Abstract
OBJECTIVE
Insulin in pancreatic β-cells is a target of autoimmunity in type 1 diabetes. In the NOD mouse model of type 1 diabetes, oral or nasal administration of insulin induces immune tolerance to insulin and protects against autoimmune diabetes. Evidence for tolerance to mucosally administered insulin or other autoantigens is poorly documented in humans. Adults with recent-onset type 1 diabetes in whom the disease process is subacute afford an opportunity to determine whether mucosal insulin induces tolerance to insulin subsequently injected for treatment.
RESEARCH DESIGN AND METHODS
We randomized 52 adults with recent-onset, noninsulin-requiring type 1 diabetes to nasal insulin or placebo for 12 months. Fasting blood glucose and serum C-peptide, glucagon-stimulated serum C-peptide, and serum antibodies to islet antigens were monitored three times monthly for 24 months. An enhanced ELISpot assay was used to measure the T-cell response to human proinsulin.
RESULTS
β-Cell function declined by 35% overall, and 23 of 52 participants (44%) progressed to insulin treatment. Metabolic parameters remained similar between nasal insulin and placebo groups, but the insulin antibody response to injected insulin was significantly blunted in a sustained manner in those who had received nasal insulin. In a small cohort, the interferon-γ response of blood T-cells to proinsulin was suppressed after nasal insulin.
CONCLUSIONS
Although nasal insulin did not retard loss of residual β-cell function in adults with established type 1 diabetes, evidence that it induced immune tolerance to insulin provides a rationale for its application to prevent diabetes in at-risk individuals.
Type 1 diabetes is an autoimmune disease that destroys insulin-producing β-cells in the islets of the pancreas. Studies in the NOD mouse model of spontaneous type 1 diabetes provide compelling evidence that insulin is a prime autoantigen that drives T-cell–mediated destruction of β-cells (1–3). Insulin is also a major target of the autoimmune response against β-cells in children with type 1 diabetes (4,5). Ideally, autoimmune diseases would be prevented by restoring immune tolerance to the autoantigens that are postulated to drive pathogenic immune responses. In rodent models of autoimmune disease, exposure of the mucosal immune system to soluble autoantigens has been shown to induce disease-protective immune tolerance associated with regulatory T-cells (6), for example, in the NOD mouse after oral (7,8) or aerosol (9) insulin. The potential of mucosal insulin as an immunotherapeutic agent to prevent type 1 diabetes in humans would be supported by evidence that it induces immune tolerance to insulin.
Several studies have examined the effects of mucosal insulin in type 1 diabetes. Two trials of oral insulin after the clinical onset of diabetes failed to demonstrate protection against loss of residual β-cell function (10,11). These used a very small dose of insulin (7.5-mg daily for 1 year) relative to that which protected NOD mice and did not document immune responses to oral insulin to demonstrate bioavailability. Moreover, it can be argued that even if protective immunity had been induced by oral insulin it might be ineffective in clinical, end-stage disease. The Diabetes Prevention Trial-Type 1 (DPT-1) studied asymptomatic at-risk, islet autoantibody-positive, first-degree type 1 diabetes relatives (12), using the same low dose of oral insulin. Although not a prespecified aim, oral insulin was found to significantly increase disease-free survival in participants who had circulating autoantibodies to insulin at entry. Oral insulin is rapidly degraded in the stomach, and its bioavailability in the upper small intestine is unpredictable (13). On the other hand, insulin administered nasally is intact on immediate contact with the nasopharyngeal mucosa. In asymptomatic children and young adults with islet autoantibodies at moderate risk for type 1 diabetes, nasal insulin induced an increase in antibody and a decrease in T-cell proliferative responses to insulin ex vivo (14), consistent with an immune-tolerizing effect, as observed after aerosol insulin in NOD mice (9). Subsequently, a randomized trial of nasal insulin administered daily to islet autoantibody-positive children less than 3 years of age at very high risk for type 1 diabetes found no effect on progression to diabetes (15), but evidence for an effect of nasal insulin on immune function was not reported.
A clear demonstration in humans of immune tolerance induced by nasal insulin would provide a rationale for further trials in at-risk individuals selected on the basis of immune status, disease stage, and risk. Compared with children with classic type 1 diabetes, adults with type 1 diabetes have greater residual β-cell function at diagnosis and in many cases do not initially require insulin for treatment (16). This affords an opportunity to evaluate whether nasal insulin has a tolerizing effect on immune responses to insulin subsequently injected for treatment, analogous to “antigen rechallenge” in animal models. We therefore conducted a randomized trial to determine the effect of nasal insulin on immune and metabolic parameters in adults with recent-onset type 1 diabetes.
RESEARCH DESIGN AND METHODS
Participants.
Participants were recruited nonselectively from general practice clinics, community and hospital diabetes clinics, and the registry of the National Diabetes Services Scheme (Diabetes Australia). Criteria for inclusion were 1) diabetes diagnosis in the previous 12 months, based on World Health Organization criteria (17); 2) age 30–75 years; 3) serum GAD antibody (GADA) concentration >5 units/mL; 4) fasting serum C-peptide ≥0.20 nM; 5) stable blood glucose control (random capillary blood glucose consistently <10 mmol/L) with diet and oral hypoglycemic drug therapy; and 6) no previous insulin injections. Exclusion criteria included previous diabetic ketoacidosis, pregnancy or lactation, active malignancy, alcohol or illicit drug dependence, and chronic liver or renal disease. Fifty-two participants of Caucasian background provided written informed consent. The study was approved by Melbourne Health Human Research Ethics Committee.
Protocol.
In a double-blind, placebo-controlled trial, participants were computer randomized to nasal insulin and nasal placebo groups by a hospital pharmacist who remained blinded to the allocation. After instruction, participants self-administered treatment via a metered dose nasal spray. Commercial recombinant human insulin solution (Humulin, 4 mg/mL; Eli Lilly, Indianapolis, IN) or the insulin diluent (placebo) was transferred under sterile conditions into 20-mL brown glass bottles fitted with pump spray nozzles, by the Pharmacy Department, The Royal Melbourne Hospital. Treatment consisted of two 100 μL spray doses per nostril, equivalent to a total of 40 units (1.6 mg) insulin, administered daily between 7:00 a.m. and 9:00 a.m. for 10 consecutive days and then on 2 consecutive days weekly for 12 months. The dosing schedule was based on a previous study (14). Nasal insulin is not absorbed and therefore has no systemic hormonal or metabolic effects.
Participants were assessed every 3 months for 24 months, by interview, physical examination, and blood tests for metabolic and immune parameters, and received advice on diabetes management. The treatment goal was optimal glycemic control, i.e., fasting blood glucose <7 mmol/L, postprandial blood glucose <10 mmol/L, and HbA1c ≤7%, based on standard dietary advice and oral hypoglycemic drugs (metformin, then a sulfonylurea agent). If glycemic control was suboptimal despite reported adherence to diet and maximal drug doses, treatment with a mixture of short- and intermediate-acting subcutaneous insulin was instituted. Compliance was assessed from a written diary and returned spray bottles at each follow-up.
β-Cell function.
β-Cell function was assessed by i.v. glucagon-stimulated secretion of C-peptide (a surrogate for insulin), a validated measure of β-cell function reasonably comparable to mixed meal test stimulation (18). Participants fasted for 10 h overnight, when oral hypoglycemic drugs were withheld. Blood samples were taken between 8:00 a.m. and 10:00 a.m. via an intravenous cannula in an antecubital vein. Providing fasting blood glucose was <10 mmol/L, blood was collected for serum C-peptide just before (baseline) and 6 min after 1 mg glucagon i.v. Stimulated C-peptide is the difference between 6 min and baseline serum concentrations. Serum C-peptide was measured by chemiluminescence (Immulite 1000; Siemens Healthcare Diagnostics, Deerfield, IL) in all samples from each participant in the one assay. The intra- and interassay coefficients of variation ranged from 1.5 to 3.7 and 9.4 to 10.0%, respectively.
Islet autoantibodies.
GADA and antibodies to tyrosine phosphatase-like insulinoma-associated protein 2 antigen (IA2A) were measured by precipitation of in vitro transcribed-translated GAD65 or IA2 biosynthetically labeled with [35S]methionine. Specificity and sensitivity in the Diabetes Autoantibody Standardization Program (19) were 76 and 94% for GADA and 72 and 100% for IA2A, respectively. Insulin autoantibodies (IAAs), or insulin antibodies (IAs) induced by treatment with subcutaneous insulin injections were measured by precipitation of 125I-(A14) human insulin (20). Specificity and sensitivity of the IAA assay in the Diabetes Autoantibody Standardization Program were 99 and 22%, respectively. The thresholds for GADA, IA2A, and IAA/IA positivity, 5, 3, and 1.0 units/mL, respectively, were established as the 97.5 percentiles of unselected healthy children and young adults.
Proinsulin-specific T-cells.
Tetanus toxoid was provided by CSL (Parkville, Victoria, Australia). Recombinant human proinsulin was produced as previously described (21). After refolding and purification by reversed-phase high-performance liquid chromatography, the protein was resolved as a single species of expected molecular mass by matrix-assisted laser desorption/ionization-time of flight mass spectrometry. The endotoxin concentration of proinsulin stock solution used was 0.51 EU/mg/mL. The response of T-cells to tetanus toxoid and human proinsulin was measured in an interferon (IFN)-γ ELISpot assay (22), the sensitivity of which was enhanced by incorporating agents to promote antigen-presenting cell function. Peripheral blood mononuclear cells (PBMCs) were prepared by Ficoll gradient centrifugation from five participants in each group, at baseline and 3 months, and stored over liquid nitrogen in 10% fetal calf serum/90% DMSO. Cells were rapidly thawed at 37°C, diluted in AIM-V serum-free medium (Invitrogen Australia, Mulgrave, Victoria, Australia), tested for viability by trypan blue (0.2%) exclusion and counted. PBMCs were then plated at 2.5 × 106 cells/250 μL/well in 48-well plates in AIM-V medium supplemented with granulocyte macrophage colony-stimulating factor (1,000 units/mL) and interleukin-4 (500 units/mL) (Preprotec, Rocky Hill, NJ) and containing tetanus toxoid (10 Lyons flocculating units [LfU]/mL), proinsulin (9 μg/mL), or no antigen. After 24 h (day 1), anti-CD40 monoclonal antibody (10 μg/mL; clone G28.5, produced in-house), IFN-α (1,000 units/mL) (Roferon-A; Roche, Dee Why, Australia), and interleukin-7 (0.5 ng/mL) (Preprotec) were added. On day 2, nonadherent cells were collected, washed, and resuspended in fresh AIM-V medium, counted, and 3 × 105 transferred in triplicate to wells of an ELISpot plate. The plate was incubated for 6 h at 37°C and processed for IFN-γ spots as described (22). Spots were counted electronically (AID Autoimmun Diagnostika, Strassberg, Germany), and medians of replicates were determined, subtracted for no antigen background, and expressed as spots/106 cells.
HLA.
Class II HLA-DR typing was performed by PCR-based sequence-specific oligonucleotide hybridization based on the 11th International Histocompatibility Workshop protocols (23) with minor modification to accommodate sequence polymorphisms subsequently described (24).
Statistical analysis.
Primary end points were stimulated serum C-peptide and the IA response to subcutaneous insulin. After diagnosis, glucagon-stimulated C-peptide in adults with type 1 diabetes is reported to decline by 20% per year, as reviewed by Fourlanos et al. (16). The sample size required to determine whether treatment would halve this decline, with a power of 80% in a two-sided test at a 5% level of significance, was estimated to be 21 per group. There were no previous data to estimate power for an effect of nasal insulin on the IA response to subcutaneous insulin.
Differences in continuous variables (C-peptide, fasting glucose, HbA1c, and IA concentrations) between baseline and 3-month time points were analyzed by nonparametric Mann-Whitney tests or paired t tests. Differences in categoric values (sex and HLA status) were analyzed by contingency Fisher exact test. The log-rank test was used to analyze Kaplan-Meier survival curves for progression to subcutaneous insulin treatment in the two trial arms. Correlation was analyzed by the Spearman rank test. Statistical analyses were performed with GraphPad Prism version 5.0 (GraphPad Software, San Diego, CA).
RESULTS
Characteristics of participants in the nasal insulin and placebo arms did not differ at baseline (Table 1). Blood glucose control (median HbA1c 6.6%) was satisfactory overall, but participants were overweight (median BMI 27.0 kg/m2). All had GADA, 16 of 52 (31%) had IA2A, and five of 52 (10%) had IAA. These have all been defined above. HLA class II DR 3 or 4 risk alleles for type 1 diabetes were present in 47 of 52 participants (90%).
TABLE 1.
Baseline characteristics of participants
| Characteristic | Nasal insulin (n = 26) | Nasal placebo (n = 26) | P value |
|---|---|---|---|
| Age (years) |
48.4 [40.3–51.5]* |
45.6 [38.3–55.1] |
0.71 |
| Male |
12 |
14 |
|
| Female |
14 |
12 |
0.78† |
| BMI |
26.4 [24.0–31.9] |
27.3 [24.9–31.6] |
0.82 |
| Waist circumference (cm) |
95 [88–108] |
97 [90–103] |
0.77 |
| Waist-hip ratio |
0.93 [0.88–0.98] |
0.90 [0.85–0.95] |
0.33 |
| Treatment at randomization | |||
| Diet alone |
6 |
4 |
0.80‡ |
| Metformin alone |
5 |
9 |
|
| Sulphonylurea alone |
7 |
7 |
|
| Metformin and sulphonylurea |
8 |
6 |
|
| Blood HbA1c (%) |
6.7 [5.9–7.6] |
6.6 [5.9–7.7] |
0.91 |
| Fasting serum lipids (mmol/L) | |||
| Total cholesterol |
4.5 [4.0–5.6] |
4.5 [4.1–5.3] |
0.87 |
| Triglycerides |
1.2 [0.8–1.9] |
1.5 [0.9–1.8] |
0.39 |
| HDL cholesterol |
1.1 [1.0–1.4] |
1.1 [1.0–1.3] |
0.52 |
| LDL cholesterol |
2.7 [2.2–3.5] |
2.4 [2.3–3.4] |
0.89 |
| Fasting plasma glucose (mmol/L) |
7.1 [5.8–8.0] |
6.7 [6.0–7.8] |
0.99 |
| Fasting serum C-peptide (nM) |
0.70 [0.43–1.00] |
0.75 [0.59–0.95] |
0.58 |
| Stimulated serum C-peptide (nM) |
0.50 [0.28–0.71] |
0.37 [0.25–0.54] |
0.29 |
| Serum GADA | |||
|
n |
26 |
26 |
1.0 |
| Concentration (units/mL) |
61 [29–76] |
56 [24–78] |
0.80 |
| Serum IA2A | |||
|
n |
6 |
10 |
0.25 |
| Concentration (units/mL) |
−0.5 [−0.9 to 1.5] |
0.0 [−1.0 to 17] |
0.76 |
| Serum IAA | |||
|
n |
2 |
2 |
1.0 |
| Concentration (units/mL) |
0.2 [0.0–0.4] |
0.3 [0.1–0.5] |
0.11 |
| HLA DR risk alleles | |||
| Combinations of DR3 and DR4 |
14 |
15 |
0.68‡ |
| Either DR3 or DR4 |
9 |
9 |
|
| Neither DR3 nor DR4 | 3 | 2 | |
*Values expressed as median [interquartile range].
†χ2 test.
‡χ2 test for trend.
Metabolic parameters.
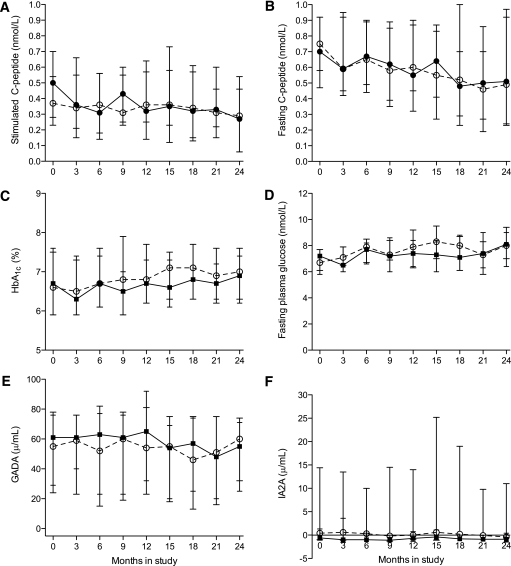
Glucagon-stimulated serum C-peptide, a measure of β-cell function, decreased overall by 35% over 24 months, from a baseline median of 0.43 nmol/L [interquartile range 0.24–0.62] to a median of 0.28 nmol/L [0.06–0.49] (P = 0.012). Likewise, fasting serum C-peptide decreased by 33% from a baseline median of 0.75 nmol/L [0.48–0.98] to 0.50 nmol/L [0.23–0.95] (P = 0.043). The decline in β-cell function did not differ between the nasal insulin and placebo groups (Fig. 1A and B). At 24 months, median stimulated serum C-peptide in the nasal insulin group was 0.27 [0.06–0.60] nM compared with 0.29 [0.06–0.48] nM in the placebo group (P = 0.86). The change in β-cell function was also assessed from the slopes of stimulated serum C-peptide during the study (nasal insulin –0.011 vs. nasal placebo –0.018; P = 0.99), confirming there was no difference between the nasal insulin and placebo groups.
FIG. 1.
Trial outcomes: stimulated serum C-peptide (A), fasting serum C-peptide (B), blood HbA1c (C), fasting plasma glucose (D), serum GADA (E), and serum IA2A (F). Median values for the nasal insulin and nasal placebo groups are indicated by closed and open symbols, respectively. Interquartile ranges are shown as vertical lines.
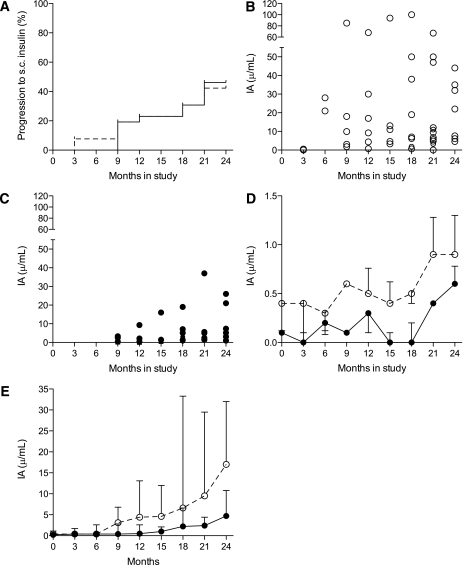
Blood glucose control did not change over the course of the study; median HbA1c at baseline was 6.6% [5.8–7.6] compared with 6.9% [6.3–7.6] at 24 months (P = 0.64, Kruskal-Wallis test). Fasting plasma glucose and HbA1c did not differ between the nasal insulin and nasal placebo groups (Fig. 1C and D). Glycemia control was attributable to close monitoring and prompt escalation of oral hypoglycemic drugs or rapid transition to treatment with subcutaneous insulin to meet control targets. Progression to insulin treatment over 24 months occurred in 23 of 52 participants (44%), being similar in the nasal insulin (12/26) and placebo (11/26) groups (log-rank test P = 0.88; Fig. 2A).
FIG. 2.
A: Progression to treatment with subcutaneous (s.c.) insulin, plotted as Kaplan-Meier survival curves (nasal insulin participants, solid line; nasal placebo participants, dashed line). B: IA concentrations in nasal placebo participants at 3-month study intervals. C: IA concentrations in nasal insulin participants at 3-month study intervals. D: Median IA concentrations for all participants at 3-month study intervals (includes values before and after commencement of subcutaneous insulin). ○, Nasal placebo and ●, nasal insulin participants. Upper quartile ranges (divided by 5 to allow fit) are shown as vertical lines. E: Median IA concentrations in nasal placebo (○) and nasal insulin (●) participants by time after commencement of subcutaneous insulin. Upper quartile ranges are shown as vertical lines.
Immune parameters.
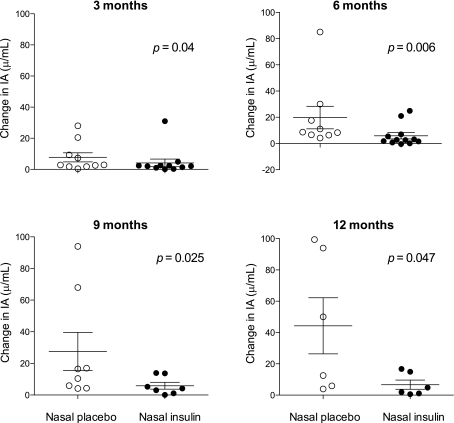
Concentrations of GADA and IA2A were similar at baseline in the nasal insulin and placebo groups and remained unchanged throughout the study (Fig. 1E and F). At baseline, only three participants in the nasal insulin group and two participants in the control group had detectable IAA (Table 2). After treatment with subcutaneous insulin, the induced IA response (Table 2) was significantly blunted in participants who had received nasal insulin. This is illustrated in several ways (Figs. 2 and 3). First, IA concentration is plotted for each participant in the nasal placebo (Fig. 2B) or nasal insulin (Fig. 2C) group at 3-month intervals and as the medians of the two groups at 3-month intervals (Fig. 2D). Second, because not all participants progressed to subcutaneous insulin and those who did commenced treatment at different times in the study, IA concentration is plotted by time after commencing subcutaneous insulin (Fig. 2E). In addition, IA concentration is plotted as the absolute change from pre-treatment baseline at 3, 6, 9, and 12 months after commencing subcutaneous insulin (Fig. 3). From areas under the curves (Fig. 2D), IA concentration was significantly lower in those from the nasal insulin group than the placebo group (4.1 vs. 12.2 units/mL, P = 0.001). By 24 months, IA concentration in participants receiving subcutaneous insulin was significantly lower in the 12 who had received nasal insulin (4.7 [2.3–11] units/mL) than in the 11 who had received nasal placebo (17 [6.0–32] units/mL) (P = 0.019) (Fig. 2E). The IA concentration in participants who had received nasal insulin remained suppressed to at least 12 months after commencing daily subcutaneous insulin treatment (Fig. 3). The relationship between subcutaneous insulin dose (shown in Table 2 as total daily dose) and the IA response was examined. The median dose of subcutaneous insulin in the nasal insulin group (26 units; [interquartile range 20–34]) was not significantly different from that in the placebo group (34 units; interquartile range 22–50) (P = 0.34). In the nasal insulin group only, insulin dose and IA concentration were significantly correlated at 3 months (r = 0.81, P = 0.001) and 6 months (r = 0.64, P = 0.024) after starting subcutaneous insulin, but in each case significance depended on the single highest paired values. Time to the start of subcutaneous insulin or HLA DR allele status did not differ significantly between the groups.
TABLE 2.
Insulin antibody concentrations (units/milliliters) in trial participants
| Participant |
Baseline |
Months in trial | Days of s.c. insulin before measurement of IA |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 3.0 |
6.0 | 9.0 | 12 | 15 | 18 | 21 | 24 | |||
| Nasal insulin | ||||||||||
| 1 |
0.6 |
0.0 |
0.5 |
0.2 |
0 |
0 |
0 |
0 |
0 |
|
| 2 |
0.0 |
0.0 |
0.0 |
0.0 |
2.4 (13)* |
1.8 (13) |
3.0 (12) |
1.9 (11) |
1.0 (11) |
90 |
| 3 |
0.0 |
0.6 |
0.6 |
0.0 |
0.4 |
0.2 |
0.0 |
0.3 |
0.3 |
|
| 4 |
0.6 |
0.8 |
0.0 |
1.1 |
0.4 |
0.0 |
0.4 |
0.4 |
0.3 |
|
| 5 |
0.0 |
0.0 |
0.4 |
0.2 (10) |
0.0 (12) |
0.5 (15) |
1.0 (14) |
1.7 (16) |
1.4 (17) |
35 |
| 6 |
0.4 |
0.4 |
0.3 |
0.4 (16) |
0.4 (18) |
1.4 (31) |
5.1 (36) |
5.6 (32) |
5.2 (33) |
75 |
| 7 |
0.0 |
0.0 |
0.0 |
0.0 |
0.2 |
0.0 |
0.0 |
1.4 (24) |
21.0 (31) |
90 |
| 8 |
0.0 |
0.0 |
0.0 |
0.5 |
0.0 |
0.0 |
0.3 (12) |
0.8 (24) |
4.1 (46) |
30 |
| 9 |
0.5 |
3.3 |
2.3 |
3.3 (10) |
9.3 (21) |
16.0 (22) |
19.0 (21) |
37.0 (24) |
26.0 (26) |
75 |
| 10 |
0.3 |
0.0 |
0.0 |
0.2 |
0.0 |
0.0 |
0.0 |
0.0 |
0.0 |
|
| 11 |
0.0 |
0.0 |
0.4 |
0.0 |
0.0 |
0.6 |
0.0 |
0.0 |
0.0 |
|
| 12 |
0.0 |
0.5 |
NA |
2.5 (50) |
2.1 (67) |
NA (67) |
1.3 (63) |
1.6 (49) |
1.0 (48) |
90 |
| 13 |
0.2 |
0.0 |
0.4 |
0.0 |
0.5 |
0.0 |
0.0 |
0.0 |
0.3 |
|
| 14 |
0.0 |
0.1 |
0.0 |
0.0 |
0.0 |
0.0 |
0.0 |
0.4 |
0.0 |
|
| 15 |
0.0 |
0.0 |
0.0 |
0.0 |
0.0 |
0.3 |
0.0 |
0.0 |
0.0 |
|
| 16 |
0.4 |
0.0 |
0.0 |
0.0 |
0.0 |
0.0 |
0.0 |
0.2 |
0.1 |
|
| 17 |
6.9 |
2.8 |
4.1 |
3.1 |
2.8 |
2.2 |
7.1 (30) |
5.0 (34) |
7.4 (36) |
90 |
| 18 |
0.3 |
0.6 |
0.4 |
0.0 |
0.3 |
0.3 |
0.0 |
0.1 |
0.2 |
|
| 19 |
0.4 |
0.1 |
0.0 |
0.3 |
0.5 |
0.0 |
0.2 |
0.0 |
0.0 |
|
| 20 |
0.0 |
NA |
0.4 |
0.0 |
0.3 |
0.0 |
0.0 |
0.0 |
0.5 |
|
| 21 |
0.3 |
0.0 |
0.0 |
0.0 |
0.0 |
0.0 |
0.0 |
2.5 (36) |
2.6 (32) |
90 |
| 22 |
5.5 |
0.4 |
0.9 |
0.4 |
0.5 |
0.5 |
0.4 |
2.3 (18) |
5.8 (23) |
90 |
| 23 |
0.0 |
0.0 |
0.0 |
0.0 |
0.0 |
0.0 |
0.0 |
0.0 |
0.6 |
|
| 24 |
0.0 |
0.0 |
0.0 |
0.3 |
0.5 |
0.0 |
NA |
2.4 (26) |
3.1 (20) |
90 |
| 25 |
2.9 |
3.1 |
3.1 |
34.0 (74) |
28.0 (92) |
17.0 (98) |
18.0 (110) |
NA (114) |
35.0 (124) |
90 |
| 26 |
0.0 |
0.0 |
0.2 |
0.0 |
0.0 |
0.0 |
0.0 |
0.0 |
0.0 |
|
| Nasal placebo | ||||||||||
| 27 |
0.4 |
0.0 |
28.0 (30) |
85.0 (42) |
68.0 (44) |
94.0 (50) |
38.0 (50) |
67.0 (55) |
44.0 (57) |
20 |
| 28 |
0.9 |
0.9 |
0.5 |
6.0 |
0.4 |
0.1 |
0.3 |
0.8 |
0.3 |
|
| 29 |
1.4 |
2.0 |
1.0 |
2.5 |
2.0 |
2.6 |
2.0 |
1.1 |
2.2 |
|
| 30 |
0.0 |
0.0 |
0.3 |
0.0 |
0.7 |
0.2 |
0.0 |
0.0 |
0.0 |
|
| 31 |
0.0 |
0.0 |
0.0 |
0.4 |
0.0 |
0.0 |
0.0 |
0.0 |
0.0 |
|
| 32 |
0.4 |
0.0 |
0.0 |
0.0 |
0.0 |
0.3 |
0.6 |
0.0 |
0.2 |
|
| 33 |
0.0 |
0.6 |
0.3 |
0.6 |
0.0 |
0.3 |
0.5 |
2.2 |
0.5 |
|
| 34 |
0.4 |
0.7 |
1.4 |
0.8 |
1.2 |
1.2 |
1.9 |
1.0 |
1.2 |
|
| 35 |
0.6 |
0.3 |
0.0 |
0.5 |
0.5 |
0.4 |
0.3 |
0.0 |
7.6 (22) |
11 |
| 36 |
0.6 |
0.5 |
0.2 |
0.5 |
0.0 |
0.4 |
0.4 |
3.3 (36) |
8.6 (32) |
30 |
| 37 |
0.5 |
0.0 |
0.4 |
0.2 |
0.3 |
0.0 |
0.3 |
0.0 |
0.2 |
|
| 38 |
0.3 |
0.3 |
0.2 |
0.3 |
0.2 |
0.3 |
0.7 |
0.6 |
0.5 |
|
| 39 |
0.4 |
0.0 |
0.4 |
0.3 |
0.7 |
3.3 (10) |
7.0 (20) |
4.8 (32) |
4.6 (35) |
14 |
| 40 |
0.0 |
0.4 |
0.0 |
0.3 |
0.0 |
1.5 |
1.8 (14) |
11.0 (22) |
17.0 (29) |
75 |
| 41 |
0.3 |
0.5 |
0.6 |
9.9 (40) |
9.2 (40) |
11.0 (30) |
100.0 (54) |
50.0 (64) |
35.0 (74) |
75 |
| 42 |
1.0 |
0.5 |
21.0 (42) |
18.0 (50) |
17.0 (55) |
13.0 (54) |
19.0 (54) |
12.0 (53) |
32.0 (57) |
20 |
| 43 |
13.0 |
4.6 |
3.7 |
3.4 |
5.0 |
5.2 |
NA |
5.6 (70) |
22.0 (82) |
90 |
| 44 |
0.0 |
0.0 |
0.0 |
0.0 |
0.3 |
0.0 |
0.0 |
0.3 |
0.0 |
|
| 45 |
0.1 |
0.4 |
0.4 |
0.3 |
0.3 |
0.4 |
0.1 |
0.0 |
0.3 |
|
| 46 |
0.0 |
0.0 |
0.1 |
0.0 |
0.5 |
0.3 |
0.3 |
0.0 |
0.0 |
|
| 47 |
0.4 |
0.5 |
1.4 |
0.8 |
0.0 |
0.2 |
0.6 (58) |
6.7 (46) |
6.1 (46) |
42 |
| 48 |
0.3 |
0.0 |
0.0 |
0.0 |
0.0 |
0.0 |
0.4 |
0.0 |
0.3 |
|
| 49 |
0.0 |
0.0 |
0.0 |
1.8 (10) |
30.0 (18) |
94.0 (20) |
50.0 (18) |
47.0 (20) |
32.0 (24) |
75 |
| 50 |
0.8 |
0.4 |
0.0 |
0.6 |
5.0 |
0.0 |
0.2 |
0.0 |
0.1 |
|
| 51 |
0.7 |
0.0 |
0.4 |
0.4 |
0.6 |
2.0 |
0.4 |
0.3 |
0.4 |
|
| 52 | 0.4 | 0.3 | 0.3 | 3.1 (24) | 4.4 (34) | 4.6 (26) | 6.2 (26) | 9.5 (30) | 6.1 (34) | 90 |
Treatment with subcutaneous insulin is indicated in boldface, and total daily insulin dose is shown in parentheses. *Subcutaneous insulin dose (units).
NA, not assayed; s.c., subcutaneous.
FIG. 3.
Changes in IA concentration from baseline at 3, 6, 9, and 12 months after commencing subcutaneous insulin in nasal placebo (○) and nasal insulin (●) participants. Medians (horizontal lines) and quartile ranges (vertical lines) are shown. IA measurements made within 30 days of commencing subcutaneous insulin were not considered.
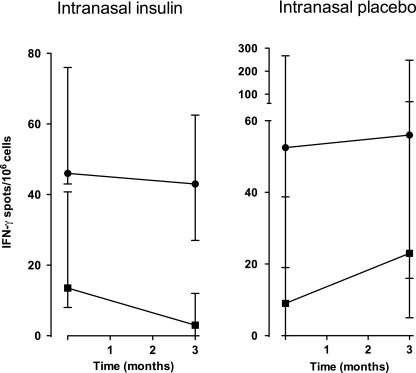
IFN-γ ELISpot responses to proinsulin and a control antigen, tetanus toxoid, were measured on frozen-thawed PBMCs available from five participants in each group at baseline and 3 months (Fig. 4). No participant required subcutaneous insulin by 3 months. Responses to tetanus toxoid were similar between the groups at baseline and 3 months. However, responses to proinsulin decreased significantly by 3 months in participants who received nasal insulin (P = 0.03; paired, one-tailed t test) but not placebo (P = 0.31).
FIG. 4.
T-cell responses to tetanus toxoid (10 LfU/mL; ●) and human recombinant proinsulin (9 μg/mL; ■) measured by an enhanced IFN-γ ELISpot assay. PBMCs were collected from five participants in each of the nasal insulin and nasal placebo groups and stored under liquid N2. After thawing, cell responses were measured as described in research design and methods. Individual assays were performed in triplicate, and data are expressed as group medians with interquartile ranges.
DISCUSSION
Mucosa-mediated immune tolerance in humans was first demonstrated to the experimental antigen, keyhole limpit hemocyanin (KLH), given orally (25) or nasally (26) to healthy volunteers. After oral KLH, T-cell but not B-cell (antibody) responses to rechallenge with subcutaneous KLH were suppressed. After nasal KLH, both T- and B-cell responses to subcutaneous KLH were suppressed, suggesting that tolerance induction via the nasal route may be more effective. Despite these findings, and a wealth of evidence for tolerogenic protective effects of oral or nasal autoantigen in mouse models of autoimmune disease, reviewed by Harrison and Hafler (6), the therapeutic promise of mucosa-mediated tolerance for human autoimmune disease has failed to meet expectations. Part of the explanation may be that earlier human studies were conducted in advanced disease. In addition, evidence that administered autoantigen was bioavailable and elicited immune responses consistent with tolerance has been lacking. Our finding that the antibody response to subcutaneous insulin was suppressed by prior treatment with nasal insulin is the first evidence for immune tolerance induction to an autoantigen demonstrated by rechallenge in humans. Tolerance was robust because the suppressive effect was sustained in the majority of individuals despite ongoing daily insulin injections. Differences between the nasal insulin and placebo groups could not be attributed to the doses of subcutaneous insulin required. Tolerance to exogenous insulin does not necessarily equate with tolerance to endogenous insulin, i.e., with suppression of autoimmune responses to insulin. This is implied, however, by evidence for tolerance at the T-cell level, namely, suppression of IFN-γ responses to proinsulin after nasal insulin in a small cohort, which must be qualified by the lack of T-cell data across the study. Proinsulin rather than insulin was used for the ELISpot assay because the serum-free medium contained insulin, but T-cells recognize epitopes in proinsulin that are not present in insulin (1,27).
There are several possible reasons why immune tolerance to insulin induced by nasal insulin might not have translated into suppression of immunity to other islet autoantigens or protection against ongoing loss of β-cell function in adults with type 1 diabetes. First, on the basis of the prevalence of autoantibodies, insulin does not seem to be a major autoantigen in this population (16), and protection may require induction of tolerance to other islet autoantigens. On the other hand, although the mechanism of nasal insulin–induced tolerance in humans remains to be defined, studies in mice (6) show that regulatory T-cells induced by mucosal administration of a single autoantigen can have a bystander effect to suppress T-cell responses to other autoantigens presented in the same microenvironment, e.g., pancreas draining lymph nodes. T-cell responses to GAD were suppressed by aerosol insulin in the NOD mouse (9), but evidence is lacking that bystander suppression induced by one antigen modifies ongoing antibody responses to other autoantigens. Irrespective whether T-cell tolerance involves induction of insulin-specific regulatory T-cells or the deletion or anergy of insulin-specific pathogenic T-cells, the outcome may be impaired T-cell “help” for IA production by B-cells. Techniques for reliably identifying and characterizing autoantigen-specific T-cells in human blood are emerging, and our findings are an impetus for their application. Second, β-cell function declined by more than 30% over 24 months with more than 40% of participants becoming insulin-dependent, indicating progressive loss of β-cell function to end-stage disease. Trials of oral insulin in adults with recent-onset type 1 diabetes (10,11), while not documenting immune outcomes, also found no effect on residual β-cell function. If the balance between pathogenic and protective immunity determines clinical outcome, then autoantigen-specific vaccination should be most effective before or soon after the onset of subclinical disease. Indeed, studies in animal models show no evidence that this approach is protective by the time clinical disease ensues. Third, it is possible that immune tolerance to insulin, even if induced early in the disease process, may be protective only in individuals with preexisting autoimmunity to insulin, as seen in the DPT-1 oral insulin trial (12). Finally, although administration of nasal insulin was associated with suppression of the antibody response to injected insulin, destruction of β-cells is primarily T-cell mediated. Further studies are required not only to determine whether nasal insulin induces insulin-specific regulatory T-cells but also to confirm that, like nasal KLH (26), nasal insulin induces changes in T-cell function to rechallenge indicative of T-cell tolerance.
An important question is whether insulin-induced tolerance would be protective in islet autoantibody-positive children at risk for type 1 diabetes in whom, in contrast with adults with type 1 diabetes, insulin seems to be a major autoantigen (4,5). In the DPT-1 randomized controlled trial of oral insulin (12), participating relatives with type 1 diabetes at entry had an interquartile age of 7–14 years and normal β-cell function. Notably, treatment with oral insulin was associated with slower progression to diabetes in the slightly younger IAA-positive cohort. In the type 1 diabetes Prediction and Prevention Project randomized trial in Finland (15), nasal insulin had no effect on progression to diabetes in islet autoantibody-positive children less than 3 years of age. Such children are a very high-risk group, and many had low first-phase insulin response to intravenous glucose. Given end-stage β-cell function, they would be less likely to exhibit a clinical response. Again, immune tolerance to insulin was not documented in either trial of oral or nasal insulin in at-risk individuals. A proposed trial (Pre-POINT) (28) aims to address questions of optimal timing, disease stage, dose, and route of administration by intervening with oral or nasal insulin in children genetically predisposed to type 1 diabetes before the appearance of islet autoantibodies. The evidence demonstrated here for nasal insulin–induced immune tolerance provides a mechanistic rationale for studies that aim to restore immune tolerance before the development of islet pathology.
ACKNOWLEDGMENTS
This work was funded by a Program Grant (305500) from the National Health and Medical Research Council of Australia (NHMRC), by a Victorian State Government Operational Infrastructure Support Grant (to L.C.H.), and by the INSERM Avenir Program (to R.M.). S.F. was a Postgraduate Scholar, and L.C.H. is a Senior Principal Research Fellow of the NHMRC. E.M. was supported by an Albert Renold Fellowship from the European Association for the Study of Diabetes.
No potential conflicts of interest relevant to this article were reported.
S.F. designed the research, performed the studies, analyzed data, and wrote the article. C.P. and S.A.G. performed the studies. E.M. performed the studies and analyzed data. R.M. contributed new reagents and tools and analyzed data. J.B. performed the studies and analyzed data. P.G.C. designed the research and performed the studies. L.C.H. designed the research, performed the studies, analyzed data, and wrote the article.
The authors thank Diabetes Australia for assistance in recruiting participants; Dr. Tom Kay of St. Vincent’s Institute of Medical Research, Melbourne, for initial discussions; Dr. Kent Jensen for producing human proinsulin; and Dr. Rhiannon Jones, Walter and Eliza Hall Institute of Medical Research, Melbourne, for administrative assistance.
Footnotes
Clinical trial reg. no. ANZ 00632, www.ANZCTR.org.au.
REFERENCES
- 1.Narendran P, Mannering SI, Harrison LC. Proinsulin-a pathogenic autoantigen in type 1 diabetes. Autoimmun Rev 2003;2:204–210 [DOI] [PubMed] [Google Scholar]
- 2.Nakayama M, Abiru N, Moriyama H, et al. Prime role for an insulin epitope in the development of type 1 diabetes in NOD mice. Nature 2005;435:220–223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Krishnamurthy B, Dudek NL, McKenzie MD, et al. Responses against islet antigens in NOD mice are prevented by tolerance to proinsulin but not IGRP. J Clin Invest 2006;116:3258–3265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Verge CF, Gianani R, Kawasaki E, et al. Prediction of type I diabetes in first-degree relatives using a combination of insulin, GAD, and ICA512bdc/IA-2 autoantibodies. Diabetes 1996;45:926–933 [DOI] [PubMed] [Google Scholar]
- 5.Achenbach P, Koczwara K, Knopff A, Naserke H, Ziegler AG, Bonifacio E. Mature high-affinity immune responses to (pro)insulin anticipate the autoimmune cascade that leads to type 1 diabetes. J Clin Invest 2004;114:589–597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Harrison LC, Hafler DA. Antigen-specific therapy for autoimmune disease. Curr Opin Immunol 2000;12:704–711 [DOI] [PubMed] [Google Scholar]
- 7.Zhang ZJ, Davidson L, Eisenbarth G, Weiner HL. Suppression of diabetes in nonobese diabetic mice by oral administration of porcine insulin. Proc Natl Acad Sci U S A 1991;88:10252–10256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bergerot I, Fabien N, Maguer V, Thivolet C. Oral administration of human insulin to NOD mice generates CD4+ T cells that suppress adoptive transfer of diabetes. J Autoimmun 1994;7:655–663 [DOI] [PubMed] [Google Scholar]
- 9.Harrison LC, Dempsey-Collier M, Kramer DR, Takahashi K. Aerosol insulin induces regulatory CD8 gamma delta T cells that prevent murine insulin-dependent diabetes. J Exp Med 1996;184:2167–2174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pozzilli P, Pitocco D, Visalli N, et al. IMDIAB Group No effect of oral insulin on residual beta-cell function in recent-onset type I diabetes (the IMDIAB VII). Diabetologia 2000;43:1000–1004 [DOI] [PubMed] [Google Scholar]
- 11.Chaillous L, Lefèvre H, Thivolet C, et al. Oral insulin administration and residual beta-cell function in recent-onset type 1 diabetes: a multicentre randomised controlled trial. Diabète Insuline Orale group. Lancet 2000;356:545–549 [DOI] [PubMed] [Google Scholar]
- 12.Skyler JS, Krischer JP, Wolfsdorf J, et al. Effects of oral insulin in relatives of patients with type 1 diabetes: The Diabetes Prevention Trial-Type 1. Diabetes Care 2005;28:1068–1076 [DOI] [PubMed] [Google Scholar]
- 13.Heinemann L, Jacques Y. Oral insulin and buccal insulin: a critical reappraisal. J Diabetes Sci Tech 2009;3:568–584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Harrison LC, Honeyman MC, Steele CE, et al. Pancreatic beta-cell function and immune responses to insulin after administration of intranasal insulin to humans at risk for type 1 diabetes. Diabetes Care 2004;27:2348–2355 [DOI] [PubMed] [Google Scholar]
- 15.Näntö-Salonen K, Kupila A, Simell S, et al. Nasal insulin to prevent type 1 diabetes in children with HLA genotypes and autoantibodies conferring increased risk of disease: a double-blind, randomised controlled trial. Lancet 2008;372:1746–1755 [DOI] [PubMed] [Google Scholar]
- 16.Fourlanos S, Dotta F, Greenbaum CJ, et al. Latent autoimmune diabetes in adults (LADA) should be less latent. Diabetologia 2005;48:2206–2212 [DOI] [PubMed] [Google Scholar]
- 17.Alberti KG, Zimmet PZ. Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: diagnosis and classification of diabetes mellitus provisional report of a WHO consultation. Diabet Med 1998;15:539–553 [DOI] [PubMed] [Google Scholar]
- 18.Greenbaum CJ, Mandrup-Poulsen T, McGee PF, et al. Type 1 Diabetes Trial Net Research Group. European C-Peptide Trial Study Group Mixed-meal tolerance test versus glucagon stimulation test for the assessment of beta-cell function in therapeutic trials in type 1 diabetes. Diabetes Care 2008;31:1966–1971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Törn C, Mueller PW, Schlosser M, Bonifacio E, Bingley PJ, Participating Laboratories Diabetes Antibody Standardization Program: evaluation of assays for autoantibodies to glutamic acid decarboxylase and islet antigen-2. Diabetologia 2008;51:846–852 [DOI] [PubMed] [Google Scholar]
- 20.Williams AJ, Bingley PJ, Bonifacio E, Palmer JP, Gale EA. A novel micro-assay for insulin autoantibodies. J Autoimmun 1997;10:473–478 [DOI] [PubMed] [Google Scholar]
- 21.Cowley DJ, Mackin RB. Expression, purification and characterization of recombinant human proinsulin. FEBS Lett 1997;402:124–130 [DOI] [PubMed] [Google Scholar]
- 22.Martinuzzi E, Scotto M, Enée E, et al. Serum-free culture medium and IL-7 costimulation increase the sensitivity of ELISpot detection. J Immunol Methods 2008;333:61–70 [DOI] [PubMed] [Google Scholar]
- 23.Kimura A, Sasazuki T. Eleventh International Histocompatibility Workshop Reference Protocol for the HLA DNA-Typing Technique. Oxford, Oxford University Press, 1992 [Google Scholar]
- 24.Varney MD, Kanaan C, Tait BD. Identification of DRB1*0422—implications for serological epitopes. Tissue Antigens 1996;47:150–152 [DOI] [PubMed] [Google Scholar]
- 25.Husby S, Mestecky J, Moldoveanu Z, Holland S, Elson CO. Oral tolerance in humans. T cell but not B cell tolerance after antigen feeding. J Immunol 1994;152:4663–4670 [PubMed] [Google Scholar]
- 26.Waldo FB, van den Wall Bake AWL, Mestecky J, Husby S. Suppression of the immune response by nasal immunization. Clin Immunol Immunopathol 1994;72:30–34 [DOI] [PubMed] [Google Scholar]
- 27.Mannering SI, Pang SH, Williamson NA, et al. The A-chain of insulin is a hot-spot for CD4+ T cell epitopes in human type 1 diabetes. Clin Exp Immunol 2009;156:226–231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Achenbach P, Barker J, Bonifacio E; Pre-POINT Study Group. Modulating the natural history of type 1 diabetes in children at high genetic risk by mucosal insulin immunization. Curr Diab Rep 2008:8;87–93 [DOI] [PubMed] [Google Scholar]