Abstract
Oxalobacter colonization of rat intestine was previously shown to promote enteric oxalate secretion and elimination, leading to significant reductions in urinary oxalate excretion (Hatch et al. Kidney Int 69: 691–698, 2006). The main goal of the present study, using a mouse model of primary hyperoxaluria type 1 (PH1), was to test the hypothesis that colonization of the mouse gut by Oxalobacter formigenes could enhance enteric oxalate secretion and effectively reduce the hyperoxaluria associated with this genetic disease. Wild-type (WT) mice and mice deficient in liver alanine-glyoxylate aminotransferase (Agxt) exhibiting hyperoxalemia and hyperoxaluria were used in these studies. We compared the unidirectional and net fluxes of oxalate across isolated, short-circuited large intestine of artificially colonized and noncolonized mice. In addition, plasma and urinary oxalate was determined. Our results demonstrate that the cecum and distal colon contribute significantly to enteric oxalate excretion in Oxalobacter-colonized Agxt and WT mice. In colonized Agxt mice, urinary oxalate excretion was reduced 50% (to within the normal range observed for WT mice). Moreover, plasma oxalate concentrations in Agxt mice were also normalized (reduced 50%). Colonization of WT mice was also associated with marked (up to 95%) reductions in urinary oxalate excretion. We conclude that segment-specific effects of Oxalobacter on intestinal oxalate transport in the PH1 mouse model are associated with a normalization of plasma oxalate and urinary oxalate excretion in otherwise hyperoxalemic and hyperoxaluric animals.
Keywords: cecum, proximal colon, distal colon, slc26a6
considerable evidence has emerged from human and animal studies suggesting that colonization of the intestinal tract by the anaerobic bacterium Oxalobacter formigenes plays an important role in degrading dietary sources of oxalate in the intestine, leading to reduced intestinal oxalate absorption and, consequently, a lower urinary oxalate excretion (9, 10, 15, 18–20, 22, 25–27, 29). Importantly, these bacteria, discovered by Allison et al. (1) in 1985, use oxalate as a sole carbon and energy source. In most of the studies involving human subjects, the approach has been to determine whether the lack of Oxalobacter colonization is associated with increased urinary oxalate excretion and stone formation (9, 10, 18–20, 22, 26, 29). Clinical findings suggest a direct correlation between the complete absence or decreased activity of luminal Oxalobacter and the development of recurrent oxalate stone disease (18), as well as the hyperoxaluria associated with conditions such as inflammatory bowel disease, jejunoileal bypass, and cystic fibrosis (9, 10, 26). Several other studies have also shown significantly higher urinary oxalate excretion in stone-forming Oxalobacter-negative than Oxalobacter-positive patients (19, 20, 22, 29). In 2002, Duncan et al. (6) showed, for the first time, that a single oral dose of O. formigenes resulted in a reduction in urinary oxalate following an oxalate load in four human subjects, and in two of these individuals, who were Oxalobacter-negative prior to the loading study, colonization was evident many months later. More recently, Hoppe et al. (16) reported very encouraging results: sizable, but transient, reductions in urinary oxalate in various small studies in patients with primary hyperoxaluria type 1 (PH1) who received Oxalobacter in the form of a paste/capsule.
We previously suggested that, in addition to degrading dietary sources of oxalate, Oxalobacter may be able to derive oxalate from systemic sources by initiating or enhancing active secretion of endogenously produced oxalate. Subsequently, using various approaches in rats, we demonstrated that Oxalobacter can modulate intestinal oxalate transport by inducing colonic oxalate excretion, and a consistent and beneficial consequence of this bacterial-enterocyte interaction was a significant reduction in urinary oxalate excretion due to this enteric oxalate shunt (15). These results are especially encouraging in the context of enteric oxalate elimination in PH1, where a deficiency in the liver enzyme alanine-glyoxylate aminotransferase (AGT) results in an enhanced endogenous burden of oxalate, leading to hyperoxaluria, oxalosis, and renal failure (5). Although vitamin B6 administration is effective in reducing hyperoxaluria in a small subset of PH1 patients, the only known cure for PH1 is a liver or liver-kidney transplant (21). Clearly, other treatment options require attention, and the goal of the present study was to determine whether Oxalobacter colonization of a mouse model of PH1 can enhance enteric oxalate secretion and effectively reduce hyperoxaluria. To our knowledge, this is also the first report examining oxalate handling in the mouse large intestine.
MATERIALS AND METHODS
Animals.
All animal experimentation was approved by the University of Florida Institutional Animal Care and Use Committee and was conducted in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals. Male and female mice (25–30 g) were used in the following studies; they were given free access to a standard mouse chow (diet 2018S, Harlan Teklad) and drinking water before initiation of the colonization procedure. Several experimental series involved use of an AGT knockout mouse model on a C57BL/6 background strain (Agxt), as described by Salido et al. (23), and another series included C57BL/6 [wild-type (WT)] mice as controls. At the time of the flux studies, the mice were euthanized by 100% CO2 inhalation, and the entire large intestine, including the cecum, proximal colon, and distal colon, was removed.
Urine collection and analytic methods.
Mice were housed in pairs in metabolic cages, and 24-h collections (2 mice per urine pool) were made under mineral oil into vessels containing 10 μl of 2% sodium azide as a preservative. Urinary oxalate was determined in acidified (HCl) samples collected from all the mice over a 24-h period on several occasions using a kit assay (catalog no. 591, Trinity Biotech, St. Louis, MO). The oxalate assay, which was appropriately scaled down for smaller mouse sample volumes, was initially validated by a comparison with a double enzymatic assay frequently used in our laboratory (13, 14). The latter, more sensitive, assay was used for the measurement of oxalate in mouse plasma pools (4 mice per pool). Since failure to account for oxalate losses incurred throughout the sample preparation has been shown to lead to significant underestimation of plasma/serum oxalate concentrations (13), [14C]oxalate (0.4 μCi/ml; New England Nuclear, Boston, MA) was added to each sample for the purpose of correcting for these cumulative losses. Urinary calcium concentration was determined using a calcium assay kit (Point Scientific, Canton, MI), and urinary creatinine was determined using a modification of the Jaffé reaction, as previously described (11). Although the design of the metabolic cage is such that urine and feces are collected separately and the urine receptacle contains sodium azide, we examined the possibility of fecal contamination of urine during the 24-h collection period, since this could result in degradation of urinary oxalate. The first approach involved the addition of a fecal pellet (∼20 mg) from a colonized mouse (n = 3) to an aliquot of the mouse urine sample (n = 3, 250 μl each) containing azide and spiked with [14C]oxalate. Appropriate controls included urine without (n = 3) and urine with (n = 3) a fecal pellet (∼20 mg) obtained from a noncolonized mouse. The samples were incubated at room temperature for 24 h and revealed no loss of [14C]oxalate after acidification of the sample, and volatilization of 14CO2 was determined. The second approach involved inoculating a mouse urine aliquot containing azide and [14C]oxalate tracer (as described above) with ∼10 μg wet weight of bacteria from an actively growing pure culture of a wild rat strain of O. formigenes (OxWR). The samples, which were prepared in triplicate, included controls that were not inoculated. All samples were incubated at room temperature for 24 h and revealed no loss of [14C]oxalate. Together, these results provide assurance that the changes in 24-h urinary oxalate excretion observed in the whole animal studies are not due to contamination of urine by fecal Oxalobacter activity.
Colonization studies.
Laboratory mice and rats are typically not found to be colonized with Oxalobacter, and the protocol for Oxalobacter colonization has been outlined previously (15). Because a mouse isolate is not available, OxWR (4) was used in these colonization studies. Since oxalate is an essential growth substrate for Oxalobacter sp., mice were primed by administration of a 1.5% oxalate-supplemented diet (0.5% calcium; diet 89222, Harlan Teklad) for ∼5 days prior to an esophageal gavage of a 0.5-ml inoculum containing an average of 35 mg wet wt of bacteria from a 24-h culture of OxWR. After 2 days, the mice were similarly inoculated a second time. Fresh fecal specimens were collected ∼7 days after the second gavage for detection of Oxalobacter, which was routinely determined by an anaerobic culture method (15). Approximately 20 mg of freshly collected fecal material were inoculated into anaerobically sealed vials containing a 20 mM oxalate medium, and after incubation at 37°C for ∼6–7 days, loss of most of the oxalate in the medium was indicative of the colonization status of the mouse. This simple method was validated during the present study on several occasions by two other approaches: 1) the degradation of oxalate was measured by spiking the inoculated anaerobic vials containing oxalate medium with [14C]oxalate tracer (0.01 μCi) and determining the loss of radioactivity due to 14CO2 volatilization after acidification; and 2) the presence of Oxalobacter was determined by DNA analyses using PCR, as previously described (24). Fecal material was also collected directly from the lumen of each of the three large intestinal segments when the mice were euthanized for the flux studies to confirm colonization status at that time. Prior to study, all animals were confirmed to be noncolonized.
Flux studies.
Unidirectional fluxes of oxalate across isolated segments of the mouse large intestine were measured using [14C]oxalate (Amersham, Piscataway, NJ), as previously described (7). The transport studies were conducted 7–11 days following the gavage procedure. Immediately following euthanasia, the large intestine was removed, and one pair of tissues from each of three segments, including the cecum, proximal colon, and distal colon, was prepared for the flux studies in Ussing chambers. The magnitude and direction of the net flux (JnetOx) were determined by calculation of the difference between two unidirectional fluxes [mucosal-to-serosal (JmsOx) and serosal-to-mucosal (JsmOx)] measured for 45 min at 15-min intervals under short-circuit conditions. The specific activity of the radioisotope, which remained constant throughout the flux period, was determined at the beginning and end of the experiment by sampling (50 μl) from the labeled compartment. The electrical parameters of the tissue were also recorded at 15-min intervals throughout the experiment. Tissue conductance (mS/cm2) was calculated as the ratio of the open-circuit potential (mV) to the short-circuit current (μA/cm2), and net fluxes were determined on conductance-matched tissues.
Immunoblotting.
Protein extracts (50 μg) of mucosal scrapings quantified using the bicinchoninic acid protein assay kit (Thermo Scientific, Rockford, IL) were separated on a 10% precast Tris·HCl polyacrylamide gel (Criterion, Bio-Rad, Hercules, CA). The proteins were electrophoretically transferred to a nitrocellulose membrane. Immunodetection of slc26a6 transport protein on Western blots was performed using a custom-made primary slc26a6 antibody (Alpha Diagnostic International, San Antonio, TX) and a commercial secondary goat anti-rabbit antibody (Thermo Scientific). Membranes were incubated for 1 h in 5% (wt/vol) blocking solution containing nonfat dry milk and Tris-buffered saline with 0.1% Tween 20. The blots were incubated for 3 h at room temperature in the blocking solution containing the primary antibody and then for 1 h with the secondary antibody. After each incubation period with primary or secondary antibody, the blots were extensively washed with Tris-buffered saline with 0.1% Tween 20. For detection, the membranes were reacted for 1 min with chemiluminescence reagent (Amersham) and exposed to autoradiographic Hyperfilm-ECL (Amersham). The intensity of the resulting band in each case was quantified using ImageJ (National Institutes of Health). Membranes were reprobed with a monoclonal antibody to GAPDH (Ambion, Austin, TX) to ensure equal loading of the total protein.
Statistical analyses.
Statistical analysis of the data was performed using a paired or unpaired t-test for the comparison of two means. Multiple means were compared by a one-way ANOVA followed by Bonferroni's t-test. For all analyses, differences were considered significant if P ≤ 0.05.
RESULTS
Agxt urinary phenotype.
The hyperoxaluric phenotype reported (12, 23) for Agxt mice was confirmed in the present study. Agxt mice of either sex in the pure C57BL/6 background do not form kidney stones, nor do they show any other pathology (23). Baseline urinary excretion values for oxalate, calcium, and creatinine compiled for the WT and Agxt mice used in different experimental series in this study are tabulated in Table 1. We observed that basal urinary oxalate excretion was ∼2.5-fold higher in Agxt than in WT mice fed the same standard diet. We also found that urinary calcium excretion was significantly higher (>60%) in Agxt than WT mice. However, this hypercalciuria was eliminated when Agxt mice were fed a low-calcium (0.5%) diet compared with the standard chow (1% calcium). In two of the experimental series below, urinary calcium excretion in Agxt mice fed a low-calcium diet (0.81 ± 0.07 μmol/24 h, n = 8 pools) was comparable to that of WT mice fed the same diet (0.83 ± 0.09 μmol/24 h, n = 9 pools), suggesting that the hypercalciuric phenotype of Agxt mice has a large dietary component. Finally, a significant (50%) elevation in plasma oxalate concentration was detected in Agxt compared with WT mice.
Table 1.
Selected urinary parameters and plasma oxalate in WT and Agxt mice fed standard mouse chow containing 1% calcium
| WT | Agxt | |
|---|---|---|
| Urine volume, ml/24 h | 2.46 ± 0.22 | 3.03 ± 0.24 |
| Urinary solute excretion, μmol/24 h | ||
| Ox | 0.54 ± 0.06 | 1.30 ± 0.15* |
| Ca2+ | 1.65 ± 0.18 | 2.66 ± 0.36* |
| Cr | 3.80 ± 0.29 | 4.28 ± 0.20 |
| Excretion ratio | ||
| Ox-to-Cr | 0.08 ± 0.01 | 0.26 ± 0.03* |
| Ca2+-to-Cr | 0.35 ± 0.04 | 0.63 ± 0.07* |
| Plasma Ox, μmol/l | 20.7 ± 3.9 | 40.7 ± 4.4* |
Values are means ± SE of duplicate measurements of 20-30 urine pools in each group with 2 mice per pool. Plasma oxalate (Ox) was measured in 5 wild-type (WT) pools and 6 pools from mice deficient in liver alanine-glyoxylate aminotransferase (Agxt), with 4 mice per pool. Cr, creatinine.
Statistically significantly different from WT (P ≤ 0.05).
Colonization of AGT-deficient mice reduces plasma and urinary oxalate and promotes enteric oxalate secretion.
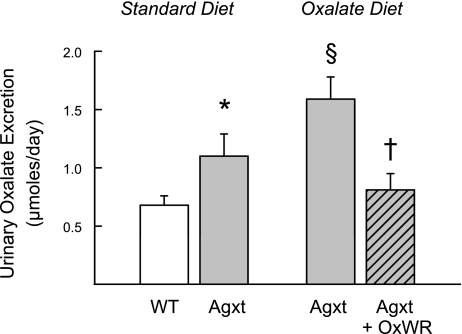
Urinary oxalate excretion in Agxt mice was shown to increase further (∼45%) when these mice were fed the oxalate-supplemented diet necessary for initiating and sustaining Oxalobacter colonization (Fig. 1). However, when Agxt mice are colonized with Oxalobacter, urinary oxalate excretion is significantly reduced to levels that are not statistically different from WT mice fed a standard diet (also shown in Fig. 1 for comparison). The significant difference in urinary oxalate excretion between the two Agxt groups, colonized vs. noncolonized, at the time of flux studies appears to be a consequence of the activity of Oxalobacter in the large intestine. In addition, colonization of Agxt results in a normalization of plasma oxalate concentrations from 40.7 ± 4.4 μM (6 plasma pools from noncolonized mice) to 20.3 ± 2.6 μM (7 plasma pools from colonized mice), revealing a 50% reduction in circulating oxalate concentrations. Colonized and noncolonized Agxt mice fed a 1.5% oxalate-0.5% calcium diet exhibited comparable urinary excretion rates for creatinine (4.23 ± 0.24 and 5.13 ± 0.64 μmol/24 h in Agxt and Agxt + OxWR, n = 5 and 6 pools, respectively) and calcium (0.89 ± 0.03 and 0.90 ± 0.09 μmol/24 h in Agxt and Agxt + OxWR, n = 5 and 6 pools, respectively) in the collections obtained immediately before the flux studies were conducted. As noted above (see Agxt urinary phenotype), the hypercalciuric phenotype of Agxt mice was eliminated when these mice were fed a diet containing 0.5% calcium. It is also clear that ∼50% of basal urinary calcium excretion in WT mice is of dietary origin.
Fig. 1.
Comparison of 24-h urinary oxalate excretion in wild-type (WT, n = 18 pools) mice and mice deficient in liver alanine-glyoxylate aminotransferase (Agxt, n = 11 pools) fed standard mouse chow. Hyperoxaluria in Agxt mice (*P < 0.05) is significantly increased when the mice (n = 5 pools) are fed 1.5% oxalate-0.5% calcium diet (§P < 0.05). In colonized Agxt mice that are also fed the oxalate-supplemented diet, urinary oxalate excretion (n = 6 pools) is significantly reduced compared with noncolonized Agxt mice fed the same diet (†P < 0.05) and not statistically different from WT mice fed the standard diet. OxWR, wild rat strain of Oxalobacter formigenes.
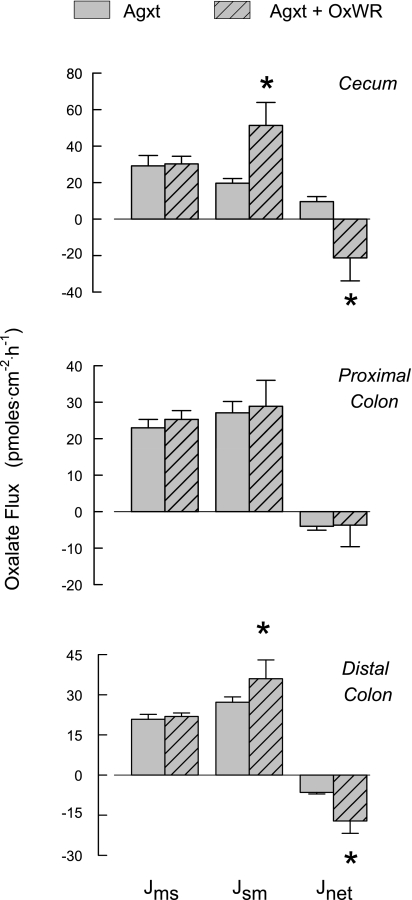
Figure 2 shows oxalate fluxes across the cecum, proximal colon, and distal colon of colonized and noncolonized Agxt mice. Of the three segments, only the noncolonized cecum supports a net absorptive flux of oxalate, which was reversed to a significant net secretory flux in the colonized mouse. Whereas a basal net secretion of oxalate was observed in the proximal and distal colonic segments removed from noncolonized mice, net oxalate secretion increased significantly in the colonized distal colon, while oxalate fluxes across the proximal colon were unaffected by colonization. The Oxalobacter-induced changes in oxalate transport in the cecum and distal colon were accompanied by significant increases in short-circuit current in these two tissue segments, indicating that the movement of some other ion(s) was also affected by colonization. No changes in transepithelial conductance were observed in any segment due to Oxalobacter colonization of Agxt mice.
Fig. 2.
Unidirectional [mucosal-to-serosal (Jms) and serosal-to-mucosal (Jsm)] and net transepithelial fluxes of oxalate across isolated, short-circuited segments of cecum, proximal colon, and distal colon from noncolonized (n = 11 tissue pairs) and artificially colonized (n = 9 tissue pairs) Agxt mice fed a diet containing 1.5% oxalate-0.5% calcium. *Significant difference between the two groups. Transepithelial conductance was not affected by colonization of any segment (20.2 ± 1.2, 19.2 ± 0.9, and 14.9 ± 0.7 mS/cm2 across cecum, proximal colon, and distal colon, respectively). Compared with noncolonized segments, short-circuit current (Isc) was significantly higher in colonized Agxt cecum (from 0.9 ± 0.1 to 1.6 ± 0.3 μeq/cm2) and distal colon (from 3.2 ± 0.4 to 5.8 ± 0.7 μeq/cm2) but remained unchanged in proximal colon (from 2.2 ± 0.3 to 2.9 ± 0.4 μeq/cm2).
Oxalobacter colonization of WT (C57BL/6) mice.
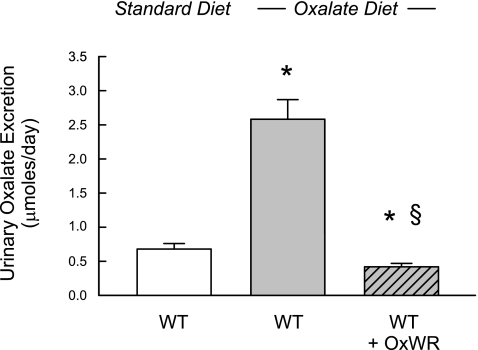
The effect of colonization on intestinal oxalate fluxes and urinary oxalate excretion was also examined in WT mice in a separate experimental series. As shown in Fig. 3, urinary oxalate excretion was almost fourfold higher in noncolonized WT mice fed the oxalate-supplemented diet than in WT mice fed the standard diet, and this dietary-induced hyperoxaluria was more than eliminated (reduced by 80%) by Oxalobacter colonization. We did not detect any changes in plasma oxalate concentrations in colonized WT mice compared with their noncolonized counterparts. As noted above, urinary calcium excretion in WT mice fed the standard mouse chow (Table 1) is reduced significantly (∼50%, to 0.83 ± 0.09 μmol/24 h, n = 9 pools) in noncolonized WT mice fed the oxalate-0.5% calcium diet and comparable to that observed in the colonized WT group fed the same low-calcium diet (0.80 ± 0.08 μmol/24 h, n = 9 pools). Urinary creatinine excretion was similar in colonized and noncolonized WT mice.
Fig. 3.
Normal 24-h urinary oxalate excretion in WT mice (n = 18 pools) fed standard mouse chow and WT mice (n = 8 pools) fed oxalate-supplemented diet (1.5% oxalate-0.5% calcium). Asterisk above the middle bar denotes a significant difference (P < 0.05) between these two groups. In colonized WT mice that are also fed the oxalate-supplemented diet (n = 10 pools), urinary oxalate is significantly reduced compared with the noncolonized group (§P < 0.05) and compared with WT mice fed the standard diet (*P < 0.05).
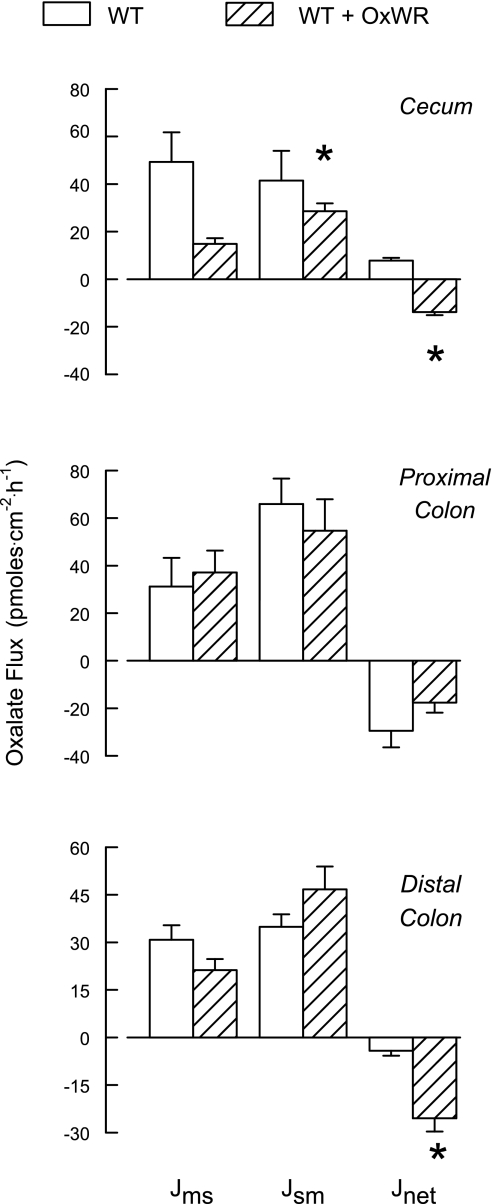
The results of the oxalate transport studies in colonized and noncolonized WT mice in Fig. 4 illustrate a similar pattern compared with the results of the Agxt series described above. Again, of the three segments examined, the noncolonized cecum supported a net absorptive flux of oxalate, which was reversed to net secretion following colonization. Similarly, both colonic segments exhibited net secretion of oxalate in noncolonized mice, and colonization significantly enhanced this secretory flux in the distal colon. While oxalate fluxes across the proximal colon were not altered by Oxalobacter colonization of WT or Agxt mice, it was clear that the magnitude of JsmOx and JnetOx was greater in WT than Agxt proximal colon whether the mice were colonized or noncolonized. No significant changes in short-circuit current or tissue conductance associated with colonization were observed in this series.
Fig. 4.
Unidirectional and net transepithelial fluxes of oxalate across isolated, short-circuited segments of cecum, proximal colon, and distal colon from noncolonized (n = 7 tissue pairs) and artificially colonized (n = 9 tissue pairs) WT mice fed 1.5% oxalate-0.5% calcium diet. *Significant difference between the two groups. Transepithelial conductance was not affected by colonization of any segment (19.9 ± 2.7, 24.1 ± 2.7, and 14.5 ± 1.5 mS/cm2, across cecum, proximal colon, and distal colon, respectively). Compared with the noncolonized segments, short-circuit current was not affected by colonization (3.1 ± 0.3, 4.6 ± 1.1, and 1.9 ± 0.3 μeq/cm2 in cecum, proximal colon, and distal colon, respectively).
Expression of slc26a6 protein in colonized Agxt and WT mice.
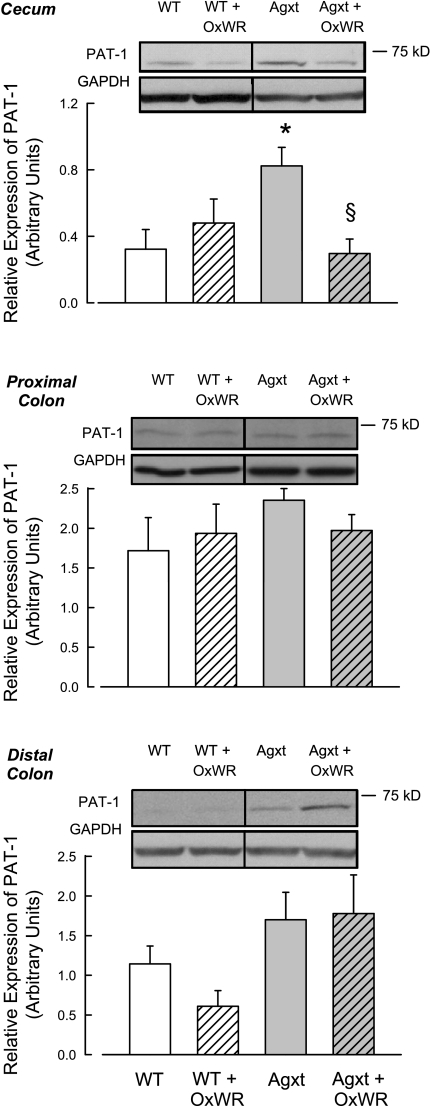
Next, we addressed whether any of the Oxalobacter-induced changes in intestinal oxalate transport could be correlated with alterations in the abundance of slc26a6, a known oxalate transporter (7). As shown in Fig. 5, slc26a6 was differentially expressed, depending on the intestinal segment examined, the Oxalobacter colonization status of the segment, and whether the tissue originated from an Agxt or a WT mouse. Interestingly, we found that the basal abundance of slc26a6 protein is greater in the cecum and distal colon of the noncolonized Agxt mouse than the noncolonized WT mouse. However, there were no differences in slc26a6 abundance in the proximal colon of the two groups. Oxalobacter colonization of the mouse large intestine did not consistently affect slc26a6 abundance in the segments examined, and there was no consistent correlation between slc26a6 abundance and the functional changes in oxalate flux. In the cecum of Agxt mice, Oxalobacter colonization led to a 64% decrease in slc26a6 protein abundance, an effect that was not evident in WT mice. In contrast, in the distal colon, Oxalobacter colonization resulted in a 46% reduction in slc26a6 protein expression in WT mice, an effect that was not evident in Agxt mice. Finally, colonization did not alter slc26a6 expression patterns in the proximal colon from either mouse group, an observation that did correlate with no changes in oxalate transport (Figs. 2 and 4).
Fig. 5.
Immunoblot analyses of slc26a6 in protein extracts of large intestine from colonized (Agxt + OxWR and WT + OxWR) and noncolonized (Agxt and WT) mice. Membranes were reprobed for GAPDH as an internal loading control. Results were quantified by densitometry from 4–12 tissues in each group. *Significant difference between noncolonized Agxt and noncolonized WT mice. §Significant difference between colonized and noncolonized Agxt mice. PAT-1, protein associated with topoisomerase II.
Sustaining OxWR colonization in mice.
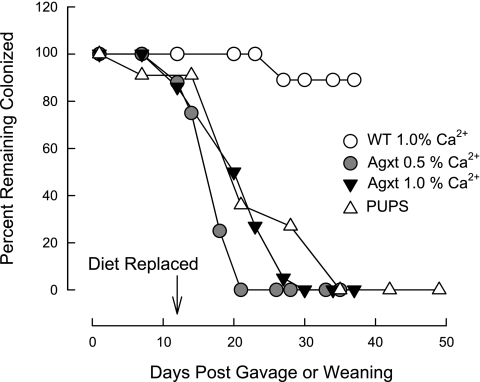
We have demonstrated that WT and Agxt mice are readily colonized with OxWR, and, unexpectedly, we found differences between the two groups in terms of the length of time colonization was sustained in the absence of exogenous dietary oxalate supplementation. The experimental design in the first series of the study involved colonizing WT and Agxt mice (n = 12 in each group) fed a 1.5% oxalate-0.5% calcium diet. After confirmation of colonization, the oxalate-supplemented diet was replaced by regular mouse chow (containing 1% calcium and 0.25% oxalate, as determined by us) at 12 days after the gavage procedure (G + 12 days). Fresh fecal specimens were obtained from the mice at intervals up to G + 35 days for the determination of Oxalobacter activity, and urinary collections were also obtained for oxalate and creatinine measurements. The results presented in Fig. 6 clearly show that, in the absence of exogenous dietary oxalate, Agxt mice lose colonization rapidly compared with WT mice. Given this unanticipated result, the study was repeated with additional animals (n = 6 WT and n = 10 Agxt mice), and, because the results were the same, they were combined for presentation in Fig. 6. Every WT (18 of 18) and Agxt (22 of 22) mouse tested positive for Oxalobacter activity on G + 7 days. By G + 30 days, 100% of Agxt mice had lost colonization compared with ∼10% of the WT mice. It is noteworthy that a subset (n = 6) of colonized animals in the WT group were followed for a longer period, and all six animals remained colonized for up to G + 75 days, at which time the study was terminated (Table 3). A reasonable addendum to this study included the reintroduction of oxalate-supplemented food to the mice that lost colonization to determine whether Oxalobacter colonization could be revived. The results in all cases, however, were negative.
Fig. 6.
Temporal characterization of loss of Oxalobacter colonization in Agxt and WT mice. In the first series, 18 artificially colonized WT mice (○) and 22 artificially colonized Agxt mice (▾) were fed 1.5% oxalate-0.5% calcium diet prior to gavage (G) with OxWR. On G + 12 days, this diet was replaced with standard chow containing 1% calcium and no oxalate supplementation. In the next series, artificially colonized Agxt mice (n = 8, ●) were fed 1.5% oxalate-0.5% calcium diet prior to gavage with OxWR. On G + 12 days, this diet was replaced with chow containing 0.5% calcium and no oxalate supplementation. The last series involved naturally colonized Agxt pups (n = 11) fed 1.5% oxalate-0.5% calcium diet before and after the day of weaning (W). On W + 12 days, this diet was replaced with standard chow containing 1% calcium and no oxalate supplementation.
Table 3.
Oxalate and creatinine concentrations in urine of WT mice before and after artificial colonization with Oxalobacter
| Urinary Excretion, μmol/24 h |
||||
|---|---|---|---|---|
| Colonization, % | Ox | Cr | Ox-to-Cr Ratio | |
| Baseline | 0 | 2.58 ± 0.29 | 4.48 ± 0.44 | 0.59 ± 0.05 |
| G + 7 | 100 | 0.35 ± 0.04* | 3.60 ± 0.17 | 0.10 ± 0.01* |
| G + 12 | 100 | 0.30 ± 0.15* | 3.71 ± 0.37 | 0.09 ± 0.01* |
| G + 27 | 89 | 0.28 ± 0.07* | 4.13 ± 0.51 | 0.05 ± 0.01* |
| G + 35 | 89 | 0.21 ± 0.05* | 4.89 ± 0.51 | 0.04 ± 0.01* |
| G + 75 | 100 | 0.11 ± 0.03* | 5.32 ± 0.45 | 0.02 ± 0.004* |
Values are means ± SE for mean of duplicate determinations on 9 urine pools. Baseline values reflect Ox excretion when the mice are fed Ox-supplemented (1.5% Ox-0.5% Ca2+) diet. After urine collection on G + 12, Ox-supplemented diet was replaced by standard (1% Ca2+) mouse chow. Value on G + 75 represents mean excretion of a subset of 6 mice.
Significantly different from baseline (P < 0.05).
Urinary oxalate excretion was tracked in Agxt and WT mice during this series of colonization studies, and the results are presented in Tables 2 and 3. A progressive reduction in urinary oxalate excretion was observed in Agxt mice following colonization, and it was maximal (∼47% reduction) at G + 27 days, when only 5% of this group of mice remained colonized (Table 2). By G + 35 days, when 100% of the mice had lost colonization, urinary oxalate excretion was not different from the precolonization baseline value. Similarly, progressive and marked reductions in urinary oxalate excretion were observed in WT mice following colonization, and by G + 35 days, when 10% of WT mice had lost colonization, urinary oxalate excretion was reduced by 90%. The subset of six mice that sustained colonization through G + 75 days exhibited even lower (∼95% reduction) oxalate excretion values (Table 3).
Table 2.
Oxalate and creatinine concentrations in urine of Agxt mice before and after artificial colonization with Oxalobacter
| Urinary Excretion, μmol/24 h |
||||
|---|---|---|---|---|
| Colonization, % | Ox | Cr | Ox-to-Cr Ratio | |
| Baseline | 0 | 1.66 ± 0.27 | 4.67 ± 0.29 | 0.38 ± 0.05 |
| G + 7 | 100 | 1.19 + 0.31* | 3.71 ± 0.28 | 0.27 ± 0.06 |
| G + 12 | 86 | 0.92 ± 0.27* | 3.96 ± 0.28 | 0.17 ± 0.06* |
| G + 19 | 50 | 0.94 ± 0.26* | 3.59 ± 0.33 | 0.17 ± 0.06* |
| G + 27 | 5 | 0.87 ± 0.21* | 4.33 ± 0.42 | 0.17 ± 0.04* |
| G + 35 | 0 | 1.46 ± 0.12 | 4.83 ± 0.33 | 0.30 ± 0.04 |
Values are means ± SE for mean of duplicate determinations on 8 urine pools. Baseline values reflect Ox excretion when the mice are fed Ox-supplemented (1.5% Ox-0.5% Ca2+) diet. After urine collection on day 12 after the gavage (G) procedure (G + 12), Ox-supplemented diet was replaced by standard (1% Ca2+) mouse chow.
Significantly different from baseline (P < 0.05).
The next series involved a similar design using Agxt mice (n = 8), but with one modification. We addressed whether the duration of colonization could be lengthened in Agxt mouse intestine if the replacement diet on G + 12 days contained 0.5% calcium, rather than the 1% calcium standard chow. In previous rat studies (15), we had demonstrated that the maintenance of Oxalobacter colonization was exquisitely sensitive to the balance between intraluminal calcium and oxalate availability. The results of this mouse study, also presented in Fig. 6, show that 100% of Agxt mice lost colonization by G + 21 days, demonstrating that this maneuver of reducing intraluminal calcium to 0.5% did not improve the duration of colonization.
In the final series of this study addressing the sustainability of Oxalobacter colonization, we determined whether naturally colonized Agxt mice sustained colonization for a longer period of time than artificially colonized mice. Two artificially colonized Agxt females (fed the oxalate-supplemented 0.5% calcium diet) gave birth to 11 pups, which were found to be 100% colonized at the time of weaning. The oxalate-supplemented diet was replaced by standard chow at 12 days postweaning (W + 12 days), and the mice began to lose colonization rapidly. Within ∼22 days after removal of oxalate from the diet, 100% of the naturally colonized mice had lost colonization (Fig. 6). Thus the loss of colonization in Agxt mice does not appear significantly different between animals that are naturally colonized and those that are artificially colonized with Oxalobacter. In addition, the results confirm previous observations in rats (3) that intestinal colonization can occur in mice from mother to offspring prior to weaning. Urinary collections were not conducted in this series.
DISCUSSION
On the basis of Oxalobacter colonization studies in rats (15), we proposed that a physiological interaction between these bacteria and the transporting colonic mucosa results in the induction of enteric secretion and elimination of oxalate, leading to significant reductions in urinary oxalate. The results of the present study using mice confirm earlier observations in rats (15). The focus of this study was to determine whether Oxalobacter colonization of a mouse model of PH1 would promote enteric oxalate secretion/elimination and, thus, reduce the oxalate burden associated with this genetic disease. As shown here, the presence of Oxalobacter in the large intestine of Agxt and WT mice is consistently associated with changes in intestinal oxalate transport and significant reductions in urinary oxalate excretion. The results obtained from this study are significant, because they show that Oxalobacter 1) modulates intestinal oxalate transport in a segment-specific manner, 2) can derive oxalate from systemic sources, in addition to degrading dietary oxalate sources, and 3) can normalize plasma and urinary oxalate excretion in a mouse model of PH1.
Oxalobacter colonization and intestinal oxalate transport.
The segmental pattern of oxalate transport in the mouse large intestine was similar in Agxt and WT groups, showing that the noncolonized mouse cecum uniquely supports a net absorptive flux compared with a net secretory flux in proximal and distal colonic segments. Whereas the magnitude of oxalate fluxes across the cecum and distal colon of Agxt and WT mice was found to be comparable, unidirectional and net oxalate fluxes across the proximal colonic segment were considerably lower in Agxt mice than in their WT counterparts. In proximal colon, net secretion of oxalate was 90% lower in noncolonized Agxt than noncolonized WT mice and 80% lower in colonized Agxt than colonized WT mice. Thus, in WT mice, the proximal colonic segment has a considerable capacity to contribute to enteric elimination of oxalate.
The segmental changes induced in oxalate transport when Oxalobacter was a component of the mucosal bacterial population were also similar in Agxt and WT mice and were confined to the cecum and distal colon, since fluxes across the proximal segment were not altered. The apparent lack of involvement of the proximal colonic segment in this regard was observed previously in studies using Oxalobacter-colonized rats (15), a result that is not readily explained. For all mice that were designated as colonized, Oxalobacter was confirmed to be present in the luminal contents of each of the large intestinal segments at the time of the flux studies; however, it is possible that the nature of the physiological-physical interaction between the bacteria and the transporting mucosa is different in the proximal colon. Nonetheless, a more robust net secretion of oxalate appears to be supported in the entire large intestine of colonized WT than Agxt mice, correlating with a larger reduction in urinary oxalate excretion of up to 95% vs. a 50% reduction in colonized Agxt mice. It is clear from our studies in rats (15) and, now, in mice that Oxalobacter can modulate the balance between renal and enteric excretion of oxalate.
One of the questions addressed was whether the Oxalobacter-induced changes in intestinal oxalate transport were due to a change in the relative abundance of the apical oxalate transporter slc26a6. Slc26a6, a multifunctional anion exchanger that mediates apical oxalate efflux in the mouse small intestine (7, 17), is reported to be more abundant in the small intestine than in the colon (28, 30). While we detected slc26a6 in the large intestine of WT and Agxt mice, it was clear that the protein tended to be more abundant in the noncolonized cecum and distal colon of Agxt than noncolonized WT mice, although this trend was not manifest in the proximal colon. Despite the differences in basal expression of slc26a6 in Agxt and WT mouse cecum and distal colon, there was no correlation between the abundance of this oxalate transporter and the pattern and magnitude of oxalate fluxes in these two segments. Furthermore, in the proximal colon, the similar pattern of slc26a6 protein expression in WT and Agxt mice did not reflect the significant difference in the magnitude of unidirectional and net oxalate fluxes that was evident between these two groups. There was also no consistent effect of Oxalobacter colonization on slc26a6 expression that could be correlated with directional changes in oxalate flux in the cecum or distal colon, and the proximal colon appeared refractory to the presence of the bacteria. While we did not detect any changes in the relative abundance of slc26a6 that correlated with functional changes, slc26a6 has been shown to mediate a significant fraction of oxalate efflux across the ileal apical membrane (7) and makes an important contribution to transepithelial oxalate transport in Caco-2 monolayers (8). Indeed, other oxalate transporters in the slc26a gene family, such as slc26a1, slc26a3, and slc26a4, are potential candidates for future study in this regard. In also addressing this issue, it is important to note that, in the intestine, for example, compensatory intraorgan differences in oxalate handling (cecum vs. colon) or, indeed, compensatory interorgan oxalate handling (intestine vs. kidney) may occur in the absence of any detectable change in protein abundance or mRNA expression. We also note that the cellular location of the transport protein cannot be ascertained by the immunoblot assay we conducted on protein extracts of intestinal scrapings and that trafficking of slc26a6 to and from the apical membrane could be influenced. Finally, it is possible that the changes in vectorial oxalate transport observed in colonized cecum and distal colon are primarily dependent on the magnitude and direction of counterion gradients in vivo, as demonstrated in in vitro studies conducted by us using Caco-2 monolayers (8). The latter study showed that vectorial transport of oxalate mediated specifically by slc26a6 is more dependent on the magnitude and direction of counterion gradients than an intrinsic property of the protein. Clearly, a better understanding of the prevailing counterion driver gradients, in addition to the relative affinities of the ions transported by these anion exchangers, is required.
Sustaining Oxalobacter colonization in mice.
In previous studies in rats (4, 15, 25), it was demonstrated that the success and maintenance of colonization depend on the availability of intraluminal oxalate and, in particular, the balance between intraluminal oxalate and calcium (15). In the present study, we have shown that Agxt and WT mice are readily colonized by esophageal gavage of a live rat strain of Oxalobacter cells, but there was a striking difference between the two groups in terms of the duration of colonization after the oxalate-supplemented diet (0.5% calcium) was replaced by regular chow (1% calcium). A subset of six WT mice remained 100% colonized ∼11 wk later, whereas 100% of Agxt mice had lost colonization within 18 days of removal of dietary oxalate supplementation. These results were consistent whether the Agxt mice were colonized artificially or naturally, and 50% reduction of dietary calcium did not improve the length of time colonization was sustained in these mice. It is possible, however, that further more extreme reductions in dietary calcium could be effective in sustaining colonization for a longer period in this knockout mouse. Although little or nothing is known about the nature of Oxalobacter colonization and the factors involved, intraluminal calcium activity in Agxt mice may indeed be a contributing factor. Clearly, calcium handling in Agxt mice is different from that in WT mice, as indicated by their dietary-dependent hypercalciuric phenotype. Other factors are not immediately obvious, however.
A consistent feature of Oxalobacter colonization in all the experimental series involving Agxt and WT mice was a reduction in urinary oxalate excretion. We have observed progressive reductions over time when the mice sustain colonization, and this adaptive response is best illustrated in WT mice (Table 3) that remain colonized for ∼11 wk in the absence of exogenously supplied oxalate. Urinary oxalate excretion is 80–95% lower in colonized than noncolonized WT mice fed the same diet, and the induction of enteric oxalate secretion/excretion provides the simplest explanation for these results. In Agxt mice, the reduction in urinary oxalate excretion was reversed when colonization was lost, a result consistent with observations of Hoppe et al. (16) in various small studies of PH patients treated with Oxalobacter in the form of a paste/capsule on a short-term basis. If Agxt mice had sustained colonization for a longer period, it is highly likely that this would correlate with even further reductions in urinary oxalate in these mice. Nonetheless, Oxalobacter colonization of Agxt mice normalizes urinary oxalate excretion and plasma oxalate concentrations, and the results obtained here provide compelling evidence for the potential role of Oxalobacter in effectively reducing the endogenous oxalate load generated in PH. Whether there are differences between PH patients and normal healthy individuals in sustaining Oxalobacter colonization or whether this phenomenon is peculiar to Agxt mice is unknown. Certainly, an understanding of Oxalobacter colonization, including the factors that impact colonization, is necessary to better interpret the results of this animal study.
In conclusion, the present study is the first to show that when WT mice are colonized, there is a progressive lowering of urinary oxalate excretion with time in the absence of repeated treatments. Furthermore, this study is the first to show that dietary oxalate is not required for sustaining colonization in these WT animals, which was, heretofore, thought to be the case. The results in this report are especially important, because they highlight the fact that there are differences (due to as-yet-unknown factors) in the luminal environment (Agxt vs. WT) impacting colonization independent of oxalate availability. Importantly, we have demonstrated that segment-specific effects of Oxalobacter on intestinal oxalate transport in a PH1 mouse model are associated with a normalization of plasma oxalate and urinary oxalate excretion in otherwise hyperoxalemic and hyperoxaluric animals. The ramifications of these observations made in PH1 may be highly relevant in idiopathic calcium oxalate stone disease, as well as in PH2 (5) and the newly classified PH3 (2). Whether Oxalobacter or products of Oxalobacter can be used therapeutically to treat PH patients, as well as influence oxalate stone formation in other patient populations, warrants long-term investigations.
GRANTS
This work was supported by National Institute of Diabetes and Digestive and Kidney Diseases Grant DK-081624 and a grant from the Oxalosis and Hyperoxaluria Foundation.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
ACKNOWLEDGMENTS
The technical assistance of Jonas Reid, Sonia Gabrilovich, and Shannon Moore is greatly appreciated.
REFERENCES
- 1. Allison MJ, Dawson KA, Mayberry WR, Foss JG. Oxalobacter formigenes gen. nov, sp. nov: oxalate-degrading anaerobes that inhabit the gastrointestinal tract. Arch Microbiol 141: 1–7, 1985 [DOI] [PubMed] [Google Scholar]
- 2. Belostotsky R, Seboun E, Idelson GH, Milliner DS, Becker-Cohen R, Rinat C, Monico CG, Feinstein S, Ben-Shalom E, Magen D, Weissman I, Charon C, Frishberg Y. Mutations in DHDPSL are responsible for primary hyperoxaluria type III. Am J Hum Genet 87: 392–399, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Cornelius JG, Peck AB. Colonization of the neonatal rat intestinal tract from environmental exposure to the anaerobic bacterium Oxalobacter formigenes. J Med Microbiol 53: 249–254, 2004 [DOI] [PubMed] [Google Scholar]
- 4. Daniel SL, Hartman PA, Allison MJ. Microbial degradation of oxalate in the gastrointestinal tracts of rats. Appl Environ Microbiol 53: 1793–1797, 1987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Danpure CJ. Primary hyperoxaluria. In: Metabolic Basis of Inherited Disease ( 8th ed.), edited by Scriver C, Beaudet AL, Sly WS, Valle D. New York: McGraw-Hill, 2001, p. 3323–3367 [Google Scholar]
- 6. Duncan SH, Richardson AJ, Kaul P, Holmes RP, Allison MJ, Stewart CS. Oxalobacter formigenes and its potential role in human health. Appl Environ Microbiol 68: 3841–3847, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Freel RW, Hatch M, Green M, Soleimani M. Ileal oxalate absorption and urinary oxalate excretion are enhanced in Slc26a6 null mice. Am J Physiol Gastrointest Liver Physiol 290: G719–G728, 2006 [DOI] [PubMed] [Google Scholar]
- 8. Freel RW, Morozumi M, Hatch M. Parsing apical oxalate exchange in Caco-2BBe1 monolayers: siRNA knockdown of SLC26A6 reveals the role and properties of PAT-1. Am J Physiol Gastrointest Liver Physiol 297: G918–G929, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Goldkind L, Cave DR, Jaffin B, Bliss CM, Allison MJ. Bacterial oxalate metabolism in the human colon: a possible factor in enteric hyperoxaluria (Abstract). Gastroenterology 90: 1431, 1986 [Google Scholar]
- 10. Goldkind L, Cave DR, Jaffin B, Robinson W, Bliss CM. A new factor in enteric hyperoxaluria: Oxalobacter formigenes (Abstract) Am J Gastroenterol 80: 860, 1985 [Google Scholar]
- 11. Green ML, Hatch M, Freel RW. Ethylene glycol induces hyperoxaluria without metabolic acidosis in rats. Am J Physiol Renal Physiol 289: F536–F543, 2005 [DOI] [PubMed] [Google Scholar]
- 12. Grujic D, Salido EC, Shenoy BC, Langman CB, McGrath ME, Patel RJ, Rashid A, Mandapati S, Jung CW, Margolin AL. Hyperoxaluria is reduced and nephrocalcinosis prevented with an oxalate-degrading enzyme in mice with hyperoxaluria. Am J Nephrol 29: 86–93, 2009 [DOI] [PubMed] [Google Scholar]
- 13. Harris AH, Freel RW, Hatch M. Serum oxalate in human beings and rats as determined with the use of ion chromatography. J Lab Clin Med 144: 45–52, 2004 [DOI] [PubMed] [Google Scholar]
- 14. Hatch M. Spectrophotometric determination of oxalate in whole blood. Clin Chim Acta 193: 199–202, 1990 [DOI] [PubMed] [Google Scholar]
- 15. Hatch M, Cornelius J, Allison M, Sidhu H, Peck A, Freel RW. Oxalobacter sp. reduces urinary oxalate excretion by promoting enteric oxalate secretion. Kidney Int 69: 691–698, 2006 [DOI] [PubMed] [Google Scholar]
- 16. Hoppe B, Beck B, Gatter N, von Unruh G, Tischer A, Hesse A, Laube N, Kaul P, Sidhu H. Oxalobacter formigenes: a potential tool for the treatment of primary hyperoxaluria type 1. Kidney Int 70: 1305–1311, 2006 [DOI] [PubMed] [Google Scholar]
- 17. Jiang Z, Asplin JR, Evan AP, Rajendran VM, Velazquez H, Nottoli TP, Binder HJ, Aronson PS. Calcium oxalate urolithiasis in mice lacking anion transporter Slc26a6. Nat Genet 38: 474–478, 2006 [DOI] [PubMed] [Google Scholar]
- 18. Kleinschmidt K, Mahlmann A, Hautmann R. Microbial degradation of dietary oxalate in the human gut and urinary oxalate concentrations in patients with calcium oxalate urolithiasis and control persons. Investig Urol (Berl) 5: 222–224, 1994 [PubMed] [Google Scholar]
- 19. Kwak C, Kim HK, Kim EC, Choi MS, Kim HH. Urinary oxalate levels and the enteric bacterium Oxalobacter formigenes in patients with calcium oxalate urolithiasis. Eur Urol 44: 475–481, 2003 [DOI] [PubMed] [Google Scholar]
- 20. Mikami K, Akakura K, Takei K, Ueda T, Mizoguchi K, Noda M, Miyake M, Ito H. Association of absence of intestinal oxalate degrading bacteria with urinary calcium oxalate stone formation. Int J Urol 10: 293–296, 2003 [DOI] [PubMed] [Google Scholar]
- 21. Monico CG, Milliner DS. Combined liver-kidney and kidney-alone transplantation in primary hyperoxaluria. Liver Transpl 7: 954–963, 2001 [DOI] [PubMed] [Google Scholar]
- 22. Neuhaus TJ, Belzer T, Blau N, Hoppe B, Sidhu H, Leumann E. Urinary oxalate excretion in urolithiasis and nephrocalcinosis. Arch Dis Child 82: 322–326, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Salido EC, Li XM, Lu Y, Wang X, Santana A, Roy-Chowdhury N, Torres A, Shapiro LJ, Roy-Chowdhury J. Alanine-glyoxylate aminotransferase-deficient mice, a model for primary hyperoxaluria that responds to adenoviral gene transfer. Proc Natl Acad Sci USA 103: 18249–18254, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Sidhu H, Allison M, Peck AB. Identification and classification of Oxalobacter formigenes strains by using oligonucleotide probes and primers. J Clin Microbiol 35: 350–353, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Sidhu H, Allison MJ, Chow JM, Clark A, Peck AB. Rapid reversal of hyperoxaluria in a rat model after probiotic administration of Oxalobacter formigenes. J Urol 166: 1487–1491, 2001 [PubMed] [Google Scholar]
- 26. Sidhu H, Hoppe B, Hesse A, Tenbrock K, Bromme S, Rietschel E, Peck AB. Absence of Oxalobacter formigenes in cystic fibrosis patients: a risk factor for hyperoxaluria. Lancet 352: 1026–1029, 1998 [DOI] [PubMed] [Google Scholar]
- 27. Sidhu H, Schmidt ME, Cornelius JG, Thamilselvan S, Khan SR, Hesse A, Peck AB. Direct correlation between hyperoxaluria/oxalate stone disease and the absence of the gastrointestinal tract-dwelling bacterium Oxalobacter formigenes: possible prevention by gut recolonization or enzyme replacement therapy. J Am Soc Nephrol 10 Suppl 14: S334–S340, 1999 [PubMed] [Google Scholar]
- 28. Simpson JE, Schweinfest CW, Shull GE, Gawenis LR, Walker NM, Boyle KT, Soleimani M, Clarke LL. PAT-1 (Slc26a6) is the predominant apical membrane Cl−/HCO3− exchanger in the upper villous epithelium of the murine duodenum. Am J Physiol Gastrointest Liver Physiol 292: G1079–G1088, 2007 [DOI] [PubMed] [Google Scholar]
- 29. Troxel SA, Sidhu H, Kaul P, Low RK. Intestinal Oxalobacter formigenes colonization in calcium oxalate stone formers and its relation to urinary oxalate. J Endourol 17: 173–176, 2003 [DOI] [PubMed] [Google Scholar]
- 30. Wang Z, Petrovic S, Mann E, Soleimani M. Identification of an apical Cl−/HCO3− exchanger in the small intestine. Am J Physiol Gastrointest Liver Physiol 282: G573–G579, 2002 [DOI] [PubMed] [Google Scholar]