Abstract
OBJECTIVE
Diabetic retinopathy (DR) is a leading cause of blindness. Increased vascular endothelial growth factor (VEGF), promoting angiogenesis and increased permeability, is a key mechanistic abnormality in DR. We investigated microRNA (miRNA) alterations in DR with specific focus on miR-200b, and its downstream target, VEGF.
RESEARCH DESIGN AND METHODS
miRNA expression profiling microarray was used to examine the retinas of streptozotocin-induced diabetic rats. Expressions of specific miRNAs were verified with PCR in the rat retina and in glucose-exposed endothelial cells. A target search, based on sequence complementarities, identified specific targets. We analyzed mRNA levels and protein expression in endothelial cells from large vessels and retinal capillaries and in the rat retina, with or without injection of miR-200b mimic or antagomir. Localization of miR-200b and its functional analysis in the rat and human retinas were performed.
RESULTS
Alteration of several miRNAs, including downregulation of miR-200b, were observed in the retina in diabetes. Such downregulation was validated in the retina of diabetic rats and in endothelial cells incubated in glucose. In parallel, VEGF (target of miR-200b) mRNA and protein were elevated. In the retina, miR-200b was localized in neuronal, glial, and vascular elements. Transfection of endothelial cells and intravitreal injection of miR-200b mimic prevented diabetes-induced increased VEGF mRNA and protein. Also prevented were glucose-induced increased permeability and angiogenesis. Furthermore, transfection of miR-200b antagonists (antagomir) led to increased VEGF production. Similar alterations were seen in the human retina.
CONCLUSIONS
These studies show a novel mechanism involving miR-200b in DR. Identification of such mechanisms may lead to the development of novel miRNA-based therapy.
Despite the identification of multiple pathogenetic mechanisms in a complex problem like diabetic retinopathy (DR), limited success has been achieved in its medical treatment. Vascular endothelial cells (ECs) undergo a series of metabolic changes in response to sustained hyperglycemia in diabetes. These metabolic derangements cause the activation of transcription factors and augmented expression of several growth factors and vasoactive factors, including vascular endothelial growth factor (VEGF), resulting in structural and functional alteration in the retina (1,2). Small, nonprotein-coding microRNAs (miRNAs) are endogenous regulators of gene expression and have been implicated in a variety of cellular physiologic and pathologic processes (3,4). The miRNA molecules are synthesized in the nucleus through RNA polymerase II. They are then processed to precursor miRNAs (70–100 nucleotides, hairpin-shaped) in the nucleus by RNAse III Dorsha and DiGeorge syndrome critical region 8 (DGCR8). The miRNAs are then exported to the cytoplasm by exportin 5. Finally, an active form (∼20–25 nucleotides) is produced in the cytoplasm after further processing by Dicer. MiRNAs have significant effects on the regulation of gene expression. They bind to the specific mRNA targets, causing their degradation or translational repression (3–5). Several investigators have used overexpression experiments to demonstrate the importance of miRNAs in diverse cellular processes (6). Other epigenetic mechanisms may play a fundamental role in the regulation of miRNAs (3–5).
Hyperglycemia is responsible for the initiation and the progression of chronic diabetes complications contributing to the structural and functional changes in the retina and other organs (1,2,7). A number of growth factors and vasoactive factors are increased in response to hyperglycemia, including endothelins and VEGF (7). Dysfunction of ECs is the primary cellular pathology in chronic diabetes complications. ECs undergo functional alterations, including increased proliferation that causes neovascularization in response to VEGF (8). VEGF has various isoforms, ranging from 121 to 206 amino acids in length, with VEGF165 the predominant form in humans (8,9). VEGF165 binds to cell surface receptor tyrosine kinases: VEGFR-1 (flt1) and VEGFR-2 (flk-1; KDR) (10). These receptors are mainly found on ECs, where VEGFR-1 mediates vessel permeability and VEGFR-2 is involved in angiogenesis (10,11). Hence, VEGF-mediated alterations are of significance in the early (vascular permeability) as well as the late stages (neovascularization) in DR.
No studies have specifically examined the role of miRNAs in DR. We investigated whether miRNA alterations are involved in DR. On the basis of our initial findings, we further investigated the role specific miRNA (i.e., miR-200b in DR). Because VEGF is a target of miR-200b, we explored this relationship. We have previously demonstrated that diabetes-induced vasoactive factor production is also mediated by transcription coactivator p300 (12). Because this epigenetic alteration may play a role in miRNA action, we further explored the relationship. To examine these changes, we used a combination of in vivo and in vitro approaches. We used cultured ECs and streptozotocin (STZ)-induced diabetic rats. We further used human archival material to examine the possibilities of such changes in human DR.
RESEARCH DESIGN AND METHODS
Animal studies.
All animals were cared for according to the Guiding Principle in the Care and Use of Animals. All experiments were approved by the University of Western Ontario Council on Animal Care Committee. Male SD rats (200–250 g) were obtained from the Charles River Colony (Wilmington, MA) and were randomly divided into control and diabetic groups. Methods of diabetes induction and monitoring have previously been described (13). After 4 weeks, the animals (n = 6/group) were killed. The retinal tissues were snap-frozen for gene expression and miRNA analysis or were placed in 10% formalin for embedding in paraffin.
Intraocular injection of miRNA mimic and antagomir.
After the onset of STZ-induced diabetes miRIDIAN miRNA mimic or antagomir (Dharmacon, Lafayette, CO) for miR-200b and the negative controls (scrambled) were intravitreally injected (1.4 μg/week, 4 weeks) using the Lipofectin Reagent (Life Technologies, Carlsbad, CA) (14). Control rats were injected with the same volume of saline and Lipofectin Reagent. Custom miRNA mimics or antagomir were synthesized by Dharmacon based on mature miRNA sequences of hsa-miR-200b (5′-UAAUACUGCCUGGUAAUGAUGAC-3′) and scrambled control (5′-UCACAACCUCCUAGAAAGAGUAGA-3′). Intravitreal p300 small interfering RNA (siRNA) injection has previously been described (14). The animals were killed in week 5, and the retinal tissues were collected, as above.
Cell culture.
Human umbilical vein ECs (HUVECs; ATCC, Manassas, VA) were used. The details of cell culture and p300 siRNA transfection have previously been described (12,14). Similar methods were used for transfection of miRNA mimics and antagomir. Bovine retinal capillary ECs (BRECs; VEC Technologies, Rensselaer, NY) were grown in the fibronectin-coated flask in a defined EC growth medium (MCDB-131 complete, VEC Technologies). At 24 h before transfection, the cells were passaged in the six-well plate coated with fibronectin (Sigma, St. Louis, MO). The culture conditions have previously been described (15,16). For transfection of BRECs, a 780-bp fragment containing mouse miR-200b locus on chromosome 4 was cloned from mouse genomic DNA into the pcDNA3.1(+) vector using restriction enzymes KpnI and XbaI. The plasmids (2 μg) were transfected into the BRECs using lipofectamin 2000 for 24 h, and then the cells were collected for analysis.
Human embryonic kidney (HEK293A) cells (ATCC) were used as previously described (17). All cell culture experiments were performed in triplicate for four times or more. All reagents were obtained from Sigma Chemicals (Oakville, ON, Canada), unless otherwise specified.
MiRNA microarray analysis.
MiRNAs were extracted using the mirVana miRNA isolation kit (Ambion, Austin, TX) according to the manufacturer’s instruction. Custom analysis of miRNA expression profiling from rat retinal samples (n = 3/group) were performed by Asuragen Inc. (Austin, TX).
MiRNA target search.
Open-sourced software using different algorithms based on sequence complementarity, such as TargetScan 5.1 (www.targetscan.org) and miRanda (www.microrna.org), were used for miRNA target predictions.
Luciferase assay.
The 3′-untranslated regions (UTR) of VEGF from rat and human genomes were used with appended SacI and HindIII restriction sites in the forward and reverse position, respectively. The primers for human VEGF 3′UTR cloning are: sense, AGAGCTCCCCGGCGAAGAGAAGAGAC; antisense, TCAAGCTTGGAGGGCAGAGCTGAGTGTTA. The primers for rat VEGF 3′UTR cloning are: sense, AGAGCTCGGGTCCTGGCAAAGAGAAG; antisense, TCAAGCTTGGAGGGCAGAGCTGAGTGTTA.
The target gene insert was then ligated into the pMIR-REPORT vector (Ambion), which was used to transform DH5α competent cells (Invitrogen, Burlington, ON, Canada). The DNA sequence of the cloned product was confirmed by sequencing. The pMIR_VEGF 3′UTR, miR-200b mimic (or scrambled) and pMIR REPORT luciferase vector reporter and control vector containing β-galactosidase with cytomegalovirus promoter were then cotransfected into the 293A cells. Nucleotide substitutions were inserted by PCR. The primers for human and rat VEGF 3′UTR mutation cloning are listed in the Supplementary Table. Luciferase activity was measured 48 h after transfection using the Dual-Light Chemiluminescent Reporter Gene Assay System (Applied Biosystems, Carlsbad, CA) following the manufacturer’s instructions. Luciferase activity was read using Chemiluminescent SpectraMax M5 (Molecular Devices, Sunnyvale, CA) (17).
MiRNA analysis.
RNA was extracted with the mirVana miRNA isolation kit (Ambion). Total RNA (50 ng) was used for cDNA synthesis with TaqMan MicroRNA Assay Reverse Transcription Primer and MultiScribe reverse transcriptase (Applied Biosystems). Specific primers were custom synthesized based on the mature miR-200b sequence (see above), mature miR-144 sequence (UACAGUAUAGAUGAUGUACU), mature miR-30a-3p sequence (CUUUCAGUCGGAUGUUUGCAGC), mature miR-429 sequence (UAAUACUGUCUGGUAAAACCGU), mature miR-200c sequence (UAAUACUGCCGGGUAAUGAUGG), and mature miR-200a sequence (UAACACUGUCUGGUAACGAUGU). Real-time qRT-PCR was performed with TaqMan MicroRNA Assay using the LightCycler (Roche Diagnostic, Laval, QC, Canada) as described, and the data were normalized to RNU6B (18).
mRNA analysis.
RNA was extracted with TRIzol reagent (Invitrogen) as previously described. Real time RT-PCR for mRNAs was done in the LightCycler (Roche Diagnostics). The data were normalized to the housekeeping gene β-actin or 18S rRNA. These methods have previously been described (12,14).
ELISA.
ELISA for VEGF was performed using a commercially available kit for rat VEGF (ALPCO, Salem, NH; R&D Systems, Minneapolis, MN) according to the manufacturers’ instructions (12).
Permeability assay.
HUVECs were seeded onto inserts (1-μm pores) in 24-well plates, with or without incubation with specific reagents for 24 h, and were tested for vascular permeability using the In Vitro Vascular Permeability Assay Kit (Millipore, Billerica, MA) according to the manufacturer’s instructions (19).
Angiogenesis assay.
An in vitro Angiogenesis Assay Kit (Chemicon, Billerica, MA) was used to evaluate tube formation of HUVECs. Tube formation was quantified using branch point counting with Infinity Capture Application 3.5.1 software on a Leica Microsystems (Bannockburn, IL) inverted microscope (20).
Immunohistochemistry.
Rat and human retinal sections were immunocytochemically stained for albumin to examine for increased vascular permeability using anti-human albumin antibody (1:500; Abcam, Cambridge, MA). These methods have previously been described (21,22). Quantification of diffuse retinal staining was done by an investigator in a masked fashion using an arbitrary scale (score 0 = no extravascular stain of retinal tissues, score 1 = mild extravascular stain of retinal tissues, score 2 = moderate extravascular stain of retinal tissue, score 3 = marked extravascular stain of retinal tissues). “No antibody” and isotype-matched IgG controls were used. CD34 (1:200; Dako, Glostrup, Denmark) immunostain was used to identify capillary endothelium.
In situ hybridization.
Rat and human retinal sections were labeled for miR-200b expression. Retinal tissue sections (5-μm thick) from formalin-fixed, paraffin-embedded blocks were transferred to positively charged slides to be used for labeling. A 5′ and 3′ double DIG-labeled custom-made mercury Locked Nucleic Acid (LNA) miRNA detection probe (Exiqon, Vedbaek, Denmark) was used to detect miR-200b expression along with the In Situ Hybridization (ISH) Kit (Biochain Institute, Hayward, CA) (23). Scrambled probes and no-probe controls were used as controls.
Statistical analysis.
Data are expressed as mean ± SEM and were analyzed by ANOVA, followed by Student t test with Bonferroni corrections or by Student t test only, where appropriate. Differences were considered significant at values of P < 0.05.
RESULTS
Diabetes causes miRNA alterations in the retina.
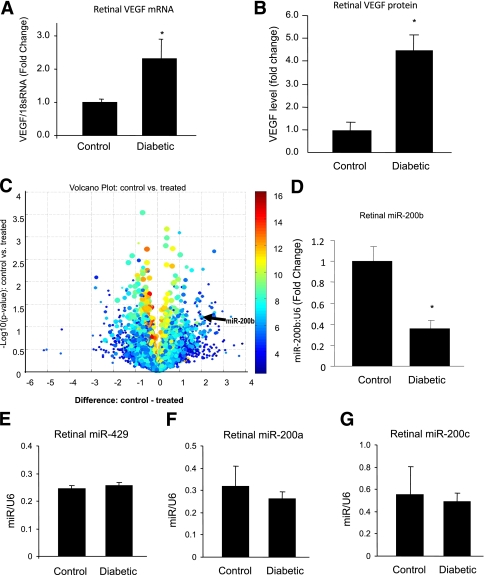
Speculating that the altered expression of key genes in diabetes may be partly regulated by miRNAs, we first searched for miRNAs whose expression changed in diabetes. To this end, we used an animal model of chronic diabetes. STZ-induced diabetic rats exhibit molecular and early structural and functional changes of DR (1,7,21). To examine miRNA alteration in DR, we did a microarray analysis of the retinal tissues from male STZ-induced diabetic rats and age- and sex-matched controls after 1 month of diabetes. Diabetic animals showed hyperglycemia (serum glucose of rats with diabetes 19.2 ± 4.7 mmol/L vs. controls 7.0 ± 0.8 mmol/L, P < 0.005) and reduced body weight (rats with diabetes 372.0 ± 34.7 g vs. controls 445.7 ± 17.4 g, P < 0.01). Retinal tissues of the diabetic animals showed increased levels of VEGF mRNA and protein as measured by qRT-PCR and ELISA (Fig. 1A and B). This is in keeping with our previous studies (21). We then extracted miRNAs from these tissues and performed microarray analysis, which showed alterations of multiple miRNAs in the retina of these animals (Fig. 1C).
FIG. 1.
MiRNA and VEGF alteration in the retina in diabetes. Quantitative RT-PCR (A) and ELISA (B) analysis from nondiabetic control and diabetic rat retinal tissue samples after 1 month of follow-up show increased levels of VEGF mRNA and protein. C: Volcano plot shows miRNA alteration in control vs. diabetic (treated) rat retina after 1 month. Circles to the left of −1 difference line and to the right of the 1 difference line are considered to have a fold-change >2× (x-axis is the log2 of the fold-change between two groups). The arrow indicates the location of miR-200b. (Custom analysis using Asuragen miRNA system. Some of the highly expressed miRNAs represent putative miRNA sites, which require biological characterization). Data array were validated with qRT-PCR analyses, which confirmed downregulation of miR-200b in the retina of diabetic rats compared with the nondiabetic controls (D) and no significant changes in the expression of miR-429 (E), miR-200a (F), and miR-200c (G). The miRNA data are expressed as a ratio to RNU6B (U6); mRNA levels are expressed as a ratio to 18S RNA, and normalized to controls. The error bars show the SEM. *Significantly different from the other group. (A high-quality color representation of this figure is available in the online issue.)
We used open sourced software (www.TargetScan.org, www.microrna.org, www.ebi.ac.uk1) for miRNA target predictions to identify miRNAs associated with known genes/proteins that are altered in DR. A VEGF targeting miRNA, miR-200b, was significantly downregulated in the diabetic animals. We verified the downregulation of miR-200b with qRT-PCR in the retina of diabetic animals (Fig. 1D). Other members of miR-200b cluster, namely miR-200a and miR-429, were not significantly altered (miR-429, miR-200a) in diabetic rat retina or do not target VEGF (miR-200a). No significant alterations of miR-200a, miR-200c, and miR-429 were seen in the array. However, an additional RT-PCR assay confirmed that there were no alterations of miR-429, miR-200a, and miR-200c in the retina in diabetes (Fig. 1E–G). Hence, an association was established between miR-200b downregulation and VEGF upregulation in DR. To test the specificity of miR-200b regarding VEGF, we examined whether fibronectin (FN), another bioinformatics-based target of miR-200b and a protein of interest in DR, is regulated by miR-200b. However, no direct regulation of FN by miR-200b was observed (data not shown). We also validated several other miRNAs (miR-144 and miR-30a-3p), which qRT-PCR showed were upregulated in the retina of diabetic animals (data not shown).
miR-200b regulates glucose-induced VEGF upregulation in the ECs.
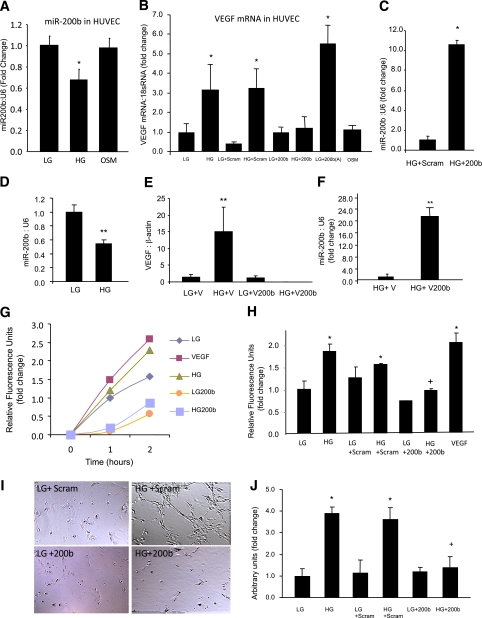
To establish a cause-effect relationship, we first used an in vitro model system. Because ECs are the primary cellular targets in DR, we used HUVECs in culture to study the mechanistic aspects and the functional significance of miR-200b alterations. ECs exposed to high levels of glucose (simulating hyperglycemia) recapitulate molecular and functional features of diabetic vascular pathologies (12,24). We first determined whether exposure of HUVECs to high levels of glucose causes changes in miR-200b levels. Our results show that 25 mmol/L d-glucose (HG), compared with 5 mmol/L d-glucose (LG), causes a significant downregulation of miR-200b (Fig. 2A). These levels of glucose were established using a dose-response analysis of VEGF expression (data not shown) and by previous experiments (12,24,25). No change in the miR-200b level was observed when the cells were challenged with 25 mmol/L l-glucose (OSM; Fig. 2A).
FIG. 2.
Effects of glucose-induced miR-200b downregulation in the endothelial cells. A: miR-200b was downregulated in the endothelial cells when exposed to 25 mmol/L d-glucose (HG) compared with 5 mmol/L d-glucose (LG), but not by 25 mmol/L l-glucose (osmotic control, OSM). B: Transfection of endothelial cells with miR-200b mimics (but not the scrambled mimics) normalized HG-induced upregulation of VEGF. Glucose-like effect (VEGF upregulation) was seen when cells in LG were transfected with miR-200b antagomir. OSM had no effect on VEGF expression. C: Efficiency of miR-200b mimic transfection as shown by increased miR-200b expression in the ECs after miR-200b mimic transfection compared with scrambled mimics. D: Similar to HUVECs, BRECs showed glucose-induced miR-200b downregulation. E: Transfection of BRECs with miR-200b mimics (using miR-200b cloned in PcDNA3.1 vector, but not by empty vector) normalized HG-induced upregulation of VEGF mRNA expression. F: Efficiency of miR-200b mimic transfection in the BREC was shown by increased miR-200b expression in these cells after miR-200b mimic transfection compared with vector control. HG-induced and VEGF-mediated increased transendothelial permeability (G), duration-dependent data and at the end point (H) were prevented by miR-200b mimic transfection. I: Similarly, glucose-induced EC tube formation was prevented by miR-200b mimic transfection. (Original magnification ×400.) J: The quantification of the tube formation assay is shown. Scram, scrambled miRNA; 200b, miR-200b mimic; 200b(A), 200b antagomir; V, PcDNA3.1 vecto. *Significantly different from LG or LG scram. +Significantly different from HG or HG scram. **Significantly different from other group(s). The miRNA levels are expressed as a ratio of RNU6B (U6) and normalized to LG; mRNA is expressed as a ratio to 18S RNA and normalized to LG. The error bars show the SEM. (A high-quality color representation of this figure is available in the online issue.)
In parallel to decreased miR-200b upon exposure to HG, mRNA levels of VEGF (measured by qRT-PCR) were increased. Such increases were prevented by miR-200b mimic transfection. Transfection efficiencies, assessed by measuring miR-200b expression showed >10-fold increase in intracellular miR-200b expression compared with scrambled miRNA transfection (Fig. 2C). However, transfection of miR-200b antagomir in the cells in LG demonstrated glucomimetic effects by upregulating VEGF transcripts (Fig. 2B)
To further establish a direct relevance of our findings in the context of diabetic retinopathy, we examined whether similar changes occur in the retinal capillary ECs. Our results show that HG, compared with LG, causes a significant downregulation of miR-200b (Fig. 2D) in BRECs. In parallel, VEGF mRNA was upregulated after exposure to HG. Transfection of miR-200b mimics prevented glucose-induced VEGF upregulation (Fig. 2E). Transfection efficiencies for miR-200b was confirmed by analyzing the abundance of miR-200b in these cells, which showed >20-fold increase in intracellular miR-200b expression compared with vector only transfection (Fig. 2F).
miR-200b regulates glucose-induced functional alterations in the endothelial cells.
We next examined endothelial permeability and tube formation, two characteristic functional effects of VEGF in this system. HUVECs showed increased permeability and tube formation after treatment with HG and VEGF peptide (Fig. 2G–J). To examine functional significance of miR-200b, we transfected miR-200b mimics (and scrambled controls) in HUVECs exposed to HG. Transfection efficiency was confirmed by analyzing the abundance of miR-200b in these cells (Fig. 2C). Glucose-induced increased endothelial permeability is a characteristic alteration in early diabetic retinal microangiopathy, which is mediated by VEGF. Upon miR-200b mimic transfection, we observed a normalization of glucose-induced upregulation of VEGF. Glucose-induced increased endothelial permeability was further prevented by such transfection (Fig. 2G and H). However, no effects were seen after scrambled mimic transfection. In parallel, incubation of the cells with VEGF peptide showed increased permeability (Fig. 2G and H). Similarly, we did a tube formation assay as a surrogate for angiogenesis. HG caused increased tube formation in the ECs. Such changes were also prevented by miR-200b mimic transfection but not by scrambled mimic transfection (Fig. 2I and J). These results established a direct regulatory relationship between miR-200b on HG-induced VEGF expression and its functional consequences.
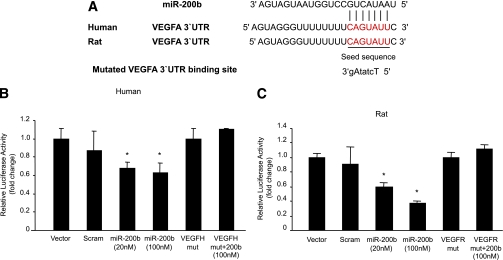
To further validate miR-200b targeting of VEGF, we examined the binding of miR-200b with 3′UTR of the VEGF gene. Luciferase reporter plasmids were generated with a constitutively active promoter containing cloned miR-200b binding sites from human or rat VEGF 3′-UTR or mutated 3′-UTR. They were cotransfected in HEK-293A cells with miR-200b mimic. Such transfection lead to ectopic overexpression of miR-200b and a significant reduction of luciferase reporter activity compared with control vector transfection, indicating its direct binding (Fig. 3B and C). Scrambled miRNA elicited no effects. Mutated target sequence VEGF 3′-UTR was also not responsive to miR-200b (Fig. 3B and C).
FIG. 3.
A: Alignment of VEGF 3′-UTR (and mutated VEGF 3′-UTR) sequence with mature miR-200b based on bioinformatics predictions (www.TargetScan.org, www.microrna.org, www.ebi.ac.uk1). The 5′ end of the mature miR-200b is the seed sequence and has perfect complementarity with seven nucleotides of the 3′-UTR of VEGF. In the mutated sequence (small caps identifying mutated nucleotides) such complementarity was lost. B and C: Binding of miR-200b with VEGF promoter luciferase reporter assay shows dose-dependent binding of VEGF 3′-UTR with miR-200b, whereas mutated (mut) VEGF 3′-UTR abrogated the inhibitory effects of miR-200b. Relative promoter activities were expressed as luminescence units normalized for β-galactosidase expression. *Significantly different from vector, scrambled (scram) or mut. VEGFH, human VEGF; VEGFR, rat VEGF. (A high-quality color representation of this figure is available in the online issue.)
miR-200b is present in the retina and regulates diabetes-induced retinal VEGF upregulation.
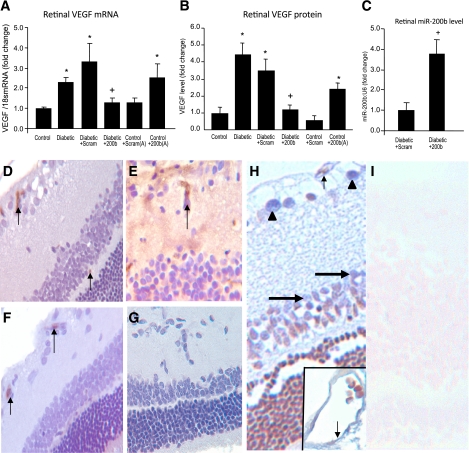
Having established VEGF targeting by miR-200b in vitro, we tested whether miR-200b targets VEGF in the diabetic animal model. The miR-200b mimic was injected in the vitreous cavity of one eye of the diabetic rats at 1.4 μg/week for 4 weeks, and the other eye received the same dose of scrambled control. In a separate set of experiments, we injected intravitreal miR-200b antagomir to nondiabetic animals. This injection led to increased VEGF expression. The level of VEGF mRNA and protein showed a significant decrease in miR-200b mimic-injected diabetic retinas compared with the retinas injected with the scrambled control (Fig. 4A and B). However, the nondiabetic rat retinas injected with antigomir showed increased VEGF mRNA and protein levels (Fig. 4A and B). Injection efficiency for the miR-200b mimic was confirmed by demonstrating the abundance of miR-200b in the retina after mimic injection compared with the scramble control (Fig. 4C).
FIG. 4.
miR-200b–mediated alteration of retinal VEGF and its functional consequences. Levels of VEGF mRNA (A) and protein (B) in the control and diabetic rat retina, with or without intravitreal injection of miR-200b mimic (200b) and scrambled mimic (Scram) show that upregulated VEGF expression in the diabetic rat retina can be prevented by miR-200b mimics (but not by scrambled mimics). Intravitreal injection of miR-200b antagomir (200b[A]) produced VEGF upregulation. C: Efficiency of intravitreal delivery as demonstrated by increased retinal miR-200b expression after intravitreal injection of miR-200b mimic compared with scrambled mimic. *Significantly different from control. +Significantly different from diabetic or diabetic scram. The error bars show the SEM. D: Immunocytochemical stain on the control rat retina using antialbumin antibody shows only intravascular albumin (brown chromogen arrow; score = 0). E: Similar stain in the diabetic rat retina resulted in intravascular reactivity (arrow) and diffuse staining of the retina (score = 3), indicating increased vascular permeability. F: After intravitreal miR-200b injection, albumin staining was only present in the intravascular compartment (arrow; score = 0). G: Negative control of E shows specificity of staining. No such effects were seen after scrambled miR-200b injection (not shown). H: LNA-ISH study of retinal tissues in a control rat retina shows localization of miR-200b (blue chromogen) in the retinal capillaries (arrow), ganglion cells (arrowheads), and in the cells of inner nuclear layer (large arrow), both in the glial and neuronal elements. The inset shows an enlarged view of a capillary with miR-200b localization (arrow). I: LNA-ISH study of retinal tissues in a diabetic rat retina (in similar orientation) show minimum (if any) expression of miR-200b (ALK Phos was used as chromogen [blue] with no counterstain in LNA-ISH; DAB chromogen [brown] and hematoxylin counterstain in albumin stain). (Original magnification ×400 for D–I.) (A high-quality digital representation of this figure is available in the online issue.)
To study permeability changes, we measured albumin permeation from the retinal vasculature using an albumin immunostaining, as previously described (21,22). Our findings show that diabetes-induced increased vascular permeability was prevented by the miR-200b mimic injection (Fig. 4D–G). No extravascular albumin was seen in the control animals (score = 0). Diabetic animals demonstrated a score of 2 to 3, indicating increased extravasated albumin. However, scores of 0 to 1 were noted in eyes injected with the miR-200b mimic, indicating prevention of diabetes-induced increased vascular permeability by such treatment. In situ hybridization studies using LNA probes showed localization of miR-200b in the vascular endothelium and in neuronal and glial elements in the retina of nondiabetic rats. The expression of miR-200b was reduced in the diabetic retina (Fig. 4H and I). No stains were seen when scrambled or no-probe controls were used.
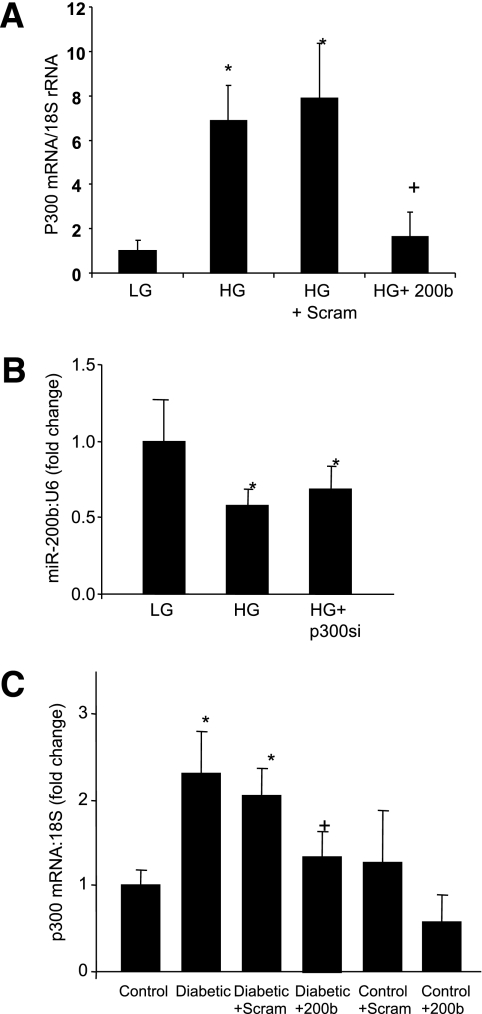
Glucose-induced reduced miR-200b mediates upregulation of transcriptional coactivator p300.
Research has shown that miR-200b regulates epithelial to mesenchymal transition in malignancies by controlling p300, a transcription coactivator (26–28). We have previously reported increased p300 in DR and glucose-exposed endothelial cells (12–14), so we studied whether hyperglycemia changes p300 through miR-200b. Interestingly, miR-200b mimic transfection prevented HG-induced p300 upregulation in the endothelial cells (Fig. 5A). However, glucose-induced downregulation of miR-200b in the endothelial cells was not corrected by p300 silencing (Fig. 5B). To further examine whether some of the mechanisms of miR-200b action is mediated through regulation of p300 in vivo, we examined p300 mRNA expression in the retinal tissues after intravitreal injection of miR-200b mimics. Diabetes-induced upregulation of retinal p300 mRNA was prevented by miR-200b injection, suggesting another mechanisms by which miR-200b may act on vasoactive factors (Fig. 5C).
FIG. 5.
miR-200b regulates diabetes-induced p300 alteration. A: miR-200b mimic (but not the scrambled mimics) transfection prevented glucose-induced p300 mRNA upregulation in the HUVECs (please see Fig. 2C regarding the efficiency of miR-200b mimic transfection). B: No effects of p300 siRNA transfection on miR-200b expression were seen. C: Augmented retinal p300 mRNA expression in the diabetic rats (compared with the controls) was prevented by intravitreal injection of miR-200b mimic (200b) but not by scrambled mimic (Scram). (Please see Fig. 4C regarding efficiency of intravitreal delivery). *Significantly different from control or LG (5 mmol/L d-glucose). +Significantly different from diabetic or HG (25 mmol/L d-glucose). The error bars show the SEM.
miR-200b downregulation is present in human DR.
Finally, we examined human retinas in the enucleated eyes from archival sources using in situ hybridization and immunostains. We found reduced miR-200b (Fig. 6A and B), and increased extravascular albumin (Fig. 6C and D) in the retinas from patients with diabetes. The cellular distribution of miR-200b was similar to that in the rat eyes (Fig. 6A).
FIG. 6.
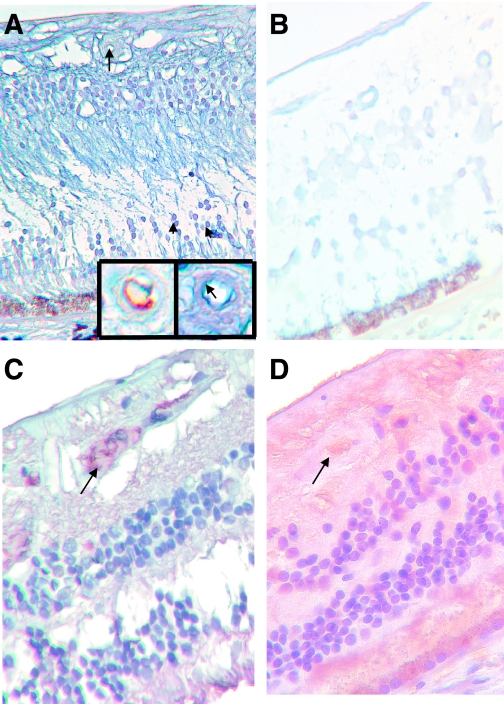
miR-200b alteration present in human diabetic retinopathy. A: LNA-ISH study of retinal tissues from nondiabetic human retina shows localization of miR-200b in the cells of the inner nuclear layer (arrowheads) and retinal capillaries (arrow). The inset (right) shows an enlarged view of the same capillary with endothelial localization of miR-200b (blue chromogen, arrow) and CD34 stain (brown chromogen) from an adjacent section showing endothelium of the capillary. B: Retinal tissues in a diabetic human retina (in similar orientation) show minimal (if any) expression of miR-200b. C: Immunocytochemical stain on the nondiabetic human retina using antialbumin antibody shows intravascular albumin (arrow). D: Diabetic human retina showed intravascular albumin staining (arrow) and diffuse staining of the retina, indicating increased vascular permeability. (ALK Phos was used as chromogen [blue] with no counterstain in LNA-ISH; DAB chromogen [brown] and hematoxylin counterstain in albumin stain.) (Original magnification ×400.) (A high-quality digital representation of this figure is available in the online issue.)
DISCUSSION
This study demonstrates a novel pathway causing VEGF expression and subsequent alterations in the retina in diabetes. We have shown that high levels of glucose in diabetes causes downregulation of miR-200b, which controls VEGF mRNA and protein levels, and increased permeability both in vivo and in vitro. We were also able to prevent diabetes-induced, VEGF-mediated functional changes in the ECs and in the retina by miR-200b mimic treatment.
We investigated the mechanisms at multiple levels of complexities. After the initial identification of miR-200b alteration in the retina in diabetes, we used HUVECs to identify the in vitro biologic significance. Although these cells are not of retinal origin, they are widely used as a model for the study of endothelial abnormalities in several diseases, including DR (12,14,19). However, in parallel we investigated retinal capillary ECs and demonstrated similar changes. After the in vitro studies, we used a well-established animal model to identify the in vivo significance. Finally, we examined human tissue to investigate whether similar changes are present in the human retina. This is the first study to investigate miRNAs in DR and directly demonstrates a functional and potential therapeutic implication of miR-200b in DR. We previously demonstrated the role of miR-133a in diabetic cardiomyopathy (18), other investigators have shown the role of miR-192 in diabetic nephropathy (29,30), and miR-200a was recently found to be of importance in diabetic nephropathy (31).
Alterations of these miRNAs were not seen in this study. Although our array analysis or PCR failed to demonstrate alteration of VEGF targeting miR-429 or other molecules with sequence similarities such as miR-200c, possibilities of VEGF regulation by these or other miRNAs cannot be completely excluded. In a complicated disease process like DR, several regulatory mechanisms may come into play, depending on the duration or severity of the disease, or both. Further investigations are necessary to address such issues.
The mechanisms by which hyperglycemia causes cellular damage are not fully understood. However, evidence indicates that increased oxidative stress causing DNA damage may be involved (2,26,32). Increased oxidative stress causes the activation of the redox-sensitive transcription factors and altered expression of a number of genes, including VEGF. VEGF is a potent growth factor responsible in mediating several abnormalities in the retina in diabetes. Under diabetic conditions, it acts to increase vascular permeability in the early stages of DR and fluid accumulates in the retinal tissue, causing macular edema and exudate. VEGF also causes an increase in angiogenesis, as seen in the late stages of DR (21,33–36). Here we have shown that miR-200b, through VEGF, influences both of these functional effects of VEGF in vitro and in vivo.
MiRNAs are endogenous regulators produced as small, nonprotein-coding RNAs and are highly conserved among species (3,4,37). They mostly negatively regulate gene expression at the post-transcriptional level by interacting with their target mRNA 3′-UTR (3,37). Most target mRNA predictions for miRNAs stem from computational analysis examining sequence complementarity (3,37,38). With their widespread involvement in several biologic processes, miRNAs may have an important modulatory role in DR.
In keeping with our data, a previous study demonstrated the presence of miR-200b in human and rat retina (39). This study and our study demonstrating miR-200b expression in humans and rats both suggest evolutionary conservation and may reflect a possible conserved functional role within the mammalian retina. The potential role of miRNAs in nondiabetic angiogenesis has further been investigated in mice homozygous for a hypomorphic allele of Dicer. These mice lacked angiogenesis and died in utero (40). Another study found that a nonlethal Dicer hypomorphism caused female mice to be sterile due to the failure of angiogenesis in the corpus luteum (41). In a model of ischemic ocular neovascularization, seven miRNAs were increased and three were decreased in the retina (41). However, neovascularization in DR may be different from nondiabetic neovascularization with respect to miRNA. No alteration of miR-200b was identified in such condition (42).
The regulatory role of miR-200b with p300 is further interesting. It has been shown that miR-200 may regulate p300, a histone aceylator and transcription coactivator in malignancies (43). Other research indicated that in pancreatic ductal adenocarcinoma, six p300 targeting miRNAs, including miR-200b, were downregulated in the highly metastatic group (28). We have previously shown the role of p300 in DR and other diabetes complications and that it regulates multiple gene and protein expression in diabetes (12,14). Such effects of p300 are mediated by its capacity to control actions of a large number of transcription factors (12). This study showed novel miR-200b–mediated mechanisms by which p300 is regulated in diabetes. Hence, in addition to its direct inhibitory effects on hyperglycemia-induced VEGF expression, miRNA may mediate such effects indirectly through p300. Such p300-mediated action of miR-200b may potentially affect gene expression of multiple vasoactive factors (12).
Further experiments are needed to establish such a notion, however. DR is a complex problem in which multiple transcripts are altered, kicking off multiple abnormal pathways. Targeting individual proteins for treatments of DR have been tried for a long time and have failed in clinical trials. From a mechanistic standpoint, one miRNA regulates multiple genes, and targeting one or a few miRNAs provides potential unique opportunities to prevent multiple gene expression. We must point out that although our initial studies showed alterations of multiple miRNAs in the retina of diabetic rats, several of these were putative miRNAs and their roles in gene regulation have not been established. The challenge for miRNA research is also to define the function of miRNAs in various tissues and disease states by identifying and validating target mRNAs (44). The analysis of miRNA alteration in pathologic disease states is becoming increasingly more common in research.
In summary, we identified miR-200b as a specific miRNA in DR. We demonstrated, using investigations at several levels of complexities, that miR-200b is important in regulation VEGF-mediated abnormalities in DR. Identification of such novel mechanisms will help us to better understand the pathogenesis of DR and will eventually be helpful in the formulation of specific adjuvant treatment.
ACKNOWLEDGMENTS
This work was supported by grants from the Canadian Diabetes Association.
No potential conflicts of interest relevant to this article were reported.
K.M. and B.F. researched data, contributed to discussion, and reviewed and edited the manuscript. Y.W. researched data and reviewed and edited the manuscript. S.Che. researched data and contributed to discussion. S.Cha. researched data, contributed to discussion, and wrote, reviewed, and edited the manuscript.
Footnotes
This article contains Supplementary Data online at http://diabetes.diabetesjournals.org/lookup/suppl/doi:10.2337/db10-1557/-/DC1.
REFERENCES
- 1.Khan ZA, Farhangkhoee H, Chakrabarti S. Towards newer molecular targets for chronic diabetic complications. Curr Vasc Pharmacol 2006;4:45–57 [DOI] [PubMed] [Google Scholar]
- 2.Brownlee M. Biochemistry and molecular cell biology of diabetic complications. Nature 2001;414:813–820 [DOI] [PubMed] [Google Scholar]
- 3.Chuang JC, Jones PA. Epigenetics and microRNAs. Pediatr Res 2007;61:24R–29R [DOI] [PubMed] [Google Scholar]
- 4.Latronico MV, Catalucci D, Condorelli G. MicroRNA and cardiac pathologies. Physiol Genomics 2008;34:239–242 [DOI] [PubMed] [Google Scholar]
- 5.Liu J, Valencia-Sanchez MA, Hannon GJ, Parker R. MicroRNA-dependent localization of targeted mRNAs to mammalian P-bodies. Nat Cell Biol 2005;7:719–723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen JF, Mandel EM, Thomson JM, et al. The role of microRNA-1 and microRNA-133 in skeletal muscle proliferation and differentiation. Nat Genet 2006;38:228–233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Khan ZA, Chakrabarti S. Growth factors in proliferative diabetic retinopathy. Exp Diabesity Res 2003;4:287–301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Aiello LP. Vascular endothelial growth factor and the eye: biochemical mechanisms of action and implications for novel therapies. Ophthalmic Res 1997;29:354–362 [DOI] [PubMed] [Google Scholar]
- 9.Magnussen AL, Rennel ES, Hua J, et al. VEGF-A165b is cytoprotective and anti-angiogenic in the retina. Invest Ophthalmol Vis Sci 2010;51:4273–4281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shibuya M. Differential roles of vascular endothelial growth factor receptor-1 and receptor-2 in angiogenesis. J Biochem Mol Biol 2006;39:469–478 [DOI] [PubMed] [Google Scholar]
- 11.Shalaby F, Rossant J, Yamaguchi TP, et al. Failure of blood-island formation and vasculogenesis in Flk-1-deficient mice. Nature 1995;376:62–66 [DOI] [PubMed] [Google Scholar]
- 12.Chen S, Feng B, George B, Chakrabarti R, Chen M, Chakrabarti S. Transcriptional coactivator p300 regulates glucose-induced gene expression in endothelial cells. Am J Physiol Endocrinol Metab 2010;298:E127–E137 [DOI] [PubMed] [Google Scholar]
- 13.Chiu J, Khan ZA, Farhangkhoee H, Chakrabarti S. Curcumin prevents diabetes-associated abnormalities in the kidneys by inhibiting p300 and nuclear factor-kappaB. Nutrition 2009;25:964–972 [DOI] [PubMed] [Google Scholar]
- 14.Kaur H, Chen S, Xin X, Chiu J, Khan ZA, Chakrabarti S. Diabetes-induced extracellular matrix protein expression is mediated by transcription coactivator p300. Diabetes 2006;55:3104–3111 [DOI] [PubMed] [Google Scholar]
- 15.Miller EC, Capps BE, Sanghani RR, Clemmons DR, Maile LA. Regulation of igf-I signaling in retinal endothelial cells by hyperglycemia. Invest Ophthalmol Vis Sci 2007;48:3878–3887 [DOI] [PubMed] [Google Scholar]
- 16.Maile LA, Allen LB, Veluvolu U, et al. Identification of compounds that inhibit IGF-I signaling in hyperglycemia. Exp Diabetes Res 2009;2009:267107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.van Rooij E, Sutherland LB, Liu N, et al. A signature pattern of stress-responsive microRNAs that can evoke cardiac hypertrophy and heart failure. Proc Natl Acad Sci USA 2006;103:18255–18260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Feng B, Chen S, George B, Feng Q, Chakrabarti S. miR133a regulates cardiomyocyte hypertrophy in diabetes. Diabetes Metab Res Rev 2010;26:40–49 [DOI] [PubMed] [Google Scholar]
- 19.Chen S, Apostolova MD, Cherian MG, Chakrabarti S. Interaction of endothelin-1 with vasoactive factors in mediating glucose-induced increased permeability in endothelial cells. Lab Invest 2000;80:1311–1321 [DOI] [PubMed] [Google Scholar]
- 20.Khan ZA, Chan BM, Uniyal S, et al. EDB fibronectin and angiogenesis — a novel mechanistic pathway. Angiogenesis 2005;8:183–196 [DOI] [PubMed] [Google Scholar]
- 21.Cukiernik M, Hileeto D, Evans T, Mukherjee S, Downey D, Chakrabarti S. Vascular endothelial growth factor in diabetes induced early retinal abnormalities. Diabetes Res Clin Pract 2004;65:197–208 [DOI] [PubMed] [Google Scholar]
- 22.Zhang X, Bao S, Lai D, Rapkins RW, Gillies MC. Intravitreal triamcinolone acetonide inhibits breakdown of the blood-retinal barrier through differential regulation of VEGF-A and its receptors in early diabetic rat retinas. Diabetes 2008;57:1026–1033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang M, Tan LP, Dijkstra MK, et al. miRNA analysis in B-cell chronic lymphocytic leukaemia: proliferation centres characterized by low miR-150 and high BIC/miR-155 expression. J Pathol 2008;215:13–20 [DOI] [PubMed] [Google Scholar]
- 24.Chen S, Mukherjee S, Chakraborty C, Chakrabarti S. High glucose-induced, endothelin-dependent fibronectin synthesis is mediated via NF-kappa B and AP-1. Am J Physiol Cell Physiol 2003;284:C263–C272 [DOI] [PubMed] [Google Scholar]
- 25.Li Y, Wu H, Khardori R, Song YH, Lu YW, Geng YJ. Insulin-like growth factor-1 receptor activation prevents high glucose-induced mitochondrial dysfunction, cytochrome-c release and apoptosis. Biochem Biophys Res Commun 2009;384:259–264 [DOI] [PubMed] [Google Scholar]
- 26.Adam L, Zhong M, Choi W, et al. miR-200 expression regulates epithelial-to-mesenchymal transition in bladder cancer cells and reverses resistance to epidermal growth factor receptor therapy. Clin Cancer Res 2009;15:5060–5072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li Y, VandenBoom TG, 2nd, Kong D, et al. Up-regulation of miR-200 and let-7 by natural agents leads to the reversal of epithelial-to-mesenchymal transition in gemcitabine-resistant pancreatic cancer cells. Cancer Res 2009;69:6704–6712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mees ST, Mardin WA, Wendel C, et al. EP300—a miRNA-regulated metastasis suppressor gene in ductal adenocarcinomas of the pancreas. Int J Cancer 2010;126:114–124 [DOI] [PubMed] [Google Scholar]
- 29.Kato M, Zhang J, Wang M, et al. MicroRNA-192 in diabetic kidney glomeruli and its function in TGF-beta-induced collagen expression via inhibition of E-box repressors. Proc Natl Acad Sci USA 2007;104:3432–3437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Krupa A, Jenkins R, Luo DD, Lewis A, Phillips A, Fraser D. Loss of MicroRNA-192 promotes fibrogenesis in diabetic nephropathy. J Am Soc Nephrol 2010;21:438–447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang B, Koh P, Winbanks C, et al. miR-200a Prevents renal fibrogenesis through repression of TGF-β2 expression. Diabetes 2011;60:280–287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.King GL, Loeken MR. Hyperglycemia-induced oxidative stress in diabetic complications. Histochem Cell Biol 2004;122:333–338 [DOI] [PubMed] [Google Scholar]
- 33.Bausero P, Cavaillé F, Méduri G, Freitas S, Perrot-Applanat M. Paracrine action of vascular endothelial growth factor in the human endometrium: production and target sites, and hormonal regulation. Angiogenesis 1998;2:167–182 [DOI] [PubMed] [Google Scholar]
- 34.Dejana E. Endothelial adherens junctions: implications in the control of vascular permeability and angiogenesis. J Clin Invest 1997;100(Suppl.):S7–S10 [PubMed] [Google Scholar]
- 35.Jackson MW, Roberts JS, Heckford SE, et al. A potential autocrine role for vascular endothelial growth factor in prostate cancer. Cancer Res 2002;62:854–859 [PubMed] [Google Scholar]
- 36.Kevil CG, Payne DK, Mire E, Alexander JS. Vascular permeability factor/vascular endothelial cell growth factor-mediated permeability occurs through disorganization of endothelial junctional proteins. J Biol Chem 1998;273:15099–15103 [DOI] [PubMed] [Google Scholar]
- 37.Bartel DP. MicroRNAs: target recognition and regulatory functions. Cell 2009;136:215–233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.He L, Hannon GJ. MicroRNAs: small RNAs with a big role in gene regulation. Nat Rev Genet 2004;5:522–531 [DOI] [PubMed] [Google Scholar]
- 39.Arora A, McKay GJ, Simpson DA. Prediction and verification of miRNA expression in human and rat retinas. Invest Ophthalmol Vis Sci 2007;48:3962–3967 [DOI] [PubMed] [Google Scholar]
- 40.Yang WJ, Yang DD, Na S, Sandusky GE, Zhang Q, Zhao G. Dicer is required for embryonic angiogenesis during mouse development. J Biol Chem 2005;280:9330–9335 [DOI] [PubMed] [Google Scholar]
- 41.Otsuka M, Zheng M, Hayashi M, et al. Impaired microRNA processing causes corpus luteum insufficiency and infertility in mice. J Clin Invest 2008;118:1944–1954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shen J, Yang X, Xie B, et al. MicroRNAs regulate ocular neovascularization. Mol Ther 2008;16:1208–1216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hu X, Macdonald DM, Huettner PC, et al. A miR-200 microRNA cluster as prognostic marker in advanced ovarian cancer. Gynecol Oncol 2009;114:457–464 [DOI] [PubMed] [Google Scholar]
- 44.Tang X, Tang G, Ozcan S. Role of microRNAs in diabetes. Biochem Biophys Acta 2008;114:897–701 [DOI] [PMC free article] [PubMed] [Google Scholar]