Abstract
Cancer-related inflammation recently has been proposed as a major physiological hallmark of malignancy. Some genetic alterations known to promote cellular proliferation and induce malignant transformation also may participate in an intrinsic inflammatory pathway that produces a cancer-promoting inflammatory microenvironment. Little is known about this intrinsic inflammatory pathway in Barrett's esophagus. We have used a series of nontransformed and transformed human Barrett's epithelial cell lines developed in our laboratory to explore the potential contribution of interleukin (IL)-6 and signal transducer and activator of transcription (STAT3) (key molecules in the intrinsic inflammatory pathway) to Barrett's carcinogenesis. We determined IL-6 mRNA expression and protein secretion and protein expression of activated phospho-STAT3 and its downstream target myeloid cell leukemia (mcl)-1 (Mcl-1). We used an IL-6 blocking antibody and two JAK kinase inhibitors (AG490 and JAK inhibitor I) to assess whether STAT3 activation is IL-6 dependent. We also used small interfering RNAs (siRNAs) to STAT3 and Mcl-1 to assess effects of STAT3 pathway inhibition on apoptosis. Phospho-STAT3 was expressed only by transformed Barrett's cells, which also exhibited higher levels of IL-6 mRNA and of IL-6 and Mcl-1 proteins than nontransformed Barrett's cells. STAT3 phosphorylation could be blocked by IL-6 blocking antibody and by AG490 and JAK inhibitor I. In transformed Barrett's cells, rates of apoptosis following exposure to deoxycholic acid were significantly increased by transfection with siRNAs for STAT3 and Mcl-1. We conclude that activation of the IL-6/STAT3 pathway in transformed Barrett's epithelial cells enables them to resist apoptosis. These findings demonstrate a possible contribution of the intrinsic inflammatory pathway to carcinogenesis in Barrett's esophagus.
Keywords: Barrett's esophagus, malignant transformation
chronic gastroesophageal reflux disease (GERD) can cause inflammation of the esophageal mucosa, a condition called reflux esophagitis. In some cases, reflux esophagitis heals with the replacement of reflux-damaged esophageal squamous cells by intestinal-type, columnar cells, resulting in the metaplastic condition called Barrett's esophagus (20). Barrett's metaplasia is predisposed to the development of esophageal adenocarcinoma for reasons that remain unclear. Chronic inflammation is known to contribute to cancer development, and chronic reflux esophagitis is judged to play a role in promoting the neoplastic progression of Barrett's esophagus.
Recently, it has been proposed that the link between inflammation and cancer can involve two pathways: 1) the familiar extrinsic pathway in which clinical disorders like GERD cause local tissue inflammation that contributes to carcinogenesis; and 2) an intrinsic pathway in which the genetic abnormalities acquired by precancerous cells produce an inflammatory tumor microenvironment that contributes to neoplastic progression (16). Patients with Barrett's esophagus usually are treated with acid-suppressing medications like proton pump inhibitors, a clinical strategy aimed exclusively at the extrinsic inflammatory pathway (GERD) in Barrett's carcinogenesis. Little is known about the role of the intrinsic inflammatory pathway in Barrett's esophagus and, consequently, there are no clinical strategies aimed at this potentially important therapeutic target.
A key concept of the intrinsic cancer-related inflammation pathway is that some of the same genetic events that endow the cancer cell with growth advantages also produce an inflammatory microenvironment (16). In human papillary thyroid cancer, for example, alterations in RET (rearranged during transfection), a key growth-promoting gene that encodes a protein tyrosine kinase, have been found to activate an “inflammatory program” that results in the expression of inflammatory chemokines, chemokine receptors, cytokines, matrix-degrading enzymes, and adhesion molecules (2). Other oncogenes such as Ras and MYC, which are activated in a number of human tumors, also induce the production of tumor-promoting inflammatory cytokines (reviewed in Ref. 16). The inflammatory environment also can be influenced by inactivation of tumor suppressor genes such as von Hippel-Lindau, transforming growth factor-β, and phosphatase and tensin homolog (PTEN) (reviewed in Ref. 16). Thus a number of genetic alterations that promote cellular proliferation also can promote inflammation.
Some of the key molecules involved in cancer-related inflammation are transcription factors such as signal transducer and activator of transcription (STAT) 3, and cytokines such as interleukin (IL)-6 (3). However, it is not clear which component(s) of the intrinsic cancer-related inflammation program contribute importantly to carcinogenesis.
Recently, we induced the malignant transformation of telomerase-immortalized, p16-deficient, human Barrett's epithelial cells by knocking down the p53 pathway and activating the oncogenic Ras pathway (12, 23). In the process, we developed a number of nontransformed cell lines that have well-defined, growth-promoting genetic changes that appear to recapitulate various stages of neoplastic progression in Barrett's esophagus. Using these cell lines, we have explored the contribution of two key molecules in the intrinsic inflammatory pathway (IL-6 and STAT3) to Barrett's carcinogenesis.
METHODS
Cell culture.
We used a series of nontransformed and transformed Barrett's epithelial cell lines developed in our laboratory (12, 23). In brief, we started with a telomerase-immortalized, p16-deficient Barrett's epithelial cell line (BAR-T) that we had established from endoscopic biopsy specimens of nondysplastic Barrett's metaplasia. The BAR-T cells were infected with the retroviral vector pSUPER-RNAi-p53 (OligoEngine, Seattle, WA) to knock down p53, the retroviral vector pBabe- H-RasG12V to express human oncogenic H-RasG12V (obtained from Dr. Robert Weinberg, Whitehead Institute, Cambridge, MA) (15), or the combination of both as previously described (23). Through these experiments, we generated BAR-T cells containing 1) pSUPER-p53RNAi (not transformed); 2) pBabe- H-RasG12V-zeocin (not transformed); and 3) pSUPER-p53RNAi and pBabe-H-RasG12V-zeocin (transformed). The nontransformed BAR-T, BAR-T p53RNAi, and BAR-T H-RasG12V-expressing (clone R6 and R7) cell lines were cocultured with a fibroblast feeder layer as previously described (12, 19). The transformed BAR-T p53RNAi H-RasG12V-expressing cell lines (clones R1 and R2) could be cultured without a fibroblast feeder layer. All cell lines were maintained in monolayer culture at 37°C in humidified air with 5% CO2 in growth media as previously described. For individual experiments, the nontransformed cell lines were equally seeded into collagen IV-coated wells (BD Biosciences, San Jose, CA), whereas the transformed cell lines were equally seeded onto standard culture dishes.
Western blotting.
Cells were washed in cold PBS followed by immediate cell lysis. Cells were lysed in 1× cell lysis buffer (Cell Signaling Technology, Beverly, MA); equal amounts of protein were separated by SDS-polyacrylamide gel electrophoresis; protein concentrations were determined using the BCA-200 protein assay kit (Pierce, Rockford, IL). After separation and transfer to nitrocellulose membranes, the membranes were incubated with primary antibodies. Primary antibodies used for Western blot were total Stat3 and phospho-Stat3 (Tyr705) (D3A7) (both from Cell Signaling Technology), myeloid cell leukemia (mcl)-1 (Mcl-1) (from Santa Cruz Biotechnology, Santa Cruz, CA), and anti-β-actin (Sigma-Aldrich, St. Louis, MO). Horseradish peroxidase secondary antibodies were used and chemiluminescence was determined by using the Super Signal West Dura detection system (Pierce); β-actin or β-tubulin was used to confirm equal loading. All Western blots were performed in duplicate. Densitometry was performed by using the MultiAnalyst software package (Bio-Rad, Hercules, CA). Results were expressed as relative intensity units and normalized to β-actin or β-tubulin.
Reverse transcription-PCR.
Total cellular RNA was extracted with TRIzol (Invitrogen) according to the manufacturer's instructions. The samples were DNase treated and the first strand cDNA was synthesized by using M-MLV reverse transcriptase (Invitrogen) per the manufacturer's instructions. The sequences of primers for IL-6, IL-6R, and gp130 were 1) IL-6 (forward) 5′-CATGTGTGAAAGCAGCAAAGAGGC-3′ and IL-6 (reverse) 5′-CACCAGGCAAGTCTCCTCATTGAA-3′, 112 bp; 2) IL-6R (forward) 5′-CCTGCCAACATCACAGTCACTG-3′ and IL-6R (reverse) 5′-TTTGACCGTTCAGCCCGAT-3′, 134 bp; and 3) gp130 (forward) 5′-AGAGGAGCGGCCAGAAGATCTA-3′ and gp130 (reverse) 5′-GACTGGATTCATGCTGACTGCA-3′, 110 bp. PCR was performed by using GoTaq DNA polymerase (Promega, Madison, WI) according to the manufacturer's instructions using the iCycler (Bio-Rad). PCR conditions consisted of 95°C for 5 min followed by 35 cycles (IL-6) or 40 cycles (IL-6R and gp130) at 95°C for 30 s, 53°C for 30 s, and 72°C for 30 s. PCR products were then electrophoresed on 2% agarose gels and stained with ethidium bromide; β-actin transcripts served as internal controls as previously described (11).
IL-6 ELISA.
Conditioned media were collected from equally seeded wells of cells. The concentration of IL-6 protein (pg/ml) in the conditioned media was detected using the IL-6 human ELISA assay (Invitrogen, Carlsbad, CA) per the manufacturer's instructions. The ELISA assays were performed in duplicate.
Treatment with DCA.
The unconjugated bile acid deoxycholic acid (DCA; Sigma) has been shown to induce apoptosis in Barrett's epithelial cells and cancer cells (4). In preliminary experiments, we treated transformed Barrett's cells with 3 h of DCA at doses of 250, 375, and 500 μM. We determined that DCA in doses greater than 250 μM had significant effects on cell viability compared with non-DCA-treated control cells. On the basis of these preliminary findings, we selected to use DCA at a dose of 250 μM for 3 h to induce apoptosis in our transformed Barrett's cells.
Detection of apoptosis.
Apoptosis was detected by using the Cell Death Detection ELISAPLUS assay (Roche Applied Science, Indianapolis, IN) per the manufacturer's instructions. Using monoclonal antibodies against DNA and histones, this assay detects the presence of mono- and oligonucleosomes in the cell cytoplasm. All assays were performed in duplicate. Apoptosis rates were expressed as a percentage of those in cells containing nontargeting small interfering RNA (siRNA).
Inhibition of the IL-6/STAT3 signaling pathway.
Equally seeded wells of cells were placed in KBM-2 media without growth factors or serum for 1, 4, and 24 h, after which STAT3 phosphorylation levels were determined. To inhibit IL-6-mediated activation of STAT3, cells were cultured in KBM-2 media without growth factors or serum for 4 h in the presence of an IL-6 blocking antibody (2 μg/ml; R&D Systems, Minneapolis, MN) followed by the detection of STAT3 phosphorylation levels; a mouse IgG antibody (2 μg/ml) was used as a control. To inhibit the JAK kinases, we used two different pharmacological inhibitors, AG490 and JAK inhibitor I (Calbiochem, San Diego, CA); JAK inhibitor I, a pan-JAK inhibitor has been shown to be more specific and sensitive than AG490 (18, 22). Transformed Barrett's cells were treated with either 50 or 100 μM AG490 for 24 h, or 10 μM of the JAK inhibitor I for durations ranging from 3–24 h, and STAT3 phosphorylation levels and expression of Mcl-1 were assayed. Since pharmacological agents can have nonspecific effects, we determined the role of STAT3 in mediating effects on apoptosis using a specific STAT3 siRNA. We also used a specific siRNA against Mcl-1, a downstream STAT3 target gene implicated in apoptotic resistance. Cells were equally plated in 6- or 24-well tissue culture plates. After 24 h, the cells were transfected using Lipofectamine 2000 (Invitrogen) with 20 nM of the siGenome SMART pool STAT3 or Mcl-1 siRNA (Thermo Scientific, Waltham, MA), per the manufacturer's instructions. 48 h after transfection, cells were treated with 250 μM DCA. As a control, cells were transfected with siGenome NonTargeting siRNA no. 2 (Thermo Scientific). All experiments were performed in duplicate.
Statistical analyses.
Quantitative data are expressed as means + SE. Statistical analysis was performed using an unpaired Student's t-test or an ANOVA and the Student-Newman-Keuls multiple-comparison test with the Instat for Windows statistical software package (GraphPad Software, San Diego, CA). P values <0.05 were considered significant for all analyses.
RESULTS
Expression of phospho-STAT3 (Tyr705) is increased in transformed, but not in nontransformed, Barrett's epithelial cells.
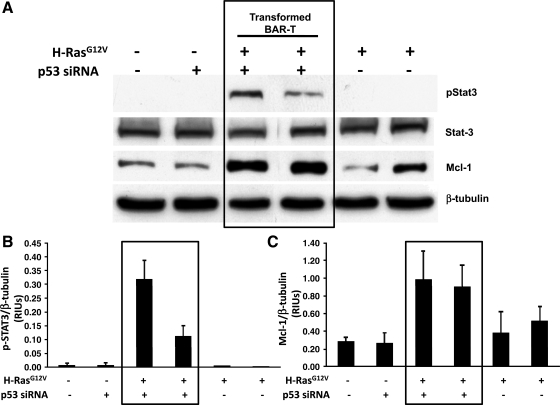
NF-κB and STAT3 are key transcription factors in cancer-related inflammation. NF-κB is well known to mediate inflammation and tumor progression, and we have previously found that activation of NF-κB causes resistance to apoptosis in Barrett's epithelial cells (10). In these experiments, we focused on STAT3 and explored its activation in our cell lines by performing Western blotting for phospho-STAT3 and Mcl-1, a survival protein that is a downstream target of STAT3. We found similar levels of expression for total STAT3 in all of our cell lines (transformed and nontransformed). In contrast, expression of phospho-STAT3 was detected only in the transformed Barrett's cells, which also expressed higher levels of Mcl-1 (Fig. 1). These data suggest that the STAT3 pathway is active in transformed, but not in nontransformed, Barrett's epithelial cells.
Fig. 1.
Phospho-STAT3 and Mcl-1 expression are increased in transformed, but not in nontransformed BAR-T cells. A: Western blot for phospho-STAT3, total STAT3, and Mcl-1. Note the expression of phospho-STAT3 only in the transformed Barrett's cells, which also express higher levels of Mcl-1. β-Tubulin served as a loading control. Bar graphs show the results of densitometry depicted as mean (+SE) relative intensity units (RIUs, normalized to β-tubulin) for p-STAT3 (B) and Mcl-1 (C) expression from 2 individual experiments. siRNA, small interfering RNA.
IL-6 mRNA levels and protein secretion are increased in transformed Barrett's epithelial cells.
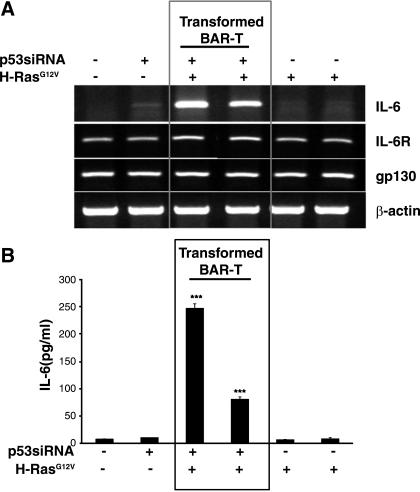
IL-6 is a key cytokine that has been implicated in cancer-related inflammation, and STAT3 is the main downstream effector molecule for IL-6. Therefore, we determined whether mRNA levels of IL-6 and its receptors (IL-6R and gp130) were increased in our transformed Barrett's cells. We found similar levels of expression for IL-6R and gp130 mRNAs in all of our cell lines (Fig. 2A). In contrast, only the transformed Barrett's cells expressed high levels of IL-6 mRNA. We also determined levels of IL-6 protein in the media of the cells and found that those levels were significantly higher in the transformed Barrett's cells (Fig. 2B).
Fig. 2.
IL-6 mRNA and protein levels are increased in transformed BAR-T cells. A: mRNA expression of IL-6 and its receptor subunits (IL-6R and gp130) in transformed and nontransformed Barrett's cells. Note the high levels of IL-6 mRNA expression only in the transformed BAR-T cells; β-actin served as a loading control. B: IL-6 protein levels in transformed and nontransformed Barrett's cells determined by ELISA. Note that IL-6 levels are significantly higher in the transformed Barrett's cells. Bar graphs depict means + SE from 2 individual experiments. ***P < 0.001 compared with vector control, p53 RNAi-only clone, and both H-RasG12V-only clones.
STAT3 activation in transformed Barrett's cells is dependent on IL-6/JAK signaling.
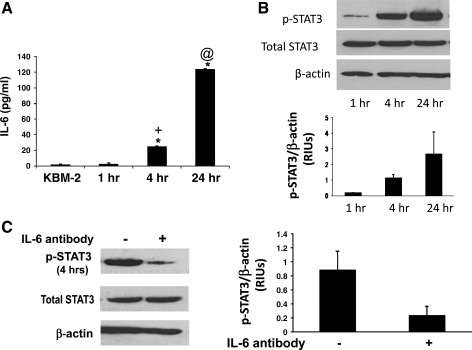
STAT3 activation in tumors can be either dependent on or independent of IL-6 signaling (reviewed in Ref. 8). To determine whether STAT3 phosphorylation is IL-6 dependent in our transformed Barrett's cells, we explored the time course for IL-6 secretion in BAR-T p53 RNAi+ H-RasG12V+ clone 1. Fresh media was placed on the transformed Barrett's cells and then collected 4 and 24 h later. We found that levels of IL-6 in the media significantly increased in a time-dependent manner, as did the levels of phospho-STAT3 expression (Fig. 3, A and B). Treatment with an IL-6 blocking antibody (2 μg/ml) caused a marked decrease in STAT3 phosphorylation, suggesting that activation of STAT3 is dependent on IL-6 signaling in transformed Barrett's cells (Fig. 3C).
Fig. 3.
STAT3 phosphorylation is IL-6 dependent in transformed Barrett's cells. A: IL-6 protein secretion increases in a time-dependent fashion; KBM-2 media-only as a control. Bar graphs depict means + SE from 3 individual experiments (*P < 0.001 compared with KBM-2 media; +P < 0.001 compared with 1 h; @P < 0.001 compared with 4 h). B: Western blot for phospho-STAT3. Note that expression levels of phospho-STAT3 increase in a time-dependent fashion; β-actin served as a loading control. Bar graphs show the results of densitometry depicted as mean (+SE) RIUs (normalized to β-actin) for phospho-STAT3 expression from 2 individual experiments. C: Western blot for phospho-STAT3 expression levels at 4 h in the presence and absence of an IL-6 blocking antibody; IgG isotype antibody served as a control. Note that the blocking antibody markedly decreases STAT3 phosphorylation levels. Bar graphs show the results of densitometry depicted as mean (+SE) RIUs (normalized to β-actin) for phospho-STAT3 expression from 2 individual experiments.
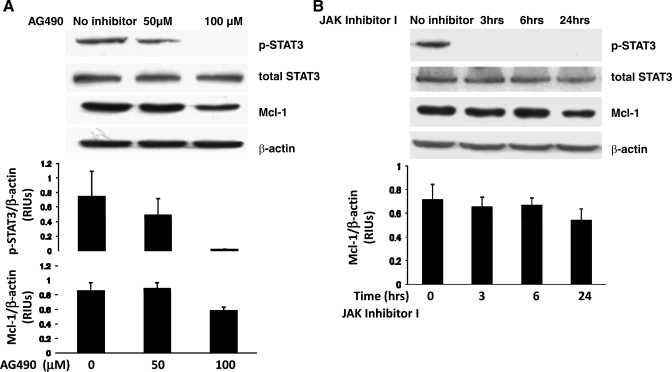
Activation and phosphorylation of the gp130 receptor subunit by IL-6 causes activation of the JAK tyrosine kinases, which in turn phosphorylate STAT3. To further demonstrate that STAT3 phosphorylation is IL-6 dependent, we treated our transformed Barrett's cell line with two different JAK inhibitors (18, 22). As shown in Fig. 4A, AG490 caused a dose-dependent decrease in STAT3 phosphorylation and Mcl-1 expression. JAK inhibitor I also decreased STAT3 phosphorylation by 3 h (an effect that persisted at 24 h) and decreased Mcl-1 protein expression by 24 h (Fig. 4B). Taken together, these data suggest that STAT3 activation in transformed Barrett's cells is indeed dependent on IL-6/JAK signaling.
Fig. 4.
Inhibition of JAK kinases decreases STAT3 phosphorylation and Mcl-1 expression in transformed Barrett's cells. A: Western blots for phospho-STAT3 and Mcl-1 after treatment with either 50 or 100 μM of the JAK inhibitor AG490 for 24 h. Note that AG490 decreases STAT3 phosphorylation and expression of Mcl-1 in a dose-dependent fashion. Bar graphs show the results of densitometry depicted as mean (+SE) RIUs (normalized to β-actin) for p-STAT3 and Mcl-1 expression from 2 individual experiments. B: Western blots for phospho-STAT3 and Mcl-1 after treatment with JAK inhibitor I for 3, 6, or 24 h. Note that JAK inhibitor I decreases STAT3 phosphorylation by 3 h and decreases Mcl-1 expression by 24 h. Bar graphs show the results of densitometry depicted as mean (+SE) RIUs (normalized to β-actin) for Mcl-1 expression from 2 individual experiments.
Inhibition of STAT3 sensitizes transformed Barrett's cells to bile acid-induced apoptosis.
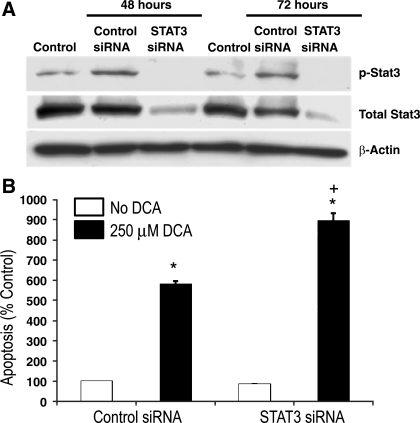
Having found activation of STAT3 and expression of its downstream antiapoptotic protein (Mcl-1) in transformed Barrett's cells, we next sought to determine whether activation of STAT3 contributes to apoptotic resistance. We inhibited STAT3 activity by transfecting transformed Barrett's cells (BAR-T p53 RNAi+ H-RasG12V+ clone 1) with a STAT3 siRNA. The bile acid DCA has been shown to induce apoptosis in Barrett's epithelial cells and cancer cells (4). Therefore, we treated cells with 250 μM DCA for 3 h, after which apoptosis was determined by a Cell Death ELISA assay. To determine the efficiency of STAT3 siRNA on inhibiting STAT3 expression, Western blotting was performed at 48 and 72 h following transfection. We found a decrease in total STAT3 expression and a complete loss of phospho-STAT3 expression following transfection with STAT3 siRNA at both time points; in contrast, total STAT3 expression was unchanged and phospho-STAT3 expression was slightly increased at both time points in cells containing a control siRNA (Fig. 5A). As expected, treatment with DCA caused a significant increase in apoptosis in transformed Barrett's cells containing the control siRNA (Fig. 5B). In the STAT3-knockdown cells, treatment with DCA caused a significantly greater increase in apoptosis than in the control cells (Fig. 5B). These observations suggest that STAT3 activation contributes to apoptotic resistance in transformed Barrett's cells.
Fig. 5.
Inhibition of STAT3 increases deoxycholic acid (DCA)-induced apoptosis in transformed Barrett's cells. A: Western blots for phospho-STAT3 and total STAT3 in control cells (without siRNA transfection), cells transfected with control siRNA, and cells transfected with STAT3 siRNA. Note that STAT3 siRNA decreases total STAT3 protein expression and eliminates phospho-STAT3 expression at 48 and 72 h. B: rates of apoptosis in cells transfected with control siRNA and in cells transfected with STAT3 siRNA at baseline and after treatment with 250 μM DCA for 3 h. Bar graphs depict means + SE from 1 of 2 individual experiments. *P < 0.001 compared with control siRNA containing cells; +P < 0.001 compared with DCA-treated control siRNA containing cells.
Inhibition of Mcl-1 sensitizes transformed Barrett's cells to bile acid-induced apoptosis.
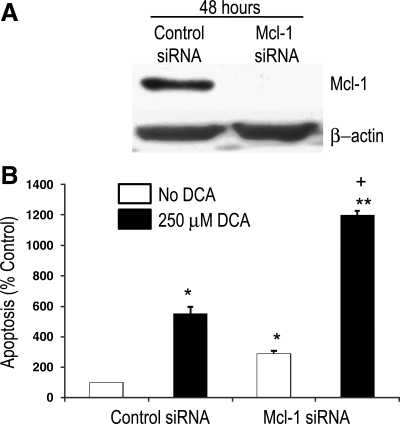
We next sought to determine whether expression of Mcl-1, the downstream target of STAT3, contributes to the apoptotic resistance of transformed Barrett's cells. We transfected BAR-T p53 RNAi+ H-RasG12V+ clone 1 with Mcl-1 siRNA oligonucleotides and determined the rates of apoptosis following treatment with 250 μM DCA for 3 h. To determine the efficiency of siRNA for inhibiting Mcl-1 expression, Western blotting was performed. We noted complete elimination of Mcl-1 expression 48 h after transfection with the Mcl-1 siRNA; Mcl-1 expression was unchanged in transformed cells containing a control siRNA (Fig. 6A). We found that transfection of the transformed Barrett's cells with siRNA against Mcl-1 significantly increased basal rates of apoptosis in the Mcl-1 knockdown cells (Fig. 6B). In agreement with our earlier findings, treatment with DCA caused an increase in apoptosis in cells containing the control siRNA (Fig. 6B). In the Mcl-1 knockdown cells, treatment with DCA caused a significantly greater increase in apoptosis than in cells containing control siRNA (Fig. 6B). These findings suggest that Mcl-1 contributes to apoptotic resistance in transformed Barrett's cells.
Fig. 6.
Inhibition of Mcl-1 increases apoptosis in transformed Barrett's cells. A: Western blots for Mcl-1 in cells transfected with control siRNA and in cells transfected with Mcl-1 siRNA. Note that Mcl-1 siRNA eliminates Mcl-1 protein expression at 48 h. B: rates of apoptosis in cells transfected with control siRNA and in cells transfected with Mcl-1 siRNA at baseline and after treatment with 250 μM DCA for 3 h. Bar graphs depict means + SE from 1 of 2 individual experiments. *P < 0.05 compared with control siRNA containing cells; **P < 0.001 compared with control siRNA containing cells; +P < 0.001 compared with DCA-treated control siRNA containing cells.
DISCUSSION
In earlier experiments, we induced the malignant transformation of benign Barrett's epithelial cells in vitro through the stepwise disruption of several key growth regulatory pathways including the p16/Rb and p53 checkpoint arrest pathways, the mitogenic Ras signaling pathway, and the telomerase-dependent replicative senescence pathway (23). As a result of these experiments, we established a series of transformed and nontransformed Barrett's epithelial cell lines with well-defined genetic alterations similar to those that have been described in esophageal biopsy specimens from patients with various stages of neoplasia in Barrett's esophagus. In the present experiments, we used our unique cell lines to explore the role of the recently proposed 7th hallmark of cancer, cancer-related inflammation, in Barrett's carcinogenesis (3). Although antisecretory agents like proton pump inhibitors have been used for decades to control GERD, the extrinsic inflammatory pathway in Barrett's esophagus, little is known about the role of the intrinsic inflammatory pathway in Barrett's carcinogenesis.
Transcription factors such as NF-κB and STAT3 are key molecules implicated in cancer-related inflammation (3). NF-κB is well known to mediate both inflammation and tumor progression, and we have previously reported that activation of NF-κB contributes to apoptotic resistance in nontransformed Barrett's epithelial cells (10). STAT3 is also known to mediate both inflammation and tumorigenesis, and we have now explored STAT3 activation in our Barrett's cells by performing Western blotting for phospho-STAT3 and its downstream target survival protein Mcl-1. We found that STAT-3 is phosphorylated only in transformed Barrett's cells, which also express higher levels of Mcl-1. This indicates that STAT3 signaling is increased in transformed Barrett's epithelial cells. The active, phosphorylated form of STAT3 has been demonstrated in biopsy specimens of dysplastic Barrett's epithelia and in esophageal adenocarcinoma (4). Moreover, phospho-STAT3 expression levels have been found to increase as the severity of dysplasia increases, suggesting that our findings in vitro reflect in vivo neoplastic processes (4). Thus signaling via STAT3 may contribute to the link between inflammation and carcinogenesis in Barrett's esophagus.
TNF-α, IL-1β, and IL-6 are among the key inflammatory cytokines implicated in carcinogenesis (3). Inflamed Barrett's epithelium has been found to express proinflammatory cytokines including IL-1β and IL-8 (6, 17). Moreover, the infiltrating inflammatory cells may not be the sole source of those cytokines, because Barrett epithelial cells have been shown to express IL-8, IL-1β, and IL-10 (7, 17). In preliminary studies, we found no increase in the secretion of TNF-α, IL-1β, and IL-8 as Barrett's cells were transformed in vitro (data not shown).
IL-6 is a potent inflammatory cytokine that appears to play a key role in cancer growth and metastasis (13). IL-6 signals via a receptor complex comprised of IL-6Rα and glycoprotein 130 (gp130) (13). Activation of this complex causes gp130 to phosphorylate and thereby activate the Janus (JAK) kinases 1 and 2 (9, 13, 14). Subsequent phosphorylation of STATs by JAKs results in activation of specific target genes implicated in carcinogenesis including Mcl-1 (1). We found similar levels of mRNA expression for IL-6 receptors (IL-6R and gp130) in our transformed and nontransformed Barrett's cell lines. In contrast, only the transformed Barrett's cells exhibited a significant increase in IL-6 mRNA and protein secretion. Biopsy specimens of Barrett's metaplasia have been shown to exhibit higher levels of IL-6 mRNA and protein secretion than normal esophageal squamous epithelium, and these levels were found to be highest in one specimen from an esophageal adenocarcinoma (5).
STAT3 activation in tumors can be either dependent on or independent of IL-6 signaling (reviewed in Ref. 8). To determine whether STAT3 phosphorylation is IL-6-dependent in our transformed Barrett's cells, we explored the time course for IL-6 secretion and STAT3 phosphorylation. We found that levels of IL-6 in the media significantly increased in a time-dependent manner, as did the levels of phospho-STAT3 expression. Moreover, STAT3 phosphorylation could be markedly decreased by treatment with an IL-6 blocking antibody, suggesting that activation of STAT3 indeed is dependent on IL-6 signaling in transformed Barrett's cells. To confirm this, we treated our transformed Barrett's cell line with two different JAK inhibitors (AG490 and JAK inhibitor I, a pan-JAK inhibitor found to be more specific and sensitive than AG490) (18, 22). Treatment with AG490 induced a dose-dependent decrease in STAT3 phosphorylation and Mcl-1 expression; treatment with the JAK inhibitor I decreased STAT3 phosphorylation as early as 3 h and Mcl-1 protein expression by 24 h. Taken together, these data demonstrate that STAT3 activation in transformed Barrett's cells is dependent on IL-6/JAK signaling.
STAT3 target genes are known to regulate many of the physiological processes involved in tumorigenesis including cell cycle progression, apoptosis, tumor angiogenesis, invasion, and metastasis (1). Mcl-1 is an antiapoptotic member of the Bcl-2 family and is a known downstream target gene of STAT3 (21). Biopsy specimens of Barrett's metaplasia demonstrate higher phospho-STAT3 expression and a greater intensity of Mcl-1 protein expression than normal esophageal squamous or duodenal epithelia (5). In our transformed Barrett's cells, we found that inhibition of IL-6/STAT3 signaling with either the IL-6 blocking antibody or the JAK inhibitors also blocked Mcl-1 expression, suggesting that Mcl-1 is a STAT3 target gene in our transformed cell lines.
To demonstrate that IL-6/STAT3 signaling is physiologically relevant in transformed Barrett's epithelial cells, we transfected the cells with siRNAs against STAT3 or Mcl-1 and determined their ability to resist apoptosis. We treated the cells with unconjugated DCA at a dose and duration previously shown to induce apoptosis in Barrett's epithelial cells and cancer cells (4). We found that transfection of the transformed cells with either of these siRNAs significantly increased DCA-induced apoptosis, suggesting that the STAT3/Mcl-1 pathway mediates apoptotic resistance of transformed Barrett's cells (Fig. 7). Further studies are warranted to elucidate other specific proteins regulated by STAT3 that may be involved in Barrett's tumorigenesis.
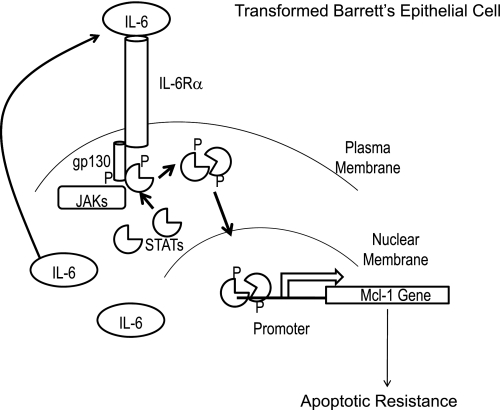
Fig. 7.
Schematic representation of how IL-6 and STAT3 mediate apoptotic resistance in transformed Barrett's epithelial cells. IL-6 produced by the cells is secreted into the extracellular space, where it binds to its receptor complex (IL-6Rα and gp130). This recruits JAKs to the receptor complex, where they phosphorylate gp130. This allows STATs to dock on the receptor where they, in turn, are phosphorylated. Dimerization of the phospho-STATs enables them to translocate into the nucleus where they bind to promoters to initiate the transcription of target genes such as Mcl-1 (a potent antiapoptotic gene). Expression of Mcl-1 allows the cells to resist bile acid-induced apoptosis.
In conclusion, we have shown that the same genetic events that induce the malignant transformation of p16-deficient, telomerase-immortalized Barrett's epithelial cells (i.e., knockdown of p53 and expression of oncogenic H-Ras) also are associated with activation of the IL-6/STAT3 pathway. We have demonstrated that inhibitors of STAT3 and Mcl-1 can significantly increase the rates of DCA-induced apoptosis in transformed Barrett's cells, suggesting that STAT3 signaling is physiologically relevant in those cells. These findings support a role for the intrinsic inflammatory pathway in Barrett's carcinogenesis and suggest that the IL-6/STAT3 pathway may be a novel target for chemoprevention in Barrett's esophagus.
GRANTS
This work was supported by the Office of Medical Research, Department of Veterans Affairs (R. F. Souza, S. J. Spechler) and the National Institutes of Health (R01-DK63621 to R. F. Souza, R01-CA134571 to R. F. Souza and S. J. Spechler).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
REFERENCES
- 1. Alvarez JV, Febbo PG, Ramaswamy S, Loda M, Richardson A, Frank DA. Identification of a genetic signature of activated signal transducer and activator of transcription 3 in human tumors. Cancer Res 65: 5054–5062, 2005 [DOI] [PubMed] [Google Scholar]
- 2. Borrello MG, Alberti L, Fischer A, Degl'innocenti D, Ferrario C, Gariboldi M, Marchesi F, Allavena P, Greco A, Collini P, Pilotti S, Cassinelli G, Bressan P, Fugazzola L, Mantovani A, Pierotti MA. Induction of a proinflammatory program in normal human thyrocytes by the RET/PTC1 oncogene. Proc Natl Acad Sci USA 102: 14825–14830, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Colotta F, Allavena P, Sica A, Garlanda C, Mantovani A. Cancer-related inflammation, the seventh hallmark of cancer: links to genetic instability. Carcinogenesis 30: 1073–1081, 2009 [DOI] [PubMed] [Google Scholar]
- 4. Dvorak K, Chavarria M, Payne CM, Ramsey L, Crowley-Weber C, Dvorakova B, Dvorak B, Bernstein H, Holubec H, Sampliner RE, Bernstein C, Prasad A, Green SB, Garewal H. Activation of the interleukin-6/STAT3 antiapoptotic pathway in esophageal cells by bile acids and low pH: relevance to Barrett's esophagus. Clin Cancer Res 13: 5305–5313, 2007 [DOI] [PubMed] [Google Scholar]
- 5. Dvorakova K, Payne CM, Ramsey L, Holubec H, Sampliner R, Dominguez J, Dvorak B, Bernstein H, Bernstein C, Prasad A, Fass R, Cui H, Garewal H. Increased expression and secretion of interleukin-6 in patients with Barrett's esophagus. Clin Cancer Res 10: 2020–2028, 2004 [DOI] [PubMed] [Google Scholar]
- 6. Fitzgerald RC, Abdalla S, Onwuegbusi BA, Sirieix P, Saeed IT, Burnham WR, Farthing MJ. Inflammatory gradient in Barrett's oesophagus: implications for disease complications. Gut 51: 316–322, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Fitzgerald RC, Onwuegbusi BA, Bajaj-Elliott M, Saeed IT, Burnham WR, Farthing MJ. Diversity in the oesophageal phenotypic response to gastro-oesophageal reflux: immunological determinants. Gut 50: 451–459, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Frank DA. STAT3 as a central mediator of neoplastic cellular transformation. Cancer Lett 251: 199–210, 2007 [DOI] [PubMed] [Google Scholar]
- 9. Greenhalgh CJ, Miller ME, Hilton DJ, Lund PK. Suppressors of cytokine signaling: relevance to gastrointestinal function and disease. Gastroenterology 123: 2064–2081, 2002 [DOI] [PubMed] [Google Scholar]
- 10. Hormi-Carver K, Zhang X, Zhang HY, Whitehead RH, Terada LS, Spechler SJ, Souza RF. Unlike esophageal squamous cells, Barrett's epithelial cells resist apoptosis by activating the nuclear factor-kappaB pathway. Cancer Res 69: 672–677, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Huo X, Zhang HY, Zhang XI, Lynch JP, Strauch ED, Wang JY, Melton SD, Genta RM, Wang DH, Spechler SJ, Souza RF. Acid and bile salt-induced CDX2 expression differs in esophageal squamous cells from patients with and without Barrett's esophagus. Gastroenterology 139: 194–203, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Jaiswal KR, Morales CP, Feagins LA, Gandia KG, Zhang X, Zhang HY, Hormi-Carver K, Shen Y, Elder F, Ramirez RD, Sarosi GA, Jr, Spechler SJ, Souza RF. Characterization of telomerase-immortalized, non-neoplastic, human Barrett's cell line (BAR-T). Dis Esophagus 20: 256–264, 2007 [DOI] [PubMed] [Google Scholar]
- 13. Kishimoto T. Interleukin-6: from basic science to medicine–40 years in immunology. Annu Rev Immunol 23: 1–21, 2005 [DOI] [PubMed] [Google Scholar]
- 14. Lin WW, Karin M. A cytokine-mediated link between innate immunity, inflammation, and cancer. J Clin Invest 117: 1175–1183, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lundberg AS, Randell SH, Stewart SA, Elenbaas B, Hartwell KA, Brooks MW, Fleming MD, Olsen JC, Miller SW, Weinberg RA, Hahn WC. Immortalization and transformation of primary human airway epithelial cells by gene transfer. Oncogene 21: 4577–4586, 2002 [DOI] [PubMed] [Google Scholar]
- 16. Mantovani A, Allavena P, Sica A, Balkwill F. Cancer-related inflammation. Nature 454: 436–444, 2008 [DOI] [PubMed] [Google Scholar]
- 17. O'Riordan JM, Abdel-latif MM, Ravi N, McNamara D, Byrne PJ, McDonald GS, Keeling PW, Kelleher D, Reynolds JV. Proinflammatory cytokine and nuclear factor kappa-B expression along the inflammation-metaplasia-dysplasia-adenocarcinoma sequence in the esophagus. Am J Gastroenterol 100: 1257–1264, 2005 [DOI] [PubMed] [Google Scholar]
- 18. Pedranzini L, Dechow T, Berishaj M, Comenzo R, Zhou P, Azare J, Bornmann W, Bromberg J. Pyridone 6, a pan-Janus-activated kinase inhibitor, induces growth inhibition of multiple myeloma cells. Cancer Res 66: 9714–9721, 2006 [DOI] [PubMed] [Google Scholar]
- 19. Ramirez RD, Morales CP, Herbert BS, Rohde JM, Passons C, Shay JW, Wright WE. Putative telomere-independent mechanisms of replicative aging reflect inadequate growth conditions. Genes Dev 15: 398–403, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Spechler SJ, Fitzgerald RC, Prasad GA, Wang KK. History, molecular mechanisms, and endoscopic treatment of Barrett's esophagus. Gastroenterology 138: 854–869, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Thomas LW, Lam C, Edwards SW. Mcl-1; the molecular regulation of protein function. FEBS Lett 584: 2981–2989, 2010 [DOI] [PubMed] [Google Scholar]
- 22. Thompson JE, Cubbon RM, Cummings RT, Wicker LS, Frankshun R, Cunningham BR, Cameron PM, Meinke PT, Liverton N, Weng Y, DeMartino JA. Photochemical preparation of a pyridone containing tetracycle: a Jak protein kinase inhibitor. Bioorg Med Chem Lett 12: 1219–1223, 2002 [DOI] [PubMed] [Google Scholar]
- 23. Zhang X, Yu C, Wilson K, Zhang H, Melton SD, Huo X, Wang DH, Genta RM, Spechler SJ, Souza RF. Malignant transformation of nonneoplastic Barrett's epithelial cells through well-defined genetic manipulations. PLoS One 5: e13093, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]