized n-3 PUFA could be β-cell protective in MLD-STZ, and we then evaluated the mechanisms involved at the pancreatic level in such diabetes prevention.
RESEARCH DESIGN AND METHODS
Animals and diets.
Transgenic fat-1 mice were generated as described previously (24) and backcrossed onto a C57BL/6 J background. We used male fat-1 transgenic mice and nontransgenic littermate controls (14 weeks old, 20–25 g) since male and female mice have different susceptibilities to STZ (25). The presence of the fat-1 gene in each mouse was confirmed both by genotyping and tail fatty acid analysis profiles. Transgenic and WT animals were maintained on a 10% safflower oil diet (INRA Jouy-en-Josas, France) ad libitum and housed in temperature- and humidity-controlled conditions with a 12-h light/dark cycle, under pathogen-free conditions. The diet contained (g/100 g diet) 4.5 sucrose, 18.8 casein, 51 corn starch, 0.3 dl-methionine, 3.8 mineral mix, 2 vitamin mix, and 10 safflower oil. Safflower oil is high in linoleic acid (18:2n-6) with very little n-3 fatty acids (less than 0.1% of the total fat supplied). Under the 10% safflower oil regimen, all the transgenic animals presented a total n-6–to–n-3 PUFA ratio greater than (but close to) 1 in their tail tissue (n-6/n-3 = 1.5 ± 0.2 in fat-1 mice vs. 34.1 ± 3.8 in WT control animals; n = 11 per group). All procedures followed institutional guidelines for the use and care of laboratory animals and were approved by the Ethical Committee of the University of Burgundy (#Bk 0611).
MLD-STZ administration.
Diabetes was induced by MLD-STZ, as described previously (26). Briefly, STZ (2-deoxy-2–3-[methyl-3-nitrosoureido]-d-glucopyranose; Sigma, St. Louis, MO) was dissolved in 0.1 mol/L sodium citrate buffer (pH 4.5) and injected intraperitoneally, within 15 min of preparation, at a dose of 45 mg/kg/day for 5 consecutive days to produce a β-cell destruction model. Control WT and transgenic mice were injected with citrate buffer as vehicle. Blood glucose level was measured, in nonfasted animals, in venous blood using a glucometer (One Touch Vita; LifeScan, Issy les Moulineaux, France). Mice were evaluated every 2 days at 2:00 p.m. and were considered diabetic when blood glucose levels exceeded 250 mg/dL, usually 7 to 9 days after the last STZ injection. All mice were killed in blind for tissue collection as follows: 3 days after the fifth STZ injection, four mice per group were killed and cytokines, monocyte chemoattractant protein (MCP)-1, GLUT2 pancreas mRNA expression, and NF-κB p65 and inhibitor of κB (IκBα) pancreas protein expression were determined; 9 days after the fifth STZ injection, three mice per group were killed and the pancreatic islets were immunostained for insulin and cleaved caspase-3; the remaining animals (four mice) were killed 20 days after the fifth STZ injection and NF-κB p65 protein expression was determined.
Blood collection and plasma insulin assays.
Before mice were killed, 800 μL of blood were collected from each mouse anesthetized by Isofluran (TEM, Bordeaux, France) from the retro-orbital vein. Plasma was snap-frozen in liquid nitrogen and stored at −80°C until plasma insulin concentration determination was performed using a mouse ELISA kit (Abcys SA, Paris, France).
Tail, pancreas, and plasma fatty acid composition.
The fatty acid composition in tail (to perform the phenotyping), pancreatic tissue, and plasma was determined by gas chromatography as described previously (27–29).
Lipidomic analysis.
Lipidomic analyses were performed according to Masoodi et al. (22). All pancreatic tissue was homogenized in ice-cold methanol and diluted with ice-cold water to 15% (vol/vol). Internal standards (40 ng PGB2-d4 and 80 ng12-HETE-d8) (Cayman Chemicals, Ann Arbor, MI) were added to each sample. These suspensions were centrifuged; the clear supernatants acidified to pH 3 and immediately applied to preconditioned solid phase extraction cartridges (C18-E, Phenomenex, Macclesfield, U.K.) to extract the lipid mediators. Chromatographic analysis was performed on a C18 column (Luna 5μ, Phenomenex) using a Waters Alliance 2695 high-performance liquid chromatography (HPLC) pump coupled to an electrospray (ESI) triple quadrupole Quattro Ultima mass spectrometer (Waters, Elstree, Hertsfordshire, U.K.). Instrument control and data acquisition were performed using MassLynx V4.0 software. The following multiple reaction monitoring transitions were used for the assay of lipid mediators and internal standards: prostaglandin E2 (PGE2) m/z 351 > 271; PGE3 m/z 349 > 269; LXA4 m/z 351 > 217; 12-HETE m/z 319 > 179; 18-hydroxyeicosapentaenoic acid (18-HEPE) m/z 317 > 133; PGB2-d4 m/z 337 > 179; 12-HETE-d8 m/z 327 > 184. Results are expressed as picograms metabolite per milligram of protein based on calibration lines constructed with commercially available eicosanoid standards (Cayman Chemicals). Protein content was estimated using the Bio-Rad protein assay kit with BSA as standard (Bio-Rad, Hemel Hempstead, U.K.).
Histological and immunohistochemical analysis.
Immunohistochemistry was performed on formalin-fixed, paraffin-embedded pancreas sections stained with hematoxylin and eosin. Sections were deparaffinized and dehydrated in a graded series of ethanol washes. Tissue sections were subjected to heat-induced epitope retrieval (0.1 M citrate buffer boiled in a 600-watt microwave), then cooled at room temperature for 20 min. After washing in PBS (pH 7.4), endogenous peroxidase was quenched using a 3% solution (v/v) of hydrogen peroxidase and PBS. After blocking nonspecific staining with PBS/3%BSA, sections were incubated with primary antibodies (overnight at 4°C for both cleaved caspase-3 and insulin antibodies). Sections were then incubated with secondary antibody, washed again in PBS, and incubated for 20 min with horseradish peroxidase-3-amino-9-ethylcarbazole detection solution. Slides were counterstained with hematoxylin. Antibodies for insulin (C27C9) and cleaved caspase-3 (Asp 175) were obtained from Cell Signaling Technology (Ozyme, Saint-Quentin-en-Yvelines, France) and used at a concentration of 1/800 and 1/100, respectively.
The islet size from STZ-induced and untreated animals has been compared using the following procedure: images of islets were acquired on the Cell Station of CellImaP Plateform (IFR100, Dijon, France). Briefly, this station is made of an inverted motorized microscope (Axiovert 200M, Carl Zeiss, Le Pecq, France) equipped with an Axiocam. Image analysis was done using Axiovision software. More precisely, islets were surrounded using polygonal lasso and surfaces were recorded. Only islets bigger than 20,000 μm2 were counted.
RT-PCR analysis.
Total RNA was extracted from snap-frozen pancreata using Tri-Reagent (Euromedex, Souffelweyersheim, France). RNA quantity was measured at 260 nm and purity estimated by the 260-to-280 ratio. Extracted RNAs were reverse-transcribed to cDNA using random primers, and a reverse transcriptase system (Invitrogen, Cergy Pontoise, France) and amplified by PCR. RT-PCR products were quantified using a densitometer and image analyzer (Bio-Rad, Ivry-sur-Seine, France). β-Actin gene primers were used as an internal control. RT-PCR was carried out using the following mouse specific primers: TNF-α(F-5′CCACATCTCCCTCCAGAAAA3′, R-5′AGGGTCTGGGCCATAGAACT3′), IL-1β (F-5′CTCACAAGCAGAGCACAAGC-3′, R-5′CTCAGTGCAGGCTATGACCA-3′), inducible nitric oxide synthase (iNOS) (F-5′ACAGCCTCAGAGTCCTTCAT3′, R-5′TTGTCACCACCAGCAGTAGT-3′), MCP-1 (F-5′AGCACCAGCCAACTCTCACT-3′, R-5′TCTGGACCCATTCCTTCTTG-3′), GLUT2 (F-5′CGGAATGGTCGCCTCATT-3′, R-5′CAGTCCTGATACACTTCGTC-3′), FAT-1 (F-5′CACTCTTCTCTCCCTACTTCC-3′, R-5′AGCTCCATTCATCAGCCTC-3′), and β-actin (F-5′GAAATCGTGCGTGACATC-3′, R-5′GCTTGCTGATCCACATCT-3′).
Western blot analysis.
Pancreatic tissue (30 mg) was homogenized in Triton protein lysis buffer (20 mM Tris, 150 mM NaCl, 200 μM EDTA, 200 μM EGTA, 1% Triton X-100) containing protease and phosphatase inhibitors (Sigma). Proteins (40 μg) were separated by 10% SDS-PAGE and electroblotted to Protran nitrocellulose membranes (Whatman, Dassel, Germany). After blocking nonspecific binding sites with 5% nonfat milk in Tris buffered saline (TBS) (0.1% Tween-20 in TBS), blots were probed overnight at 4°C with primary antibody against NF-κB p65 and IκBα (Cell Signaling, Ozyme) and β-actin (Sigma-Aldrich, Saint-Quentin Fallavier, France) at a concentration of 1/200, 1/800, and 1/5,000, respectively, washed in T-TBS, incubated 1 h at room temperature with horseradish peroxidase-conjugated goat anti-rabbit IgG for NF-κB p65 and goat anti mouse IgG for IκBα and β-actin (Jackson ImmunoResearch Laboratories, West Grove, PA). Detection was performed using the enhanced chemiluminescence (ECL) Western blotting analysis procedure (ECL Plus, Amersham, Freiburg, Germany).
Statistical analysis.
Results were expressed as the arithmetical mean and SE (mean ± SE) for each group. To determine significant differences, means ± SE were analyzed using ANOVA and the Newman-Keuls test. Statistical significance in the pancreas major fatty acid composition, total n-6, total n-3, and the n-6–to–n-3 ratio between WT and fat-1 transgenic mice was determined using a Student t test.
RESULTS
MLD-STZ–induced fat-1 transgenic mice do not develop hyperglycemia.
No significant body weight change was observed in WT and fat-1 mice after STZ treatment. One week after the last injection of STZ, STZ-induced mice started to develop hyperglycemia, which persisted for the entire observation period (25 days). As shown Fig. 1, glycemia increased in all the WT STZ-induced mice throughout the study period; in contrast, glycemia in all the fat-1 STZ-induced mice remained unchanged and was identical to that in WT and fat-1 citrate-treated mice. In the WT STZ-induced group, the mean blood glucose concentration was 320 mg/dL, with no body weight change, whereas in the STZ-induced fat-1 mice and the control citrate-treated mice this mean was 130 mg/dL.
FIG. 1.
n-3 fatty acid enrichment protects animals from MLD-STZ–induced hyperglycemia. Blood glucose level was measured in nonfasted WT and transgenic animals given STZ or STZ-vehicle as control (n = 4). Results are presented as a mean ± SE. Differences were analyzed by the Newman-Keuls test. Means assigned a superscript letters (a, b, c) were statistically different at P < 0.05. WC, citrate-treated WT mice; WS, STZ-induced WT mice; FC, citrate-treated fat-1 mice; FS, STZ-induced fat-1 mice.
n-3 enrichment protects fat-1 transgenic mice from MLD-STZ–induced β-cell damage.
STZ induced severe degenerative and necrotic changes and islet shrinkage in WT mice compared with the citrate; in contrast, no histological changes were observed in STZ-induced fat-1 mice compared with the WT or fat-1 citrate-treated mice (Fig. 2A).
FIG. 2.
n-3 fatty acid enrichment protects animals from MLD-STZ–induced β-cell damage. A: Representative hematoxylin and eosin (H&E)-stained sections analysis showing islet morphology of WT (top) and fat-1 transgenic (bottom) mice (n = 3). B: Representative effect of STZ administration on pancreas GLUT2 gene expression (n = 4). C: Representative immunohistochemistry for insulin in the pancreatic islets of WT (top) and fat-1 transgenic (bottom) mice (n = 3). Islet quantification of WT and fat-1 mice presented as percentage of islets with an area bigger than 20,000 μm2. Plasma insulin concentration in control or STZ-injected WT and transgenic mice (n = 4) is shown. D: Representative immunohistochemistry for cleaved caspase-3 in the pancreatic islets of the WT (top) and fat-1 transgenic (bottom) mice (n = 3). Results are presented as a mean ± SE. Differences were analyzed by the Newman-Keuls test. Means assigned different superscript letters (a, b, c) (D) were statistically different at P < 0.05. For A, C, and D, results were obtained at day 9 after the fifth STZ injection. WC, citrate-treated WT mice; WS, STZ-induced WT mice; FC, citrate-treated fat-1 mice; FS, STZ-induced fat-1 mice. (A high-quality digital representation of this figure is available in the online issue.)
The effect of STZ administration on pancreatic GLUT2 gene expression was also assessed, since STZ is taken up via GLUT2. GLUT2 mRNA expression was decreased in STZ-induced WT mice compared with citrate-treated animals (Fig. 2B).
β-Cell insulin level was assessed by immunostaining pancreatic tissue, and pictures of representative islets are shown (Fig. 2C). Pancreas sections from citrate-treated WT and fat-1 mice showed islets with normal insulin content of insulin-expressing cells. As expected, diabetic STZ-induced WT mice showed lower insulin content and increased islet destruction. In contrast, STZ-induced fat-1 mice showed large islets with normal insulin content similar to the one observed in vehicle-treated animals. The number of islets bigger than 20,000 μm2 was decreased by 70% in WT STZ-induced mice compared with WT citrate-treated animals. The percentage of islets bigger than 20,000 μm2 was much higher in citrate-treated fat-1 mice compared with WT (+120%) and decreased by 33% when fat-1 mice were given STZ. Nevertheless, this percentage remained higher in fat-1 STZ-induced mice than in WT citrate-treated animals.
On day 25, the plasma insulin level (Fig. 2C) was dramatically decreased in WT STZ-induced mice, whereas this was prevented in fat-1 mice. Meanwhile, the plasma insulin level was significantly higher in citrate-treated transgenic mice compared with WT; however, when fat-1 mice were given STZ, the insulin level decreased to that of WT citrate-treated mice.
To further determine whether fat-1 mice were protected against MLD-STZ–induced pancreatic damage, the presence of apoptotic β-cells in pancreas sections was assessed by immunostaining for cleaved caspase-3, and pictures of representative islets are shown (Fig. 2D). Islets from STZ-induced WT mice showed a marked increase in apoptotic β-cells compared with islets of citrate-treated mice (Fig. 2D). However, STZ-induced fat-1 mice showed that the levels of apoptotic cells were identical to those of citrate-treated fat-1 mice and were even lower than in sections from citrate-treated WT mice.
Pancreas n-3 fatty acid enrichment inhibits proinflammatory cytokines, NF-κB protein expression and increases IκBα protein expression in MLD-STZ–induced fat-1 transgenic mice.
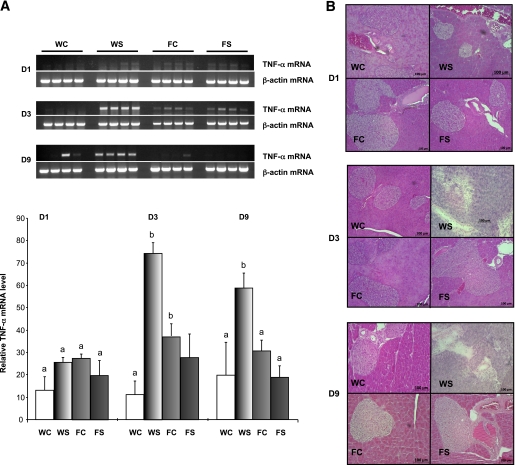
A preliminary time-course study (days 1, 3, and 9 after the last STZ injection) has been performed to estimate the initiation and development of the inflammatory process and β-cell damage. Our results show that TNF-α mRNA expression was increased from day 3 and remained highly expressed at day 9 in the WT STZ-induced mice, when no expression of this proinflammatory cytokine was observed in the fat-1 animals, whatever the treatment (Fig. 3A). Moreover, in parallel to the induction of TNF-α expression, we observed an islet damage progression (Fig. 3B).
FIG. 3.
A: Time course mRNA level for TNF-α in pancreas collected 1, 3, and 9 days after the last STZ injection, from WT and transgenic animals injected with multiple low doses of streptozotocin or citrate as vehicle. B: Representative hematoxylin and eosin (H&E)-stained pancreas sections analysis, showing islet morphology of WT (top) and fat-1 transgenic (bottom) mice (n = 3). Differences were analyzed by Newman-Keuls test. Bars assigned different superscript letters (a, b, c) were statistically different at P < 0.05; n = 4 mice in each group. WC, citrate-treated WT mice; WS, STZ-induced WT mice; FC, citrate-treated fat-1 mice; FS, STZ-induced fat-1 mice. (A high-quality digital representation of this figure is available in the online issue.)
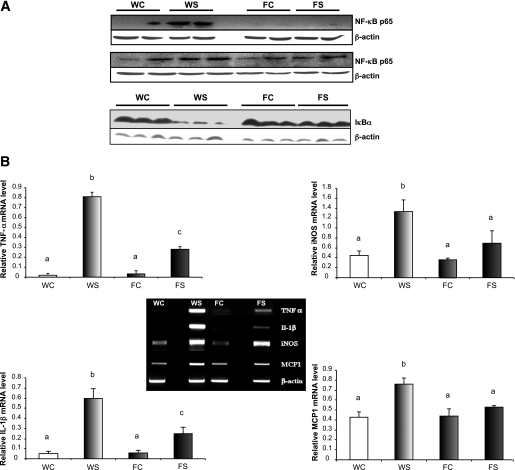
NF-κB activation plays a critical role in proinflammatory cytokine expression. We, therefore, examined pancreatic expression of the NF-κB p65 protein to determine the underlying mechanisms involved in inhibition of STZ-induced inflammatory responses by fat-1 transgenic mice. Our results show (Fig. 4A) that the expression of the NF-κB p65 subunit was greatly increased in STZ-induced WT mice but not in STZ-induced fat-1 mice, suggesting that the endogenous n-3 fatty acid enrichment in the latter inhibited induction of NF-κB p65. Moreover, IκB protein expression, the NF-κB repressor, was highly repressed in WT STZ-induced mice but not in STZ-induced fat-1 mice.
FIG. 4.
Pancreas n-3 fatty acid enrichment inhibits expression of NF-κB p65 and proinflammatory cytokines and increases expression of IκBα in fat-1 transgenic mouse model. A: Representative effect of STZ administration on pancreas NF-κB (p65) and IκBα protein expression. B: mRNA levels for proinflammatory cytokines and chemokine in pancreas from WT and transgenic animals. TNF-α, IL-1β, iNOS, MCP-1, and β-actin mRNA levels from pancreas of animals that have been injected with MLD-STZ or citrate as control are shown. Differences were analyzed by Newman-Keuls test. Bars assigned different superscript letters (a, b, c) were statistically different at P < 0.05; n = 4 mice in each group. In A, for NF-κB (p65) results were obtained at day 3 (top) and day 20 (bottom) after the fifth STZ injection. For IκBα, results were obtained at day 3 after the fifth STZ injection. For B, results were obtained at day 3 after the fifth STZ injection. WC, citrate-treated WT mice; WS, STZ-induced WT mice; FC, citrate-treated fat-1 mice; FS, STZ-induced fat-1 mice.
In view of the preliminary results shown in Fig. 3A and in order to gain insight into the mechanisms underlying the protection of fat-1 mice from STZ-induced diabetes, we next examined at day 3 pancreatic mRNA expression of the inflammatory cytokines TNF-α/IL-1β, the expression of the chemokine MCP-1, and iNOS. As shown in Fig. 4B, the fat-1 mice showed a significant reduction in TNF-α, IL-1β, and iNOS mRNA expression compared with WT mice. Although MCP-1 gene expression was significantly increased in STZ-induced WT mice, it remained quite unchanged in STZ-induced fat-1 mice, as compared with citrate-treated groups.
Pancreas n-3 fatty acid enrichment and formation of PUFA-derived mediators.
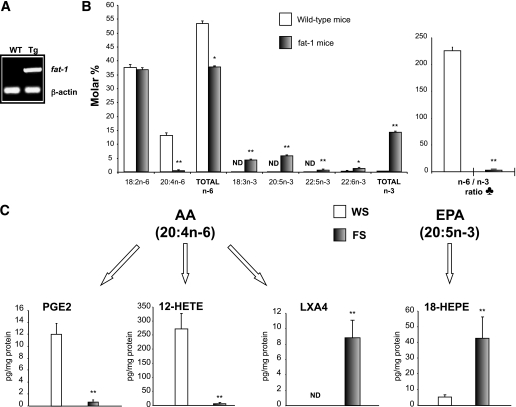
As shown in Fig. 5A, the fat-1 gene was well expressed in the pancreatic tissue of transgenic animals. Pancreas total lipid fatty acid compositional analysis revealed high increases in alpha-linolenic acid (18:3 n-3) EPA (20:5 n-3), and docosapentaenoic acid (DPA; 22:5 n-3) in fat-1 transgenic mice compared with WT mice, whereas AA (20:4 n-6) was decreased by 97% (Fig. 5B). In addition, the ratio of n-6 PUFA to the long chain n-3 PUFA was drastically reduced (P < 0.01) in fat-1 pancreatic tissue (2.6 ± 0.4), compared with WT mice (225.8 ± 6.7). These data indicate that n-3 fatty acid desaturase expression enriched fat-1 pancreatic tissue in n-3 PUFA at the expense of n-6, giving a more balanced n-6–to–n-3 ratio.
FIG. 5.
A: Representative RT-PCR for fat-1 and β-actin pancreas expression. Tg, fat-1 transgenic mice. B: Pancreas major fatty acids composition, total n-6, total n-3, and n-6–to–n-3 ratio are indicated for untreated WT and fat-1 transgenic mice as white and gray bars, respectively (mean ± SE). *P < 0.05; **P < 0.01 (Student t test); n = 11 per group. ND, not detected. ♣, The n-6–to–n-3 ratio is given by (18:2 n-6 + 20:4 n-6 + 22:4 n-6 + 22:5 n-6)/(18:3 n-3 + 20:5 n-3 + 22:5 n-3 + 22:6 n-3). C: Presence of different lipid mediators in pancreas samples of STZ-induced WT (n = 4) and fat-1 transgenic mice (n = 4). **P < 0.01 (Student t test).
We assessed PUFA-derived bioactive mediators, particularly 12- and 15-LO products from the pancreas (Fig. 5C). Although no n-3 PUFA–derived mediators were detected in the WT pancreas, some were present in the fat-1 pancreas. Regarding AA-derived mediators, the proinflammatory PGE2 was decreased by 95% in the transgenic STZ-induced mice and the toxic β-cell LO product, 12-HETE, was reduced by 97% in the fat-1 when compared with the STZ-induced WT mice. Moreover, the anti-inflammatory LXA4, undetected in WT, was detected in the fat-1 STZ-induced mice. Among the EPA-derived mediators, only 18-HEPE, precursor for the biosynthetic pathway of the anti-inflammatory resolvin E1, was detected in STZ-induced fat-1 mice, and its concentration was increased eightfold compared with the WT animals.
Plasma n-3 fatty acid enrichment and total lipid level.
Plasma total lipid fatty acid compositional analysis revealed huge increases in EPA and DPA in fat-1 transgenic mice compared with WT mice, whereas AA (20:4 n-6) was highly decreased (Fig. 6A). STZ administration did not affect the fatty acid composition of both WT and fat-1 mice. In addition, the ratio of n-6 PUFA to the n-3 PUFA was drastically reduced (P < 0.05) in fat-1 plasma, compared with WT mice (Fig. 6B). This data confirm that plasma was enriched in n-3 PUFA at the expense of n-6 PUFA, giving a more balanced n-6–to–n-3 ratio, when plasma total lipid level was not statistically changed in all groups (Fig. 6C).
FIG. 6.
A: Plasma major fatty acids composition, total n-6, and total n-3 are indicated for untreated and STZ-induced WT and fat-1 mice (n = 5). B: Plasma fatty-acids ratios in untreated and STZ-induced WT and fat-1 mice (n = 5). C: Plasma total lipid level in untreated and STZ-induced animals. Results are presented as a mean ± SE. Differences were analyzed by the Newman-Keuls test. Means assigned different superscript letters (a, b, c) were statistically different at P < 0.05.
DISCUSSION
These results clearly demonstrate that increasing the pancreatic levels of endogenously synthesized n-3 PUFA prevents MLD-STZ–induced diabetes in fat-1 transgenic mice and that this effect is associated with less activation of markers of the inflammatory response. Furthermore, the protection from type 1 diabetes in fat-1 mice is correlated with the formation of anti-inflammatory derivatives of n-3 fatty acids and with downregulation of NF-κB p65 and proinflammatory cytokine expression in the pancreatic tissue of these animals.
More than alleviating chemically induced diabetes, our results show that endogenous production of n-3 fatty acids in fat-1 transgenic mice totally prevents hyperglycemia (Fig. 1). Animal studies have already suggested that PUFA might reduce the risk of chemically induced diabetes and attenuate the oxidant stress in animal models (30,31). Nevertheless, n-3 feeding is unable to mimic the protection observed in the fat-1 mice (19,32). Indeed, in these reports WT animals fed n-3 did not show similar phenotype than the fat-1 mice, which were totally resistant to MLD-STZ–induced diabetes. This can be explained by the fact that the fat-1 mice present the ideal n-6–to–n-3 ratio of about 1—only achievable in WT animals by consuming foods containing this ratio—without introducing stringent dietary changes, which is not the case in the above dietary experiments. Additionally, inconsistent and/or controversial outcomes may be the result of confounding dietary factors. Many variables can arise from the diet and feeding procedure, including impurities in the oils used, food storage, and diet duration, all of which can affect the tissue fatty acid profile. In contrast, the genetic approach presented in this study allows us to generate two different plasma and pancreatic fatty acid profiles exhibiting a balanced ratio of n-6–to–n-3 PUFA (Fig. 5B, 6A and B) using a single diet rich in linoleic acid (18:2 n-6) but lacking n-3 fatty acids.
STZ is taken up by pancreatic β-cells via GLUT2, which is expressed at high levels in β-cells. We found that GLUT2 was expressed similarly in fat-1 and in WT mice (Fig. 2B). Moreover, GLUT2 mRNA expression was decreased in STZ-induced WT mice compared with citrate-treated animals, which agree with an alteration of β-cells. Nevertheless, we observed a slight decrease (not statistically different) of GLUT2 mRNA expression in fat-1 STZ-induced mice versus fat-1 controls, consistent with studies reporting that a reduced GLUT2 expression can prevent the diabetogenic action of STZ (33,34). Additionally, STZ itself restricts GLUT2 expression in vivo and in vitro when administered in multiple doses (35).
The dramatic decrease in WT STZ-induced mice plasma insulin level (Fig. 2C) observed in the current study is consistent with the usually described STZ fragmentation of DNA, which induces destruction of the insulin-producing β-cells (mainly by apoptosis), leading to reduced insulin secretion and thus to hyperglycemia (1,25). STZ-induced fat-1 mice showed large islets with no apoptotic β-cells and no decrease in insulin level (Fig. 2C and D). The higher plasma insulin level as well as bigger islets observed in the fat-1 transgenic mice compared with WT agrees with data reported recently on mfat-1 isolated islets (23). These authors explained this phenomenon by reduced level of PGE2, being known as a negative regulator of insulin production. Accordingly, in our study, such reduced PGE2 level is also observed in the fat-1 mice pancreas (Fig. 5C), in relation to higher insulin level. This suggests that the control of inflammation through reduction of n-6 PUFAs can be beneficial on β-cell function.
This demonstrates that n-3 fatty acid enrichment of plasma and the pancreas protects the fat-1 mice from STZ-induced β-cell destruction. We can then conclude that there is a relationship between n-3 PUFA levels and protection from hyperglycemia.
Type 1 diabetes is thought to result from perturbed immune regulation. STZ promotes immune cell invasion of the islet and generally causes pancreatic inflammation known as insulitis (36), generated by cytokines and free radicals. Despite C57Bl/6 J background being sensitive to hyperglycemic action of STZ, it has been reported that this model is resistant to STZ-induced insulitis (25). The present experiment confirms such data, and recent observations on mouse insulinoma 6 cells (data not shown) revealed higher TNF-α expression after STZ administration indicating increased inflammation, independently to immune cell invasion. In the current study, prevention of hyperglycemia in fat-1 mice was accompanied by downregulation of NF-κB p65, TNF-α, IL-1β, MCP-1, and iNOS mRNA expression (Fig. 4), suggesting that NF-κB plays an important proapoptotic role in cytokine-induced β-cell destruction. Interestingly, our results also show a higher expression of IκBα in the fat-1 mice than in WT, suggesting that a high n-3 PUFA pancreatic level would be able to overexpress IκBα to prevent NF-κB activation. Recently, Eldor et al. (37) reported that a conditional and specific NF-κB blockade protects pancreatic β-cells from diabetogenic agents, showing that β-cell–specific activation of NF-κB is key in the progressive loss of β-cells in diabetes.
It is becoming increasingly clear that n-3 PUFA exert their effect on inflammatory gene expression through direct actions on the intracellular signaling pathways that lead to NF-κB activation (38). Our results can be related to a significant decrease in NF-κB activity observed in the colon of fat-1 mice with colitis (21) and colon tumors (39). Moreover, EPA-derived resolvin E1 is able to inhibit TNF-α–induced NF-κB activation (20).
TNF-α and IL-1β play a central role in regulating β-cell destruction in the pancreas (40), and TNF-α and IL-1β inhibitors prevent diabetes in mouse models (41,42). Our findings are consistent with previous studies in animal models and humans, showing that n-3 PUFA decrease TNF-α and IL-6 production (15,43,44), and with recent studies showing a lower TNF-α production in fat-1 mice during inflammation (21,38,45), confirming the function of the n-3 PUFA–derived compounds as anti-inflammatory mediators (20,21).
Both EPA and DHA decrease agonist-induced activation of NF-κB, which might play a role in reducing MCP-1 gene expression (46). We found decreased MCP-1 mRNA expression in transgenic mice (Fig. 4B); we propose that this MCP-1 downregulation is critically involved in the beneficial effects of endogenous n-3 fatty acids, along with downregulation of proinflammatory cytokines. Several of the deleterious effects of cytokines on rodent pancreatic islets are mediated by NO, which is produced by the inducible form of iNOS (47). Our results agree with previous reports showing attenuation of iNOS in an lipopolysaccharide-stimulated macrophages model by n-3 PUFA through NF-κB inhibition (48). Here we show that cytokine-induced NF-κB–mediated iNOS expression was significantly lower in the pancreas of fat-1 transgenic mice compared with WT (Fig. 4B).
The inflammatory process alleviation observed in the current study occurs via mechanisms similar to data recently obtained in vitro on islets, isolated from mfat-1 mice and then exposed to proinflammatory cytokines (23). Such islets showed a strong resistance to cytokine-caused destruction comparable with the present one. These interesting data observed in vitro needed to be explored in vivo in chemically induced diabetic conditions; our present data evidence that fat-1 expression and its consequent pancreas enrichment in n-3 fatty acids is efficient in deterring diabetes by protecting from the STZ-cellular destruction.
Another significant finding of the current study was large differences in the levels of the AA (notably 12-HETE) and EPA-derived mediators in fat-1 mice compared with controls. 12-HETE production has been linked to diabetes (9,49,50). It can activate NF-κB and is directly toxic to β-cells, markedly decreasing insulin secretion and increasing β-cell death (8). These observations can be related to the huge difference in n-6–to–n-3 fatty acid ratio between the transgenic and WT mice (Fig. 5B). Furthermore, the increased production of AA-derived LXA4, in the fat-1 tissue, indicates an overall shift from AA-derived proinflammatory metabolites to an anti-inflammatory and proresolving profile. LXA4 is formed by either transcellular metabolism of AA through two sequential lipoxygenation steps or from 15-HETE esterified in cellular phospholipids (51), a mechanism that has been linked to disease or host defense and that may be preferentially activated in the fat-1 mice. Additionally, EPA can compete with AA as substrate for cyclooxygenase (COX)-2, resulting in reduced levels of PGE2 and increased levels of PGE3. PGE3 was detected, albeit at very low levels, in fat-1 mice pancreatic tissue although it was not found in WT mice. However, the concentration of PGE3 did not reach that of PGE2, suggesting that there is a role for AA-derived lipid mediators that cannot be totally replaced by EPA-derived lipid mediators.
Taken together, our results evidence for the first time that fat-1 expression and its consequent pancreas enrichment in n-3 fatty acid prevents chemically induced diabetes. This prevention occurs by downregulating proinflammatory cytokine gene expression, blocking NF-κB activation, and highly repressing proinflammatory PUFA-derived lipid mediators in the pancreas of fat-1 mice versus WT. If pancreatic n-3 fatty acid enrichment is found to be effective in preventing insulin-dependent diabetes in humans, as was the case in mice in the current study, a nutritional cost-effective intervention could benefit young people affected by this disease, since there is currently no clinically useful preventive measure against developing autoimmune type 1 diabetes.
ACKNOWLEDGMENTS
J.B. received support from the French Ministry of Education and Research, INSERM, and from the Région Bourgogne. This work was also partially supported by a grant from the International Foundation for the Promotion of Nutrition Research and Nutrition Education (ISFE).
No potential conflicts of interest relevant to this article were reported.
J.B. conceived and designed the experiments, researched data, wrote the manuscript, contributed to discussion, and reviewed and edited the manuscript. S.B. researched data and contributed to discussion. A.B. and K.A.M. researched data. A.N. contributed to discussion and reviewed and edited the manuscript. M.R. and C.T. contributed to discussion. J.X.K. contributed to discussion and reviewed and edited the manuscript. M.N. conceived and designed the experiments, wrote the manuscript, contributed to discussion, and reviewed and edited the manuscript.
J.B. thanks Joseph Gresti (UMR 866 Physiopathologie des Dyslipidémies, Dijon, France) for his experience in chromatography and his help in lipid analysis, André Bouchot (CellImap Plateform, IFR100, Dijon, France) for immunohistochemistry pictures and islet size quantification, and Olivier Michaud (UMR 866, Dijon, France) for his help in the complementary experiments required for the study of NF-κB pathway. The authors also thank Fanny Guinot (UMR 866 Physiopathologie des Dyslipidémies, Dijon, France) for technical assistance in glycemia and plasma insulin level measurements.
REFERENCES
- 1.Pighin D, Karabatas L, Pastorale C, et al. Role of lipids in the early developmental stages of experimental immune diabetes induced by multiple low-dose streptozotocin. J Appl Physiol 2005;98:1064–1069 10.1152/japplphysiol.00559.2004 [DOI] [PubMed] [Google Scholar]
- 2.Karabatas LM, Pastorale C, de Bruno LF, et al. Early manifestations in multiple-low-dose streptozotocin-induced diabetes in mice. Pancreas 2005;30:318–324 10.1097/01.mpa.0000161888.02244.7a [DOI] [PubMed] [Google Scholar]
- 3.Kim WH, Lee JW, Gao B, Jung MH. Synergistic activation of JNK/SAPK induced by TNF-alpha and IFN-gamma: apoptosis of pancreatic beta-cells via the p53 and ROS pathway. Cell Signal 2005;17:1516–1532 10.1016/j.cellsig.2005.03.020 [DOI] [PubMed] [Google Scholar]
- 4.Emre Y, Hurtaud C, Karaca M, Nubel T, Zavala F, Ricquier D. Role of uncoupling protein UCP2 in cell-mediated immunity: how macrophage-mediated insulitis is accelerated in a model of autoimmune diabetes. Proc Natl Acad Sci USA 2007;104:19085–19090 10.1073/pnas.0709557104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Makarov SS. NF-kappaB as a therapeutic target in chronic inflammation: recent advances. Mol Med Today 2000;6:441–448 10.1016/S1357-4310(00)01814-1 [DOI] [PubMed] [Google Scholar]
- 6.Knip M, Veijola R, Virtanen SM, Hyöty H, Vaarala O, Akerblom HK. Environmental triggers and determinants of type 1 diabetes. Diabetes 2005;54(Suppl. 2):S125–S136 10.2337/diabetes.54.suppl_2.S125 [DOI] [PubMed] [Google Scholar]
- 7.Simopoulos AP. The importance of the ratio of omega-6/omega-3 essential fatty acids. Biomed Pharmacother 2002;56:365–379 10.1016/S0753-3322(02)00253-6 [DOI] [PubMed] [Google Scholar]
- 8.Chen M, Yang ZD, Smith KM, Carter JD, Nadler JL. Activation of 12-lipoxygenase in proinflammatory cytokine-mediated beta cell toxicity. Diabetologia 2005;48:486–495 10.1007/s00125-005-1673-y [DOI] [PubMed] [Google Scholar]
- 9.McDuffie M, Maybee NA, Keller SR, et al. Nonobese diabetic (NOD) mice congenic for a targeted deletion of 12/15-lipoxygenase are protected from autoimmune diabetes. Diabetes 2008;57:199–208 10.2337/db07-0830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Machado FS, Esper L, Dias A, et al. Native and aspirin-triggered lipoxins control innate immunity by inducing proteasomal degradation of TRAF6. J Exp Med 2008;205:1077–1086 [retracted in: J Exp Med 2009;206:2573] 10.1084/jem.20072416 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 11.Yacoubian S, Serhan CN. New endogenous anti-inflammatory and proresolving lipid mediators: implications for rheumatic diseases. Nat Clin Pract Rheumatol 2007;3:570–579 10.1038/ncprheum0616 [DOI] [PubMed] [Google Scholar]
- 12.Serhan CN. Resolution phase of inflammation: novel endogenous anti-inflammatory and proresolving lipid mediators and pathways. Annu Rev Immunol 2007;25:101–137 10.1146/annurev.immunol.25.022106.141647 [DOI] [PubMed] [Google Scholar]
- 13.De Caterina R, Madonna R, Bertolotto A, Schmidt EB. n-3 Fatty acids in the treatment of diabetic patients: biological rationale and clinical data. Diabetes Care 2007;30:1012–1026 10.2337/dc06-1332 [DOI] [PubMed] [Google Scholar]
- 14.Weylandt KH, Kang JX. Rethinking lipid mediators. Lancet 2005;366:618–620 10.1016/S0140-6736(05)67119-X [DOI] [PubMed] [Google Scholar]
- 15.Endres S, Ghorbani R, Kelley VE, et al. The effect of dietary supplementation with n-3 polyunsaturated fatty acids on the synthesis of interleukin-1 and tumor necrosis factor by mononuclear cells. N Engl J Med 1989;320:265–271 10.1056/NEJM198902023200501 [DOI] [PubMed] [Google Scholar]
- 16.Calder PC. N-3 polyunsaturated fatty acids and inflammation: from molecular biology to the clinic. Lipids 2003;38:343–352 10.1007/s11745-003-1068-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.De Caterina R, Madonna R, Massaro M. Effects of omega-3 fatty acids on cytokines and adhesion molecules. Curr Atheroscler Rep 2004;6:485–491 10.1007/s11883-004-0090-x [DOI] [PubMed] [Google Scholar]
- 18.Jolly CA, Jiang YH, Chapkin RS, McMurray DN. Dietary (n-3) polyunsaturated fatty acids suppress murine lymphoproliferation, interleukin-2 secretion, and the formation of diacylglycerol and ceramide. J Nutr 1997;127:37–43 [DOI] [PubMed] [Google Scholar]
- 19.Suresh Y, Das UN. Long-chain polyunsaturated fatty acids and chemically induced diabetes mellitus. Effect of omega-3 fatty acids. Nutrition 2003;19:213–228 10.1016/S0899-9007(02)00855-9 [DOI] [PubMed] [Google Scholar]
- 20.Arita M, Bianchini F, Aliberti J, et al. Stereochemical assignment, antiinflammatory properties, and receptor for the omega-3 lipid mediator resolvin E1. J Exp Med 2005;201:713–722 10.1084/jem.20042031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hudert CA, Weylandt KH, Lu Y, et al. Transgenic mice rich in endogenous omega-3 fatty acids are protected from colitis. Proc Natl Acad Sci USA 2006;103:11276–11281 10.1073/pnas.0601280103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Masoodi M, Mir AA, Petasis NA, Serhan CN, Nicolaou A. Simultaneous lipidomic analysis of three families of bioactive lipid mediators leukotrienes, resolvins, protectins and related hydroxy-fatty acids by liquid chromatography/electrospray ionisation tandem mass spectrometry. Rapid Commun Mass Spectrom 2008;22:75–83 10.1002/rcm.3331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wei D, Li J, Shen M, et al. Cellular production of n-3 PUFAs and reduction of n-6-to-n-3 ratios in the pancreatic β-cells and islets enhance insulin secretion and confer protection against cytokine-induced cell death. Diabetes 2010;59:471–478 10.2337/db09-0284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kang JX, Wang J, Wu L, Kang ZB. Transgenic mice: fat-1 mice convert n-6 to n-3 fatty acids. Nature 2004;427:504 10.1038/427504a [DOI] [PubMed] [Google Scholar]
- 25.Leiter EH. Multiple low-dose streptozotocin-induced hyperglycemia and insulitis in C57BL mice: influence of inbred background, sex, and thymus. Proc Natl Acad Sci USA 1982;79:630–634 10.1073/pnas.79.2.630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Friesen NT, Büchau AS, Schott-Ohly P, Lgssiar A, Gleichmann H. Generation of hydrogen peroxide and failure of antioxidative responses in pancreatic islets of male C57BL/6 mice are associated with diabetes induced by multiple low doses of streptozotocin. Diabetologia 2004;47:676–685 10.1007/s00125-004-1367-x [DOI] [PubMed] [Google Scholar]
- 27.Narce M, Ausdrubal P, Delachambre MC, Gresti J, Poisson JP. Influence of spontaneous hypertension on n-3 delta-6-desaturase activity and fatty acid composition of rat hepatocytes. Mol Cell Biochem 1995;152:7–12 [DOI] [PubMed] [Google Scholar]
- 28.Narce M, Asdrubal P, Delachambre MC, Véricel E, Lagarde M, Poisson JP. Age-related changes in linoleic acid bioconversion by isolated hepatocytes from spontaneously hypertensive and normotensive rats. Mol Cell Biochem 1994;141:9–13 10.1007/BF00935585 [DOI] [PubMed] [Google Scholar]
- 29.Bellenger J, Bellenger S, Clément L, et al. A new hypotensive polyunsaturated fatty acid dietary combination regulates oleic acid accumulation by suppression of stearoyl CoA desaturase 1 gene expression in the SHR model of genetic hypertension. FASEB J 2004;18:773–775 [DOI] [PubMed] [Google Scholar]
- 30.Sailaja Devi MM, Das UN. Effect of prostaglandins against alloxan-induced diabetes mellitus. Prostaglandins Leukot Essent Fatty Acids 2006;74:39–60 10.1016/j.plefa.2005.09.003 [DOI] [PubMed] [Google Scholar]
- 31.Kleemann R, Scott FW, Wörz-Pagenstert U, Nimal Ratnayake WM, Kolb H. Impact of dietary fat on Th1/Th2 cytokine gene expression in the pancreas and gut of diabetes-prone BB rats. J Autoimmun 1998;11:97–103 10.1006/jaut.1997.0179 [DOI] [PubMed] [Google Scholar]
- 32.Linn T, Noke M, Woehrle M, et al. Fish oil-enriched diet and reduction of low-dose streptozocin-induced hyperglycemia. Inhibition of macrophage activation. Diabetes 1989;38:1402–1411 10.2337/diabetes.38.11.1402 [DOI] [PubMed] [Google Scholar]
- 33.Schnedl WJ, Ferber S, Johnson JH, Newgard CB. STZ transport and cytotoxicity. Specific enhancement in GLUT2-expressing cells. Diabetes 1994;43:1326–1333 10.2337/diabetes.43.11.1326 [DOI] [PubMed] [Google Scholar]
- 34.Thulesen J, Orskov C, Holst JJ, Poulsen SS. Short-term insulin treatment prevents the diabetogenic action of streptozotocin in rats. Endocrinology 1997;138:62–68 10.1210/en.138.1.62 [DOI] [PubMed] [Google Scholar]
- 35.Wang Z, Gleichmann H. GLUT2 in pancreatic islets: crucial target molecule in diabetes induced with multiple low doses of streptozotocin in mice. Diabetes 1998;47:50–56 10.2337/diabetes.47.1.50 [DOI] [PubMed] [Google Scholar]
- 36.Rabinovitch A, Suarez-Pinzon WL. Cytokines and their roles in pancreatic islet beta-cell destruction and insulin-dependent diabetes mellitus. Biochem Pharmacol 1998;55:1139–1149 10.1016/S0006-2952(97)00492-9 [DOI] [PubMed] [Google Scholar]
- 37.Eldor R, Yeffet A, Baum K, et al. Conditional and specific NF-kappaB blockade protects pancreatic beta cells from diabetogenic agents. Proc Natl Acad Sci USA 2006;103:5072–5077 10.1073/pnas.0508166103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bhattacharya A, Chandrasekar B, Rahman MM, Banu J, Kang JX, Fernandes G. Inhibition of inflammatory response in transgenic fat-1 mice on a calorie-restricted diet. Biochem Biophys Res Commun 2006;349:925–930 10.1016/j.bbrc.2006.08.093 [DOI] [PubMed] [Google Scholar]
- 39.Nowak J, Weylandt KH, Habbel P, et al. Colitis-associated colon tumorigenesis is suppressed in transgenic mice rich in endogenous n-3 fatty acids. Carcinogenesis 2007;28:1991–1995 10.1093/carcin/bgm166 [DOI] [PubMed] [Google Scholar]
- 40.Lamhamedi-Cherradi SE, Zheng S, Tisch RM, Chen YH. Critical roles of tumor necrosis factor-related apoptosis-inducing ligand in type 1 diabetes. Diabetes 2003;52:2274–2278 10.2337/diabetes.52.9.2274 [DOI] [PubMed] [Google Scholar]
- 41.Holstad M, Sandler S. A transcriptional inhibitor of TNF-alpha prevents diabetes induced by multiple low-dose streptozotocin injections in mice. J Autoimmun 2001;16:441–447 10.1006/jaut.2001.0506 [DOI] [PubMed] [Google Scholar]
- 42.Cailleau C, Diu-Hercend A, Ruuth E, Westwood R, Carnaud C. Treatment with neutralizing antibodies specific for IL-1β prevents cyclophosphamide-induced diabetes in nonobese diabetic mice. Diabetes 1997;46:937–940 10.2337/diabetes.46.6.937 [DOI] [PubMed] [Google Scholar]
- 43.Babcock TA, Helton WS, Hong D, Espat NJ. Omega-3 fatty acid lipid emulsion reduces LPS-stimulated macrophage TNF-alpha production. Surg Infect (Larchmt) 2002;3:145–149 10.1089/109629602760105817 [DOI] [PubMed] [Google Scholar]
- 44.Weylandt KH, Nadolny A, Kahlke L, et al. Reduction of inflammation and chronic tissue damage by omega-3 fatty acids in fat-1 transgenic mice with pancreatitis. Biochim Biophys Acta 2008;1782:634–641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schmöcker C, Weylandt KH, Kahlke L, et al. Omega-3 fatty acids alleviate chemically induced acute hepatitis by suppression of cytokines. Hepatology 2007;45:864–869 10.1002/hep.21626 [DOI] [PubMed] [Google Scholar]
- 46.Viedt C, Orth SR. Monocyte chemoattractant protein-1 (MCP-1) in the kidney: does it more than simply attract monocytes? Nephrol Dial Transplant 2002;17:2043–2047 10.1093/ndt/17.12.2043 [DOI] [PubMed] [Google Scholar]
- 47.Eizirik DL, Pavlovic D. Is there a role for nitric oxide in beta-cell dysfunction and damage in IDDM? Diabetes Metab Rev 1997;13:293–307 [DOI] [PubMed] [Google Scholar]
- 48.Razzak A, Aldrich C, Babcock TA, Saied A, Espat NJ. Attenuation of iNOS in an LPS-stimulated macrophage model by omega-3 fatty acids is independent of COX-2 derived PGE2. J Surg Res 2008;145:244–250 10.1016/j.jss.2007.07.003 [DOI] [PubMed] [Google Scholar]
- 49.Bleich D, Chen S, Zipser B, Sun D, Funk CD, Nadler JL. Resistance to type 1 diabetes induction in 12-lipoxygenase knockout mice. J Clin Invest 1999;103:1431–1436 10.1172/JCI5241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Laybutt DR, Sharma A, Sgroi DC, Gaudet J, Bonner-Weir S, Weir GC. Genetic regulation of metabolic pathways in beta-cells disrupted by hyperglycemia. J Biol Chem 2002;277:10912–10921 10.1074/jbc.M111751200 [DOI] [PubMed] [Google Scholar]
- 51.Maderna P, Godson C. Lipoxins: resolutionary road. Br J Pharmacol 2009;158:947–959 10.1111/j.1476-5381.2009.00386.x [DOI] [PMC free article] [PubMed] [Google Scholar]