Abstract
OBJECTIVE
We tested the hypotheses that in nondiabetic individuals, partial inhibition of insulin secretion with the ATP-sensitive K+ channel agonist (opener) diazoxide, compared with placebo, results in higher plasma glucose and higher plasma glucagon concentrations after a mixed meal and after administration of the sulfonylurea glimepiride.
RESEARCH DESIGN AND METHODS
Plasma glucose, insulin, C-peptide, and glucagon concentrations were measured every 30 min from −60 through 180 min with random-sequence, double-blind administration of diazoxide (6.0 mg/kg) or placebo at −30 and 1 min, ingestion of a formula mixed meal (Ensure Plus) at 0 min after diazoxide and after placebo and, on a separate occasion, ingestion of glimepiride (4.0 mg) at 0 min (with glucose infused to prevent hypoglycemia) after diazoxide and after placebo in 11 healthy young adults.
RESULTS
With diazoxide administration, insulin (P = 0.0016) and C-peptide (P = 0.0287) concentrations were decreased and glucose concentrations were increased (e.g., 180-min values of 106 ± 4 mg/dL [5.9 ± 0.2 mmol/L] compared with 87 ± 2 mg/dL [4.8 ± 0.1 mmol/L] with placebo; P < 0.0001), but glucagon concentrations were no different after the mixed meal. Similarly, with diazoxide, C-peptide concentrations were decreased (P = 0.0015) and glucose concentrations were increased (P < 0.0001), but glucagon concentrations declined similarly after glimepiride administration.
CONCLUSIONS
Partial inhibition of insulin secretion results in impairment of glucose tolerance after a mixed meal and after glimepiride administration in the absence of a difference in glucagon secretion. They underscore the primary glucoregulatory role of insulin and support the evidence that β-cell secretion is not the only regulator of α-cell glucagon secretion.
Reduced pancreatic β-cell insulin secretion causes hyperglycemia in diabetes. The extent to which that is the result of reduced β-cell mass, reduced function per β-cell, or both, in type 2 diabetes is unknown (1,2), but it has been suggested that both are required (1). Selective inhibition of β-cell secretion, in the absence of a reduction in β-cell mass, has not been shown to produce diabetes in humans (1). However, intravenous and oral administration of diazoxide, an ATP-sensitive K+ (KATP) channel agonist (opener), partially inhibited insulin secretion and raised postabsorptive plasma glucose concentrations in nondiabetic humans (3). We therefore tested the hypothesis that partial inhibition of insulin secretion with diazoxide, compared with placebo, would result in higher plasma glucose concentrations after a mixed meal and, on a separate occasion, after administration of the sulfonylurea glimepiride (with glucose infused to prevent hypoglycemia) in nondiabetic individuals.
The regulation of pancreatic α-cell glucagon secretion is complex and incompletely understood (4–13). It involves direct signaling of α-cells through an KATP channel-dependent mechanism (4) and indirect signaling of α-cells by β-cell (5–9) and δ-cell (10) secretory products, the autonomic nervous system (11,12) and gut incretins (13). Furthermore, the functional relationship between β-cells and α-cells might be altered by distorted architecture of the islets in diabetes. Among the array of signals, there is increasing evidence that β-cell secretory products (5–9), including insulin (14–16), normally restrain α-cell glucagon secretion and that 1) a decrease in β-cell secretion, in concert with a low plasma glucose concentration, stimulates glucagon secretion during hypoglycemia (16–20), and 2) an increase in β-cell secretion inhibits glucagon secretion during euglycemia and hyperglycemia (21) in humans. With respect to the latter, a mixed meal suppresses α-cell glucagon secretion when it can stimulate β-cell insulin secretion (i.e., in nondiabetic individuals) but stimulates α-cell glucagon secretion when it cannot stimulate β-cell insulin secretion (i.e., in individuals with type 1 diabetes) (21). Similarly, the sulfonylurea glimepiride suppresses α-cell glucagon secretion when it can stimulate β-cell insulin secretion but stimulates α-cell glucagon secretion when it cannot stimulate β-cell insulin secretion (21). Therefore, we also tested the hypothesis that partial inhibition of insulin secretion with the KATP channel agonist (opener) diazoxide, compared with placebo, would result in higher plasma glucagon and plasma glucose concentrations after a mixed meal and, on a separate occasion, after the sulfonylurea glimepiride (with glucose infused to prevent hypoglycemia) in nondiabetic individuals.
RESEARCH DESIGN AND METHODS
Subjects.
The study recruited 11 healthy individuals (six women, five men), who were a mean ± SD age 29.8 ± 6.2 years and had a BMI of 26.3 ± 5.3 kg/m2. This study was approved by the Washington University Human Research Protection Office and conducted at the Washington University Clinical Research Unit (CRU). All participants provided written consent to participate. They were in good health, as determined by their medical history and a physical examination, were taking no drugs (aside from an oral contraceptive or a stable dose of thyroxine), and had fasting plasma glucose and creatinine concentrations, hematocrits, and electrocardiogram (ECG) results that were within normal reference ranges.
Experimental design.
Subjects reported to the CRU in the morning after an overnight fast on four occasions. They remained supine throughout each study. A catheter was inserted into vein in a hand vein, with that hand kept in an ∼55°C plexiglas box, for arterialized venous sampling every 30 min from −60 to 180 min. For the glimepiride studies, a catheter was also inserted into an antecubital vein for glucose infusion. Heart rates and blood pressures were also monitored (GE Dash 3000, Fairfield, CT) every 30 min, and the ECG was monitored throughout.
On two occasions, participants received oral 6.0 mg/kg diazoxide (Proglycem, Gate Pharmaceuticals, Sellersville, PA) or placebo in random sequence, in a double-blind fashion at −30 min after a formula mixed meal (Ensure Plus, 350 kcal, 50 g carbohydrate, 13 g protein, and 11 g fat) at 0 min. On two other occasions, they also received oral diazoxide or placebo at −30 min, followed by glimepiride (Amaryl, sanofi-aventis, U.S., Bridgewater, NJ), 4.0 mg at 0 min. Glucose was infused intravenously, determined by bedside plasma glucose measurements every 5 min, to prevent hypoglycemia after glimepiride ingestion.
Analytic methods.
Plasma glucose concentrations were measured with a glucose oxidase method (YSI Glucose Analyzer, Yellow Springs Instruments, Yellow Springs, OH). Plasma insulin and C-peptide concentrations were measured with two site chemiluminescent assays (Immulite 1000, Siemens Corp, Los Angeles, CA). Plasma glucagon concentrations were measured with the Linco radioimmunoassay (Millipore, Temecula, CA). Plasma epinephrine and norepinephrine concentrations were measured with a single isotope derivative (radioenzymatic) method (22).
Statistical methods.
Except where the SD is specified, data are reported as the mean ± SE. Data related to time and condition (placebo or diazoxide) were analyzed by repeated-measures mixed-model ANOVA. Modeling was performed with SAS 9.2 software (SAS Institute, Cary, NC) with the MIXED procedure. Condition-related P values are reported; values of P < 0.05 were considered to indicate significant differences.
RESULTS
Meal studies.
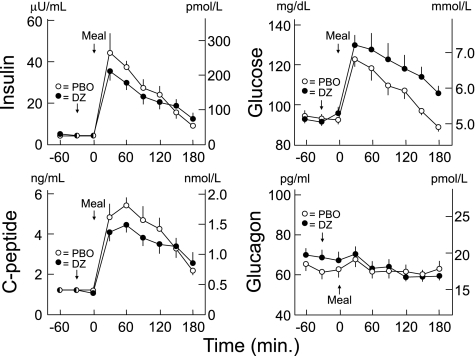
With diazoxide, compared with placebo, plasma insulin (P = 0.0016) and C-peptide (P = 0.0287) concentrations were decreased after the mixed meal, plasma glucose concentrations were increased (P = 0.0001), and plasma glucagon concentrations were no different (P = 0.6602; Fig. 1, Tables 1 and 2). Plasma epinephrine concentrations were unaltered (P = 0.1392), but plasma norepinephrine concentrations increased (P < 0.0001) after diazoxide administration (Table 1). Heart rates were slightly higher (P < 0.0001) and diastolic blood pressures were slightly lower (P = 0.0039) after diazoxide (Table 2).
FIG. 1.
Mean ± SE plasma insulin, C-peptide, glucose, and glucagon concentrations following a mixed meal after oral administration of placebo (PBO; ○) or 6.0 mg/kg diazoxide (DZ, ●) in healthy humans. Insulin (P = 0.0016), C-peptide (P = 0.0287), and glucose (P < 0.0001) levels differed, but glucagon levels did not (P = 0.6602).
TABLE 1.
Plasma epinephrine and norepinephrine concentrations*
| Time (min) | Mixed meal |
Glimepiride |
||
|---|---|---|---|---|
| Placebo | Diazoxide | Placebo | Diazoxide | |
| Epinephrine (pg/mL)† | ||||
| −60 | 29 ± 6 | 30 ± 8 | 36 ± 8 | 30 ± 5 |
| −30 | 29 ± 5 | 27 ± 8 | 35 ± 8 | 24 ± 6 |
| 0 | 25 ± 4 | 23 ± 7 | 31 ± 7 | 29 ± 6 |
| 30 | 18 ± 2 | 20 ± 5 | 29 ± 7 | 23 ± 5 |
| 60 | 25 ± 8 | 23 ± 5 | 36 ± 8 | 24 ± 6 |
| 90 | 26 ± 4 | 27 ± 7 | 36 ± 8 | 24 ± 7 |
| 120 | 22 ± 4 | 30 ± 8 | 34 ± 8 | 29 ± 7 |
| 150 | 26 ± 5 | 31 ± 7 | 36 ± 8 | 32 ± 8 |
| 180 | 29 ± 5 | 34 ± 6 | 37 ± 8 | 37 ± 8 |
| (P = 0.1392) | (P = 0.0733) | |||
| Norepinephrine (pg/mL)‡ | ||||
| −60 | 175 ± 12 | 184 ± 13 | 188 ± 13 | 174 ± 9 |
| −30 | 167 ± 10 | 179 ± 15 | 191 ± 12 | 173 ± 8 |
| 0 | 176 ± 13 | 162 ± 15 | 185 ± 16 | 188 ± 12 |
| 30 | 189 ± 13 | 220 ± 21 | 188 ± 8 | 199 ± 14 |
| 60 | 189 ± 13 | 233 ± 23 | 186 ± 10 | 224 ± 11 |
| 90 | 180 ± 12 | 247 ± 28 | 189 ± 14 | 238 ± 19 |
| 120 | 163 ± 14 | 223 ± 21 | 187 ± 12 | 236 ± 18 |
| 150 | 162 ± 10 | 223 ± 21 | 187 ± 12 | 237 ± 14 |
| 180 | 168 ± 11 | 225 ± 16 | 183 ± 12 | 265 ± 22 |
| (P < 0.0001) | (P < 0.0001) | |||
*Mean ± SE data are shown with oral administration of placebo or diazoxide (6.0 mg/kg) at −30 min and a mixed meal (Ensure Plus) at 0 min on two occasions (left) or oral glimepiride (4.0 mg) at 0 min on another two occasions (right) in healthy humans.
†To convert epinephrine to pmol/L, multiply pg/mL by 5.458.
‡To convert norepinephrine to nmol/L, multiply pg/mL by 0.005911.
TABLE 2.
Heart rates and systolic and diastolic blood pressures*
| Time (min) | Mixed meal |
Glimepiride |
||
|---|---|---|---|---|
| Placebo | Diazoxide | Placebo | Diazoxide | |
| Heart rate (bpm) | ||||
| −60 | 69 ± 4 | 71 ± 2 | 70 ± 4 | 71 ± 3 |
| −30 | 70 ± 3 | 70 ± 3 | 72 ± 4 | 70 ± 3 |
| 0 | 73 ± 4 | 77 ± 3 | 69 ± 4 | 70 ± 4 |
| 30 | 79 ± 5 | 86 ± 4 | 68 ± 4 | 73 ± 4 |
| 60 | 78 ± 5 | 81 ± 4 | 67 ± 5 | 78 ± 4 |
| 90 | 77 ± 4 | 84 ± 4 | 68 ± 4 | 74 ± 3 |
| 120 | 77 ± 6 | 85 ± 4 | 69 ± 5 | 77 ± 4 |
| 150 | 76 ± 5 | 80 ± 4 | 70 ± 4 | 77 ± 4 |
| 180 | 74 ± 5 | 81 ± 4 | 75 ± 4 | 84 ± 4 |
| (P < 0.0001) | (P < 0.0001) | |||
| Systolic blood pressure (mmHg) | ||||
| −60 | 118 ± 2 | 121 ± 3 | 118 ± 2 | 118 ± 3 |
| −30 | 116 ± 3 | 121 ± 3 | 122 ± 2 | 126 ± 3 |
| 0 | 120 ± 3 | 121 ± 3 | 122 ± 2 | 118 ± 2 |
| 30 | 118 ± 3 | 118 ± 3 | 119 ± 2 | 118 ± 2 |
| 60 | 119 ± 3 | 117 ± 3 | 120 ± 2 | 118 ± 2 |
| 90 | 116 ± 3 | 117 ± 3 | 119 ± 2 | 115 ± 3 |
| 120 | 116 ± 3 | 114 ± 2 | 122 ± 3 | 114 ± 2 |
| 150 | 115 ± 3 | 112 ± 3 | 119 ± 3 | 115 ± 3 |
| 180 | 118 ± 3 | 113 ± 3 | 122 ± 3 | 114 ± 3 |
| (P = 0.2173) | (P < 0.0001) | |||
| Diastolic blood pressure (mmHg) | ||||
| −60 | 65 ± 2 | 68 ± 2 | 69 ± 2 | 68 ± 2 |
| −30 | 66 ± 2 | 69 ± 2 | 68 ± 1 | 73 ± 3 |
| 0 | 65 ± 3 | 67 ± 2 | 66 ± 2 | 68 ± 2 |
| 30 | 61 ± 3 | 58 ± 2 | 65 ± 2 | 66 ± 2 |
| 60 | 60 ± 2 | 59 ± 3 | 68 ± 2 | 61 ± 3 |
| 90 | 58 ± 2 | 60 ± 2 | 65 ± 2 | 60 ± 3 |
| 120 | 62 ± 2 | 57 ± 2 | 66 ± 2 | 60 ± 2 |
| 150 | 63 ± 2 | 58 ± 3 | 68 ± 2 | 59 ± 3 |
| 180 | 62 ± 2 | 57 ± 2 | 66 ± 2 | 60 ± 3 |
| (P = 0.0339) | (P < 0.0001) | |||
*Mean ± SE data are shown after oral administration of placebo or diazoxide (6.0 mg/kg) at −30 min and a mixed meal (Ensure Plus) at 0 min on two occasions (left) or oral glimepiride (4.0 mg) on another two occasions (right) in healthy humans.
Glimepiride studies.
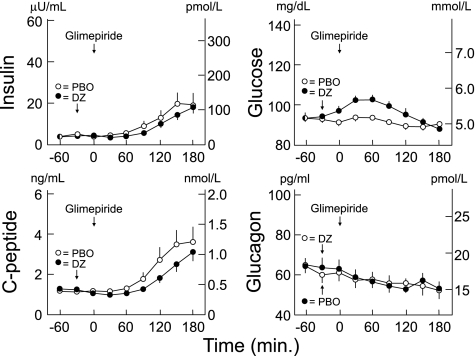
With diazoxide, compared with placebo, plasma insulin concentrations after glimepiride tended to be decreased (P = 0.0649), plasma C-peptide concentrations were decreased (P = 0.0015), and plasma glucose concentrations were increased (P < 0.0001; Fig. 2, Tables 1–3), despite glucose infusion rates that were ∼50% of those after glimepiride with placebo (P < 0.0001; Table 3). Plasma glucagon concentrations were no different (P = 0.7482). Again, plasma epinephrine concentrations were unaltered, but plasma norepinephrine concentrations increased (P < 0.0001) after diazoxide administration (Table 1). Heart rates were slightly higher (P < 0.0001), and systolic and diastolic blood pressures were slightly lower (both P < 0.0001) after diazoxide administration (Table 2).
FIG. 2.
Mean ± SE plasma insulin, C-peptide, glucose, and glucagon concentrations after glimepiride (4.0 ng) ingestion after oral administration of placebo (PBO; ○) or 6.0 mg/kg diazoxide (DZ, ●) in healthy humans. Glucose was infused intravenously to prevent hypoglycemia after glimepiride. Insulin levels tended to differ (P = 0.0649), and C-peptide (P = 0.0015) and glucose (P < 0.0001) differed, but glucagon levels did not (P = 0.7482).
TABLE 3.
Glucose infusion rates*
| Time (min) | Glimepiride |
|
|---|---|---|
| Placebo | Diazoxide | |
| −60 | 0.0 | 0.0 |
| −30 | 0.0 | 0.0 |
| 0 | 0.1 ± 0.1 | 0.2 ± 0.1 |
| 15 | 0.2 ± 0.1 | 0.2 ± 0.1 |
| 30 | 0.3 ± 0.2 | 0.2 ± 0.1 |
| 45 | 0.5 ± 0.2 | 0.1 ± 0.1 |
| 60 | 0.7 ± 0.2 | 0.3 ± 0.2 |
| 75 | 0.8 ± 0.2 | 0.3 ± 0.2 |
| 90 | 0.9 ± 0.2 | 0.2 ± 0.1 |
| 105 | 1.3 ± 0.2 | 0.5 ± 0.2 |
| 120 | 1.8 ± 0.4 | 0.9 ± 0.3 |
| 135 | 2.0 ± 0.4 | 1.3 ± 0.4 |
| 150 | 2.4 ± 0.5 | 1.4 ± 0.4 |
| 165 | 2.5 ± 0.5 | 2.2 ± 0.2 |
| 180 | 2.5 ± 0.5 | 2.0 ± 0.2 |
| (P < 0.0001) | ||
*Mean ± SE data (mg · kg−1 · min−1) are shown for oral administration of placebo or diazoxide (6.0 mg/kg) at −30 min and oral glimepiride (4.0 mg) at 0 min. To convert mg · kg−1 · min.−1 to μmol · kg−1 · min−1, multiply by 5.551.
DISCUSSION
Reduced pancreatic β-cell insulin secretion causes hyperglycemia in diabetes (1,2). Relative hyperglucagonemia is thought to also be involved in the pathogenesis of hyperglycemia (23–26). Here we demonstrate that partial inhibition of insulin secretion, with the KATP channel agonist (opener) diazoxide, results in glucose intolerance after a mixed meal with no difference in plasma glucagon concentrations in nondiabetic individuals. It also results in higher plasma glucose concentrations, despite lower glucose infusion rates, after ingestion of the sulfonylurea glimepiride, with no difference in plasma glucagon concentrations in such individuals. These data uniquely (1,2) document that impairment of insulin secretion, in the absence of a reduction of β-cell mass and of prior elevated glucose or fatty acid levels (i.e., glucolipotoxicity), results in glucose intolerance in humans. Our results also underscore the primary glucoregulatory role of insulin.
Oral glucose tolerance tests in individuals with normal glucose tolerance, impaired glucose tolerance, and type 2 diabetes have demonstrated an inverse relationship between increasing plasma glucose concentrations and suppression of plasma glucagon concentrations (27). On the basis of our studies of the regulation of glucagon secretion in nondiabetic and type 1 diabetic individuals, we suggested that progressively diminishing early β-cell insulin secretion might account not only for progressively increasing plasma glucose concentrations but also for progressively decreasing suppression of plasma glucagon concentrations across this spectrum from normal glucose tolerance to type 2 diabetes (21). Thus, given evidence that β-cell secretory products, including insulin, normally restrain α-cell glucagon secretion (5–9,14–21), we anticipated that partial inhibition of insulin secretion would also result in higher plasma glucagon, as well as glucose, concentrations after the mixed meal and after glimepiride. That hypothesis was not confirmed.
Despite partially inhibited insulin secretion and higher plasma glucose levels following the mixed meal after diazoxide, compared with after placebo, plasma glucagon concentrations were the same. Similarly, despite decreased insulin secretion and higher plasma glucose levels following the sulfonylurea glimepiride after diazoxide, compared with placebo, plasma glucagon concentrations declined similarly. (Glucose was infused intravenously to prevent hypoglycemia after glimepiride ingestion, but the glucose infusion rates with diazoxide were ∼50% of those with placebo.) In an earlier study (3), partial inhibition of insulin secretion with diazoxide also raised postabsorptive plasma glucose concentrations but not postabsorptive plasma glucagon concentrations.
These findings do not exclude an inhibitory effect of β-cell secretion on α-cell glucagon secretion, but suggest that that the effect is not as potent as other factors. A greater degree of inhibition of insulin secretion might have resulted in both hyperglycemia and hyperglucagonemia after the meal and after glimepiride (as observed in individuals with absolute endogenous insulin deficiency [21]), but that was not demonstrated in this study of mild insulin deficiency.
Among the weaknesses of this study is that only partial inhibition of insulin secretion was accomplished with the dose of diazoxide that was used. However, diazoxide is a nonselective KATP channel agonist (3), and its dosing is therefore limited by hypotension. The dose of 6.0 mg/kg used in this study approximates the maximum tolerable single dose (3); indeed, heart rates were slightly higher and blood pressures were slightly lower after diazoxide administration. The latter undoubtedly resulted in sympathetic neural activation, as evidenced by a small but statistically significant increase in the plasma norepinephrine concentration. That did not alter basal insulin or C-peptide levels but may have contributed to the partial suppression of insulin secretion after the meal and after glimepiride. Notably, however, there was no increase in the plasma concentration of the adrenomedullary hormone epinephrine.
In addition, the glucagon radioimmunoassay used reacts with species in addition to biologically active 3500-d glucagon, although increments in measured values are thought to represent biologically active glucagon (26). Therefore, it is conceivable that small differences in hepatic portal venous glucagon concentrations were missed in our peripheral arterialized venous measurements. However, our data showed no trends toward higher glucagon levels. Furthermore, it is conceivable that higher plasma glucose concentrations might have masked the effects of lower insulin levels to increase glucagon secretion, but that would not support a role of increased glucagon secretion in the pathogenesis of hyperglycemia.
A direct effect, if any, of diazoxide on glucagon secretion in vivo in humans is not known. It is difficult to predict because diazoxide, a KATP channel opener, and the sulfonylurea tolbutamide, a KATP channel closer, have both been reported to stimulate glucagon release from isolated islets at low concentrations and to inhibit glucagon release from isolated islets at higher concentrations (4). Diazoxide administration did not alter plasma glucagon concentrations before ingestion of a mixed meal or of glimepiride in the current study, or during a more prolonged postabsorptive period in our earlier study (3).
Glimepiride raised plasma glucagon concentrations in the virtual absence of insulin secretion in patients with type 1 diabetes (21), which implies that KATP channel closure stimulates α-cell glucagon secretion in humans. If so, KATP channel opening, with diazoxide, might inhibit α-cell glucagon secretion in vivo. Diazoxide has been reported to decrease glucagon release from isolated rat α-cells (5). Thus, it is theoretically conceivable that an inhibitory effect of diazoxide precluded increased glucagon secretion triggered by partial inhibition of insulin secretion after a mixed meal and after glimepiride.
In conclusion, these data indicate that partial inhibition of insulin secretion results in impairment of glucose tolerance after a mixed meal and in higher glucose levels after glimepiride in the absence of a difference in measured glucagon secretion in humans. They underscore the primary glucoregulatory role of insulin. For the various reasons discussed, this is not a definitive negative study of the effect of reduced insulin secretion to increase glucagon secretion. Nonetheless, the data support the evidence that β-cell secretion is not the only regulator of α-cell glucagon secretion.
ACKNOWLEDGMENTS
This study was partly supported by National Institutes of Health (NIH) grants R37-DK-27085, UL1-RR-24992, and KL2-RR-24994 (to A.M.A.) and by a fellowship award from the American Diabetes Association (ADA). The content of this article is solely the responsibility of the authors and does not necessarily represent the official view of NIH or ADA.
P.E.C. has served as a consultant to Bristol-Meyers Squibb/AstraZeneca, Merck & Co., MannKind Corp., and Novo Nordisk in the past year. No other potential conflicts of interest relevant to this article were reported.
R.P.R., A.M.A., and P.E.C. planned the study. R.P.R. performed the study. R.P.R., A.M.A., and P.E.C. analyzed data and wrote the manuscript.
The authors acknowledge the assistance of the staff of the Washington University Clinical Research Unit; the technical assistance of Krishan Jethi, MS, in Dr. Cryer's Lab, and the members of the Core Laboratory for Clinical Studies, including Licia Rowe, Laura Karsteter, Nilima Parikh, Tanya Eden, Shirley Frei, Janice Bathon, Paula Blood, and David Gibson; and the assistance of Ling Chen, PhD, MPH, of the Washington University Institute for Clinical and Translational Science, with the statistical analysis. Janet Dedeke, assistant to P.E.C., prepared this manuscript.
REFERENCES
- 1.Kahn SE, Zraika S, Utzschneider KM, Hull RL. The beta cell lesion in type 2 diabetes: there has to be a primary functional abnormality. Diabetologia 2009;52:1003–1012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ferrannini E. The stunned beta cell: a brief history. Cell Metab 2010;11:349–352 [DOI] [PubMed] [Google Scholar]
- 3.Raju B, Cryer PE. Mechanism, temporal patterns, and magnitudes of the metabolic responses to the KATP channel agonist diazoxide. Am J Physiol Endocrinol Metab 2005;288:E80–E85 [DOI] [PubMed] [Google Scholar]
- 4.MacDonald PE, De Marinis YZ, Ramracheya R, et al. A K ATP channel-dependent pathway within alpha cells regulates glucagon release from both rodent and human islets of Langerhans. PLoS Biol 2007;5:e143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gromada J, Franklin I, Wollheim CB. α-cells of the endocrine pancreas: 35 years of research but the enigma remains. Endocr Rev 2007;28:84–116 [DOI] [PubMed] [Google Scholar]
- 6.Maruyama H, Hisatomi A, Orci L, Grodsky GM, Unger RH. Insulin within islets is a physiologic glucagon release inhibitor. J Clin Invest 1984;74:2296–2299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Samols E, Stagner JI, Ewart RBL, Marks V. The order of islet microvascular cellular perfusion is B----A----D in the perfused rat pancreas. J Clin Invest 1988;82:350–353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brunicardi FC, Kleinman R, Moldovan S, et al. Immunoneutralization of somatostatin, insulin, and glucagon causes alterations in islet cell secretion in the isolated perfused human pancreas. Pancreas 2001;23:302–308 [DOI] [PubMed] [Google Scholar]
- 9.Zhou H, Tran PO, Yang S, et al. Regulation of α-cell function by the β-cell during hypoglycemia in Wistar rats: the “switch-off” hypothesis. Diabetes 2004;53:1482–1487 [DOI] [PubMed] [Google Scholar]
- 10.Hauge-Evans AC, King AJ, Carmignac D, et al. Somatostatin secreted by islet δ-cells fulfills multiple roles as a paracrine regulator of islet function. Diabetes 2009;58:403–411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Taborsky GJ, Jr, Ahrén B, Havel PJ. Autonomic mediation of glucagon secretion during hypoglycemia: implications for impaired alpha-cell responses in type 1 diabetes. Diabetes 1998;47:995–1005 [DOI] [PubMed] [Google Scholar]
- 12.Marty N, Dallaporta M, Thorens B. Brain glucose sensing, counterregulation, and energy homeostasis. Physiology (Bethesda) 2007;22:241–251 [DOI] [PubMed] [Google Scholar]
- 13.Holst JJ. The physiology of glucagon-like peptide 1. Physiol Rev 2007;87:1409–1439 [DOI] [PubMed] [Google Scholar]
- 14.Meier JJ, Kjems LL, Veldhuis JD, Lefèbvre P, Butler PC. Postprandial suppression of glucagon secretion depends on intact pulsatile insulin secretion: further evidence for the intraislet insulin hypothesis. Diabetes 2006;55:1051–1056 [DOI] [PubMed] [Google Scholar]
- 15.Bansal P, Wang Q. Insulin as a physiological modulator of glucagon secretion. Am J Physiol Endocrinol Metab 2008;295:E751–E761 [DOI] [PubMed] [Google Scholar]
- 16.Cooperberg BA, Cryer PE. Insulin reciprocally regulates glucagon secretion in humans. Diabetes 2010;59:2936–2940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Banarer S, McGregor VP, Cryer PE. Intraislet hyperinsulinemia prevents the glucagon response to hypoglycemia despite an intact autonomic response. Diabetes 2002;51:958–965 [DOI] [PubMed] [Google Scholar]
- 18.Raju B, Cryer PE. Loss of the decrement in intraislet insulin plausibly explains loss of the glucagon response to hypoglycemia in insulin-deficient diabetes: documentation of the intraislet insulin hypothesis in humans. Diabetes 2005;54:757–764 [DOI] [PubMed] [Google Scholar]
- 19.Gosmanov NR, Szoke E, Israelian Z, et al. Role of the decrement in intraislet insulin for the glucagon response to hypoglycemia in humans. Diabetes Care 2005;28:1124–1131 [DOI] [PubMed] [Google Scholar]
- 20.Israelian Z, Gosmanov NR, Szoke E, et al. Increasing the decrement in insulin secretion improves glucagon responses to hypoglycemia in advanced type 2 diabetes. Diabetes Care 2005;28:2691–2696 [DOI] [PubMed] [Google Scholar]
- 21.Cooperberg BA, Cryer PE. β-cell-mediated signaling predominates over direct α-cell signaling in the regulation of glucagon secretion in humans. Diabetes Care 2009;32:2275–2280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shah SD, Clutter WE, Cryer PE. External and internal standards in the single-isotope derivative (radioenzymatic) measurement of plasma norepinephrine and epinephrine. J Lab Clin Med 1985;106:624–629 [PubMed] [Google Scholar]
- 23.Müller WA, Faloona GR, Aguilar-Parada E, Unger RH. Abnormal alpha-cell function in diabetes. Response to carbohydrate and protein ingestion. N Engl J Med 1970;283:109–115 [DOI] [PubMed] [Google Scholar]
- 24.Reaven GM, Chen YD, Golay A, Swislocki AL, Jaspan JB. Documentation of hyperglucagonemia throughout the day in nonobese and obese patients with noninsulin-dependent diabetes mellitus. J Clin Endocrinol Metab 1987;64:106–110 [DOI] [PubMed] [Google Scholar]
- 25.Shah P, Basu A, Basu R, Rizza R. Impact of lack of suppression of glucagon on glucose tolerance in humans. Am J Physiol 1999;277:E283–E290 [DOI] [PubMed] [Google Scholar]
- 26.Dunning BE, Gerich JE. The role of α-cell dysregulation in fasting and postprandial hyperglycemia in type 2 diabetes and therapeutic implications. Endocr Rev 2007;28:253–283 [DOI] [PubMed] [Google Scholar]
- 27.Abdul-Ghani M, DeFronzo RA. Fasting hyperglycemia impairs glucose- but not insulin-mediated suppression of glucagon secretion. J Clin Endocrinol Metab 2007;92:1778–1784 [DOI] [PubMed] [Google Scholar]