Abstract
OBJECTIVE
Circulating angiogenic progenitor cells (APCs) participate in endothelial repair after arterial injury. Type 2 diabetes is associated with fewer circulating APCs, APC dysfunction, and impaired endothelial repair. We set out to determine whether insulin resistance adversely affects APCs and endothelial regeneration.
RESEARCH DESIGN AND METHODS
We quantified APCs and assessed APC mobilization and function in mice hemizygous for knockout of the insulin receptor (IRKO) and wild-type (WT) littermate controls. Endothelial regeneration after femoral artery wire injury was also quantified after APC transfusion.
RESULTS
IRKO mice, although glucose tolerant, had fewer circulating Sca-1+/Flk-1+ APCs than WT mice. Culture of mononuclear cells demonstrated that IRKO mice had fewer APCs in peripheral blood, but not in bone marrow or spleen, suggestive of a mobilization defect. Defective vascular endothelial growth factor–stimulated APC mobilization was confirmed in IRKO mice, consistent with reduced endothelial nitric oxide synthase (eNOS) expression in bone marrow and impaired vascular eNOS activity. Paracrine angiogenic activity of APCs from IRKO mice was impaired compared with those from WT animals. Endothelial regeneration of the femoral artery after denuding wire injury was delayed in IRKO mice compared with WT. Transfusion of mononuclear cells from WT mice normalized the impaired endothelial regeneration in IRKO mice. Transfusion of c-kit+ bone marrow cells from WT mice also restored endothelial regeneration in IRKO mice. However, transfusion of c-kit+ cells from IRKO mice was less effective at improving endothelial repair.
CONCLUSIONS
Insulin resistance impairs APC function and delays endothelial regeneration after arterial injury. These findings support the hypothesis that insulin resistance per se is sufficient to jeopardize endogenous vascular repair. Defective endothelial repair may be normalized by transfusion of APCs from insulin-sensitive animals but not from insulin-resistant animals.
Insulin resistance, the metabolic abnormality underpinning type 2 diabetes and obesity, is an important risk factor for the development of atherosclerotic cardiovascular disease (1). Type 2 diabetes is predicted to affect 300 million people worldwide by 2025 (2); as a result, the cardiovascular complications of type 2 diabetes will represent a major burden on global health care systems. Despite optimal medical therapy, patients with impaired glucose tolerance or type 2 diabetes have substantially worse outcomes after an acute myocardial infarction than patients without these metabolic abnormalities (3–6).
Recent clinical trials in which intensive lowering of blood glucose failed to improve cardiovascular outcomes in patients with type 2 diabetes (7,8) highlighted the importance of identifying novel targets or approaches to prevent or retard the development of atherosclerosis in insulin-resistant individuals. Several components of the type 2 diabetes phenotype have been implicated in the pathogenesis of type 2 diabetes-related cardiovascular disease (9); among these, insulin resistance has emerged as an independent risk factor for the development of atherosclerosis (1). The mechanisms underlying this effect, however, remain only partially understood (10), and cardiovascular benefits of treatments directly targeting insulin resistance have proved to be disappointing (11,12)
It is now an accepted pathophysiologic paradigm that cardiovascular risk factors damage the endothelium and that endogenous repair mechanisms are instituted to repair this damage (13). The classical mechanism thought to underpin endothelial cell (EC) repair and replacement is that local ECs divide to re-endothelialize vessels. Evidence now supports a complementary role to resident ECs for circulating vascular progenitor cells derived from bone marrow (BM), and possibly other tissues, in the maintenance of EC function (13). Direct evidence that angiogenic progenitor cells (APCs)—early outgrowth endothelial progenitor cells or circulating angiogenic cells—may contribute to endothelial regeneration has emerged from animal studies demonstrating that APCs incorporate into neoendothelium after endothelium-denuding injury (14), bypass graft surgery (15), and hyperlipidemia (16) and reduce the development of neointima, endothelial dysfunction, and atherosclerosis (17,18). These early-outgrowth APCs are likely to be of monocytic origin and enhance vascular repair by secreting proangiogenic cytokines (19). Conversely, a less abundant population of late outgrowth “endothelial colony-forming cells” may differentiate and proliferate to repopulate the endothelium (19).
Individuals with type 2 diabetes and obesity, and insulin-resistant men of South Asian origin, all have EC dysfunction characterized by a reduction in the bioavailability of the antiatherosclerotic signaling radical nitric oxide (NO) (20–22). In mice hemizygous for knockout of the insulin receptor (IRKO), in which normoglycemia is maintained by compensatory hyperinsulinemia, we have shown that a modest decline in insulin signaling results in substantially reduced endothelial NO bioavailability, independent of hyperglycemia (23). Humans with diabetes have fewer circulating endothelial progenitors, which are associated with multiple functional defects that impair their regenerative capacity (24–31).
A recent study in genetically diabetic db/db mice demonstrated that type 2 diabetes is associated with reduced APC numbers, APC dysfunction, and delayed endothelial regeneration after injury (14). However, the effect of insulin resistance per se on APCs and endothelial regeneration after arterial injury remains unexplored. We have shown that insulin-resistant young South Asian men have fewer circulating APCs and impaired APC mobilization in response to physiologic stimuli than insulin-sensitive white European men (21,32). Here we investigate the effects of insulin resistance on APCs and endothelial regeneration in IRKO mice, a nonobese, nondiabetic model in which insulin signaling in insulin-sensitive tissues is reduced by 30% (33).
RESEARCH DESIGN AND METHODS
Animals.
IRKO mice (34) were obtained from the Medical Research Council Mammalian Genetics Unit (Harwell, Oxfordshire, U.K.). Animals were maintained as heterozygotes on a C57BL/6 J background in a conventional animal facility with a 12-h light/dark cycle and received a standard laboratory diet. Male IRKO mice (aged 8–12 weeks) were compared with age- and sex-matched wild-type (WT) littermates. Genotyping was performed using PCR on ear notch genomic DNA, with primers specific for the gene-targeting cassette, as previously described (23). All procedures were approved by the Ethical Review Committee at the University of Leeds and were done in accordance with the Animals (Scientific Procedures) Act of 1986. Because of inherent difficulties in culturing late outgrowth endothelial colony cells from the blood of insulin-resistant mice (35), we restricted our investigation to “early outgrowth” APCs.
Metabolic assessment.
Intraperitoneal glucose- and insulin-tolerance tests were performed in conscious, fasted animals, as previously described (23). At 30-minute intervals after an intraperitoneal injection of glucose (1 mg/g body wt) or insulin (0.75 units/kg; Actrapid, Novo Nordisk, Bagsvaerd, Denmark), blood glucose was measured using a glucometer (Accu-Chek, Aviva, Roche, Basel, Switzerland).
Fluorescence-activated cell sorter enumeration of APCs.
Saphenous vein blood samples (100 μL) were incubated with PharmLyse (BD Biosciences, San Jose, CA) at room temperature. After centrifugation, mononuclear cells (MNCs) were resuspended in fluorescence-activated cell sorter (FACS) buffer and incubated with FcR blocker (BD Biosciences) at 4°C. Appropriate volumes of the following antibodies, or their respective isotype controls, were then added for 10 minutes at 4°C: fluorescein isothiocyanate (FITC) anti-mouse Sca-1 and PE anti-mouse Flk-1 (BD Biosciences). APCs were enumerated using flow cytometry (BD FACS Calibur) to quantify dual-stained Sca-1/Flk-1 cells. Isotype control specimens were used to define the threshold for antigen presence and to subtract nonspecific fluorescence. The cytometer was set to acquire 100,000 events within the lymphocyte gate, defined by typical light scatter properties.
To assess APC mobilization, mice received an intraperitoneal injection of 5 μg of vascular endothelial growth factor (VEGF) on four consecutive days, as previously described (36). Sca-1/Flk-1 cells were quantified using FACS analysis at baseline and at day 4 after treatment.
Western blot analysis of BM lysates.
BM, which was isolated by flushing harvested femurs and tibias with ice-cold Dulbecco’s modified Eagle’s medium, was resuspended by pipetting through a 70-μm cell strainer and centrifuged briefly. Erythrocytes were removed using Erythrocyte Lysis Buffer (Sigma-Aldrich, St. Louis, MO). Protein was extracted in lysis buffer and quantified using the protein BCA assay (Sigma-Aldrich). Then, 50 μg of protein were separated by electrophoresis through 4–12% SDS-PAGE gels (Invitrogen Life Technologies, Carlsbad, CA) and blotted onto polyvinylidene fluoride membranes. Blots were probed with 1:1000 eNOS, 1:1000 Ser1177phospho eNOS, and 1:3000 actin antibodies (BD Bioscience), as previously described (37). Human umbilical vein EC lysates were used as a positive control for all blots.
Aortic eNOS activity.
We have previously demonstrated that IRKO mice have reduced aortic NO production in organ bath studies and in studies assessing serine phosphorylation of eNOS in response to systemic insulin (23). Here we assessed the effect of insulin (100 nmol/L) on eNOS activity in aortas by quantifying the conversion of [14C]l-arginine to [14C]l-citrulline, as previously described (37).
Preparation of MNCs.
Spleens were harvested and mechanically minced. MNCs were isolated using density-gradient centrifugation (Histopaque-1083, Sigma) and were then stained with Cell-Tracker CM-DiI (2 μg/mL; C7000, Invitrogen). A total of 30 × 106 cells were resuspended in 200 μL of PBS for intravenous injection.
In a subset of experiments, femurs and tibias were flushed with magnetic cell sorting (MACS) buffer to collect BM. BM-derived cells were subjected to magnetic bead separation to collect CD117+ (C-kit receptor) cells. In brief, BM-derived cells were washed, resuspended, and magnetically labeled with CD117 microbeads (MACS Microbeads, Miltenyi Biotech GmbH, Bergisch Gladbach, Germany). After incubation and additional washing, magnetic cell separation was performed using a separation column placed in a magnetic field of a magnetic bead separator (MACS Separation Columns, Miltenyi). The attached CD117+ cells were collected in buffer and were resuspended in 200 μL of MACS buffer for intravenous injection.
APC isolation and culture.
MNCs from 1 mL of blood, obtained from the vena cava under terminal anesthesia, were isolated by Histopaque-1083 (Sigma) density gradient centrifugation. MNCs were seeded on fibronectin 24-well plates (BD Biosciences) at a density of 5 × 106 cells/well. Cells were cultured in EC growth (EGM-2) medium supplemented with EGM-2 Bullet kit (Lonza, Basel, Switzerland) in addition to 20% FCS.
Spleens obtained from mice under terminal anesthesia were mechanically minced. MNCs were isolated by density gradient centrifugation, as described above. After washing steps, cells were seeded on fibronectin 24-well plates at a seeding density of 8 × 106 cells/well and cultured as described above.
Femurs and tibias were flushed three times in DMEM with a 26-gauge needle to collect BM. MNCs were isolated by density gradient centrifugation as described above. After washing steps, cells were seeded on fibronectin 24-well plates at a seeding density of 1 × 106 cells/well and cultured as described above.
APC characterization.
After 4 days of incubation at 37°C in 5% CO2, nonadherent cells were discarded by gentle washing with PBS and adherent cells resuspended in medium. At day 7, attached cells from peripheral blood, spleen, and BM were stained for the uptake of 1,1’-dioctadecy-3,3,3′,3′-tetramethyllindocarbocyanine-labeled acetylated low-density lipoprotein (DiI-Ac-LDL) (Molecular Probes, Invitrogen, Carlsbad, CA) and lectin from Ulex europaeus FITC conjugate (Sigma). Cells were first incubated with DiI-Ac-LDL at 37°C for 3 h and later fixed with 4% paraformaldehyde for 10 minutes. Cells were washed and reacted with lectin for 1 h. After staining, cells were quantified by examining 10 random high-power fields (HPF) and double-positive cells were identified as APCs and counted, as previously reported (38).
APC function: adhesion and in vitro angiogenesis assay.
To evaluate adhesion, 50,000 APCs were resuspended in EGM-2 medium, plated onto fibronectin 24-well plates, and incubated for 1 h at 37°C. After washing three times with PBS, attached cells were counted. Adhesion was evaluated as the mean number of attached cells per HPF (×100). The potential for APCs to stimulate angiogenesis by secreting paracrine factors was assessed, as previously described (39). Briefly, conditioned media were obtained by replacing the medium of day 4 APC cultures with serum-free EGM-2 supplemented with 1% FCS and culturing the cells for an additional 24 h. APCs were counted, and conditioned media was diluted to correct for cell numbers. After 24 h, tube formation by human umbilical vein endothelial cell (HUVEC) on matrigel-coated 24-well plates (BD Bioscience), in the presence of conditioned media, was measured by staining the viable cells with hematoxylin-eosin (Sigma). HUVEC cultures were used at passage 3–5. The number of endothelial tubes was quantified per HPF (×100) (39).
Vascular injury.
Mice were anesthetized with isoflurane (2.5–5%) before a small incision was made in the midthigh and extended. Having carefully isolated the femoral artery, an arteriotomy was made using iris scissors (World-Precision Instruments, Sarasota, FL), and a 0.014-inch-diameter angioplasty guidewire with tapered tip (Hi-torque Cross-it XT, Abbott-Vascular, Abbott, IL), was introduced. The guidewire was advanced 3 cm, and three passages were performed per mouse, resulting in complete arterial denudation. The guidewire was removed completely and the suture was tightened rapidly. The vessel was then ligated, and the skin was closed with a continuous suture. The contralateral artery underwent an identical sham operation, without passage of the wire. Animals received postoperative analgesia with buprenorphine (0.25 mg/kg s.c.).
Splenectomy.
Mice were anesthetized with isoflurane, and the spleen was dissected through a lateral incision of the left abdomen. Vessels were carefully ligated using 6-0 silk. After removal of the spleen, the abdomen was closed with single sutures using 6-0 silk.
Transfusion regimen.
After splenectomy and vascular injury, IRKO and WT mice received an intravenous injection of 30 × 106 fluorescently labeled spleen-derived MNCs from WT donors, as previously described (17). In a separate set of experiments, IRKO mice received an intravenous injection of 6 × 106 c-kit receptor-positive BM-derived cells from WT and IRKO donors on 2 consecutive days after splenectomy and vascular injury. Control animals underwent splenectomy and vascular injury and received a corresponding amount of normal saline without cells. Femoral arteries were examined at 5 days for Evans blue staining and for en face fluorescence microscopy.
En face microscopy.
Mice were anesthetized at 3, 5, 7, and 14 days after wire injury, and 50 μL of 5% Evans blue dye was injected into the vena cava. The mice were perfused/fixed with formaldehyde before the femoral arteries (injured and uninjured) were harvested. The vessels were opened longitudinally. The areas stained and unstained in blue were measured in the injured area 5 mm from the proximal suture, and the percentage areas were calculated using ImagePro Plus 6.0 software (Media Cybernetics, Bethesda, MD).
A subset of perfusion-fixed femoral arteries were opened longitudinally for en face fluorescence microscopy at day 5 after transfusion, embedded in mounting medium containing the nuclear stain DAPI (VECTASHIELD, Vector Laboratories, Burlingame, CA), and directly assessed for DiI and DAPI fluorescence using a Zeiss ApoTome imager Z1 microscope (Carl Zeiss, Oberkochen, Germany). The images were assessed using Axiovision 4.8.1 software (Zeiss).
Aortic ring angiogenesis assay.
Descending thoracic aortas were excised and flushed with ice-cold PBS until free of blood. Surrounding fibroadipose tissue was dissected free, and the aorta sectioned into 1-mm rings. Culture plates (24-well) were coated with 200 μL/well of growth factor–reduced Matrigel (BD Bioscience) and then allowed to polymerize for 30 minutes at 37°C. Rings were embedded on the growth factor–reduced Matrigel and incubated with Endothelial Cell Growth Medium MV2 (PromoCell, Heidelberg, Germany), which was replaced daily. Quantitative analysis of endothelial sprouting was performed using images from day 7. The greatest distance from the aortic ring body to the end of the vascular sprouts was measured at three distinct points per ring and in three different rings per treatment group, as previously described (40).
Statistics.
Data are presented as arithmetic mean and SEM, unless stated otherwise. Graphic analyses were performed using Prism 5 software (GraphPad Software, San Diego, CA). Continuous data were compared using unpaired, two-tailed t tests for difference in means. Time-dependent re-endothelialization was compared using two-way ANOVA. Post hoc t tests were performed with application of a Bonferroni correction. Statistical significance was accepted at P < 0.05.
RESULTS
Metabolic homeostasis in IRKO mice.
Blood glucose concentrations were similar in IRKO and WT mice in the fasting state (5.9 ± 0.3 vs. 5.8 ± 0.6 mmol/L; P = 0.89) and after a glucose challenge (10.9 ± 0.6 vs. 9.7 ± 0.5 mmol/L; P = 0.21). Insulin sensitivity, assessed by the hypoglycemic response to intraperitoneal insulin administration, was similar in IRKO and WT mice, with a glucose concentration 30 minutes after insulin administration of 5.2 ± 0.4 vs 5.4 ± 0.4 mmol/L (P = 0.63).
Quantification of APCs.
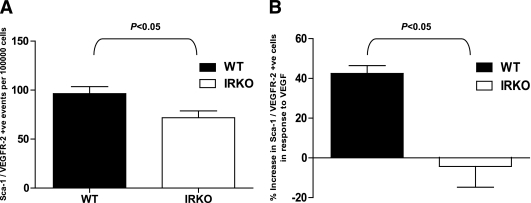
Sca-1/Flk-1+ APCs in peripheral blood were significantly reduced in IRKO mice compared with WT controls (P < 0.05; Fig. 1A). To investigate whether the reduced abundance of APCs we observed in IRKO mice was attributable to impaired APC mobilization from the BM, mice were treated with VEGF (5 μg i.p.) on 4 consecutive days, and Sca-1/Flk-1+ cells were quantified before and after treatment. IRKO mice demonstrated significantly impaired APC mobilization in response to VEGF compared with WT (P < 0.05; Fig. 1B).
FIG. 1.
Enumeration of APCs in blood using flow cytometry. A: Numbers of Sca-1/Flk-1+ cells at baseline (P < 0.05; n = 9 per group). B: Mobilization of Sca-1/Flk-1+ cells in response to VEGF administration (P < 0.05; n = 5 mice per group). Data are presented as mean and SEM.
Phenotypic analysis of in vitro APC culture.
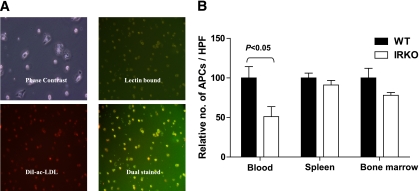
As a second method of APC quantification, DiI-ac-LDL/lectin+ APCs were expanded from peripheral blood-, spleen-, and BM-derived MNCs, resulting in the growth of early-outgrowth APCs. These exhibited typical spindle-shaped morphology on day 4 of culture. On day 7, cultivated cells were incubated with DiI-ac-LDL and lectin. Cells staining positive for both lectin and DiI-ac-LDL were quantified in 10 randomly selected HPFs (Fig. 2A).
FIG. 2.
Enumeration of APCs derived from blood, spleen, and BM by cell culture. A: Fluorescence microscopy revealing the spindle-shaped APCs (phase contrast image), lectin-bound (green), DiI-ac-LDL uptake (red), and dual-stained APCs (yellow; magnification ×100). B: Numbers of peripheral blood-, spleen- and BM-derived cultured APCs from uninjured mice are expressed as the percentage of cells derived from WT mice (P < 0.05; n = 6 mice per group). Data are presented as mean and SEM. (A high-quality digital representation of this figure is available in the online issue.)
Significantly fewer APCs were cultured from blood of IRKO mice compared with WT controls (Fig. 2B). In contrast, there were no significant differences in the numbers of spleen- or BM-derived APCs cultured from the two groups of mice (Fig. 2B).
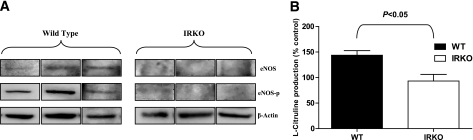
BM eNOS expression and aortic eNOS activity.
Because APC mobilization is critically dependent on NO bioactivity, we investigated eNOS expression in BM and eNOS activity in the vasculature. eNOS and Ser1177phospho eNOS expression was demonstrated in BM from WT animals but was undetectable in BM from IRKO mice (Fig. 3A). In aorta, insulin-stimulated eNOS activity, as assessed by conversion of [14C]l-arginine to [14C]l-citrulline, was significantly blunted in the IRKO mice compared with WT controls (Fig. 3B).
FIG. 3.
Expression and activity of eNOS. A: eNOS and Ser-1177 phospho eNOS expression was confirmed in BM of WT animals but was undetectable in BM of IRKO mice (n = 3 mice per group). B: Insulin-stimulated eNOS activity was measured in aortic rings from IRKO and WT mice (n = 6 mice per group). Data are presented as mean and SEM.
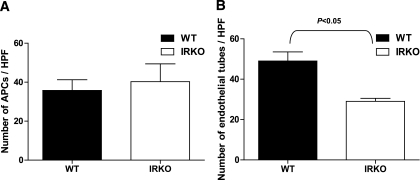
APC function: adhesion and in vitro angiogenesis assay.
There was no difference between IRKO and WT in adhesive capacity of APCs to fibronectin, an extracellular matrix component involved in the initial steps of angiogenesis (Fig. 4A). To further evaluate the effect of insulin resistance on APC function, an in vitro angiogenesis assay was performed with conditioned medium of cultured APCs from IRKO and WT mice. As shown in Fig. 4B, we observed a significant reduction in tube formation when HUVECs were subjected to conditioned media of APCs derived from IRKO mice compared with that from APCs derived from WT (P < 0.05), indicating reduced paracrine angiogenic capacity of the IRKO cells. Importantly, the difference in paracrine function was not a reflection of lower APC numbers in IRKO, because the conditioned medium was diluted to normalize for APC number.
FIG. 4.
APC functional assays. A: Adhesion capacity of APCs cultured from IRKO and WT mice expressed as number of cells adhering to fibronectin-coated plates (n = 4 mice per group). B: Endothelial tube formation in response to conditioned medium from WT and IRKO APCs (P < 0.05; n = 4 mice per group). Data are presented as mean and SEM.
Endothelial regeneration.
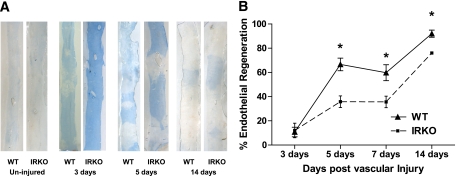
IRKO and WT mice were subjected to femoral artery-denuding wire injury. Evans blue staining of whole-mounted femoral arteries confirmed that the entire injured segment was denuded of endothelium immediately after wire injury (not shown). Evans blue staining at intervals after the experimental injury demonstrated that endothelial regeneration was significantly delayed in IRKO animals compared with WT (Fig. 5).
FIG. 5.
Endothelial regeneration after wire injury of the femoral artery. A: Representative in situ Evans blue staining in an uninjured vessel and vessels at 3, 5, and 14 days after vascular injury (blue staining indicates denuded endothelium) in IRKO and WT mice (magnification ×20). B: Time-dependent endothelial regeneration is shown after vascular injury (n = 5 mice per group). *P < 0.05. Data are presented as mean and SEM. (A high-quality digital representation of this figure is available in the online issue.)
APC transfusion.
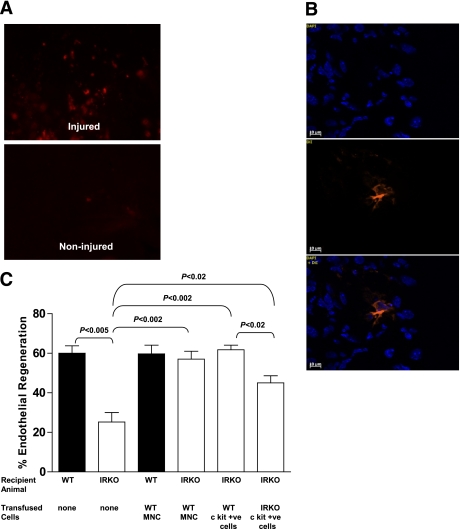
To examine the potential therapeutic effect of systemic administration of APCs on endothelial repair, we performed APC transfusion experiments. IRKO and WT mice initially received an intravenous femoral vein injection of 30 × 106 fluorescently labeled spleen-derived MNCs from WT mice after vascular injury. To enhance homing of the transfused cells to the injury site, mice were splenectomized before injury. Control animals underwent splenectomy and vascular injury and received a corresponding volume of 0.9% saline vehicle. En face fluorescence microscopy 5 days after femoral artery injury revealed that homing of transfused DiI-labeled cells was restricted to the injury site, with no detectable fluorescent cells in noninjured regions or in the contralateral vessel (Fig. 6A). Nuclear staining with DAPI and overlay experiments confirmed the presence of transfused cells in the injured endothelium (Fig. 6B).
FIG. 6.
Effects of cell transfusion on endothelial regeneration. A: En face microscopy demonstrates the presence of DiI-labeled MNCs at the injury site 5 days after wire injury with no detectable cells in the uninjured vessel (magnification ×100). B: Corresponding nuclear staining of the endothelium with DAPI revealing native endothelial cells and DiI-labeled transfused MNCs (magnification ×400). C: Effects of transfusion of spleen-derived MNCs or BM-derived c-kit(CD117)+ cells from WT or IRKO mice are shown on endothelial regeneration 5 days after wire injury (n = 4 mice per group). Data are presented as mean and SEM. (A high-quality digital representation of this figure is available in the online issue.)
To evaluate the effect of transfused spleen-derived labeled MNCs on re-endothelialization in transfused and placebo-treated mice, the de-endothelialized areas were determined by Evans blue staining in a whole-vessel preparation 5 days after vascular injury. Consistent with our earlier observations, vehicle-treated IRKO mice who underwent splenectomy and vascular injury displayed impaired endothelial regeneration compared with WT controls (25 ± 5 vs. 60 ± 4%; P < 0.005). Transfusion of WT MNCs into IRKO mice led to a significant increase in re-endothelialization (57 ± 4%) compared with vehicle alone (25 ± 5%; P < 0.002; Fig. 6C).
To further explore the therapeutic effect of APCs, BM-derived cells from WT and IRKO mice that were positive for c-kit receptor (stem cell marker CD 117) were obtained by magnetic bead separation. Transfusion of WT cells positive for c-kit receptor into IRKO mice (6 × 106/day for 2 days) significantly improved endothelial regeneration compared with placebo-treated IRKO (62 ± 2 vs. 25 ± 5%; P < 0.002). Transfusion of IRKO cells positive for c-kit receptor into IRKO mice also improved endothelial regeneration compared with the vehicle-treated IRKO mice (45 ± 4 vs. 25 ± 5%; P < 0.02), although this treatment was not as effective as treatment with WT cells positive for c-kit receptor (62 ± 2 vs. 45 ± 4%; P < 0.02; Fig. 6C).
Aortic ring angiogenesis assay.
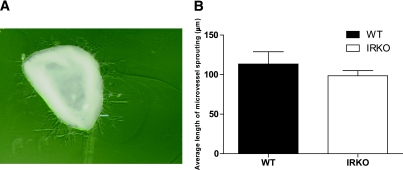
This in vitro assay closely simulates in vivo angiogenesis, not only because it includes the surrounding non-ECs but also because the ECs have not been preselected by ex vivo expansion (41). There was no difference in the length of aortic microvessel sprouting between aortic explants from WT and IRKO mice, indicating no impairment in angiogenic potential of native mature ECs in the vasculature of IRKO mice (Fig. 7A and B).
FIG. 7.
Aortic ring angiogenesis assay. A: Representative image demonstrates sprouting microvessels from an aortic ring cultured in Matrigel (magnification ×40). B: Quantification of microvessels sprouting from aortic rings from IRKO and WT mice (n = 4 mice per group). Data are presented as mean and SEM. (A high-quality digital representation of this figure is available in the online issue.)
DISCUSSION
This report provides a number of novel insights into the effect of whole-body insulin resistance on endothelial regenerative mechanisms:
we show that IRKO mice have impaired endothelial reparative capacity after endothelium-denuding arterial injury;
consistent with our recent findings in insulin-resistant humans, APC mobilization is blunted in IRKO mice;
in vitro APC functionality is impaired in IRKO mice;
transfusion of different APC subsets from WT mice enhances endothelial regeneration in IRKO mice; and
transfusion of IRKO APCs into IRKO mice improves endothelial regeneration but not to the same extent as transfusion of WT cells.
Compelling evidence now supports the concept that in response to vascular injury, circulating progenitor cells are mobilized from the BM—and possibly other tissues—into the circulation, home to sites of EC damage, and contribute to vascular repair (42).
Our study provides evidence of reduced numbers of APCs in the peripheral blood and impaired APC mobilization in mice with mild whole-body insulin resistance but normoglycemia. Emerging data support a role of insulin in modulating progenitor cell function (43,44). Although a reduced number of basal APCs was previously demonstrated in a mouse model of diabetes (db/db mice) (14), this is the first evidence that insulin resistance per se is associated with a reduction in peripheral APCs and impaired APC mobilization. We have shown that mice with haploinsufficiency of the insulin receptor have reduced basal and insulin-mediated endothelial NO production and a progressive deterioration in NO bioavailability with age (23,45).
It is interesting to note that although there was a reduction in the number of peripheral APCs in the IRKO mice, there was no difference in the number of spleen- or BM-derived APCs, potentially consistent with impaired APC mobilization secondary to a reduction in NO bioavailability. There is convincing evidence to suggest that NO has a critical role to play in APC mobilization (46). In keeping with this, we found reduced expression of eNOS in BM in IRKO mice along with reduced eNOS activity in the aorta. The finding of reduced APC numbers in this mouse model of insulin resistance is in line with previously published human data from our group showing diminished APC numbers and impaired NO-dependent APC mobilization in healthy insulin-resistant volunteers of South Asian descent compared with normoglycemic insulin-sensitive white European control subjects (21,32).
In addition to the circulating numbers of APCs, the functional capacity of these cells is arguably of greater pathophysiologic relevance. Although APCs enhance new vessel formation, the process is likely to involve the participation of mature resident ECs. After adhering to the vessel wall, it has been suggested that a major function of APCs could be the secretion of a portfolio of angiogenic factors that activate resident mature EC-mediated repair (19). Here we show that despite similar adhesion capacity of the cells, the angiogenic capacity of conditioned media from IRKO-derived APCs is reduced. Because we restricted our studies to APCs, we cannot rule out the possibility that alterations in late outgrowth endothelial colony-forming cells may also have contributed to dysfunctional endothelial repair.
We have clearly demonstrated that endothelial regeneration is significantly impaired in IRKO mice compared with WT littermates. Delayed endothelial repair has previously been demonstrated in a mouse model of diabetes (14); however, this is the first evidence directly linking whole-body insulin resistance with impaired endothelial regeneration. Although a cause-effect relationship between the reduction in APC numbers and impaired endothelial regeneration cannot be demonstrated at this stage, previous work has shown that 55% of re-endothelialization after wire injury is likely to be attributable to BM-derived cells (47). Using an aortic ring angiogenesis assay, we demonstrated no difference in the angiogenic capacity of IRKO mice. Therefore, although impaired angiogenic repair by mature ECs at sites of damage may partly account for the delayed endothelial regeneration seen in IRKO mice, the reduction in APCs and impaired APC mobilization and function seen in this model provide a compelling case for an impairment of APC-mediated repair in insulin-resistant mice.
Other groups have used lethal irradiation, followed by BM transplantation, to demonstrate the contribution of BM-derived cells to endothelial repair (14). Because irradiation potentially leads to a number of deleterious effects on recipient animals, including endothelial dysfunction and apoptosis (48), there is concern regarding the physiologic significance of the findings observed in these studies. To avoid these problems and to further investigate the potential therapeutic effect of APCs on endothelial repair, we performed APC transfusion studies. Homing signals for circulating progenitor cells mostly result from local injury and guide them to the target tissue. In our study, intravenously transfused cells were exclusively found at the injury site. Transfusion of WT spleen-derived MNCs into IRKO mice after vascular injury significantly improved endothelial repair. Because the spleen is a hematopoietic organ in the mouse, it is not possible to determine which exact cell type of the spleen-derived MNCs is responsible for the observed beneficial effect on endothelial repair. It is tempting to speculate that the complex composition of spleen-derived MNCs, including monocytes, macrophages, and other progenitor cells, all with differentiation potential into APCs, leads to enhanced paracrine interactions that ultimately lead to improved endothelial repair.
To determine whether a pure population of insulin-sensitive BM hematopoietic stem cells characterized by the expression of the cell surface marker c-kit could augment repair, we infused WT c-kit+ BM cells into IRKO mice. This led to normalization of endothelial regeneration, suggesting an important role for this cell type. Transfusion of IRKO c-kit+ cells resulted in an improvement but not normalization of endothelial repair, underlining the importance of the abnormal phenotype of progenitor cells from IRKO mice.
Our studies in whole-body and endothelial-specific insulin resistance provide potential insights into the molecular basis for the APC dysfunction seen here. Our observations that insulin resistance downregulates signaling through the PI-3-kinase/akt/eNOS pathway (23,45) and increases endothelial intracellular reactive oxygen species (49) are pertinent. PI-3-kinase/akt/eNOS signaling plays a key role in multiple facets of APC function, including mobilization, homing, and migration (44,50). Imbalance between intracellular reactive oxygen species and endogenous oxidant defense mechanisms in endothelial progenitors has recently emerged as a cause of impaired ischemic neovascularization and wound healing in diabetes (27,51–53). The consequences of insulin resistance on reactive oxygen species and oxidant defenses in progenitor cells warrant further investigation.
In summary, this dataset demonstrates that mild whole-body insulin resistance reminiscent of the human scenario reduces APC numbers, impairs APC mobilization and function, and delays endothelial regeneration after injury. Transfusion of WT but not IRKO APCs restores regenerative capacity in IRKO mice. These data may have important implications for the development of therapeutic strategies for vascular disease associated with insulin resistance.
ACKNOWLEDGMENTS
This study was funded by a British Heart Foundation Clinical Research Training Fellowship to M.B.K. A.A., A.R., and V.B. hold British Heart Foundation Clinical Research Training Fellowships. S.B.W. holds a British Heart Foundation Intermediate Clinical Research Fellowship. S.T.R. is supported by Wellcome Trust, M.G. is funded by Diabetes UK, and H.I. is supported by the Medical Research Council.
No potential conflicts of interest relevant to this article were reported.
M.B.K. and N.Y.Y. conducted experiments, analyzed data, and prepared the manuscript. R.M.C., J.S., S.T.R., H.V., H.I., A.A., A.R., A.A., V.B., P.S., and M.G. assisted with experiments and reviewed the manuscript. M.T.K. and S.B.W. secured funding, designed the experiments, supervised the project, and edited the manuscript.
REFERENCES
- 1.Pyörälä M, Miettinen H, Laakso M, Pyörälä K. Hyperinsulinemia predicts coronary heart disease risk in healthy middle-aged men: the 22-year follow-up results of the Helsinki Policemen Study. Circulation 1998;98:398–404 [DOI] [PubMed] [Google Scholar]
- 2.King H, Aubert RE, Herman WH. Global burden of diabetes, 1995-2025: prevalence, numerical estimates, and projections. Diabetes Care 1998;21:1414–1431 [DOI] [PubMed] [Google Scholar]
- 3.Cubbon RM, Wheatcroft SB, Grant PJ, et al. Temporal trends in mortality of patients with diabetes mellitus suffering acute myocardial infarction: a comparison of over 3000 patients between 1995 and 2003. Eur Heart J 2007;28:540–545 [DOI] [PubMed] [Google Scholar]
- 4.Cubbon RM, Gale CP, Rajwani A, et al. Aspirin and mortality in patients with diabetes sustaining acute coronary syndrome. Diabetes Care 2008;31:363–365 [DOI] [PubMed] [Google Scholar]
- 5.Cubbon RM, Abbas A, Wheatcroft SB, et al. EMMACE-2 investigators Diabetes mellitus and mortality after acute coronary syndrome as a first or recurrent cardiovascular event. PLoS ONE 2008;3:e3483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cubbon RM, Rajwani A, Abbas A, et al. Hyperglycaemia, in relation to sex, and mortality after acute coronary syndrome. Eur J Cardiovasc Prev Rehabil 2007;14:666–671 [DOI] [PubMed] [Google Scholar]
- 7.Gerstein HC, Miller ME, Byington RP, et al. Action to Control Cardiovascular Risk in Diabetes Study Group Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med 2008;358:2545–2559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Patel A, MacMahon S, Chalmers J, et al. ADVANCE Collaborative Group Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N Engl J Med 2008;358:2560–2572 [DOI] [PubMed] [Google Scholar]
- 9.Semenkovich CF. Insulin resistance and atherosclerosis. J Clin Invest 2006;116:1813–1822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kim JA, Montagnani M, Koh KK, Quon MJ. Reciprocal relationships between insulin resistance and endothelial dysfunction: molecular and pathophysiological mechanisms. Circulation 2006;113:1888–1904 [DOI] [PubMed] [Google Scholar]
- 11.Frye RL, August P, Brooks MM, et al. BARI 2D Study Group A randomized trial of therapies for type 2 diabetes and coronary artery disease. N Engl J Med 2009;360:2503–2515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Home PD, Pocock SJ, Beck-Nielsen H, et al. RECORD Study Team Rosiglitazone evaluated for cardiovascular outcomes in oral agent combination therapy for type 2 diabetes (RECORD): a multicentre, randomised, open-label trial. Lancet 2009;373:2125–2135 [DOI] [PubMed] [Google Scholar]
- 13.Kirton JP, Xu Q. Endothelial precursors in vascular repair. Microvasc Res 2010;79:193–199 [DOI] [PubMed] [Google Scholar]
- 14.Ii M, Takenaka H, Asai J, et al. Endothelial progenitor thrombospondin-1 mediates diabetes-induced delay in reendothelialization following arterial injury. Circ Res 2006;98:697–704 [DOI] [PubMed] [Google Scholar]
- 15.Xu Q, Zhang Z, Davison F, Hu Y. Circulating progenitor cells regenerate endothelium of vein graft atherosclerosis, which is diminished in ApoE-deficient mice. Circ Res 2003;93:e76–e86 [DOI] [PubMed] [Google Scholar]
- 16.Rauscher FM, Goldschmidt-Clermont PJ, Davis BH, et al. Aging, progenitor cell exhaustion, and atherosclerosis. Circulation 2003;108:457–463 [DOI] [PubMed] [Google Scholar]
- 17.Wassmann S, Werner N, Czech T, Nickenig G. Improvement of endothelial function by systemic transfusion of vascular progenitor cells. Circ Res 2006;99:e74–e83 [DOI] [PubMed] [Google Scholar]
- 18.Werner N, Junk S, Laufs U, et al. Intravenous transfusion of endothelial progenitor cells reduces neointima formation after vascular injury. Circ Res 2003;93:e17–e24 [DOI] [PubMed] [Google Scholar]
- 19.Hirschi KK, Ingram DA, Yoder MC. Assessing identity, phenotype, and fate of endothelial progenitor cells. Arterioscler Thromb Vasc Biol 2008;28:1584–1595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Melikian N, Chowienczyk P, MacCarthy PA, et al. Determinants of endothelial function in asymptomatic subjects with and without the metabolic syndrome. Atherosclerosis 2008;197:375–382 [DOI] [PubMed] [Google Scholar]
- 21.Murphy C, Kanaganayagam GS, Jiang B, et al. Vascular dysfunction and reduced circulating endothelial progenitor cells in young healthy UK South Asian men. Arterioscler Thromb Vasc Biol 2007;27:936–942 [DOI] [PubMed] [Google Scholar]
- 22.Williams IL, Chowienczyk PJ, Wheatcroft SB, et al. Endothelial function and weight loss in obese humans. Obes Surg 2005;15:1055–1060 [DOI] [PubMed] [Google Scholar]
- 23.Wheatcroft SB, Shah AM, Li JM, et al. Preserved glucoregulation but attenuation of the vascular actions of insulin in mice heterozygous for knockout of the insulin receptor. Diabetes 2004;53:2645–2652 [DOI] [PubMed] [Google Scholar]
- 24.Caballero S, Sengupta N, Afzal A, et al. Ischemic vascular damage can be repaired by healthy, but not diabetic, endothelial progenitor cells. Diabetes 2007;56:960–967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fadini GP, Boscaro E, de Kreutzenberg S, et al. Time course and mechanisms of circulating progenitor cell reduction in the natural history of type 2 diabetes. Diabetes Care 2010;33:1097–1102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Segal MS, Shah R, Afzal A, et al. Nitric oxide cytoskeletal-induced alterations reverse the endothelial progenitor cell migratory defect associated with diabetes. Diabetes 2006;55:102–109 [PubMed] [Google Scholar]
- 27.Sorrentino SA, Bahlmann FH, Besler C, et al. Oxidant stress impairs in vivo reendothelialization capacity of endothelial progenitor cells from patients with type 2 diabetes mellitus: restoration by the peroxisome proliferator-activated receptor-gamma agonist rosiglitazone. Circulation 2007;116:163–173 [DOI] [PubMed] [Google Scholar]
- 28.Tepper OM, Galiano RD, Capla JM, et al. Human endothelial progenitor cells from type II diabetics exhibit impaired proliferation, adhesion, and incorporation into vascular structures. Circulation 2002;106:2781–2786 [DOI] [PubMed] [Google Scholar]
- 29.Tepper OM, Carr J, Allen RJ, Jr, et al. Decreased circulating progenitor cell number and failed mechanisms of stromal cell-derived factor-1alpha mediated bone marrow mobilization impair diabetic tissue repair. Diabetes 2010;59:1974–1983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Thum T, Fraccarollo D, Schultheiss M, et al. Endothelial nitric oxide synthase uncoupling impairs endothelial progenitor cell mobilization and function in diabetes. Diabetes 2007;56:666–674 [DOI] [PubMed] [Google Scholar]
- 31.Togliatto G, Trombetta A, Dentelli P, et al. Unacylated ghrelin rescues endothelial progenitor cell function in individuals with type 2 diabetes. Diabetes 2010;59:1016–1025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cubbon RM, Murgatroyd SR, Ferguson C, et al. Human exercise-induced circulating progenitor cell mobilization is nitric oxide-dependent and is blunted in South Asian men. Arterioscler Thromb Vasc Biol 2010;30:878–884 [DOI] [PubMed] [Google Scholar]
- 33.Brüning JC, Winnay J, Bonner-Weir S, Taylor SI, Accili D, Kahn CR. Development of a novel polygenic model of NIDDM in mice heterozygous for IR and IRS-1 null alleles. Cell 1997;88:561–572 [DOI] [PubMed] [Google Scholar]
- 34.Accili D, Drago J, Lee EJ, et al. Early neonatal death in mice homozygous for a null allele of the insulin receptor gene. Nat Genet 1996;12:106–109 [DOI] [PubMed] [Google Scholar]
- 35.Somani A, Nguyen J, Milbauer LC, Solovey A, Sajja S, Hebbel RP. The establishment of murine blood outgrowth endothelial cells and observations relevant to gene therapy. Transl Res 2007;150:30–39 [DOI] [PubMed] [Google Scholar]
- 36.Pitchford SC, Furze RC, Jones CP, Wengner AM, Rankin SM. Differential mobilization of subsets of progenitor cells from the bone marrow. Cell Stem Cell 2009;4:62–72 [DOI] [PubMed] [Google Scholar]
- 37.Imrie H, Abbas A, Viswambharan H, et al. Vascular insulin-like growth factor-I resistance and diet-induced obesity. Endocrinology 2009;150:4575–4582 [DOI] [PubMed] [Google Scholar]
- 38.Galasso G, Schiekofer S, Sato K, et al. Impaired angiogenesis in glutathione peroxidase-1-deficient mice is associated with endothelial progenitor cell dysfunction. Circ Res 2006;98:254–261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Loomans CJ, de Koning EJ, Staal FJ, et al. Endothelial progenitor cell dysfunction: a novel concept in the pathogenesis of vascular complications of type 1 diabetes. Diabetes 2004;53:195–199 [DOI] [PubMed] [Google Scholar]
- 40.Wang CY, Kim HH, Hiroi Y, et al. Obesity increases vascular senescence and susceptibility to ischemic injury through chronic activation of Akt and mTOR. Sci Signal 2009;2:ra11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Auerbach R, Lewis R, Shinners B, Kubai L, Akhtar N. Angiogenesis assays: a critical overview. Clin Chem 2003;49:32–40 [DOI] [PubMed] [Google Scholar]
- 42.Cubbon RM, Kahn MB, Wheatcroft SB. Effects of insulin resistance on endothelial progenitor cells and vascular repair. Clin Sci (Lond) 2009;117:173–190 [DOI] [PubMed] [Google Scholar]
- 43.Humpert PM, Djuric Z, Zeuge U, et al. Insulin stimulates the clonogenic potential of angiogenic endothelial progenitor cells by IGF-1 receptor-dependent signaling. Mol Med 2008;14:301–308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhao L, Cao F, Yin T, et al. Moderate dose insulin promotes function of endothelial progenitor cells. Cell Biol Int 2011;35:215–220 [DOI] [PubMed] [Google Scholar]
- 45.Duncan ER, Walker SJ, Ezzat VA, et al. Accelerated endothelial dysfunction in mild prediabetic insulin resistance: the early role of reactive oxygen species. Am J Physiol Endocrinol Metab 2007;293:E1311–E1319 [DOI] [PubMed] [Google Scholar]
- 46.Aicher A, Heeschen C, Mildner-Rihm C, et al. Essential role of endothelial nitric oxide synthase for mobilization of stem and progenitor cells. Nat Med 2003;9:1370–1376 [DOI] [PubMed] [Google Scholar]
- 47.Urao N, Okigaki M, Yamada H, et al. Erythropoietin-mobilized endothelial progenitors enhance reendothelialization via Akt-endothelial nitric oxide synthase activation and prevent neointimal hyperplasia. Circ Res 2006;98:1405–1413 [DOI] [PubMed] [Google Scholar]
- 48.Tanaka K, Sata M, Natori T, et al. Circulating progenitor cells contribute to neointimal formation in nonirradiated chimeric mice. FASEB J 2008;22:428–436 [DOI] [PubMed] [Google Scholar]
- 49.Duncan ER, Crossey PA, Walker S, et al. Effect of endothelium-specific insulin resistance on endothelial function in vivo. Diabetes 2008;57:3307–3314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Everaert BR, Van Craenenbroeck EM, Hoymans VY, et al. Current perspective of pathophysiological and interventional effects on endothelial progenitor cell biology: focus on PI3K/AKT/eNOS pathway. Int J Cardiol 2010;144:350–366 [DOI] [PubMed] [Google Scholar]
- 51.Ceradini DJ, Yao D, Grogan RH, et al. Decreasing intracellular superoxide corrects defective ischemia-induced new vessel formation in diabetic mice. J Biol Chem 2008;283:10930–10938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Groleau J, Dussault S, Haddad P, et al. Essential role of copper-zinc superoxide dismutase for ischemia-induced neovascularization via modulation of bone marrow-derived endothelial progenitor cells. Arterioscler Thromb Vasc Biol 2010;30:2173–2181 [DOI] [PubMed] [Google Scholar]
- 53.Marrotte EJ, Chen DD, Hakim JS, Chen AF. Manganese superoxide dismutase expression in endothelial progenitor cells accelerates wound healing in diabetic mice. J Clin Invest 2010;120:4207–4219 [DOI] [PMC free article] [PubMed] [Google Scholar]