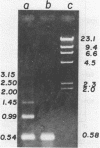
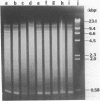
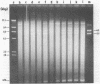
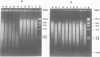
Abstract
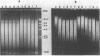
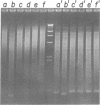
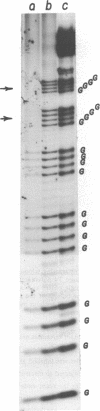
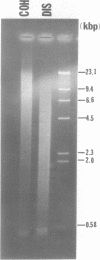
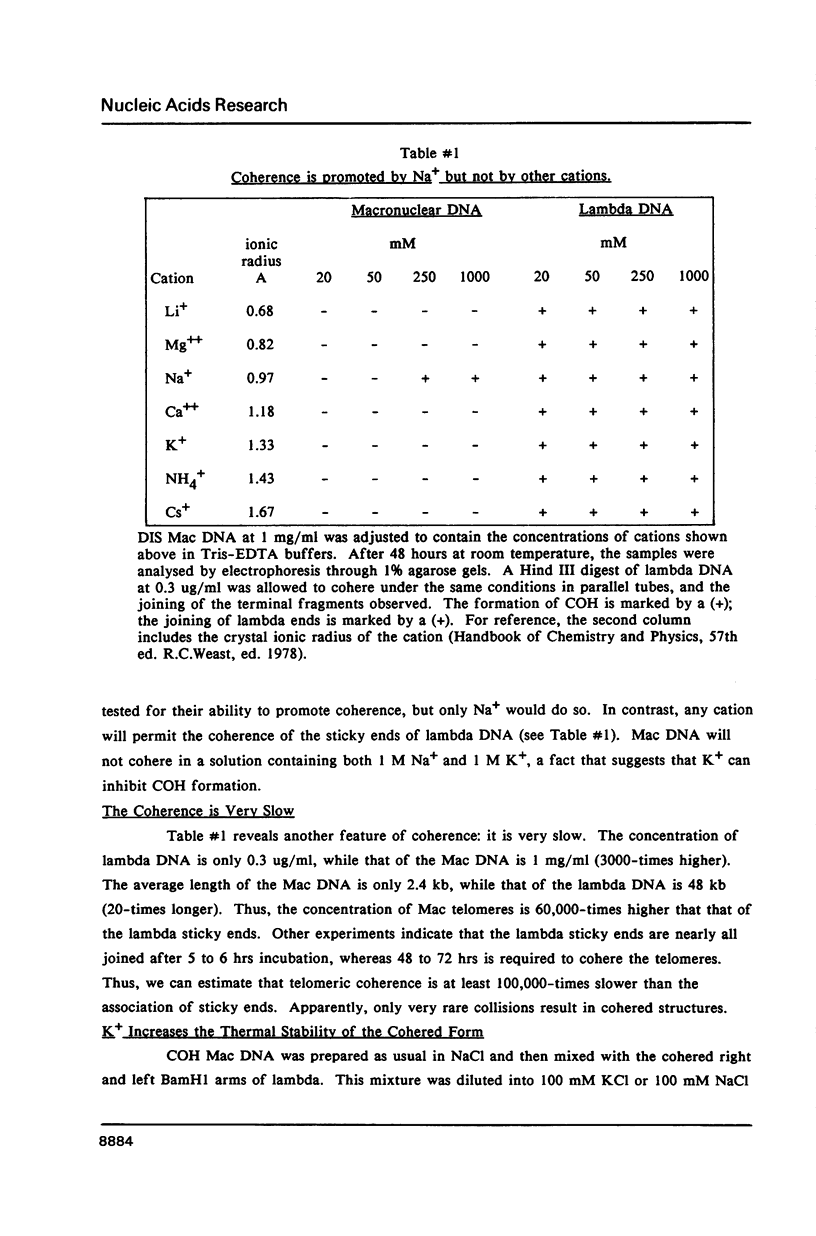
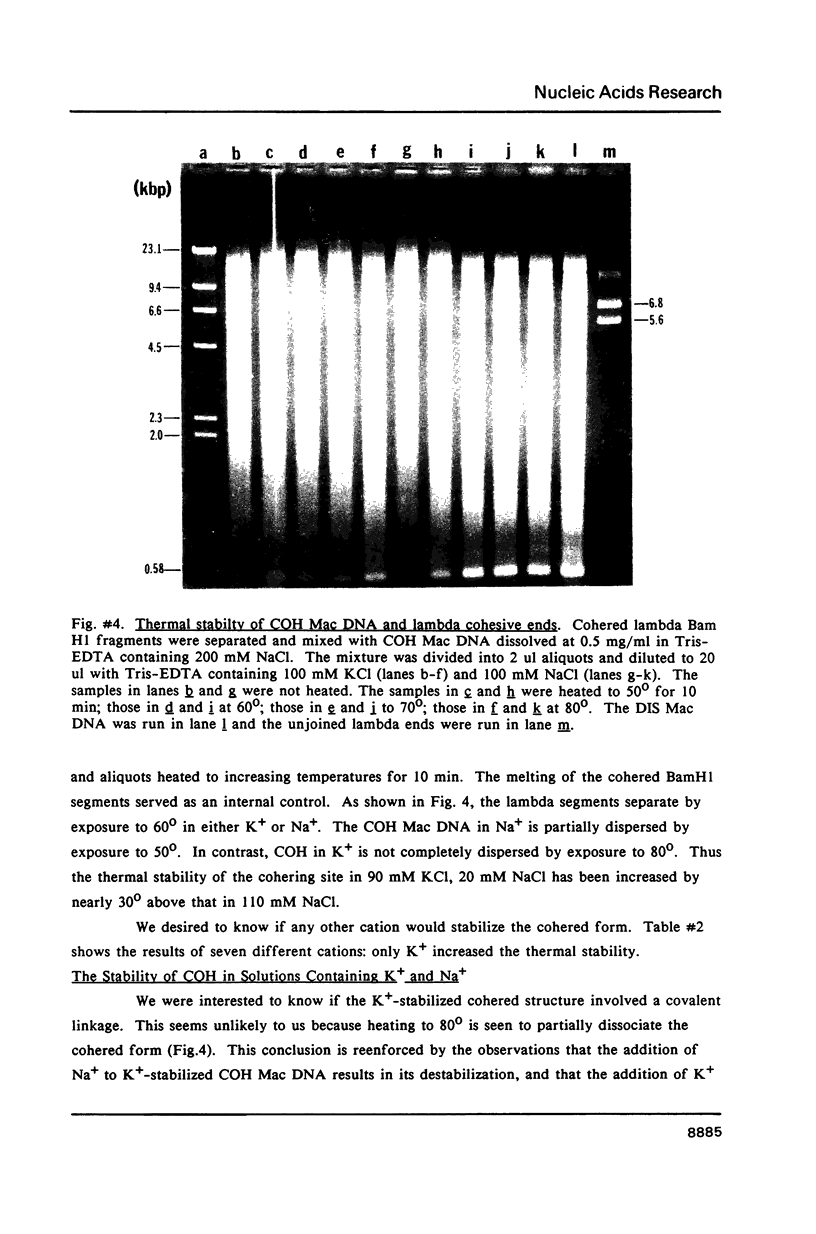
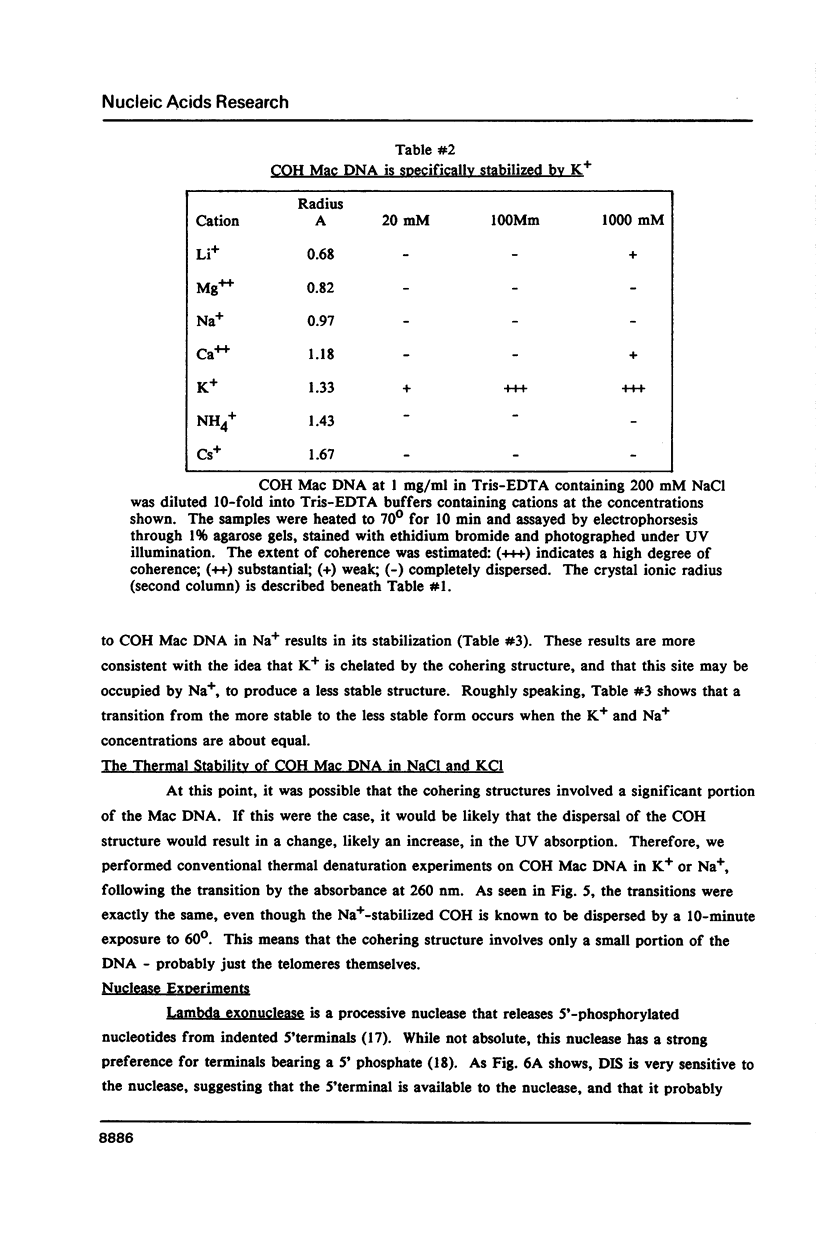
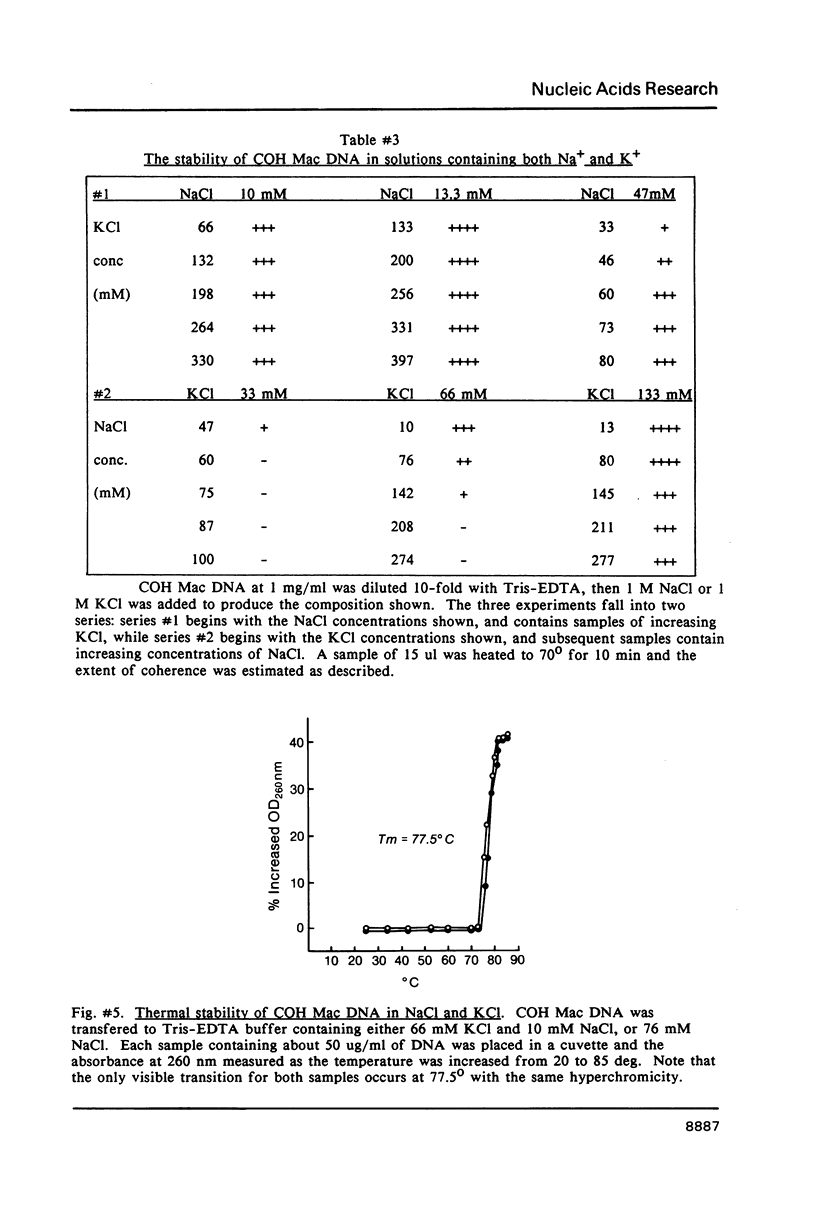
We have studied the process by which purified Oxytricha macronuclear DNA associates with itself to form large aggregates. The various macronuclear DNA molecules all have the same terminal or telomeric DNA sequences that are shown below. 5' C4A4C4A4C4--mean length----G4T4G4T4G4T4G4T4G4 G4T4G4T4G4T4G4T4G4-----2.4 kb------C4A4C4A4C4. When incubated at high concentrations, these telomeric sequences cohere with one another to form an unusual structure--one that is quite different from any DNA structure so far described. The evidence for this is the following: 1) These sequences cohere albeit slowly, in the presence of relatively high concentrations of Na+, and no other cation tested. This contrasts with the rapid coherence of complementary single-chain terminals of normal DNA (sticky ends) which occurs in the presence of any cation tested. 2) If the cohered form is transferred into buffers containing a special cation, K+, it becomes much more resistant to dissociation by heating. We estimate that K+ increases the thermal stability by 25 degrees or more. The only precedent known (to us) for a cation-specific stabilization is that seen in the quadruplex structure formed by poly I. The thermal stability of double helical macronuclear DNA depends on the cation concentration, but not the cation type. Limited treatment with specific nucleases show that the 3' and 5'-ended strands are essential for the formation of the cohering structure. Once in the cohered form, the telomeric sequences are protected from the action of nucleases. Coherence is inhibited by specific, but not by non-specific, synthetic oligomers, and by short telomeric fragments with or without their terminal single chains. We conclude that the coherence occurs by the formation of a novel condensed structure that involves the terminal nucleotides in three or four chains.
Full text
PDF



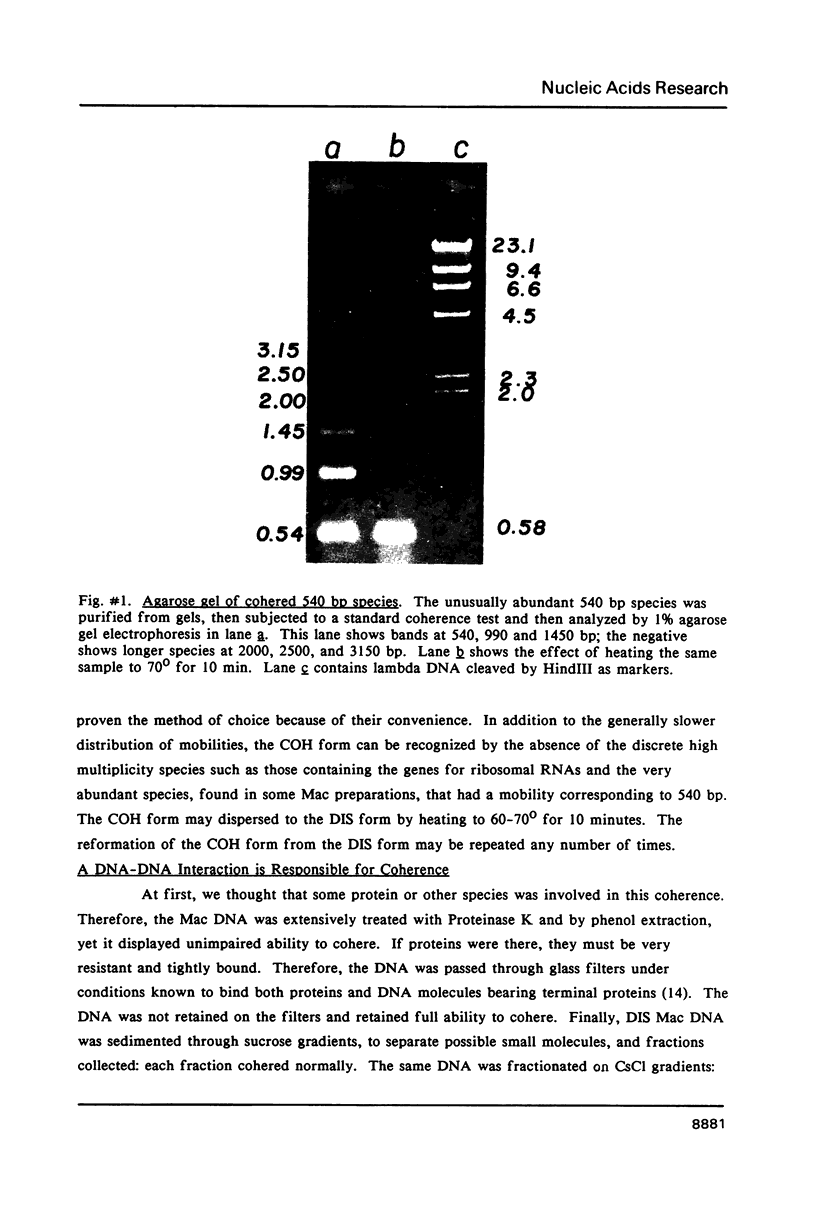
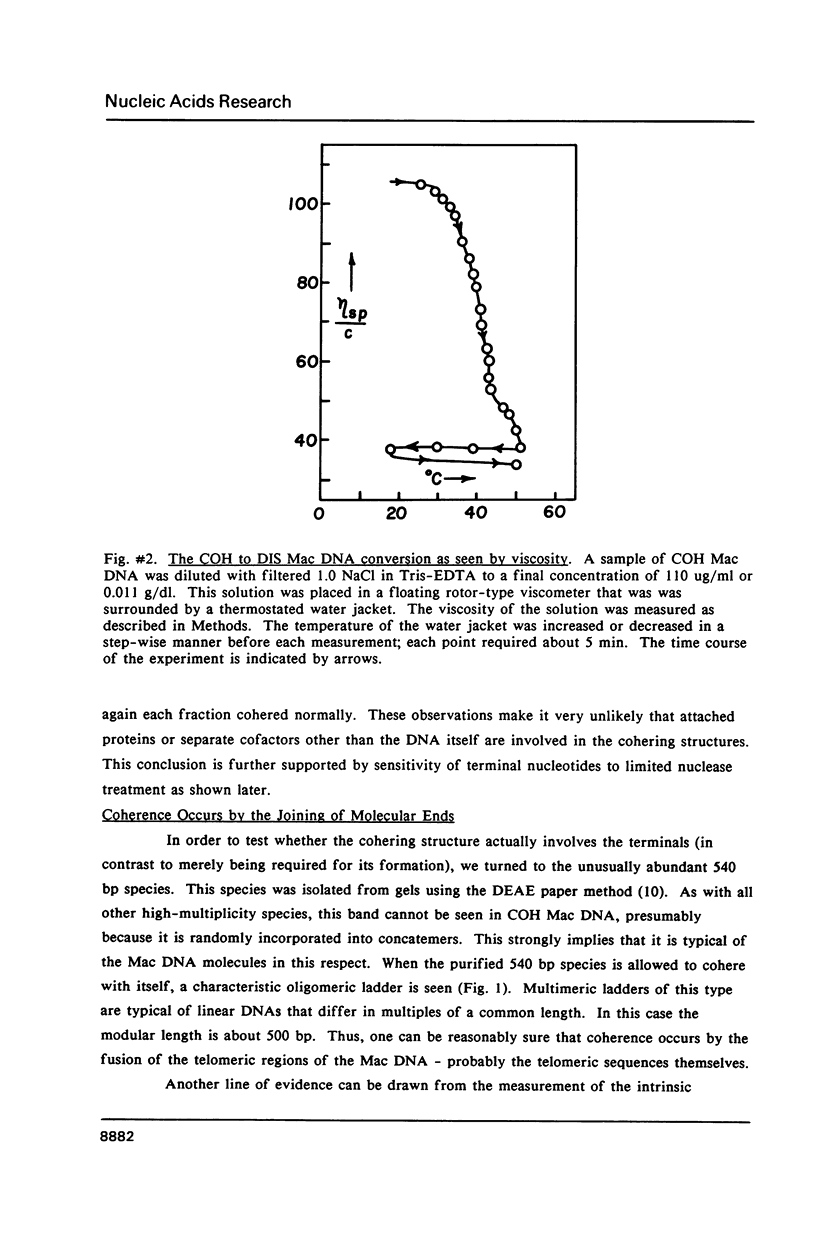
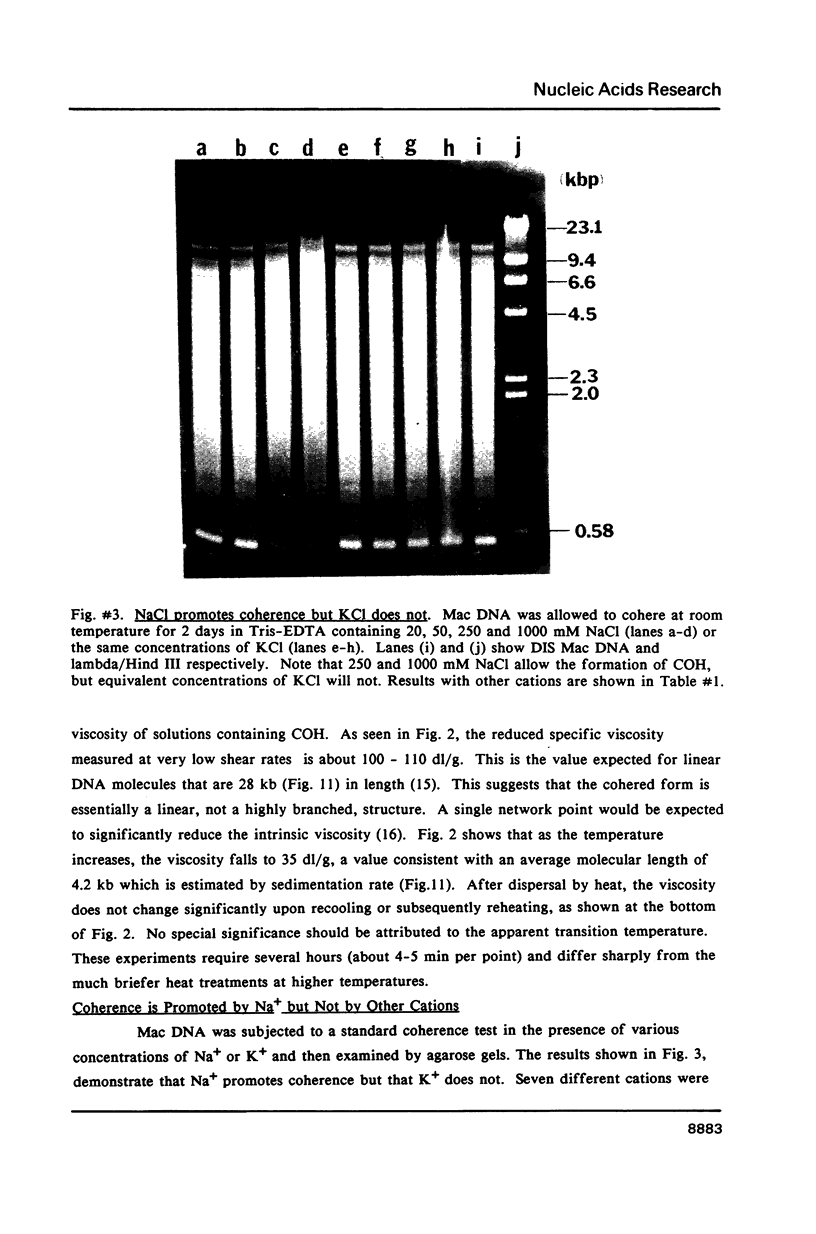



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Dretzen G., Bellard M., Sassone-Corsi P., Chambon P. A reliable method for the recovery of DNA fragments from agarose and acrylamide gels. Anal Biochem. 1981 Apr;112(2):295–298. doi: 10.1016/0003-2697(81)90296-7. [DOI] [PubMed] [Google Scholar]
- Greene P. J., Heyneker H. L., Bolivar F., Rodriguez R. L., Betlach M. C., Covarrubias A. A., Backman K., Russel D. J., Tait R., Boyer H. W. A general method for the purification of restriction enzymes. Nucleic Acids Res. 1978 Jul;5(7):2373–2380. doi: 10.1093/nar/5.7.2373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrick G., Wesley R. D. Isolation and characterization of a highly repetitious inverted terminal repeat sequence from Oxytricha macronuclear DNA. Proc Natl Acad Sci U S A. 1978 Jun;75(6):2626–2630. doi: 10.1073/pnas.75.6.2626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard F. B., Miles H. T. Poly(inosinic acid) helices: essential chelation of alkali metal ions in the axial channel. Biochemistry. 1982 Dec 21;21(26):6736–6745. doi: 10.1021/bi00269a019. [DOI] [PubMed] [Google Scholar]
- Khorana H. G. Total synthesis of a gene. Science. 1979 Feb 16;203(4381):614–625. doi: 10.1126/science.366749. [DOI] [PubMed] [Google Scholar]
- Koo H. S., Wu H. M., Crothers D. M. DNA bending at adenine . thymine tracts. Nature. 1986 Apr 10;320(6062):501–506. doi: 10.1038/320501a0. [DOI] [PubMed] [Google Scholar]
- Lipps H. J., Gruissem W., Prescott D. M. Higher order DNA structure in macronuclear chromatin of the hypotrichous ciliate Oxytricha nova. Proc Natl Acad Sci U S A. 1982 Apr;79(8):2495–2499. doi: 10.1073/pnas.79.8.2495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipps H. J. In vitro aggregation of the gene-sized DNA molecules of the ciliate Stylonychia mytilus. Proc Natl Acad Sci U S A. 1980 Jul;77(7):4104–4107. doi: 10.1073/pnas.77.7.4104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Little J. W. An exonuclease induced by bacteriophage lambda. II. Nature of the enzymatic reaction. J Biol Chem. 1967 Feb 25;242(4):679–686. [PubMed] [Google Scholar]
- Little J. W., Lehman I. R., Kaiser A. D. An exonuclease induced by bacteriophage lambda. I. Preparation of the crystalline enzyme. J Biol Chem. 1967 Feb 25;242(4):672–678. [PubMed] [Google Scholar]
- Masamune Y., Fleischman R. A., Richardson C. C. Enzymatic removal and replacement of nucleotides at single strand breaks in deoxyribonucleic acid. J Biol Chem. 1971 Apr 25;246(8):2680–2691. [PubMed] [Google Scholar]
- McGavin S. Models of specifically paired like (homologous) nucleic acid structures. J Mol Biol. 1971 Jan 28;55(2):293–298. doi: 10.1016/0022-2836(71)90201-4. [DOI] [PubMed] [Google Scholar]
- Noll H. Characterization of macromolecules by constant velocity sedimentation. Nature. 1967 Jul 22;215(5099):360–363. doi: 10.1038/215360a0. [DOI] [PubMed] [Google Scholar]
- Shenk T. E., Rhodes C., Rigby P. W., Berg P. Biochemical method for mapping mutational alterations in DNA with S1 nuclease: the location of deletions and temperature-sensitive mutations in simian virus 40. Proc Natl Acad Sci U S A. 1975 Mar;72(3):989–993. doi: 10.1073/pnas.72.3.989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanton M. T., Heumann J. M., Prescott D. M. Gene-sized DNA molecules of the macronuclei in three species of hypotrichs: size distributions and absence of nicks. DNA of ciliated protozoa. VIII. Chromosoma. 1980;77(2):217–227. doi: 10.1007/BF00329546. [DOI] [PubMed] [Google Scholar]
- Thomas C. A., Jr, Saigo K., McLeod E., Ito J. The separation of DNA segments attached to proteins. Anal Biochem. 1979 Feb;93(1):158–166. [PubMed] [Google Scholar]
- Wilson J. H. Nick-free formation of reciprocal heteroduplexes: a simple solution to the topological problem. Proc Natl Acad Sci U S A. 1979 Aug;76(8):3641–3645. doi: 10.1073/pnas.76.8.3641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimm B. H., Crothers D. M. SIMPLIFIED ROTATING CYLINDER VISCOMETER FOR DNA. Proc Natl Acad Sci U S A. 1962 Jun;48(6):905–911. doi: 10.1073/pnas.48.6.905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Massy B., Weisberg R. A., Studier F. W. Gene 3 endonuclease of bacteriophage T7 resolves conformationally branched structures in double-stranded DNA. J Mol Biol. 1987 Jan 20;193(2):359–376. doi: 10.1016/0022-2836(87)90224-5. [DOI] [PubMed] [Google Scholar]