Abstract
OBJECTIVE
The fatty acid translocase and scavenger receptor CD36 is important in the recognition and uptake of lipids. Accordingly, we hypothesized that it plays a role in saturated fatty acid–induced macrophage lipid accumulation and proinflammatory activation.
RESEARCH DESIGN AND METHODS
In vitro, the effect of CD36 inhibition and deletion in lipid-induced macrophage inflammation was assessed using the putative CD36 inhibitor, sulfosuccinimidyl oleate (SSO), and bone marrow–derived macrophages from mice with (CD36KO) or without (wild-type) global deletion of CD36. To investigate whether deletion of macrophage CD36 would improve insulin sensitivity in vivo, wild-type mice were transplanted with bone marrow from CD36KO or wild-type mice and then fed a standard or high-fat diet (HFD) for 20 weeks.
RESULTS
SSO treatment markedly reduced saturated fatty acid–induced lipid accumulation and inflammation in RAW264.7 macrophages. Mice harboring CD36-specific deletion in hematopoietic-derived cells (HSC CD36KO) fed an HFD displayed improved insulin signaling and reduced macrophage infiltration in adipose tissue compared with wild-type mice, but this did not translate into protection against HFD-induced whole-body insulin resistance. Contrary to our hypothesis and our results using SSO in RAW264.7 macrophages, neither saturated fatty acid–induced lipid accumulation nor inflammation was reduced when comparing CD36KO with wild-type bone marrow–derived macrophages.
CONCLUSIONS
Although CD36 does not appear important in saturated fatty acid–induced macrophage lipid accumulation, our study uncovers a novel role for CD36 in the migration of proinflammatory phagocytes to adipose tissue in obesity, with a concomitant improvement in insulin action.
Obesity is a key risk factor for the development of insulin resistance leading to type 2 diabetes and other metabolic pathologies. Obesity induces a state of chronic, low-grade inflammation that directly contributes to the development of insulin resistance (1). The recruitment of proinflammatory macrophages to adipose tissue is central to this inflammatory response. Accordingly, conditional deletion of CD11c+ proinflammatory (M1) macrophages normalizes insulin sensitivity and reduces inflammatory markers in diet-induced obesity (2). Furthermore, mice that have a myeloid deficiency in peroxisome proliferator–activated receptor-γ, and therefore an impaired ability to differentiate into alternatively activated anti-inflammatory macrophages (M2), are predisposed to diet-induced obesity, inflammation, and insulin resistance (3). Conversely, deletion of either inhibitor of κB kinase (IKK) β or Jun NH2-terminal kinase (JNK), critical regulators of inflammatory and cellular stress responses (4), from myeloid cells or hematopoietic-derived cells, respectively, can protect mice from obesity-induced inflammation and insulin resistance (5,6).
Although the recruitment of proinflammatory macrophages and the consequent induction of adipose tissue inflammation are important in the development of insulin resistance, the upstream signals by which nutrient excess trigger this response are unclear. A hallmark of obesity is the intracellular accumulation of ectopic lipid and, in particular, highly bioactive lipids such as diacylglycerols (DAGs) and ceramide (7,8). Elevations in these intermediates have been linked to inflammation and impaired insulin action (9–11). Importantly, reducing macrophage lipid accumulation decreases macrophage inflammation and improves systemic insulin sensitivity (12,13).
The fatty acid translocase CD36 binds fatty acids and is important for the uptake of lipids throughout the gastrointestinal tract (14), liver (15), myocardium, skeletal muscle, and adipose tissue (16,17). Accordingly, CD36 polymorphisms and deficiencies in humans (18) and CD36 knockout mice (17,19,20) are linked to perturbations in metabolism and substrate utilization. In phagocytes, CD36 primarily functions as a scavenger receptor, recognizing specific self and nonself molecular patterns and triggering internalization and inflammatory signaling pathways to eliminate pathogens and altered self components, such as apoptotic cells (21). CD36 cooperates with toll-like receptor (TLR)-4 and -6 to mediate the sterile inflammatory response to altered self components oxidized LDL (oxLDL) and amyloid-β (22). CD36 also acts as a coreceptor with TLR2 and -6 in the recognition of microbial diacylglycerides (23,24). Interestingly, TLR4 recently has been suggested to mediate lipid-induced inflammation (25,26). Given the pivotal roles of CD36 in both the recognition and uptake of lipids and its capacity to induce inflammatory signaling in macrophages, we hypothesized that hematopoietic cell–restricted deletion of CD36 would reduce lipid-induced macrophage inflammation and improve obesity-induced metabolic perturbations.
RESEARCH DESIGN AND METHODS
Cell culture experiments.
RAW264.7 macrophages were grown and passaged in low-glucose Dulbecco’s modified Eagle’s medium supplemented with 10% FBS, 100 units/mL penicillin, and 0.1 mg/mL streptomycin. Primary bone marrow macrophages were derived from hind-limb bones of killed C57BL/6 (wild-type) and CD36KO mice at 6–12 weeks of age. Cells differentiated into macrophages over 5–7 days in 20% conditioned media from L929 cells or M-CSF (25 ng/mL). 3T3-L1 fibroblasts were differentiated into adipocytes prior to experiments, as previously described (27). Thioglycollate-elicited peritoneal macrophages and primary adipocytes were isolated and differentiated as previously described (28).
All fatty acid treatments and overnight equilibration of cells were conducted in Dulbecco’s modified Eagle’s medium, 3% FBS, and 2% fatty acid–free BSA (Bovogen, Essendon, Australia). Macrophages were treated with sulfosuccinimidyl oleate (SSO) (500 μmol/L; A.Bon. and/or Anthem Biosciences, Bangalore, India) or vehicle (DMSO) for 4 h prior to cotreatment with palmitate (0.75 mmol/L; Sigma-Aldrich, St. Louis, MO), stearate (0.75 mmol/L; Sigma-Aldrich), or vehicle (100% EtOH plus BSA) for an additional 4 h. For conditioned-media studies, macrophages were only cotreated with SSO and with palmitate for 2 h. Conditioned media collected over the following 4 h was placed on 3T3-L1 adipocytes for 48 h, prior to the 30-min insulin (10 nmol/L; Sigma-Aldrich) stimulation. Macrophages were treated with lipopolysaccharide (LPS) (100 ng/mL, L8274; Sigma-Aldrich) or interferon (INF)-γ (4 ng/mL; R&D Systems) for the times indicated.
Bone marrow transplant and mouse procedures.
Wild-type C57BL/6 and CD36KO mice on a C57BL/6 background (29) were bred and housed at either the Baker IDI Alfred Medical Research and Education Precinct Animal Facility or an Association for Assessment and Accreditation of Laboratory Animal Care–accredited facility at the Lerner Research Institute under a 12-h light/dark cycle. All procedures were approved by the Alfred Medical Research and Education Precinct Animal Ethics Committee or the Cleveland Clinic Institutional Animal Care and Use Committee and were in accordance with the National Health and Medical Research Council of Australia and with the Guide for the Care and Use of Laboratory Animals published by the National Institutes of Health.
To generate radiation chimeras, 6-week-old male mice were given bone marrow transplants, as per Solinas et al. (5). Following 6 weeks of recovery, chimeras were either maintained on a normal standard diet (14.3 MJ/kg energy, containing 8% calories from fat, 21% from protein, and 71% from carbohydrate; Specialty Feeds, Glen Forrest, Australia) or switched to a high-fat diet (HFD) (19 MJ/kg energy, 42% of calories from fat [lard], 20% from protein, and 35% from carbohydrate; Specialty Feeds) for 20 weeks. Prior to procedures and blood collection, mice were fasted for 6 h. For the intraperitoneal glucose tolerance test and intraperitoneal insulin tolerance test, glucose (1.0 g/kg lean body mass [LBM]) or insulin (0.75 units/kg LBM), respectively, was injected. Tail blood was taken at indicated time points, and glucose was measured using a human ACCU-CHEK blood monitor and test strips (Roche, Basel, Switzerland). LBM and fat mass were assessed fortnightly using the four-in-one EchoMRI body composition analyzer (Columbus Instruments, Columbus, OH). Metabolic data were collected over 24 h using the Comprehensive Laboratory Animal Monitoring System (Columbus Instruments) after an initial 2-h acclimatization. Following experimentation, mice were anesthetized (80 mg/kg sodium pentobarbital) and tissues excised before and after the insulin injection (1.0 units/kg LBM) into the inferior vena cava.
Western blotting and lipid analysis.
Cells were lyzed and tissues homogenized as previously described (30). Protein was determined using the Micro BCA Protein Assay Kit (no. 23235; Thermo Scientific, Rockford, IL) and boiled in Laemelli’s buffer for 5 min. Protein was resolved by SDS-PAGE, transferred to a nitrocellulose membrane, blocked in 5% milk, and incubated overnight with primary antibody. Phosphorylated-JNK (Thr183/Tyr185), phosphorylated–c-jun (Ser73), JNK, phosphorylated-IKKβ (Ser181), IKKβ, tumor necrosis factor (TNF) α, phosphorylated-Akt (Ser473) and (Thr308), Akt, phosphorylated-IRβ (Tyr1361), IRβ, and β-actin were obtained from Cell Signaling Technology; α-tubulin was from Sigma-Aldrich; and CD36 was from R&D Systems. Following incubation with the appropriate horseradish peroxidase–conjugated secondary antibody, proteins were detected with the ECL Advance Western Blotting Detection Kit (GE Healthcare, Buckinghamshire, U.K.) and visualized using the Molecular Imager ChemiDoc System (Bio-Rad Laboratories, Hercules, CA), and band volumes were quantified using Quantity One 1-D Analysis Software 4.5.2. Initially, ceramide and diacylglycerols were assessed via thin-layer chromatography, and additional lipid measurements were made via mass spectrometry; both methods have been previously described (31,32).
Real-time PCR.
Epididymal white adipose tissue (WAT) and liver were homogenized in 1.0 mL TRIzol reagent (Invitrogen, Mulgrave, Australia); RNA was extracted according to the manufacturer’s instructions, DNAse treated using DNase 1 (Invitrogen, Mulgrave, Australia), transcribed, and converted to cDNA using TaqMan reverse transcription reagents (Applied Biosystems, Foster City, CA). Gene expression analysis was performed using SYBR Green PCR Mastermix (Applied Biosystems, Warrington, U.K.) or TaqMan gene expression assays (Applied Biosystems), including 18S probe and primers for housekeeping measurements.
Genomic PCR.
DNA was extracted from whole blood using Maxwell Whole Blood Purification Kits and the Maxwell 16 Instrument (Promega, Madison, WI). Specific primers yielded PCR products of 600 bp (wild-type) and 750–800 bp (CD36KO).
Multianalyte plasma profiling.
Plasma chemokine (C-C motif) ligand 2 (CCL2)/monocyte chemotactic protein (MCP)-1, interleukin (IL)-6, IL-1β, INF-γ, IL-4, and IL-10 were measured using a fluorokine mouse multianalyte profiling kit (R&D Systems, Minneapolis, MN) and read using a BioPlex system (Bio-Rad Laboratories).
Flow cytometry.
Six weeks after the bone marrow transplant, blood collected from Ly5.1 and Ly5.2 mice was stained with anti–CD45.2-fluorescein isothiocyanate (Ly5.2) and anti–CD45.1-phycoethrin (Ly5.1) antibodies or appropriate isotype controls (BD Biosciences, San Jose, CA). Erythrocytes were lyzed using FACS Lyzing Solution (BD Biosciences). White blood cells were analyzed via a FACSCalibur (BD Biosciences) flow cytometer using CellQuest Pro software.
Hormone and cytokine analysis.
Fasting plasma insulin was determined using a rat/mouse insulin ELISA kit (Millipore, Billerica, MA). TNFα was determined using a DuoSet ELISA Development System (R&D Systems) for mouse TNFα.
Immunohistochemistry.
Paraformaldehyde-fixed, paraffin-embedded, epididymal WAT was deparaffinized and rehydrated in histoline and alcohol and stained with Mayer’s hematoxylin and eosin and analyzed using Image-Pro Plus (6.0) software. For immunostaining, sections were boiled in sodium citrate buffer and blocked in 3% hydrogen peroxide, incubated in F4/80 antibody (1:100 dilution; AbD Serotec, Kidlington, U.K.) overnight at 4°C using an M.O.M. Immunodetection Kit (Vector Laboratories, Burlingame, CA), incubated with biotinylated anti-rat IgG and then with avidin-biotin peroxidase complex and DAB peroxidase substrate (Vector Laboratories), and, finally, stained with Mayer’s hematoxylin and dehydrated.
Binding and migration assay.
A migration assay was conducted as previously described (28). Briefly, conditioned media was collected from primary adipocytes and treated for 12 h with vehicle, oxLDL, or advanced glycation end product (AGE)-BSA; washed with PBS; and incubated with serum-free media for 8 h. Wild-type and CD36KO peritoneal macrophages were loaded into the migration chamber with adipocyte-conditioned media. Sixteen hours later, nuclei of migratory cells were stained and counted. For the binding assay, peritoneal macrophages were plated onto treated tissue-culture plastic (Corning) at 0.5 × 106 cells per mL and placed on an orbital shaker at 60 rpm for 18 h, with vehicle, NO2LDL, or MCP-1. Plates were washed and macrophages labeled with 2.5 μmol/L PKH dye in diluent B (Sigma) for 5 min at room temperature, washed again, and analyzed using a fluorescent microplate reader (Gemini EM; Molecular Devices) (551 nm excitation, 567 nm emission).
Statistical analysis.
Data are expressed as means ± SE. ANOVA with repeated measures or the Student t test were performed using SigmaStat (version 3.5), where appropriate (see figure legends). A P value of <0.05 denoted statistical significance.
RESULTS
The putative CD36 inhibitor SSO prevents saturated fatty acid–induced lipid accumulation and macrophage inflammation, resulting in improved insulin signaling in adipocytes.
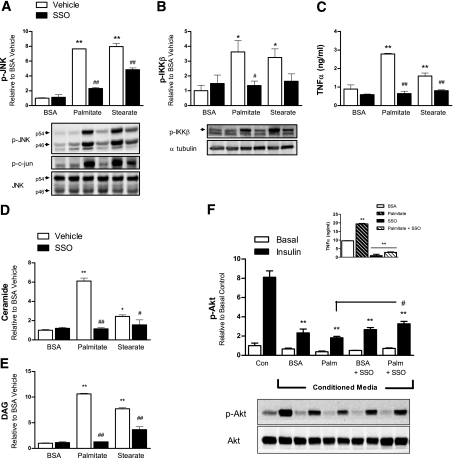
To investigate the role of CD36 in saturated fatty acid–induced macrophage activation, we inhibited CD36 using SSO, a CD36 inhibitor shown to cross-link CD36 on the cell membrane and prevent lipid transport (33). RAW264.7 macrophages were pretreated with or without SSO before being treated with the saturated fatty acids palmitate (C16:0) or stearate (C18:0). Both fatty acids induced phosphorylation of the stress-activated serine threonine kinase JNK, its substrate c-jun, and IKKβ (Fig. 1A and B) and stimulated the secretion of TNFα (Fig. 1C). These effects were reduced in macrophages pretreated with SSO (Fig. 1A–C). Both palmitate and stearate increased ceramide and DAG accumulation in RAW264.7 macrophages (Fig. 1D and E) and primary bone marrow–derived macrophages (BMDMs) (Supplementary Fig. 1A and B), an effect that was largely prevented by SSO (Fig. 1D and E; Supplementary Fig. 1A and B). These data suggested that CD36 was involved in the uptake of long-chain saturated fatty acids into macrophages. Adipose tissue macrophages secrete proinflammatory molecules that interfere with adipocyte insulin signaling (4). Using conditioned media from RAW264.7 macrophages, we investigated whether SSO treatment of macrophages could improve insulin signaling in 3T3-L1 adipocytes. Conditioned media from both control BSA and palmitate-treated macrophages reduced insulin-stimulated phosphorylation of Akt, a critical mediator of insulin signaling, compared with control cells with normal medium (Fig. 1F). Pretreating macrophages with SSO reduced TNFα secretion into conditioned media and increased Akt phosphorylation in adipocytes treated with palmitate-conditioned media (Fig. 1F, inset). These data suggested that targeting CD36 in myeloid cells, in settings of lipid oversupply, could be a viable strategy to attenuate insulin resistance in adipose tissue in vivo.
FIG. 1.
The putative CD36 inhibitor SSO prevents saturated fatty acid–induced lipid accumulation and macrophage inflammation, resulting in improved insulin signaling in adipocytes. RAW264.7 macrophages were incubated with vehicle or the putative CD36 inhibitor SSO for 4 h then with either BSA (vehicle) or the saturated fatty acids palmitate (16:0) or stearate (18:0) for an additional 4 h. Cellular extracts were analyzed for phosphorylated (p) (Thr183/Tyr185) JNK (A), its substrate phosphorylated (Ser73) c-jun total JNK (B), and phosphorylated (Ser181) IKKβ (C) by Western blotting. TNFα released into the media was measured by ELISA. Total ceramide (D) and DAG (E) were extracted and quantified via thin-layer chromatography. F: To obtain conditioned media, RAW264.7 macrophages were incubated with vehicle or SSO overnight then with BSA (vehicle) or palmitate (Palm) for 2 h and then palmitate/BSA-free media for 4 h. This conditioned media, or nonconditioned control media (Con), was incubated with differentiated 3T3-L1 adipocytes for 48 h before insulin stimulation. Cellular extracts were analyzed for phosphorylated (p) (Ser473) Akt and total Akt. TNFα released into the conditioned media was measured via ELISA (inset). Data shown are from one experiment, representative of two to four independent experiments, expressed as means ± SE (n = 3). A–E and F, inset: *P < 0.05 fatty acid vs. BSA; **P < 0.001 fatty acid vs. BSA; #P < 0.05 SSO vs. vehicle; ##P < 0.001 SSO vs. vehicle. F: **P < 0.001 insulin stimulated vs. control insulin stimulated; #P < 0.05 palmitate conditioned media insulin stimulated vs. palmitate plus SSO conditioned media insulin stimulated; two-way ANOVA with post hoc analysis.
Hematopoietic deletion of CD36 reduces macrophage content and improves insulin signaling in adipose tissue of mice fed an HFD.
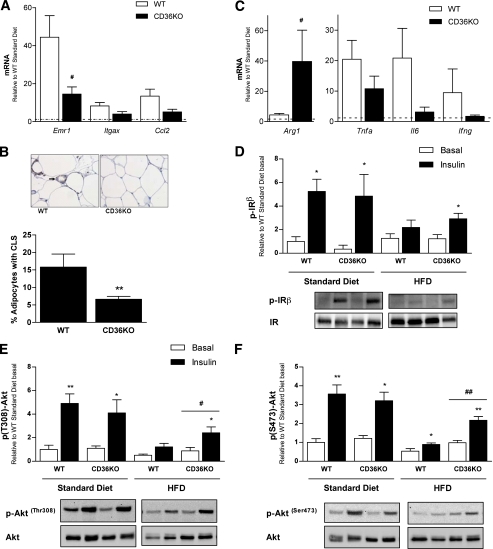
In the context of obesity-induced inflammation and insulin resistance, macrophages play an important role in the adipose tissue (1). We hypothesized that hematopoietic deletion of CD36 would improve whole-body insulin sensitivity via effects on adipose tissue. To this end, we transplanted lethally irradiated wild-type mice with bone marrow from CD36KO or background-matched (C57BL/6) wild-type mice to create chimeras whose hematopoietic-derived cells were deficient in CD36, designated wild-type or CD36KO, respectively. Consistent with previous reports (5,34), we show ~95% reconstitution of bone marrow as assessed by flow cytometry (Supplementary Fig. 2A). In addition, we also PCR genotyped experimental mice at the completion of the study (26 weeks after bone marrow transplant) and confirmed reconstitution of donor bone marrow in the CD36KO animals (Supplementary Fig. 2B). Following 6 weeks of recovery, mice either continued on a normal standard diet or were placed on an HFD for 20 weeks. To assess macrophage content of adipose tissue, we determined the mRNA expression of the macrophage markers F4/80 (Emr1), CD11c (Itgax), and chemokine (C-C motif) ligand 2 (Ccl2). The expression of emr1, itgax, and ccl2 in adipose tissue of mice fed an HFD was markedly elevated relative to the standard diet–fed mice (Fig. 2A). Importantly, the expression of emr1 was significantly lower, and itgax and ccl2 also tended to be lower, in CD36KO mice compared with wild-type mice fed an HFD (Fig. 2A). To confirm that CD36KO mice had reduced macrophage content within the adipose tissue, we performed immunohistochemistry and enumerated the number of F4/80-positive crown-like structures present in adipose tissue cross-sections. The numbers of crown-like structures present in HFD-fed CD36KO mice were substantially reduced relative to wild-type control mice (Fig. 2B and Supplementary Fig. 2C).
FIG. 2.
Hematopoietic deletion of CD36 reduces macrophage content and improves insulin signaling in adipose tissue of mice fed an HFD. Mice with hematopoietic-specific deletion of CD36 (CD36KO) were created by bone marrow transplanting stem cells from CD36KO or background-matched wild-type (WT) mice into lethally irradiated C57BL/6 mice. After 20 weeks of the standard diet or HFD, epididymal WAT depots were excised before and after insulin stimulation. A: Gene expression of F4/80 (Emr1), CD11c (Itgax), and ccl2 in the WAT of WT and CD36KO mice on an HFD was measured by real-time PCR, expressed relative to WT standard diet–fed mice indicated by the dotted line. B: Immunohistochemical staining for F4/80 (brown staining, see arrow) in WAT sections from WT and CD36KO mice on an HFD. The percentage of adipocytes with crown-like structures (CLS) was determined for both WT and CD36KO mice on an HFD (6–10 images at ×20 magnification were analyzed per mouse). C: Gene expression of the alternative macrophage activation (M2) marker, arginase 1 (Arg1), and inflammatory cytokines tnfa, il6, and ifng in the WAT of WT and CD36KO mice were measured by real-time PCR, expressed relative to WT standard diet–fed mice, indicated by the dotted line. WAT from basal and insulin-stimulated WT and CD36KO mice fed a standard diet and HFD were analyzed for phosphorylated (p) (Tyr1361) and total IR (D) and phosphorylated (p) (Ser473 and Thr308) and total Akt (E and F) by Western blotting. Data are expressed as means ± SE (n = 6–8 mice per group). *P < 0.05 insulin stimulated vs. basal; **P < 0.001 insulin stimulated vs. basal; #P < 0.05 CD36KO vs. WT; ##P < 0.001 CD36KT; Student t tests and two-way ANOVA with post hoc analysis. (A high-quality digital representation of this figure is available in the online issue.)
To assess the inflammatory profile of the adipose tissue, we performed gene expression analysis for markers of both proinflammatory (M1) and alternative/remodelling (M2) macrophages. The expression of arginase 1 (Arg1), an M2 macrophage marker, was significantly higher in the adipose tissue of CD36KO mice compared with wild-type mice (Fig. 2C). Correspondingly, we observed a trend for reduced mRNA expression of cytokines produced primarily by M1 macrophages (e.g., tnfa and il6), as well as the Th1 cytokine ifng, when comparing HFD-fed CD36KO and wild-type mice (Fig. 2C). We did not observe any differences in the mRNA expression of the anti-inflammatory cytokine IL-1 receptor antagonist (Il1rn), neither were there any differences in JNK phosphorylation in adipose tissue when comparing wild-type and CD36KO mice fed an HFD (Supplementary Fig. 2D–F).
Emr1 and itgax mRNA expression in the liver of mice fed an HFD were increased relative to standard-diet–fed mice, indicating kupffer cell accrual. Similarly, ccl2 mRNA expression was significantly elevated; no significant changes in the expression of tnfa, il1b, ifng, il6, or il10 were observed in mice fed an HFD compared with standard-diet–fed mice (Supplementary Fig. 2F and data not shown). These data suggest that the role of CD36 in macrophage accrual is restricted to the adipose tissue.
Next, we determined whether the reduced macrophage accumulation observed in CD36KO mice, relative to wild-type mice on an HFD, resulted in altered adipose tissue insulin sensitivity. Insulin failed to stimulate phosphorylation of the insulin receptor (IR) in the wild-type mice fed an HFD, but increased insulin-stimulated IR phosphorylation was observed in CD36KO mice (Fig. 2D). Furthermore, although insulin-stimulated Akt phosphorylation at Thr308 and Ser473 was reduced in HFD relative to standard diet–fed mice, insulin-stimulated Akt phosphorylation was significantly increased in CD36KO mice compared with wild-type mice on an HFD (Fig. 2E and F).
Hematopoietic deletion of CD36 does not affect body weight or whole-body insulin sensitivity.
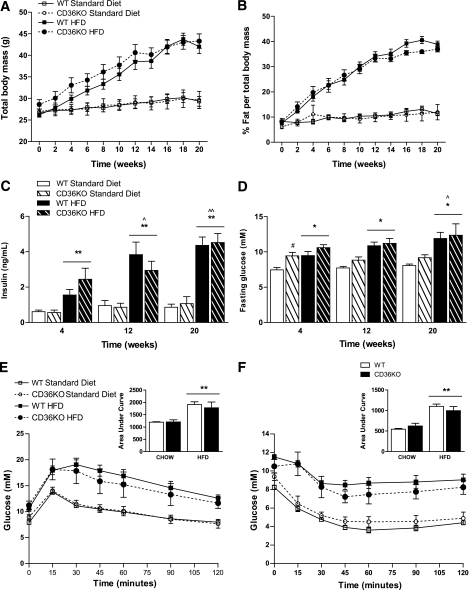
Next, we examined whether hematopoietic cell–restricted deletion of CD36 altered whole-body adiposity and/or insulin action. There were no differences in the total body weight or percentage of body fat between wild-type and CD36KO mice at any time during the 20-week dietary intervention (Fig. 3A and B). Furthermore, neither epididymal WAT weight nor adipocyte size or number was different when comparing the CD36KO with wild-type mice after 20 weeks of either standard diet or high-fat feeding (Supplementary Fig. 3A and B and data not shown). This finding is consistent with others that show unaltered adiposity despite improvements in adipose tissue insulin sensitivity (35).
FIG. 3.
Hematopoietic deletion of CD36 does not affect body weight or whole-body insulin sensitivity. Radiation chimeras with hematopoietic deletion of CD36 (HSC CD36KO) and control mice (WT) were placed on either an HFD or normal standard diet for 20 weeks. Total body mass (A) and percent fat mass (B) were assessed every fortnight over the dietary intervention using EchoMRI. At the weeks indicated, fasting insulin (C) and glucose (D) levels in the plasma were assessed via ELISA and glucometer, respectively. Intraperitoneal glucose (E) and insulin tolerance (F) tests were conducted at 20 weeks. □, WT standard diet; ○, HSC CD36KO standard diet; ■, WT HFD; ●, HSC CD36KO HFD. Area under the curve is displayed in the inset. All data are expressed as means ± SE (n = 6–8). *P < 0.05 HFD vs. standard diet; **P < 0.001 HFD vs. standard diet; #P < 0.05 WT vs. HSC CD36KO; ^P < 0.05 different to week 4; ^^P < 0.001 different to week 4; two-way repeated-measures ANOVA, where appropriate, and two-way ANOVA with post hoc analysis.
As expected, HFD-fed mice became progressively hyperinsulinemic and hyperglycemic compared with standard diet–fed animals (Fig. 3C and D). Although fasting glucose was higher in the CD36KO mice compared with wild-type mice fed a standard diet at 4 weeks, no differences in plasma insulin were observed on either the standard diet or HFD at any time point measured (Fig. 3C and D). We performed glucose and insulin tolerance tests at the end of the 20-week dietary period. As expected, mice fed an HFD displayed impaired glucose (Fig. 3E) and insulin (Fig. 3F) tolerance compared with standard diet–fed mice; however, no differences were observed when comparing CD36KO and wild-type mice on either diet (Fig. 2E and F).
Adipose tissue inflammation and insulin resistance can result in systemic insulin resistance via the secretion of inflammatory cytokines/adipokines that, in turn, alter insulin action in other peripheral tissues, such as liver and skeletal muscle (4,13). We measured a number of circulating cytokines/adipokines via multianalyte plasma profiling and found no differences in any measurements when comparing plasma taken from CD36KO or wild-type mice fed an HFD for 19 weeks (data not shown). Accordingly, there were no differences in insulin-stimulated Akt phosphorylation between the CD36KO and wild-type mice on an HFD in either the liver or skeletal muscle (data not shown). We observed no differences in food intake, oxygen consumption, respiratory exchange ratio, or activity levels when comparing CD36KO with wild-type mice on either the standard diet or the HFD (data not shown). Thus, HFD-fed mice with a hematopoietic deletion of CD36, despite enhanced adipose tissue insulin signaling and a reduction in adipose tissue inflammation, are not protected from the development of HFD-induced insulin resistance.
CD36KO BMDMs are not protected from saturated fatty acid–induced lipid accumulation.
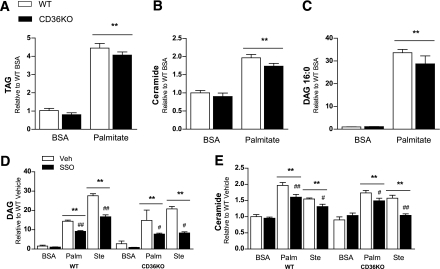
The putative CD36 inhibitor SSO prevented lipid accumulation in macrophages. Moreover, adipose tissue macrophage recruitment and insulin signaling were improved in HFD-fed mice with a hematopoietic-specific deficiency in CD36, compared with wild-type mice. To further explore these findings at the cellular level, we examined whether genetic deletion of CD36 from macrophages prevented lipid accumulation and resultant inflammation. Accordingly, we treated bone marrow–derived wild-type and CD36KO macrophages with palmitate and stearate. In contrast to pharmacological inhibition of CD36 using SSO (see Fig. 1), CD36KO macrophages accumulated triacylglycerol, DAG, and ceramide to the same extent as wild-type macrophages (Fig. 4A–C). When CD36KO macrophages were treated with SSO, the reduction in the accumulation of ceramide and DAG was comparable with that seen in wild-type macrophages (Fig. 4D and E). These results demonstrate that the intracellular accumulation of these lipids is not dependent on CD36, and, therefore, in macrophages, CD36 does not appear to act as a lipid transporter. In addition, this highlights that SSO prevents lipid accumulation in BMDM in a CD36-independent manner.
FIG. 4.
CD36KO BMDMs are not protected from saturated fatty acid–induced lipid accumulation. Bone marrow–derived macrophages from wild-type (WT) and CD36KO mice were treated with the saturated fatty acids palmitate (16:0), stearate (18:0), or BSA (control) for 4 h, with or without pretreatment with the putative CD36 inhibitor SSO (0.5 mmol/L, 4 h). Total triacylglycerol (TAG) (A), total ceramide (B and E), DAG species 16:0 (C), and the sum of 16:0 and 18:0 DAGs (D) were extracted and analyzed via electrospray ionization-tandem mass spectrometry. All data are expressed as means ± SE (n = 3–6, data shown are from one experiment, representative of two independent experiments). **P < 0.001 fatty acid vs. BSA vehicle; #P < 0.05 vehicle vs. SSO; ##P < 0.001 vehicle vs. SSO; two-way ANOVA with post hoc analysis. Palm, palmitate; Ste, stearate; Veh, vehicle.
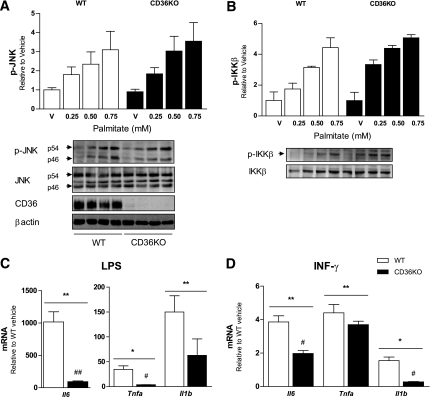
CD36KO BMDMs show a blunted proinflammatory response to LPS and INF-γ but not palmitate.
Consistent with the lack of effect of CD36 deletion on saturated fatty acid uptake in BMDMs, we observed that increasing concentrations of palmitate induced JNK and IKKβ phosphorylation to the same extent in CD36KO and wild-type macrophages (Fig. 5A and B). CD36 has been shown to cooperate with TLR4 to promote sterile inflammation (22), and TLR4 is implicated in lipid-induced inflammation (36). Therefore, we investigated whether the absence of CD36 blunted inflammatory responses to LPS, a TLR4 ligand. Deletion of CD36 did not prevent the phosphorylation of JNK in response to LPS (Supplementary Fig. 4). However, LPS-induced il6 and tnfa gene expression were blunted in CD36KO BMDMs compared with wild-type controls (Fig. 5C). Gene expression of il6 and il1b in response to INF-γ also was blunted in CD36-deficient BMDMs. These results suggest that although CD36 may not play an important role in the promotion of saturated fatty acid–induced macrophage activation, it is important in regulating responses to other proinflammatory stimuli.
FIG. 5.
CD36KO BMDMs show a blunted proinflammatory response to LPS and INF-γ but not palmitate. Bone marrow–derived macrophages from wild-type (WT) and CD36KO mice were treated with increasing concentrations of the saturated fatty acid palmitate (16:0) or BSA (vehicle) for 4 h. Cellular extracts were analyzed for phosphorylated (p) (Thr183/Tyr185) and total JNK, CD36, β-actin (A) and phosphorylated (p) (Ser181) and total IKK (B) by Western blotting. Bone marrow–derived macrophages from WT and CD36KO mice were treated with LPS (C) or INF-γ (D) for 6 h and gene expression of TNFα, IL-6, and IL-1β were assessed by real-time PCR. All data are expressed as means ± SE. A and B: n = 3–9, data are the combination of three independent experiments. C and D: n = 6, data are from one experiment. *P < 0.05 treatment vs. vehicle; **P < 0.001 treatment vs. vehicle; #P < 0.05 WT vs. CD36KO; ##P < 0.001 WT vs. CD36KO; two-way ANOVA with post hoc analysis.
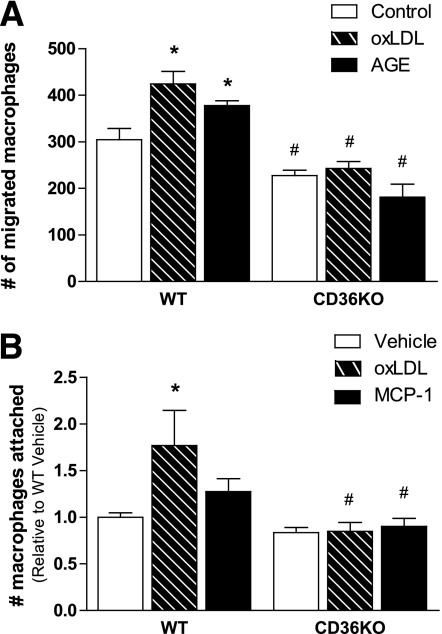
CD36KO peritoneal macrophages do not migrate in response to adipocyte-derived chemotactic factors.
Because HFD-fed hematopoietic stem cell (HSC) CD36KO mice accumulate less macrophages than wild-type mice, we inferred that CD36 may be important in macrophage migration and, accordingly, conducted an in vitro migration assay. Conditioned media was collected from primary adipocytes, which were either untreated (control) or stimulated with oxLDL or AGEs to release chemotactic cytokines. Wild-type and CD36KO macrophages were placed in wells with a porous membrane and incubated with conditioned media. Both treatments increased the migration of wild-type macrophages through the porous membrane; however, this was blunted in CD36KO macrophages (Fig. 6A). In addition, CD36KO macrophages also showed reduced migration to conditioned media from untreated adipocytes, compared with wild-type macrophages.
FIG. 6.
CD36KO peritoneal macrophages do not migrate in response to adipocyte-derived chemotactic factors. A: Wild-type (WT) and CD36KO peritoneal macrophages were placed in transwell inserts with an 8 μmol/L porous membrane and incubated with conditioned media from vehicle, oxLDL, or AGE-treated primary adipocytes for 16 h. Macrophages that migrated to the other side of the membrane were stained and counted; each replicate is the average of four fields. B: WT and CD36KO peritoneal macrophages were treated with vehicle, oxLDL, or MCP-1 for 18 h. Macrophages attached to the cell culture plate were stained with PKH dye and fluorescence quantified. All data are expressed as means ± SE. A: n = 4; B: n = 6–15. *P < 0.05 treatment vs. vehicle; #P < 0.05 WT vs. CD36KO; two-way ANOVA with post hoc analysis.
Because CD36 is a scavenger receptor important in the recognition of oxLDL, bacterial proteins, and apoptotic cells (37), we proposed that CD36KO macrophages may have altered binding capacity. Indeed, compared with wild-type macrophages, the binding of CD36KO macrophages to plastic in response to oxLDL or MCP-1 was blunted (Fig. 6B). Together with the finding that mice with hematopoietic-specific deletion of CD36 have reduced macrophage accumulation into expanded adipose tissue, these results argue that CD36 is important in obesity-induced macrophage recruitment into adipose tissue.
DISCUSSION
We hypothesized that hematopoietic cell–restricted deletion of CD36 would ameliorate obesity-induced inflammation and insulin resistance, potentially via the inhibition of fatty acid uptake into macrophages and/or via the inhibition of fatty acid–induced signaling pathways. In partial support of this hypothesis, HSC deletion of CD36 improves insulin action in adipose tissue of mice fed an HFD. Concomitantly, we observed a marked reduction in macrophage recruitment and a tendency toward a reduction in the inflammatory profile of WAT. However, these improvements did not result in improved whole-body insulin sensitivity. Surprisingly, we found that CD36 is not required for the uptake or the proinflammatory effects of long-chain saturated fatty acids in macrophages; however, it is likely involved in their migration to adipose tissue in obesity. In addition, our results strongly argue that SSO, thought to be a specific inhibitor of CD36, blocks lipid accumulation and inflammation in BMDMs in a CD36-independent manner.
Because HFD-fed mice with a hematopoietic-specific deletion of CD36 show reduced recruitment of proinflammatory macrophages into adipose tissue, this suggests that CD36 plays a role in monocyte recruitment to inflammatory tissues. In vitro, CD36KO macrophages have a reduced migratory capacity when incubated with adipose-derived chemotactic cytokines. In support of our findings, macrophages from CD36/apolipoprotein E double-knockout mice show reduced spreading in response to the adipose-derived chemotactic cytokine CCL2 in in vitro migration assays (38,39). Two additional reports also identify a role for CD36 in the regulation of monocyte migration to sites of vascular inflammation (40,41). Thus, we propose that CD36 is important in the recruitment of monocytes/macrophages to sites of inflammation and contributes to obesity-induced adipose tissue inflammation.
Adipose tissue remodeling and associated adipocyte cell death in obesity is a dynamic process that has been linked to macrophage recruitment and adipose tissue inflammation (42,43). CD36 is implicated in recognition of cellular apoptotic markers, such as oxidized phosphatidylserine, and clearance of apoptotic cells (44). CD36 deletion in macrophages may limit macrophage recruitment into the adipose tissue because of their inability to recognize apoptotic adipocytes.
Our data suggest that although strategies that reduce macrophage recruitment into adipose tissue may reduce adipose tissue inflammation and improve adipocyte insulin sensitivity, these effects in isolation may not be sufficient to improve insulin action in other tissues. This view is consistent with other studies, where the inactivation of JNK1 (a serine kinase with important roles in proinflammatory signaling) in hematopoietic cells was not sufficient to significantly affect systemic insulin sensitivity in mice on an HFD (35,45), despite significant reductions in adipose-derived cytokines (45). Altogether, it is possible that the contribution of adipose tissue macrophages to obesity-induced systemic metabolic deregulation may be limited.
Alternatively, it is possible that bone marrow ablation of CD36 is not sufficient to improve systemic insulin sensitivity because its effect on adipose tissue macrophage recruitment and proinflammatory adipokines is partial and a more potent intervention on adipose tissue macrophages could lead to significant systemic effects on glucose homeostasis. Consistent with this view, both complete ablation of activated adipose tissue macrophages (2) or hematopoietic-specific deletion of the lipid chaperone aP2 (13) is sufficient to normalize, or improve, insulin sensitivity in HFD-fed mice, respectively.
Because neither ceramide nor DAG accumulation was reduced in CD36KO macrophages treated with saturated fatty acids, it seems that CD36 itself is not critical in the transport or trafficking of saturated fatty acids into macrophages. It is unequivocal that CD36 plays an important role in the uptake of long-chain fatty acids into adipocytes, cardiac and skeletal myocytes, and other tissues (16,17). Other transport proteins, such as the lipid chaperone fatty acid–binding protein 4 (aP2), are likely to play a more critical role in macrophage lipid accumulation (13).
CD36 has gained considerable attention recently as a pattern-recognition receptor and coreceptor for specific TLRs (i.e., TLR2, -4, and -6) (22,23). In this regard, it is of interest to note that TLR4 has been suggested to sense lipids and may provide a link between dyslipidemia and obesity-induced inflammation (36). Although LPS-induced JNK activation was unaltered in CD36KO macrophages, expression of the proinflammatory genes il6 and tnfa were markedly reduced. These data argue a role for CD36 in mediating TLR4-dependent signaling and priming macrophages to take on a more proinflammatory (M1) phenotype. Given that palmitate-induced inflammatory signaling was unaffected by the absence of CD36 in macrophages, it is unlikely that CD36 cooperates with TLRs in their possible role as lipid sensors.
Our initial findings using the putative CD36 inhibitor SSO led us to hypothesize a role for CD36 in lipid transport in macrophages. However, the finding that SSO reduced ceramide and DAG accumulation in CD36KO BMDMs suggests that SSO is affecting lipid metabolism and inflammation in a CD36-independent manner. In support of this notion, it has previously been reported that SSO binds to the mitochondrial membrane and alters mitochondrial oxidation rates independently of CD36 (46). Although we do not know how SSO prevented lipid accumulation and inflammation in macrophages, our findings suggest that results obtained with SSO, in particular cell types, should be interpreted with caution.
In conclusion, although CD36 does not appear to be important in saturated fatty acid–induced macrophage lipid accumulation, or in directly mediating the proinflammatory effects of lipids, our study uncovers a novel role for CD36 in the migration of proinflammatory phagocytes into adipose tissue during diet-induced obesity.
ACKNOWLEDGMENTS
This study was supported by grants from the Diabetes Australia Research Trust, the National Health and Medical Research Council of Australia (NHMRC) (project grant no. 526619 to M.A.F. G.I.L.), the American Heart Association Great Rivers Affiliate (0825685D to D.J.K.), and the National Institutes of Health (NIH P01 HL087018 to Roy L. Silverstein, supporting D.J.K. and M.F.). M.J.W. is a Senior Research Fellow and A.Bob. and M.A.F. are Principal Research Fellows of the NHMRC. D.J.K. is supported by the Lerner Research Institute’s David and Lindsay Morgenthaler Endowed Fellowship.
No potential conflicts of interest relevant to this article were reported.
H.T.N. and G.I.L. performed experiments and wrote the manuscript. G.K., D.J.K., S.R., L.A.Z., N.W., P.K., and M.J.W. performed experiments. A.Bob., A.Bon., and M.F. provided reagents and expertise. M.A.F. wrote the manuscript. All authors contributed to discussion and reviewed and edited the manuscript.
The authors acknowledge the technical support provided by S. Turpin (Department of Physiology, Monash University); A. Selathurai (Vascular Biology and Atherosclerosis Laboratory, Baker IDI); A. Agrotis (Cell Biology, Baker IDI); and P. Miekle, G. MacIntosh, and J. Wier (Lipidomics Core, Baker IDI). The authors also thank G. Solinas (Department of Medicine, Physiology, University of Fribourg) for his guidance with the bone marrow transplantation technique.
Footnotes
This article contains Supplementary Data online at http://diabetes.diabetesjournals.org/lookup/suppl/doi:10.2337/db10-1353/-/DC1.
REFERENCES
- 1.Hotamisligil GS. Inflammation and metabolic disorders. Nature 2006;444:860–867 [DOI] [PubMed] [Google Scholar]
- 2.Patsouris D, Li PP, Thapar D, Chapman J, Olefsky JM, Neels JG. Ablation of CD11c-positive cells normalizes insulin sensitivity in obese insulin resistant animals. Cell Metab 2008;8:301–309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Odegaard JI, Ricardo-Gonzalez RR, Goforth MH, et al. Macrophage-specific PPARgamma controls alternative activation and improves insulin resistance. Nature 2007;447:1116–1120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Solinas G, Karin M. JNK1 and IKKbeta: molecular links between obesity and metabolic dysfunction. FASEB J 2010;24:2596–2611 [DOI] [PubMed] [Google Scholar]
- 5.Solinas G, Vilcu C, Neels JG, et al. JNK1 in hematopoietically derived cells contributes to diet-induced inflammation and insulin resistance without affecting obesity. Cell Metab 2007;6:386–397 [DOI] [PubMed] [Google Scholar]
- 6.Arkan MC, Hevener AL, Greten FR, et al. IKK-beta links inflammation to obesity-induced insulin resistance. Nat Med 2005;11:191–198 [DOI] [PubMed] [Google Scholar]
- 7.Itani SI, Ruderman NB, Schmieder F, Boden G. Lipid-induced insulin resistance in human muscle is associated with changes in diacylglycerol, protein kinase C, and IkappaB-alpha. Diabetes 2002;51:2005–2011 [DOI] [PubMed] [Google Scholar]
- 8.Summers SA. Ceramides in insulin resistance and lipotoxicity. Prog Lipid Res 2006;45:42–72 [DOI] [PubMed] [Google Scholar]
- 9.Holland WL, Brozinick JT, Wang LP, et al. Inhibition of ceramide synthesis ameliorates glucocorticoid-, saturated-fat-, and obesity-induced insulin resistance. Cell Metab 2007;5:167–179 [DOI] [PubMed] [Google Scholar]
- 10.Stratford S, Hoehn KL, Liu F, Summers SA. Regulation of insulin action by ceramide: dual mechanisms linking ceramide accumulation to the inhibition of Akt/protein kinase B. J Biol Chem 2004;279:36608–36615 [DOI] [PubMed] [Google Scholar]
- 11.Chibalin AV, Leng Y, Vieira E, et al. Downregulation of diacylglycerol kinase delta contributes to hyperglycemia-induced insulin resistance. Cell 2008;132:375–386 [DOI] [PubMed] [Google Scholar]
- 12.Koliwad SK, Streeper RS, Monetti M, et al. DGAT1-dependent triacylglycerol storage by macrophages protects mice from diet-induced insulin resistance and inflammation. J Clin Invest 2010;120:756–767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Furuhashi M, Fucho R, Görgün CZ, Tuncman G, Cao H, Hotamisligil GS. Adipocyte/macrophage fatty acid-binding proteins contribute to metabolic deterioration through actions in both macrophages and adipocytes in mice. J Clin Invest 2008;118:2640–2650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Drover VA, Nguyen DV, Bastie CC, et al. CD36 mediates both cellular uptake of very long chain fatty acids and their intestinal absorption in mice. J Biol Chem 2008;283:13108–13115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhou J, Febbraio M, Wada T, et al. Hepatic fatty acid transporter Cd36 is a common target of LXR, PXR, and PPARgamma in promoting steatosis. Gastroenterology 2008;134:556–567 [DOI] [PubMed] [Google Scholar]
- 16.Koonen DP, Glatz JF, Bonen A, Luiken JJ. Long-chain fatty acid uptake and FAT/CD36 translocation in heart and skeletal muscle. Biochim Biophys Acta 2005;1736:163–180 [DOI] [PubMed] [Google Scholar]
- 17.Coburn CT, Knapp FF, Jr, Febbraio M, Beets AL, Silverstein RL, Abumrad NA. Defective uptake and utilization of long chain fatty acids in muscle and adipose tissues of CD36 knockout mice. J Biol Chem 2000;275:32523–32529 [DOI] [PubMed] [Google Scholar]
- 18.Miyaoka K, Kuwasako T, Hirano K, Nozaki S, Yamashita S, Matsuzawa Y. CD36 deficiency associated with insulin resistance. Lancet 2001;357:686–687 [DOI] [PubMed] [Google Scholar]
- 19.Goudriaan JR, Dahlmans VE, Teusink B, et al. CD36 deficiency increases insulin sensitivity in muscle, but induces insulin resistance in the liver in mice. J Lipid Res 2003;44:2270–2277 [DOI] [PubMed] [Google Scholar]
- 20.Hajri T, Han XX, Bonen A, Abumrad NA. Defective fatty acid uptake modulates insulin responsiveness and metabolic responses to diet in CD36-null mice. J Clin Invest 2002;109:1381–1389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Silverstein RL, Febbraio M. CD36, a scavenger receptor involved in immunity, metabolism, angiogenesis, and behavior. Sci Signal 2009;2:re3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stewart CR, Stuart LM, Wilkinson K, et al. CD36 ligands promote sterile inflammation through assembly of a toll-like receptor 4 and 6 heterodimer. Nat Immunol 2010;11:155–161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hoebe K, Georgel P, Rutschmann S, et al. CD36 is a sensor of diacylglycerides. Nature 2005;433:523–527 [DOI] [PubMed] [Google Scholar]
- 24.Stuart LM, Deng J, Silver JM, et al. Response to staphylococcus aureus requires CD36-mediated phagocytosis triggered by the COOH-terminal cytoplasmic domain. J Cell Biol 2005;170:477–485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shi H, Kokoeva MV, Inouye K, Tzameli I, Yin H, Flier JS. TLR4 links innate immunity and fatty acid-induced insulin resistance. J Clin Invest 2006;116:3015–3025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tsukumo DM, Carvalho-Filho MA, Carvalheira JB, et al. Loss-of-function mutation in toll-like receptor 4 prevents diet-induced obesity and insulin resistance. Diabetes 2007;56:1986–1998 [DOI] [PubMed] [Google Scholar]
- 27.Petersen EW, Carey AL, Sacchetti M, et al. Acute IL-6 treatment increases fatty acid turnover in elderly humans in vivo and in tissue culture in vitro. Am J Physiol Endocrinol Metab 2005;288:E155–E162 [DOI] [PubMed] [Google Scholar]
- 28.Kennedy DJ, Kuchibhotla S, Westfall KM, Silverstein RL, Morton RE, Febbraio M. A CD36-dependent pathway enhances macrophage and adipose inflammation and impairs insulin signaling. Cardiovasc Res 2011;89:604–613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Febbraio M, Abumrad NA, Hajjar DP, et al. A null mutation in murine CD36 reveals an important role in fatty acid and lipoprotein metabolism. J Biol Chem 1999;274:19055–19062 [DOI] [PubMed] [Google Scholar]
- 30.Watt MJ, Steinberg GR, Chan S, Garnham A, Kemp BE, Febbraio MA. Beta-adrenergic stimulation of skeletal muscle HSL can be overridden by AMPK signaling. FASEB J 2004;18:1445–1446 [DOI] [PubMed] [Google Scholar]
- 31.Matthews VB, Allen TL, Risis S, et al. Interleukin-6 deficient mice develop hepatic inflammation and systemic insulin resistance. Diabetologia 2010;53:2431–2441 [DOI] [PubMed] [Google Scholar]
- 32.Turpin SM, Lancaster GI, Darby I, Febbraio MA, Watt MJ. Apoptosis in skeletal muscle myotubes is induced by ceramides and is positively related to insulin resistance. Am J Physiol Endocrinol Metab 2006;291:E1341–E1350 [DOI] [PubMed] [Google Scholar]
- 33.Coort SL, Willems J, Coumans WA, et al. Sulfo-N-succinimidyl esters of long chain fatty acids specifically inhibit fatty acid translocase (FAT/CD36)-mediated cellular fatty acid uptake. Mol Cell Biochem 2002;239:213–219 [PubMed] [Google Scholar]
- 34.Hevener AL, Olefsky JM, Reichart D, et al. Macrophage PPAR gamma is required for normal skeletal muscle and hepatic insulin sensitivity and full antidiabetic effects of thiazolidinediones. J Clin Invest 2007;117:1658–1669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sabio G, Das M, Mora A, et al. A stress signaling pathway in adipose tissue regulates hepatic insulin resistance. Science 2008;322:1539–1543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nguyen MT, Favelyukis S, Nguyen AK, et al. A subpopulation of macrophages infiltrates hypertrophic adipose tissue and is activated by free fatty acids via toll-like receptors 2 and 4 and JNK-dependent pathways. J Biol Chem 2007;282:35279–35292 [DOI] [PubMed] [Google Scholar]
- 37.Febbraio M, Silverstein RL. CD36: implications in cardiovascular disease. Int J Biochem Cell Biol 2007;39:2012–2030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kuchibhotla S, Vanegas D, Kennedy DJ, et al. Absence of CD36 protects against atherosclerosis in ApoE knock-out mice with no additional protection provided by absence of scavenger receptor A I/II. Cardiovasc Res 2008;78:185–196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Weisberg SP, Hunter D, Huber R, et al. CCR2 modulates inflammatory and metabolic effects of high-fat feeding. J Clin Invest 2006;116:115–124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Harb D, Bujold K, Febbraio M, Sirois MG, Ong H, Marleau S. The role of the scavenger receptor CD36 in regulating mononuclear phagocyte trafficking to atherosclerotic lesions and vascular inflammation. Cardiovasc Res 2009;83:42–51 [DOI] [PubMed] [Google Scholar]
- 41.Park YM, Febbraio M, Silverstein RL. CD36 modulates migration of mouse and human macrophages in response to oxidized LDL and may contribute to macrophage trapping in the arterial intima. J Clin Invest 2009;119:136–145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Strissel KJ, Stancheva Z, Miyoshi H, et al. Adipocyte death, adipose tissue remodeling, and obesity complications. Diabetes 2007;56:2910–2918 [DOI] [PubMed] [Google Scholar]
- 43.Alkhouri N, Gornicka A, Berk MP, et al. Adipocyte apoptosis, a link between obesity, insulin resistance, and hepatic steatosis. J Biol Chem 2010;285:3428–3438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Greenberg ME, Sun M, Zhang R, Febbraio M, Silverstein R, Hazen SL. Oxidized phosphatidylserine-CD36 interactions play an essential role in macrophage-dependent phagocytosis of apoptotic cells. J Exp Med 2006;203:2613–2625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Vallerie SN, Furuhashi M, Fucho R, Hotamisligil GS. A predominant role for parenchymal c-jun amino terminal kinase (JNK) in the regulation of systemic insulin sensitivity. PLoS ONE 2008;3:e3151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Drahota Z, Vrbacký M, Nůsková H, et al. Succinimidyl oleate, established inhibitor of CD36/FAT translocase inhibits complex III of mitochondrial respiratory chain. Biochem Biophys Res Commun 2010;391:1348–1351 [DOI] [PubMed] [Google Scholar]