Abstract
OBJECTIVE
Ghrelin is a stomach-derived peptide that increases food intake through the activation of hypothalamic AMP-activated protein kinase (AMPK). However, the molecular mechanisms initiated by the activation of the ghrelin receptor, which in turn lead to AMPK activation, remain unclear. Sirtuin 1 (SIRT1) is a deacetylase activated in response to calorie restriction that acts through the tumor suppressor gene p53. We tested the hypothesis that the central SIRT1/p53 pathway might be mediating the orexigenic action of ghrelin.
RESEARCH DESIGN AND METHODS
SIRT1 inhibitors, such as Ex527 and sirtinol, and AMPK activators, such as AICAR, were administered alongside ghrelin in the brain of rats and mice (wild-type versus p53 knockout [KO]). Their hypothalamic effects on lipid metabolism and changes in transcription factors and neuropeptides were assessed by Western blot and in situ hybridization.
RESULTS
The central pretreatment with Ex527, a potent SIRT1 inhibitor, blunted the ghrelin-induced food intake in rats. Mice lacking p53, a target of SIRT1 action, failed to respond to ghrelin in feeding behavior. Ghrelin failed to phosphorylate hypothalamic AMPK when rats were pretreated with Ex527, as it did in p53 KO mice. It is noteworthy that the hypothalamic SIRT1/p53 pathway seems to be specific for mediating the orexigenic action of ghrelin, because central administration of AICAR, a potent AMPK activator, increased food intake in p53 KO mice. Finally, blockade of the central SIRT1 pathway did not modify ghrelin-induced growth hormone secretion.
CONCLUSIONS
Ghrelin specifically triggers a central SIRT1/p53 pathway that is essential for its orexigenic action, but not for the release of growth hormone.
Ghrelin is the only known endogenous signal stimulating adiposity and feeding (1–3). At the hypothalamic level, ghrelin activates AMP-activated protein kinase (AMPK), causing relevant changes in hypothalamic mitochondrial respiration and production of reactive oxygen species (4–6), altering the expression of transcription factors Bsx, Forkhead box class O (FoxO1), and cAMP-responsive element–binding protein (pCREB), and leading to the final activation of agouti-related peptide/neuropeptide Y (AgRP/NPY) neurons. However, the molecular mechanisms occurring after GHS-R1a activation and before AMPK phosphorylation are completely unknown. Ghrelin is the only gut peptide with orexigenic properties in rodents and humans; thus, the ghrelin system is uniquely positioned as a drug target for the treatment of cachexia. The current study tested the hypothesis that the central sirtuin 1 (SIRT1)/p53 pathway might be mediating the orexigenic action of ghrelin.
SIRT1 is a NAD+-dependent deacetylase that acts on important tumor suppressors like p53. In addition to their biologic actions on cancer, SIRT1 and p53 are also important in several metabolically relevant tissues. SIRT1 controls divergent metabolic pathways in adipose tissue (7), liver (8), pancreatic β cells (9), and skeletal muscle (10), mainly through the regulation of rate-limiting enzymes involved in glucose and lipid metabolism. Recent reports have shown that central SIRT1 also regulates energy and glucose homeostasis (11–17). On the other hand, p53 is activated by the lack of nutrients through the activation of AMPK, and p53 senescence activity contributes to the development of insulin resistance (18).
Because the molecular mechanisms that link the effects of the ghrelin/GHS-R1a system to AMPK are unknown, the current study tested the hypothesis that the hypothalamic SIRT1/p53 pathway might be mediating the orexigenic action of ghrelin.
RESEARCH DESIGN AND METHODS
Animal models.
Male Sprague-Dawley rats (8 weeks old, 200–250 g) and C57/B6 mice (8 weeks old) were housed in air-conditioned rooms (22–24°C) under a 12/12-h light/dark cycle and fed standard chow. p53-null (8–10 weeks old, mixed background C57BL/6J and 129/Sv) mice were described previously (19). Homozygous wild-type (WT) and knockout (KO) mice were originated from heterozygous mating, so only littermate WT and KO animals were compared in each experiment. Animals were treated and killed when they were 12 to 14 weeks of age before any sign of morbidity resulting from tumor development occurred. Animals were killed by decapitation between 1000 and 1200 h. Animal experiments were conducted in accordance with the standards approved by the Faculty Animal Committee at the University of Santiago de Compostela, and the experiments were performed in agreement with the Rules of Laboratory Animal Care and International Law on Animal Experimentation.
Nutritional status.
Rats (n = 8/group), were assigned to one of the following groups: fed ad libitum, deprived of food for 48 h, and fasted during 48 h and refed during 24 h. All animals had free access to tap water.
Implantation of intracerebroventricular cannulae.
Rats were anesthetized by an intraperitoneal injection of ketamine (100 mg/kg body wt)/xylazine (15 mg/kg body wt). Mice were anesthetized by an intraperitoneal injection of tribromoethanol (480 mg/kg; Sigma-Aldrich, St Louis, MO). Intracerebroventricular cannulae were implanted stereotaxically in rats (20) or mice (21), as described previously.
Intracerebroventricular treatments.
Rats received an intracerebroventricular administration of 5 μL of vehicle or ghrelin (5 μg; Bachem, Bubendorf, Switzerland). For the inhibition of SIRT1, we used two potent specific inhibitors of SIRT1: Ex527 (1 to 5-10 μg in a total volume of 5 μL; Tocris Bioscience, St. Louis, MO) (22) and sirtinol (1 to 5-10 μg in a total volume of 5 μL; Tocris Bioscience) (23) before ghrelin administration. For the experiments involving only two groups (vehicle versus ghrelin), the vehicle was saline. For the experiments involving SIRT1 inhibitors, the vehicle was DMSO, because Ex527 and sirtinol were both diluted in DMSO. Mice received an intracerebroventricular administration of vehicle, ghrelin (5 μg), or AICAR (3 μg; Sigma-Aldrich A9978) in a total volume of 2 μL. For the experiments involving vehicle versus ghrelin and vehicle versus AICAR, the vehicle was saline.
We used the same dose of ghrelin for both rats and mice because this dose has been demonstrated to be effective in both species (2). We used eight rats per group, and the experiments were repeated at least twice. Rats were killed by cervical dislocation. Hypothalami were dissected and stored at −80°C until further processing.
Western blotting.
Hypothalami were homogenized in ice-cold lysis buffer containing 50 mmol/L Tris-HCl (pH 7.5), 1 mmol/L EGTA, 1 mmol/L EDTA, 1% Triton X-100, 1 mmol/L sodium orthovanadate, 50 mmol/L sodium fluoride, 5 mmol/L sodium pyrophosphate, 0.27 mol/L sucrose, 0.1% 2-mercaptoethanol, and Complete protease inhibitor cocktail (1 tablet/50 mL; Roche Diagnostics, Mannheim, Germany). Homogenates were centrifuged at 13,000g for 10 min at 4°C, supernatants were removed, and aliquots were snap-frozen in liquid nitrogen. Hypothalamus lysate (40 μg) was subjected to SDS-PAGE on 6% polyacrylamide gels and electrotransferred on a polyvinylidene fluoride membrane.
Membranes were blocked for 1 h in TBS-Tween 20 (TBST: 50 mmol/L Tris-HCl [pH 7.5], 0.15 mol/L NaCl, and 0.1% Tween 20) containing 5% skimmed milk or 3% BSA (for pAMPK Thr172 and pACC Ser79) and probed for 16 h at 4°C in TBST, 5% skimmed milk, or 3% BSA (for pAMPK Thr172, pACC Ser79, SIRT1, and acetyl-p53-Lys379) with the appropriate dilution of the indicated antibodies (acetyl-CoA carboxylase [ACC]: 1:1500; pACC: 1:2000; AMPKα1: 1:1000; AMPKα2: 1:1000; pAMPK: 1:2000; β-actin (loading control): 1:2000). ACC was detected using horseradish peroxidase (HRP)-conjugated–coupled streptavidin (Amersham Biosciences, Little Chalfont, U.K.).
Detection of proteins was performed using HRP-conjugated secondary antibodies and an enhanced chemiluminescence reagent (Amersham Biosciences). We used 8 to 12 hypothalami per experimental group. Acetyl-p53-Lys379 was obtained from Cell Signaling (Danvers, MA). ACCα, pACCα-Ser79, AMPKα1, and AMPKα2 were obtained from Upstate Biotechnology (Temecula, CA); pAMPKα-Thr172 from Cell Signaling; fatty acid synthase (FAS), pCREB, and FoxO1 from Santa Cruz Biotechnology (Santa Cruz, CA); and β-actin from Abcam (Cambridge, U.K.), as described previously (6).
For the blotting assays, the experiments constituted by two groups: Sprague-Dawley rats and mice (WT and p53 KO) treated with ghrelin or AICAR and analyzed using a nonparametric Mann–Whitney test. In the experiments constituted by four groups (Sprague-Dawley rats treated with vehicle, ghrelin, Ex527, and Ex527 + ghrelin, or with vehicle, ghrelin, sirtinol, and sirtinol + ghrelin), the data were analyzed by two-way ANOVA, followed by a post hoc multiple comparison test (Tukey’s test).
In situ hybridization.
Coronal hypothalamic sections (16 μm) were cut on a cryostat and immediately stored at −80ºC until hybridization. For AgRP, NPY, and Bsx mRNA detection, we used the specific antisense oligodeoxynucleotides (Table 1). These probes were 3′-end–labeled with [35S]deoxy-ATP using terminal deoxynucleotidyl transferase. The specificity of the probes was confirmed by incubating the sections with an excess of the unlabeled probes, as reported previously (24,25). In situ hybridizations were performed as reported previously (24,25).
TABLE 1.
Antisense oligonucleotides for in situ hybridization analysis
| mRNA | GenBank accession number | Sequence |
|---|---|---|
| AgRP | AF206017 | 5′-CGACGCGGAGAACGAGACTCGCGGTTCTGTGGATCTAGCACCTCTGCC-3′ |
| BSX | XM_001064837 | 5′-CCTCAACGGCTTGGGCTTGTGTAGCAGAATGTCC-3′ |
| NPY | M20373 | 5′-AGATGAGATGTGGGGGGAAACTAGGAAAAGTCAGGAGAGCAAGTTTCATT-3′ |
The frozen sections were fixed with 4% paraformaldehyde in phosphate buffer (0.1 mol/L, pH 7.4) at room temperature for 30 min. They were dehydrated using 70%, 80%, 90%, 95%, and 100% ethanol for 5 min each. The hybridization was done overnight at 37°C in a moist chamber. The hybridization solution contained 5 × 105 (AgRP and Bsx) or 1 × 106 cpm (NPY) per slide of the labeled probe, 4× standard saline citrate (SSC), 50% deionized formamide, 1× Denhardt’s solution, 10% dextran sulfate, and 10 μg/mL sheared, single-stranded salmon sperm DNA.
Afterward, the hybridization sections were sequentially washed in 1× SSC at room temperature, four times in 1× SSC at 42ºC (30 min/wash), and once in 1× SSC at room temperature (1 h) and then rinsed in water and ethanol. Finally, the sections were air-dried and exposed to Hyperfilm β-Max (Amersham Biosciences) at room temperature for 4 to 6 days for AgRP and NPY and for 21 days for Bsx. The slides were developed in Kodak D-19 developer (Eastman Kodak Co., Rochester, NY), fixed (Kodak fixer), and counterstained with methylene blue.
To compare anatomically similar regions, the slides were matched according to Paxinos and Watson (26). The slides from control and treated animals at each treatment time were always exposed to the same autoradiographic film. All sections were scanned, and the specific hybridization signal was quantified by densitometry using the Molecular Analyst digital imaging system (Bio-Rad Laboratories Inc., Richmond, CA). The optical density of the hybridization signal was determined and subsequently corrected by the optical density of its adjacent background value. For this reason, a rectangle with the same dimensions in each case was drawn enclosing the hybridization signal over each nucleus and over adjacent brain areas of each section (background). We used 16 to 20 sections for each animal (4–5 slides, 4 sections/slide). The mean of these 16 to 20 values was used as the densitometry value for each animal.
Plasma growth hormone response.
Chronic intracerebroventricular and intracardiac cannulae were implanted under ketamine/xylazine anesthesia, as described above. After surgery, the animals were placed directly in isolation test chambers for 5 days and were given free access to regular rat chow and tap water. On the day of the experiment, blood samples (0.3 mL) were withdrawn at the appropriate times. The animals (n = 7–9 rats/group) received vehicle, ghrelin (12 nmol/kg i.v.), Ex527 (1 μg i.c.v.), or Ex527 + ghrelin.
Hormone assays.
Plasma growth hormone (GH) concentrations were determined by double-antibody radioimmunoassay using materials supplied by the National Hormone Pituitary Program, as described previously (27). Values are expressed in terms of the GH reference preparation (GH-RP-2). The intra- and interassay coefficients of variation were 7 and 10%, respectively.
Statistical analysis and data presentation.
The experiments constituted by two groups: Sprague-Dawley rats and mice (WT and p53 KO) treated with ghrelin or AICAR and analyzed using a nonparametric Mann–Whitney test. In the experiments constituted by four groups (Sprague-Dawley rats treated with vehicle, ghrelin, Ex527, and Ex527 + ghrelin, and vehicle, ghrelin, sirtinol, and sirtinol + ghrelin), the data were analyzed by two-way ANOVA, followed by a post hoc multiple comparison test (Tukey’s test). Data are expressed as mean ± SEM and analyzed using PASW Statistics 18.0 software (SPSS Inc., Chicago, IL). A value of P < 0.05 was considered as being significant.
RESULTS
Regulation of SIRT1 by nutritional status.
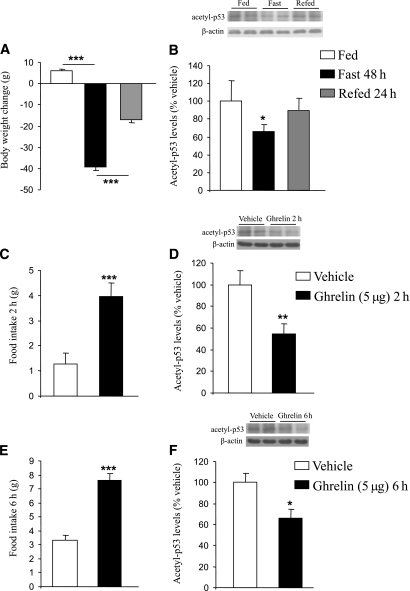
Rats fasted during 48 h exhibited a loss of body weight, whereas the refeeding during 24 h partially led to a substantial recovery (Fig. 1A). Acetyl-p53 levels, a marker of SIRT1 activity in vivo (28), were decreased in the hypothalamus of fasted rats (F2,18 = 3.651, P < 0.05), whereas those levels were similar to baseline in rats after refeeding (Fig. 1B). Because ghrelin is a key player for increasing feeding behavior, we tested the hypothesis that central ghrelin administration might stimulate hypothalamic SIRT1 activity. As previously reported, ghrelin increased food intake after 2 h (F1,13 = 24.161, P < 0.001; Fig. 1C) and 6 h (F1,13 = 36.14, P < 0.001; Fig. 1E) (29) and decreased hypothalamic acetyl-p53 levels after 2 h (F1,13 = 7.915, P < 0.01; Fig. 1D) and 6 h (F1,13 = 7.645, P < 0.05; Fig. 1F).
FIG. 1.
Effects of nutritional status on hypothalamic SIRT1. A: Fasting for 48 h caused a significant (P < 0.001) decrease in body weight, whereas refeeding during 24 h partially recovered the weight loss. B: Hypothalamic acetylated p53 levels decreased in fasted rats and recovered in refed rats. Effects of intracerebroventricular ghrelin injection (5 μg/rat) after 2 h on food intake (C), and hypothalamic acetylated p53 levels (D). Effects of intracerebroventricular ghrelin injection (5 μg/rat) after 6 h on food intake (E), and hypothalamic acetylated p53 levels (F). Values were normalized to those of the internal control β-actin, and the results are expressed as arbitrary units. Mean values were obtained from six animals per group. Values are the mean ± SEM. *P < 0.05, **P < 0.01, ***P < 0.001.
Blockade of the SIRT1/p53 pathway blunts the orexigenic action of ghrelin.
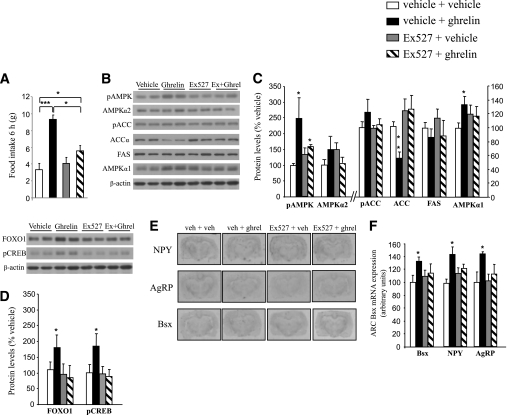
We next studied the functional relevance of these findings by assessing whether the pharmacologic blockade of SIRT1 activity might regulate the orexigenic action of ghrelin. First, we observed that the central injection of Ex527, a potent inhibitor of SIRT1 activity, increased hypothalamic acetyl-p53 levels at different doses (1, 5, and 10 μg; Supplementary Fig. 1A), indicating that this compound decreased hypothalamic SIRT1 activity in vivo. We then centrally administered ghrelin, Ex527, and Ex527 + ghrelin to the rats. We found that ghrelin increased food intake at 2 h (data not shown) and 6 h (F3,27 = 12.282, P < 0.001; Fig. 2A), but when the SIRT1 inhibitor was administered 20 min before ghrelin, the orexigenic action of ghrelin was markedly blunted after 6 h (Fig. 2A). Because ghrelin increases food intake through its effects on hypothalamic fatty acid metabolism (5,6), we next assessed the levels of several key enzymes for the synthesis of lipids. We found that 6 h after an intracerebroventricular ghrelin injection, pAMPK levels were increased (F3,28 = 2.455, P < 0.05), but ACC levels were decreased (F3,27 = 6.045, P < 0.01). Those effects were abolished when the SIRT1 inhibitor was coadministered (Fig. 2B and C).
FIG. 2.
Pharmacologic blockade of SIRT1 blunts the orexigenic action of ghrelin. A: Effects of intracerebroventricular ghrelin injection (5 μg/rat), Ex527 (1 μg/rat), and ghrelin + Ex527 on food intake after 6 h. Hypothalamic protein levels of pAMPKα, AMPKα1, AMPKα2, pACC, ACCα, FAS (B and C), pCREB, FoxO1 (D), and Bsx, NPY, and AgRP (E and F) after 6 h of ghrelin and Ex527 injection. Values were normalized to those of the internal control β-actin, and the results are expressed as arbitrary units. Mean values were obtained from six animals per group. Values are the mean ± SEM. *P < 0.05, **P < 0.01, ***P < 0.001.
It is important to note that even though pAMPK levels were increased, pACC did not reach statistical significance, probably because of the different kinetics of phosphorylation of both enzymes (6). Furthermore, the higher expression of the transcription factors FoxO1 (F3,27 = 2.509, P < 0.05), pCREB (F3,26 = 3.668, P < 0.05; Fig. 2D), and Bsx (F3,26 = 3.526, P < 0.05; Fig. 2E and F) and the neuropeptides NPY (F(3,26) = 4.362, P < 0.05) and AgRP (F3,26 = 3.33, P < 0.05; Fig. 2E and F) in the hypothalamic arcuate nucleus induced by ghrelin was also abolished when the SIRT1 inhibitor was coadministered. When we used sirtinol, another inhibitor of SIRT1 activity, results were similar to those obtained with Ex527 (Supplementary Fig. 1).
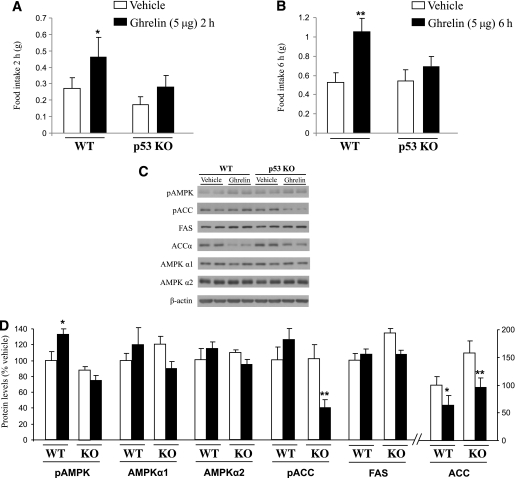
Tumor suppressor protein p53 is a substrate of SIRT1, and it is found hyperacetylated in SIRT1 KO mice (30). Because a recent report showed that p53 is involved in energy metabolism and homeostasis (31), we next inquired whether p53 could be a mediator of SIRT1-dependent effects of ghrelin. For this purpose, we treated WT and p53 KO mice with intracerebroventricular ghrelin, following the same protocol as that described above. The p53 KO mice did not show alterations in body weight, food intake, fat mass, or nonfat mass compared with WT littermates (Supplementary Fig. 2A–D). As expected, central ghrelin administration increased food intake in WT animals, whereas identical intracerebroventricular ghrelin treatment in p53 KO animals had no effect on food intake after 2 (Fig. 3A) or 6 h (Fig. 3B).
FIG. 3.
Mice lacking p53 do not respond to ghrelin injection. Effects of intracerebroventricular ghrelin injection (5 μg/mouse) on food intake after 2 h (A) and 6 h (B) in WT and p53 KO mice. Hypothalamic protein levels of pAMPKα, AMPKα1, AMPKα2, pACC, ACCα, and FAS after 6 h of ghrelin injection (C and D). Values were normalized with to those of the internal control β-actin, and the results are expressed as arbitrary units. Mean values were obtained from six animals per group. Values are the mean ± SEM. *P < 0.05, **P < 0.01.
We next assessed the levels of several key enzymes for the synthesis of lipids. No principal differences were found between WT and p53 KO mice regarding the expression of AMPKα1, AMPKα2, or FAS (Fig. 3C and D). It is noteworthy that we found that 6 h after its central injection, ghrelin increased pAMPK levels in WT mice (F1,12 = 4.466, P < 0.05) but failed to do so in p53 KO mice (Fig. 3C and D), suggesting that p53 is an essential mediator of ghrelin actions on AMPK. The levels of pAMPK and pACC are correlated in normal conditions; however, we found that the hypothalamic levels of pACC are downregulated in p53 KO mice (F1,12 = 8.576, P < 0.01) but not in WT mice (Fig. 3C and D).
Although we do not have a clear explanation for these results, it seems that ghrelin is able to activate ACC when p53 is not present, suggesting that p53 might also regulate the actions of ghrelin on different key enzymes modulating fatty acid metabolism. Further studies analyzing not only protein levels but also enzymatic activity and lipolysis/lipogenesis will be necessary to address this issue. Furthermore, we detected that central ghrelin injection decreased ACCα levels in both WT (F1,12 = 2.844, P < 0.05) and p53 KO mice (F1,12 = 6.699, P < 0.01; Fig. 3C and D), indicating that p53 is not essential for ghrelin-mediated ACC regulation.
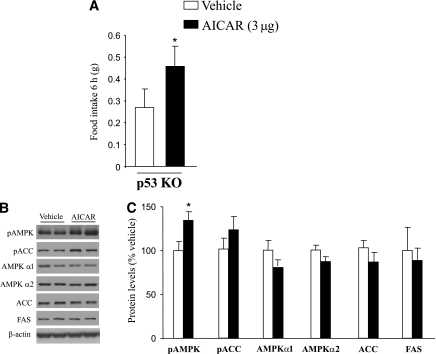
p53 does not mediate the orexigenic action of AICAR.
Central injection of AICAR, a potent activator of AMPK activity, stimulates food intake in rodents (32). To determine whether p53 is a crucial player for the orexigenic action of AICAR, we centrally treated p53 KO mice with AICAR (3 μg) and found a stimulation in food intake after 6 h (F1,12 = 3.542, P < 0.05; Fig. 4A). Hypothalamic pAMPK levels were also increased in p53 KO mice treated with AICAR (F1,12 = 4.479; Fig. 4B and C). Therefore, our data indicate that p53 is not required for the orexigenic action of direct AMPK activators.
FIG. 4.
Central injection of AICAR increases food intake in p53 KO mice. A: Effects of intracerebroventricular AICAR injection (3 μg/mouse) on food intake after 6 h in p53 KO mice. B and C: Hypothalamic protein levels of pAMPK, pACC, AMPKα1, AMPKα2, ACCα, and FAS after 6 h of AICAR injection. Values were normalized to those of the internal control β-actin, and the results are expressed as arbitrary units. Mean values were obtained from six animals per group. Values are the mean ± SEM, *P < 0.05.
The central SIRT1 pathway does not modulate ghrelin-induced GH secretion.
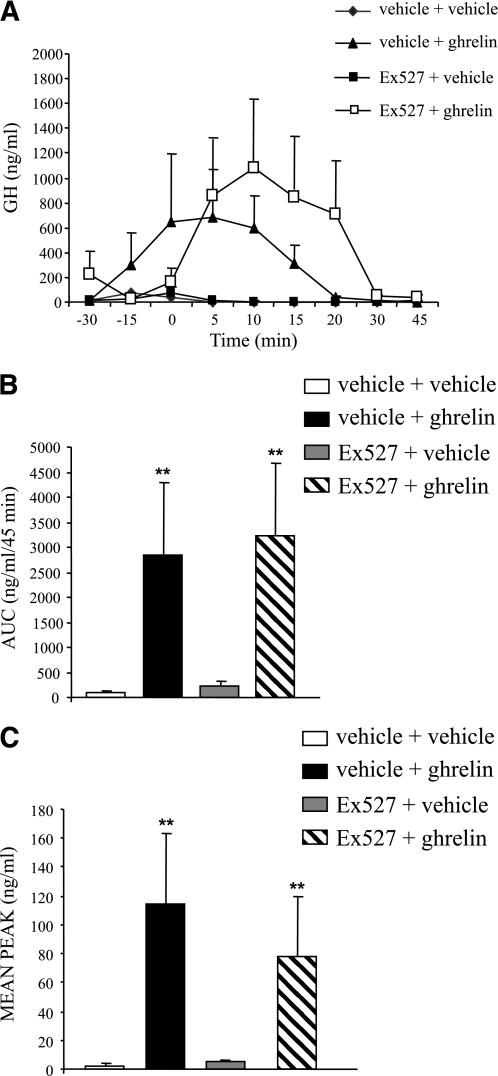
Finally, we assessed whether the SIRT1 pathway is mediating other neuroendocrine actions of ghrelin, namely GH secretion. Administration of ghrelin (27) led to the expected increase in plasma GH levels at 5, 10, and 15 min (Fig. 5A), whereas the central blockade of SIRT1 did not alter that response (Fig. 5A). Ghrelin exhibited a similar stimulatory effect in both area under the curve and mean peak GH levels (Fig. 5B and C).
FIG. 5.
Pharmacologic blockade of SIRT1 does not modify the ghrelin-induced GH secretion. A: Effects of intravenous ghrelin injection (12 nmol/kg), intracerebroventricular Ex527 (1 μg/rat), and ghrelin + Ex527 on plasma GH levels in adult male freely moving rats. Area under the curve (AUC) (B) and mean peak GH (C) levels. Mean values were obtained from six animals per group. Values are the mean ± SEM. *P < 0.05, **P < 0.01.
DISCUSSION
Our current data demonstrate that the hypothalamic SIRT1/p53 pathway is crucial for the orexigenic effect of ghrelin. Pharmacologic inhibition of SIRT1 or genetic depletion of p53 abolish the effects of ghrelin on AMPK and thereby blunt ghrelin-induced effects on transcription factors, including pCREB, FoxO1, and Bsx, and neuropeptides, such as NPY and AgRP, leading to a suppression of ghrelin-induced food intake.
SIRT1 is a deacetylase that regulates metabolism in multiple peripheral tissues. It has been reported recently that SIRT1 mRNA is located in metabolically relevant areas of the mouse neuroaxis, such as pro-opiomelanocortin neurons, which are critical for energy and glucose homeostasis (11). It seems that SIRT1 regulates the central melanocortin system (13) and that the specific lack of SIRT1 in the brain abolishes the higher physical activity induced by calorie restriction (12). More specifically, the lack of SIRT1 in pro-opiomelanocortin neurons causes hypersensitivity to diet-induced obesity because of reduced energy expenditure (17). Concurring with those data, we observed that acetylation of p53 in the hypothalamus is decreased after food deprivation, indicating that SIRT1 as a deacetylase increased its hypothalamic activity during starvation. Therefore, the regulation of hypothalamic SIRT1 activity by nutritional status is similar to its regulation in several peripheral tissues (33).
Ghrelin is a stomach-derived hormone that rapidly increases food intake and body weight (1,29). Its regulation by nutritional status was controversial because the assays detected both acyl-ghrelin and des-acyl ghrelin and thus were not specific. Studies using new technologies for separately detecting both isoforms indicate that circulating des-acyl ghrelin increases significantly with fasting, whereas blood acyl-ghrelin levels are not changed over the course of fasting (34,35). Most of the effects of ghrelin are exerted through the GH secretagogue receptor 1a (GHS-R1a) (36), which is expressed in AgRP/NPY neurons in the hypothalamic arcuate nucleus (37). The orexigenic effect of ghrelin is mediated by AMPK, a key upstream master regulator of lipid metabolism (5,6). However, the molecular events regulating AMPK phosphorylation after the activation of the GHS-R1a are unknown.
In the present work, we demonstrate that central ghrelin administration increases hypothalamic SIRT1 activity, stimulating the deacetylation of p53. More interestingly and consistent with previous findings (14), the blockade of central SIRT1 activity abolished the potent orexigenic effect of ghrelin. At molecular level, the ghrelin-induced activation of AMPK is prevented when SIRT1 activity is blocked. The interaction between SIRT1 and AMPK has been previously shown in vitro, indicating that resveratrol activates AMPK in neurons (38).
Although pharmacologic experiments based on the administration of SIRT1 inhibitors to animal models are providing important insight into principal effects, targets, and mechanisms of the SIRT1 system, understanding the function of its endogenous role requires more sophisticated approaches, such as genetic disruption of SIRT1 or its substrates. We focused on p53 because this tumor-suppressor protein is a well-known target of SIRT1 action, and there is growing evidence of its role on metabolism and energy balance in peripheral tissues (31). Our results indicate that p53 is required for the ghrelin-induced food intake, because ghrelin failed to increase food intake in p53 KO mice. In accordance with pharmacologic findings, ghrelin stimulated hypothalamic pAMPK levels in WT mice but failed to do so in p53 KO mice. On the other hand, it has been shown that SIRT1 is a target gene of p53 in some but not all of the tissues (31,39), so it might be possible that p53 mediates the changes in SIRT1 upon ghrelin treatment or calorie restriction. However, the observed correlation between acetylated p53 and SIRT1 activity in the hypothalamus on different experimental conditions indicate that p53 is an essential mediator of SIRT1-dependent effects of ghrelin on AMPK. Mice lacking p53 in specific hypothalamic areas will be essential to demonstrating which particular neuronal circuits are responsible for those actions. However, the central SIRT1/p53 pathway is not required by direct AMPK activators, because AICAR stimulated food intake in p53-deficient mice. Therefore, these findings corroborate that the central SIRT1/p53 pathway is not associated with AICAR-induced activation of AMPK.
Finally, our results indicate that ghrelin stimulated GH release as expected, but the blockade of central SIRT1 did not modify GH levels. Therefore, it seems that the central SIRT1/p53 pathway is specifically mediating the ghrelin orexigenic action and suggests that different neuronal pathways modulate ghrelin-induced food intake and GH secretion.
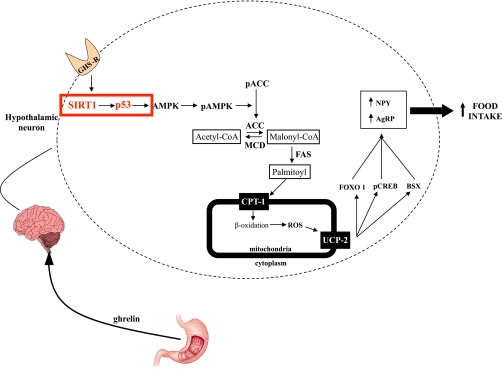
In summary, we provide a combination of pharmacologic and genetic evidence to demonstrate that the central nervous system SIRT1/p53 pathway is essential for the orexigenic response to ghrelin (Fig. 6). The molecular pathway mediating those effects involves alterations in AMPK activation, which leads to changes in hypothalamic fatty acid metabolism, and finally, modifies feeding behavior.
FIG. 6.
Schematic overview summarizing our proposed model for the molecular mechanisms initiated by the activation of the ghrelin receptor leading to AMPK activation and finally to an increased feeding behavior. ROS, reactive oxygen species; CPT, carnitine palmitoyltransferase; UCP, uncoupling protein. (A high-quality color representation of this figure is available in the online issue.)
ACKNOWLEDGMENTS
This work has been supported by the Ministerio de Educacion y Ciencia (Grants BFU2008 [to C.D.], RYC-2008-02219 and SAF2009-07049 [to R.N.], RYC-2007-00211 [to M.L.], and SAF2009-07389 [to A.V.]); Xunta de Galicia (Grants 2010/14 [to R.N.] and 10PXIB208164PR [to M.L.]; Fondo Investigationes Sanitarias (Grant PS09/01880 [to M.L.]); and European Union (Grant Health-F2-2008-223713: “Reprobesity” [to C.D]). The research leading to these results has received funding from the European Community’s Seventh Framework Programme (FP7/2007-2013) under grant agreement No. 245009. CIBER de Fisiopatología de la Obesidad y Nutrición is an initiative of ISCIII.
No potential conflicts of interest relevant to this article were reported.
D.A.V., G.M., A.R., M.J.V., K.D.B., and I.G.D.-R. contributed to researched data and wrote the manuscript. M.L., A.V., R.N., and C.D. reviewed and edited the manuscript and contributed to discussion.
The authors thank Dr. Hector J. Caruncho (University of Santiago de Compostela) for helpful comments, Carmen Cadarso (University of Santiago de Compostela) for sound statistical advice, and Luz Casas (University of Santiago de Compostela) for excellent technical assistance. The authors thank Dr. Manuel Serrano from the Spanish National Cancer Research Center (CNIO) for providing p53-deficient mice.
Footnotes
This article contains Supplementary Data online at http://diabetes.diabetesjournals.org/lookup/suppl/doi:10.2337/db10-0802/-/DC1.
REFERENCES
- 1.Tschöp M, Smiley DL, Heiman ML. Ghrelin induces adiposity in rodents. Nature 2000;407:908–913 [DOI] [PubMed] [Google Scholar]
- 2.Theander-Carrillo C, Wiedmer P, Cettour-Rose P, et al. Ghrelin action in the brain controls adipocyte metabolism. J Clin Invest 2006;116:1983–1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Egecioglu E, Jerlhag E, Salomé N, et al. Ghrelin increases intake of rewarding food in rodents. Addict Biol 2010;15:304–311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kola B, Farkas I, Christ-Crain M, et al. The orexigenic effect of ghrelin is mediated through central activation of the endogenous cannabinoid system. PLoS One 2008;3:e1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Andrews ZB, Liu ZW, Walllingford N, et al. UCP2 mediates ghrelin’s action on NPY/AgRP neurons by lowering free radicals. Nature 2008;454:846–851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.López M, Lage R, Saha AK, et al. Hypothalamic fatty acid metabolism mediates the orexigenic action of ghrelin. Cell Metab 2008;7:389–399 [DOI] [PubMed] [Google Scholar]
- 7.Picard F, Kurtev M, Chung N, et al. Sirt1 promotes fat mobilization in white adipocytes by repressing PPAR-gamma. Nature 2004;429:771–776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rodgers JT, Puigserver P. Fasting-dependent glucose and lipid metabolic response through hepatic sirtuin 1. Proc Natl Acad Sci USA 2007;104:12861–12866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bordone L, Motta MC, Picard F, et al. Sirt1 regulates insulin secretion by repressing UCP2 in pancreatic beta cells. PLoS Biol 2006;4:e31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cantó C, Gerhart-Hines Z, Feige JN, et al. AMPK regulates energy expenditure by modulating NAD+ metabolism and SIRT1 activity. Nature 2009;458:1056–1060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ramadori G, Lee CE, Bookout AL, et al. Brain SIRT1: anatomical distribution and regulation by energy availability. J Neurosci 2008;28:9989–9996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cohen DE, Supinski AM, Bonkowski MS, Donmez G, Guarente LP. Neuronal SIRT1 regulates endocrine and behavioral responses to calorie restriction. Genes Dev 2009;23:2812–2817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cakir I, Perello M, Lansari O, Messier NJ, Vaslet CA, Nillni EA. Hypothalamic Sirt1 regulates food intake in a rodent model system. PLoS One 2009;4:e8322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dietrich MO, Antunes C, Geliang G, et al. Agrp neurons mediate Sirt1’s action on the melanocortin system and energy balance: roles for Sirt1 in neuronal firing and synaptic plasticity. J Neurosci 2010;30:11815–11825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Satoh A, Brace CS, Ben-Josef G, et al. SIRT1 promotes the central adaptive response to diet restriction through activation of the dorsomedial and lateral nuclei of the hypothalamus. J Neurosci 2010;30:10220–10232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sasaki T, Kim HJ, Kobayashi M, et al. Induction of hypothalamic Sirt1 leads to cessation of feeding via agouti-related peptide. Endocrinology 2010;151:2556–2566 [DOI] [PubMed] [Google Scholar]
- 17.Ramadori G, Fujikawa T, Fukuda M, et al. SIRT1 deacetylase in POMC neurons is required for homeostatic defenses against diet-induced obesity. Cell Metab 2010;12:78–87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vousden KH, Ryan KM. p53 and metabolism. Nat Rev Cancer 2009;9:691–700 [DOI] [PubMed] [Google Scholar]
- 19.Jacks T, Remington L, Williams BO, et al. Tumor spectrum analysis in p53-mutant mice. Curr Biol 1994;4:1–7 [DOI] [PubMed] [Google Scholar]
- 20.Nogueiras R, Wiedmer P, Perez-Tilve D, et al. The central melanocortin system directly controls peripheral lipid metabolism. J Clin Invest 2007;117:3475–3488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nogueiras R, Pérez-Tilve D, Veyrat-Durebex C, et al. Direct control of peripheral lipid deposition by CNS GLP-1 receptor signaling is mediated by the sympathetic nervous system and blunted in diet-induced obesity. J Neurosci 2009;29:5916–5925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Solomon JM, Pasupuleti R, Xu L, et al. Inhibition of SIRT1 catalytic activity increases p53 acetylation but does not alter cell survival following DNA damage. Mol Cell Biol 2006;26:28–38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ota H, Tokunaga E, Chang K, et al. Sirt1 inhibitor, Sirtinol, induces senescence-like growth arrest with attenuated Ras-MAPK signaling in human cancer cells. Oncogene 2006;25:176–185 [DOI] [PubMed] [Google Scholar]
- 24.Nogueiras R, López M, Lage R, et al. Bsx, a novel hypothalamic factor linking feeding with locomotor activity, is regulated by energy availability. Endocrinology 2008;149:3009–3015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Seoane LM, López M, Tovar S, Casanueva FF, Señarís R, Diéguez C. Agouti-related peptide, neuropeptide Y, and somatostatin-producing neurons are targets for ghrelin actions in the rat hypothalamus. Endocrinology 2003;144:544–551 [DOI] [PubMed] [Google Scholar]
- 26.Paxinos GW. The Rat Brain in Stereotaxic Coordinates. Sydney, Academic Press, 1986 [Google Scholar]
- 27.Seoane LM, Tovar S, Baldelli R, et al. Ghrelin elicits a marked stimulatory effect on GH secretion in freely-moving rats. Eur J Endocrinol 2000;143:R7–R9 [DOI] [PubMed] [Google Scholar]
- 28.Kim EJ, Kho JH, Kang MR, Um SJ. Active regulator of SIRT1 cooperates with SIRT1 and facilitates suppression of p53 activity. Mol Cell 2007;28:277–290 [DOI] [PubMed] [Google Scholar]
- 29.Nakazato M, Murakami N, Date Y, et al. A role for ghrelin in the central regulation of feeding. Nature 2001;409:194–198 [DOI] [PubMed] [Google Scholar]
- 30.Han MK, Song EK, Guo Y, Ou X, Mantel C, Broxmeyer HE. SIRT1 regulates apoptosis and Nanog expression in mouse embryonic stem cells by controlling p53 subcellular localization. Cell Stem Cell 2008;2:241–251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hallenborg P, Feddersen S, Madsen L, Kristiansen K. The tumor suppressors pRB and p53 as regulators of adipocyte differentiation and function. Expert Opin Ther Targets 2009;13:235–246 [DOI] [PubMed] [Google Scholar]
- 32.Andersson U, Filipsson K, Abbott CR, et al. AMP-activated protein kinase plays a role in the control of food intake. J Biol Chem 2004;279:12005–12008 [DOI] [PubMed] [Google Scholar]
- 33.Cohen HY, Miller C, Bitterman KJ, et al. Calorie restriction promotes mammalian cell survival by inducing the SIRT1 deacetylase. Science 2004;305:390–392 [DOI] [PubMed] [Google Scholar]
- 34.Kirchner H, Gutierrez JA, Solenberg PJ, et al. GOAT links dietary lipids with the endocrine control of energy balance. Nat Med 2009;15:741–745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Prudom C, Liu J, Patrie J, et al. Comparison of competitive radioimmunoassays and two-site sandwich assays for the measurement and interpretation of plasma ghrelin levels. J Clin Endocrinol Metab 2010;95:2351–2358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kojima M, Hosoda H, Date Y, Nakazato M, Matsuo H, Kangawa K. Ghrelin is a growth-hormone-releasing acylated peptide from stomach. Nature 1999;402:656–660 [DOI] [PubMed] [Google Scholar]
- 37.Zigman JM, Jones JE, Lee CE, Saper CB, Elmquist JK. Expression of ghrelin receptor mRNA in the rat and the mouse brain. J Comp Neurol 2006;494:528–548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dasgupta B, Milbrandt J. Resveratrol stimulates AMP kinase activity in neurons. Proc Natl Acad Sci USA 2007;104:7217–7222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nemoto S, Fergusson MM, Finkel T. Nutrient availability regulates SIRT1 through a forkhead-dependent pathway. Science 2004;306:2105–2108 [DOI] [PubMed] [Google Scholar]