Abstract
Irritable bowel syndrome is characterized by colorectal hypersensitivity and contributed to by sensitized mechanosensitive primary afferents and recruitment of mechanoinsensitive (silent) afferents. Neurotrophic factors are well known to orchestrate dynamic changes in the properties of sensory neurons. Although pain modulation by proteins in the glial cell line-derived neurotrophic factor (GDNF) family has been documented in various pathophysiological states, their role in colorectal hypersensitivity remains unexplored. Therefore, we investigated the involvement of the GDNF family receptor α-3 (GFRα3) signaling in visceral hypersensitivity by quantifying visceromotor responses (VMR) to colorectal distension before and after intracolonic treatment with 2,4,6-trinitrobenzene sulfonic acid (TNBS). Baseline responses to colorectal distension did not differ between C57BL/6 and GFRα3 knockout (KO) mice. Relative to intracolonic saline treatment, TNBS significantly enhanced the VMR to colorectal distension in C57BL/6 mice 2, 7, 10, and 14 days posttreatment, whereas TNBS-induced visceral hypersensitivity was significantly suppressed in GFRα3 KO mice. The proportion of GFRα3 immunopositive thoracolumbar and lumbosacral colorectal dorsal root ganglion neurons was significantly elevated 2 days after TNBS treatment. In single fiber recordings, responses to circumferential stretch of colorectal afferent endings in C57BL/6 mice were significantly increased (sensitized) after exposure to an inflammatory soup, whereas responses to stretch did not sensitize in GFRα3 KO mice. These findings suggest that enhanced GFRα3 signaling in visceral afferents may contribute to development of colorectal hypersensitivity.
Keywords: irritable bowel syndrome, growth factors, visceral pain
irritable bowel syndrome (IBS) is characterized by recurring (and sometimes persistent) abdominal pain in the absence of a demonstrable organic cause to explain symptoms. Lowered visceral pain/discomfort thresholds to intrarectal balloon inflation have been well documented, confirming the presence of hypersensitivity in IBS (1, 6, 12, 29). Because intrarectal instillation of a local anesthetic promptly relieves the pain and discomfort of IBS as well as the associated abdominal tenderness (32), colorectal afferents and various receptors and mediators have been targeted as contributors to development and maintenance of colorectal hypersensitivity.
For example, three members of the transient receptor potential family (TRPV1, TRPV4, and TRPA1) have been shown to be expressed in colorectal afferents, and their modulation, either pharmacologically or genetically, has been shown to alter colorectal mechanosensitivity (4, 5, 7, 8, 17). Some of these receptors have been shown to be differentially expressed in lumbar splanchnic and pelvic nerve colorectal afferents [TRPV4 (4); TRPA1 (J. A. Christianson, G. F. Gebhart, and B. M. Davis, unpublished observations)] and in different functional types of pelvic nerve afferents [TRPV1 (24)]. In addition, purinergic receptors (particularly P2X3) and neurotrophins [e.g., nerve growth factor (NGF)] have been implicated in colorectal hypersensitivity (2, 11, 21, 31, 33). Although NGF has been established as contributing to nociceptor sensitization (22, 26, 27), relatively little is known about contributions of other neurotrophic growth factors.
Recently, artemin [a glial cell line-derived neurotrophic factor (GDNF) family ligand] and its preferred GDNF family receptor α-3 (GFRα3) have drawn attention as being involved in pain modulation. GFRα3 is extensively colocalized with TRPV1 and TRPA1 in dorsal root ganglion (DRG) neurons, and artemin overexpression in skin upregulates GFRα3 and TRP expression in DRG neurons and increases thermal sensitivity (13). Artemin has been reported to potentiate capsaicin-evoked Ca2+ influx in isolated DRG neurons and to produce thermal hyperalgesia (23). Thus artemin and its receptor GFRα3 appear to contribute significantly to development of hypersensitivity, at least in cutaneous models. However, their role in visceral nociception and hypersensitivity has not been reported. In the present study, we investigated the involvement of GFRα3 signaling in colorectal mechanotransduction and colorectal hypersensitivity produced by an inflammatory challenge. Colorectal inflammation and hypersensitivity was produced by intracolonic treatment with 2,4,6-trinitrobenzene sulfonic acid (TNBS), a widely used model of colitis that produces long-lasting colorectal hypersensitivity during and after resolution of colorectal inflammation (16).
MATERIALS AND METHODS
Animals.
Experiments were conducted with C57BL/6 mice (Taconic, Germantown, NY) and GFRα3 knockout (KO) mice weighing 20–30 g (backcrossed onto the C57BL/6 genetic background for >10 generations). GFRα3 KO mice were graciously provided by Dr. Jeffrey Milbrandt, Washington University School of Medicine. The genetic status of mice was confirmed by PCR genotyping as described in Honma et al. (15). Mice were housed in an American Association for the Accreditation of Laboratory Animal Care-accredited animal facility with free access to water and food. All procedures were approved by the University of Pittsburgh Institutional Animal Care and Use Committee. Behavioral assessment of colorectal hypersensitivity was carried out by the investigators in the Rodent Behavioral Assay Core in the animal facility.
Colorectal distension and sensitivity.
All surgical procedures were performed under 2–3% isoflurane anesthesia (Hospira, Lake Forest, IL). Following hair removal and disinfection of the surgical area, the lower right abdominal musculature was exposed. Stripped ends of two sets of sterilized Teflon-coated stainless steel wire (Cooner Wire Sales, Chatsworth, CA) were inserted in the peritoneal musculature and secured in place with sutures. The other ends were tunneled subcutaneously to the back of the head (see Ref. 10 for details). Wounds were sutured and treated with a local anesthetic ointment. Buprenorphine (0.9 μg/mouse) was administered subcutaneously for pain management. After electrode implantation, mice were housed separately.
Following a 4- to 5-day recovery period from surgery, baseline responses to colorectal distension (CRD) were obtained. As previously described (10, 20, 31), mice were exposed to 2% isoflurane for brief sedation to allow balloon insertion. A lubricated polyethylene balloon (length, 1.5 cm; diameter, 0.9 cm) was inserted transanally to 0.5 cm beyond the anal sphincter and taped securely to the tail. Movement of mice was restricted by placement in a plastic tube (20) and allowed to recover from isoflurane ≥30 min. CRD consisted of constant-pressure phasic balloon inflation (15, 30, 45, and 60 mmHg, 10 s) every 3 min, three times at each distension pressure. Electromyographic (EMG) activity was recorded from the peritoneal musculature, amplified, and rectified. The rectified EMG was quantified as area under the curve using the modulus program in the Spike 2 software [Cambridge Electronic Design (CED), Cambridge, UK] (see Ref. 10 for details). Responses to CRD were taken as the difference in EMG during distension minus the resting EMG (recorded in the 10-s period immediately before each distension) normalized as percentage of the baseline response to 60 mmHg CRD.
After baseline responses were determined, mice were anesthetized (2–3% isoflurane), and either 0.2 ml of saline or TNBS (10 mg/ml; Sigma-Aldrich, St. Louis, MO) in 50% ethanol was given transanally using an oral feeding tube inserted 20–30 mm in the colon from the anus. Responses to CRD were subsequently determined 2, 7, 10, and 14 days after intracolonic treatment.
Colorectal compliance was measured in GFRα3 KO and C57BL/6 mice (n = 4 each) by measuring the volume of fluid required to produce distending pressures of 15, 30, 45, and 60 mmHg. Each mouse was subjected to three trials, first while sedated with isoflurane and the third after recovery from sedation.
Immunohistochemistry.
To label DRG neurons innervating the colorectum, mice were anesthetized (2–3% isoflurane), a laparotomy was performed, and 50 mg/ml (in 100% DMSO) of 1,1′-dioctadecyl-3,3,3′,3-tetramethylindocarbocyanine methane sulfonate (DiI; Molecular Probes, Eugene, OR) was injected in one to three sites within the distal colorectum (∼6 μl/site). Visible leakage of DiI was removed with a cotton swab. All wounds were sutured, and mice were treated as above and housed separately for 14 days before intracolonic instillation of vehicle or TNBS (as above). At different times after intracolonic treatment, mice were deeply anesthetized with ketamine/xylazine (87.5/12.5 mg/kg ip) and transcardially perfused with saline followed by a cold fixative containing 4% paraformaldehyde in 0.2% picric acid and 0.1 M phosphate buffer (pH 7.4). Thoracolumbar (T11-L1) and lumbosacral (L6-S2) DRG were removed and postfixed in the same fixative for 4 h at 4°C. After cryoprotection for 12 h in 0.01 M PBS containing 20% sucrose, DRG were embedded in Tissue-Tek (Sakura Finetechnical, Tokyo, Japan) and cut on a cryostat (10 μm) at −20°C. To examine GFRα3, DRG sections were incubated with rabbit anti-mouse GFRα3 antibody (1:200; R&D Systems, Minneapolis, MN) diluted in 0.01 M PBS containing 0.3% Triton and 4% donkey serum overnight at 4°C. After being washed in 0.01 M PBS, the sections were incubated with AlexaFluor488-conjugated goat anti-rabbit IgG (1:200; Invitrogen, Carlsbad, CA) diluted in 0.01 M PBS for 2 h at room temperature. Following washes in PBS, sections were coverslipped in mounting medium (Dako, Carpinteria, CA). Immunofluorescence was detected using a fluorescence microscope (Nikon Eclipse TE 300 with a CCD spot camera) with appropriate filters. The number of DiI-labeled DRG neurons and of those also GFRα3 immunopositive in three randomly selected DRG sections at each level were counted without knowledge of intracolonic treatment. Cells twofold or more intense than average background were considered positive for GFRα3 immunoreactivity. No specific labeling was observed in the absence of primary antibody.
Western blotting.
Mice were deeply anesthetized with ketamine/xylazine (87.5/12.5 mg/kg ip) at varying times after TNBS treatment, and the distal colorectum (∼2 cm) was removed and homogenized in lysis buffer containing 0.5% SDS, 50 mM Tris·HCl, pH 7.4, and protease inhibitors (1 μg/ml pepstatin, 1 μg/ml leupeptin, 1 μg/ml aprotinin, 1 mM sodium orthovanadate, and 100 μg/ml phenylmethylsulfonyl fluoride). Samples (n = 3 for each group) were centrifuged for 10 min at 14,000 rpm at 4°C, and protein concentration of the supernatant was determined using a Pierce 660 nm protein assay kit (Thermo Scientific, Rockford, IL). Protein samples were heat-denatured in Laemmli sample buffer solution (Bio-Rad, Hercules, CA) and stored at −80°C until use.
Samples (35 μg) were subject to electrophoresis for protein separation on 10% SDS-PAGE and electroblotted onto polyvinylidene difluoride membranes (GE Healthcare, Piscataway, NJ). Membranes were incubated overnight at 4°C with artemin antibody (Santa Cruz, Santa Cruz, CA) diluted in TBS solution containing 5% skim milk. Artemin binding was visualized using a horseradish peroxidase-conjugated donkey anti-goat antibody (Santa Cruz) and SuperSignal West Pico Chemiluminescent Substrate (Thermo Scientific). Band intensity was quantified using a Fuji Las-1000 imaging system (Fuji, Valhalla, NY) and normalized to GAPDH immunoreactivity on blots reprobed with anti-GAPDH antibody (Santa Cruz) after removing artemin binding using a stripping reagent (Thermo Scientific).
In vitro colon-pelvic nerve preparation.
As previously described (14, 18), mice were killed by inhalation of CO2 followed by exsanguination after perforating the right atrium. The colorectum with major pelvic ganglion and pelvic nerve attached was removed, and the colorectum was opened longitudinally along the antimesenteric border and placed in modified Krebs solution on ice [in mM: 117.9 NaCl, 4.7 KCl, 25 NaHCO3, 1.3 NaH2PO4, 1.2 MgSO4(H2O)7, 2.5 CaCl2, 11.1 d-glucose, 2 butyrate, and 20 acetate] bubbled with carbogen (95% O2-5% CO2) to which was added the L-type calcium channel antagonist nifedipine (4 μM; to block spontaneous muscle contractions) and indomethacin (3 μM; to block endogenous prostaglandin synthesis). The tissue preparation was pinned flat mucosal-side-up in a custom-made organ bath. The pelvic nerves were extended from the tissue chamber and laid on a mirror in a separate recording chamber isolated by a movable wall with a small hole allowing passage of the nerves. The recording and the tissue chambers were filled with paraffin oil and perfused with the Krebs solution at 33–34°C, respectively. The pelvic nerve was teased into fine bundles ∼10 μm thick by fine forceps after peeling the sheath surrounding the nerve to expose the nerve trunk.
An individual nerve bundle was placed on a platinum-iridium recording electrode, and electrical signals generated by fibers were amplified, filtered, and sampled via a 1401 interface (sample rate: 20 kHz; CED), which allowed signal monitoring on Spike2 software. A reference electrode was placed in the tissue chamber. To identify mechanosensitive receptive fields, the mucosal surface was stroked with a brush, after which responses to circumferential stretch and stroking of the receptive field with calibrated nylon filaments (10 mg force) were determined. Circumferential stretch was achieved using custom-built claws attached at 1-mm intervals along the length of the antimesenteric edge of the colorectum and fixed to a rigid plastic block whose displacement was regulated by a servo-controlled force actuator (series 300B dual mode servo system; Aurora Scientific, Toronto, Canada). As described previously (14), this produces a homogeneous circumferential stretch (slow ramped force from 0 to 170 mN at 5 mN/s) and allows mathematical conversion of the circumferential force to intralumenal pressure [pressure = 2πforce/(LD), where L is colon length and D is the circumference]. The forces applied convert to pressures between 0 and 45 mmHg distension.
Pelvic nerve afferent sensitization after application of an inflammatory soup (IS) was investigated in GFRα3 KO and C57BL/6 mice. The IS consisted of bradykinin, PGE2, serotonin, and histamine (all at 10 μM) with pH adjusted to 6.0. Only stretch-sensitive receptive endings were studied. IS was applied for 3 min, and responses to stretch were compared before and 2 min after IS washout. At least 5 min separated successive ramped stretch of the colon. All recorded data were saved to a personal computer and analyzed by Spike2 software.
Myeloperoxidase activity assay.
Myeloperoxidase (MPO) activity was evaluated as a biochemical indication of inflammation. Mice were killed under deep isoflurane anesthesia, and the distal colorectum (∼2 cm) was removed as above at varying times after intracolonic treatment, rinsed in saline, and weighed. Finely minced colon was homogenized in potassium phosphate buffer (pH 6.0) containing 0.5% hexadecyltrimethylammonium bromide, and the tissue suspensions were frozen and thawed three times. The supernatant was collected after centrifugation for 5 min at 13,000 rpm and mixed with phosphate buffer containing o-dianisidine dihydrochloride (Sigma-Aldrich) and 0.0005% hydrogen peroxide. The samples underwent three freeze-thaw cycles and were centrifuged two times and loaded, along with MPO standards (Calbiochem, San Diego, CA), on a 96-well plate. The samples and standards were reacted with o-dianisidine dihydrochloride (Sigma) and read on a plate reader at 460 nm every 20 s for 15 min. The slope for each standard was calculated, plotted, and used to calculate units of MPO activity/tissue weight for each sample.
Data analysis.
Results were analyzed by one-way, two-way, or repeated-measures two-way ANOVA followed by Bonferroni or Holm-Sidak's post hoc multiple comparisons as appropriate using SigmaPlot version 9.0 (Systat Software, San Jose, CA). P < 0.05 was considered statistically significant.
RESULTS
TNBS-induced visceral hypersensitivity.
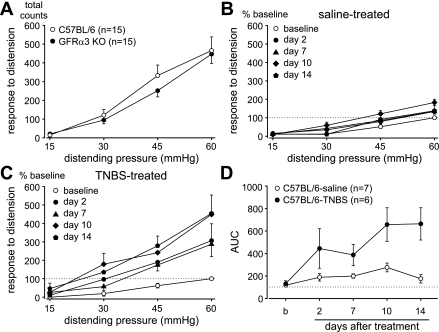
We first examined baseline responses to CRD in C57BL/6 and GFRα3 KO mice. Figure 1A reveals equivalent visceromotor responses in these two genotypes [F(1,112) = 1.07, P = 0.304]. Subsequently, the effect of intracolonic TNBS treatment on responses to distension was investigated. Figures 1 and 2 summarize data collected before (baseline) and 2, 7, 10, and 14 days after intracolonic administration of either saline (as control) or TNBS in C57BL/6 (Fig. 1, B–D) and GFRα3 (Fig. 2, A–C) KO mice. Relative to intracolonic instillation of saline, TNBS produced a robust colorectal hypersensitivity in C57BL/6 mice on all days of testing after treatment [F(4,20) = 6.32, P = 0.002; Fig. 1C]. Repeated CRD in saline-treated mice produced no colorectal hypersensitivity except on day 10 [overall F(4,24) = 5.37; Holm-Sidak post hoc test, t = 4.58, P < 0.05; Fig. 1B]. The TNBS-treated group differed significantly from the saline-treated group [F(1,44) = 8.09, P = 0.016; Fig. 1D].
Fig. 1.
Responses to colorectal distension. A: visceromotor responses to distension did not differ between C57BL/6 and glial cell line-derived neurotrophic factor family receptor α-3 (GFRα3) knockout (KO) mice [F(1,112) = 1.07, P = 0.304]. Relative to intracolonic treatment with saline (B), 2,4,6-trinitrobenzene sulfonic acid (TNBS, C) significantly increased visceromotor responses to distension on all days of testing [TNBS, n = 6, F(4,20) = 6.32, P = 0.002]. Responses to distension in saline-treated mice were slightly, but significantly, increased on day 10 of testing relative to baseline [saline, n = 7, overall F(4,24) = 5.37; Holm-Sidak post hoc test, t = 4.58, P < 0.05]. Data are expressed as means ± SE of responses normalized to the pretreatment baseline response to 60 mmHg. D: areas under the stimulus-response curves (AUC) derived from the data presented in B and C. Visceral hypersensitivity produced by TNBS significantly differed in magnitude across days after treatment [F(1,44) = 8.09, P = 0.0160].
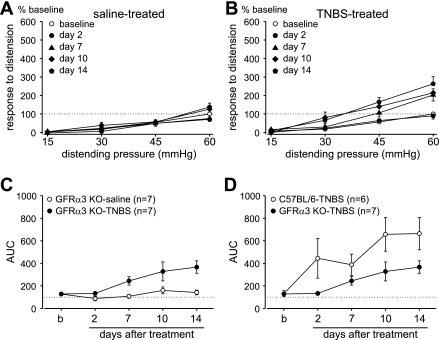
Fig. 2.
TNBS-produced visceral hypersensitivity is significantly attenuated in GFRα3 KO mice. Intracolonic treatment with TNBS (B) but not saline (A) significantly increased visceromotor responses to distension [saline, n = 7, overall F(4,24) = 3.65; Holm-Sidak post hoc test, t = 1.78, P > 0.05; TNBS, n = 7, F(4,24) = 9.10, P < 0.001]. Data are expressed as means ± SE of responses normalized to the pretreatment baseline response to 60 mmHg. C: AUC derived from the data presented in A and B. Visceral hypersensitivity produced by TNBS in GFRα3 KO mice significantly differed in magnitude across days after treatment [F(1,48) = 10.43, P = 0.007]. D: comparison of TNBS-induced stimulus-response curves (AUC) between C57BL/6 (from Fig. 1D) and GFRα3 KO mice. TNBS induced significantly greater visceral hypersensitivity in C57BL/6 mice than in GFRα3 KO mice [F(1,44) = 4.96, P = 0.048].
Similarly, intracolonic instillation of TNBS produced colorectal hypersensitivity in GFRα3 KO mice on days 7, 10, and 14 after treatment [F(4,24) = 9.10, P < 0.001; Fig. 2B], but not on day 2 after treatment. Repeated CRD in saline-treated GFRα3 KO mice produced no colorectal hypersensitivity [overall F(4,24) = 3.65; Holm-Sidak post hoc test, t = 1.78, P > 0.05; Fig. 2A]. An area under the stimulus-response curves analysis revealed a significant difference between saline- and TNBS-treated groups of GFRα3 KO mice [F(1,48) = 10.43, P = 0.007; Fig. 2C]. Although intracolonic instillation of TNBS produced colorectal hypersensitivity in both genotypes, the magnitude of hypersensitivity in GFRα3 KO mice was significantly attenuated relative to that produced in C57BL/6 mice [F(1,44) = 4.96, P = 0.048; Fig. 2D].
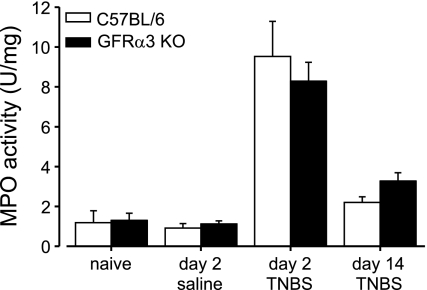
These differences in colorectal hypersensitivity produced by TNBS could reflect differences in colorectal compliance or the magnitude of inflammation produced in the two genotypes. Colorectal compliance measurements revealed no difference between GFRα3 KO and C57BL/6 mice [F(1,6) = 0.348, P = 0.577]. Figure 3 shows that neither baseline nor intracolonic treatment with either saline or TNBS reveals any differences in colorectal MPO activity. Intracolonic saline treatment did not [F(1,12) = 0.48, P = 0.501], whereas TNBS did [F(2,15) = 33.79, P < 0.001], significantly increase MPO activity in either genotype; there were no significant differences between genotypes after either saline [F(1,12) = 0.23, P = 0.639] or TNBS [F(1,15) = 0.00, P = 0.981] treatments.
Fig. 3.
TNBS-produced colorectal inflammation does not differ between genotypes. Colorectal myeloperoxidase (MPO) activity did not differ between C57BL/6 and GFRα3 KO mice either before or after intracolonic saline or TNBS treatment. TNBS produced a similar significant increase in MPO in both genotypes on day 2 after treatment [treatment, F(2,15) = 33.79, P < 0.001; genotype, F(2,15) < 0.01, P = 0.981]. Data are presented as means ± SE (n = 3–6).
GFRα3 and artemin expression following TNBS treatment.
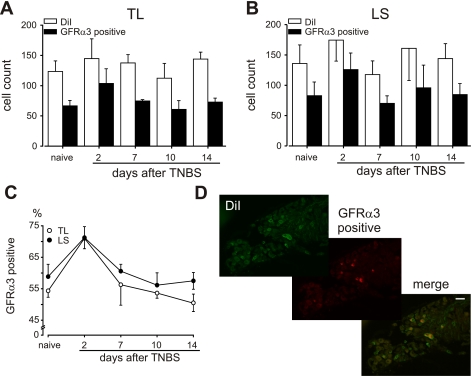
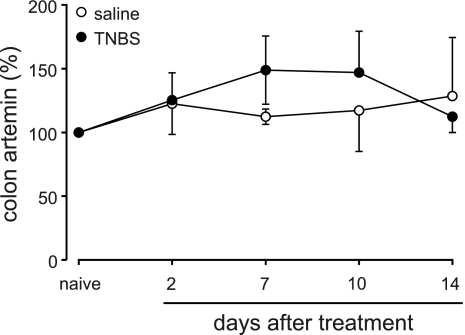
Expression of GFRα3 in colorectal DRG neurons and artemin in colorectal tissue were examined by immunohistochemistry and Western blotting, respectively. Figure 4, A and B, shows the numbers of DiI-labeled colorectal DRG neurons in thorocolumbar and lumbrosacral ganglia, respectively, before and after TNBS treatment and the numbers that were also GFRα3 immunopositive. The number of DiI-labeled colorectal neurons in thorocolumbar and lumbrosacral DRG did not significantly differ either before or after TNBS treatment. GFRα3-immunopositive neurons comprised 54.3 ± 2.0 and 58.8 ± 3.9% of thorocolumbar and lumbrosacral DiI-labeled neurons, respectively. These percentages significantly increased to 71.0 ± 1.5% [thorocolumbar: F(4,15) = 5.43, P = 0.007] and 71.2 ± 3.5% [lumbrosacral: F(4,15) = 3.35, P = 0.038] on day 2 after TNBS treatment (Fig. 4C) and returned to control thereafter. Associated with upregulation in the receptor, a modest, nonsignificant increase in colon artemin protein was observed following intracolonic TNBS treatment relative to saline treatment (Fig. 5).
Fig. 4.
Effect of intracolonic TNBS treatment on GFRα3 expression in colorectal dorsal root ganglion (DRG) neurons in C57BL/6 mice. A and B: no. of thoracolumbar (TL) and lumbosacral (LS) colorectal DRG neurons retrogradely filled with 1,1′-dioctadecyl-3,3,3′,3-tetramethylindocarbocyanine methane sulfonate (DiI) and the nos. of DiI-filled neurons that were GFRα3 immunopositive. C: TNBS produced a significant but transient increase in the proportion of GFRα3 immunopositive colorectal DRG neurons [TL, F(4,15) = 5.43, P = 0.007; LS, F(4,15) = 3.35, P = 0.038]. D: examples of DRG sections showing DiI-filled neurons, GFRα3 immunohistochemistry, and a merged image. Calibration bar = 50 μm.
Fig. 5.
Effect of intracolonic TNBS treatment on artemin content in the colorectum. Data are derived from Western blots quantified by densitometry and presented as percentage relative to artemin tissue content in naïve mice.
Single fiber recordings of colorectal afferents.
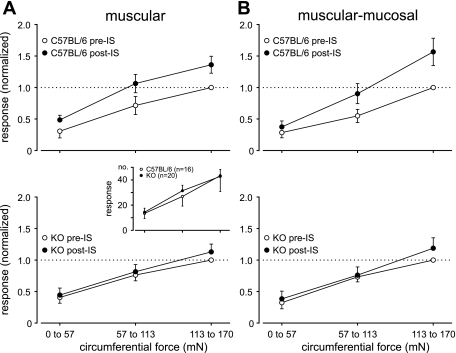
Using a colorectal-pelvic nerve in vitro preparation, 36 stretch-sensitive colorectal afferents were studied in six C57BL/6 and nine GFRα3 KO mice. Only muscular and muscular-mucosal afferent endings that responded to ramped circumferential stretch (0–170 mN at 5 mN/s) were studied (3, 14). After evaluation of baseline responses to stretch, the effect of IS applied to the receptive ending was examined. Figure 6A, inset, shows there were no significant differences in baseline responses to stretch between genotypes [F(1,102) = 0.10, P = 0.76]. Consistent with previous findings (14), the response (no. of action potentials) to circumferential stretch was significantly increased after IS application in seven muscular [F(1,6) = 137.49, P < 0.001] and nine muscular-mucosal [F(1,8) = 5.72, P = 0.044] in C57BL/6 mice (Fig. 6, A and B) fibers. In contrast, IS failed to sensitize 10 muscular or 10 muscular-mucosal pelvic nerve fibers studied in GFRα3 KO mice [F(1,9) = 0.79, P = 0.396 and F(1,9) = 1.212, P = 0.3, respectively] (Fig. 6, A and B).
Fig. 6.
Responses of muscular (A) and muscular-mucosal (B) colorectal afferent endings to ramped circumferential stretch in C57BL/6 and GFRα3 KO mice before and after application of inflammatory soup (IS) to their receptive endings. Inset shows that baseline responses of stretch-sensitive muscular and muscular-mucosal afferent endings did not differ between genotypes. IS sensitized responses of both muscular and muscular-mucosal afferents to circumferential stretch in C57BL/6 [A, top, muscular, F(1,6) = 137.48, P < 0.001 and B, muscular-mucosal, F(1,8) = 5.72, P = 0.044] but not in GFRα3 KO (A and B, bottom) mice. The horizontal axes in all panels are circumferential force as indicated in the bottom panels of A and B. The vertical axis of the inset represents the no. of action potentials; other vertical axes represent responses to circumferential stretch normalized to the maximum pre-IS response.
DISCUSSION
In the present study, we evaluated the involvement of GFRα3 signaling in the mouse colorectum. Neither baseline responses to CRD nor responses of pelvic nerve afferent fibers to circumferential stretch differed in GFRα3 KO mice relative to C57BL/6 control mice, suggesting that colorectal mechanotransduction was unaffected by the deletion of GFRα3. Accordingly, GFRα3 appears not to play an important role in normal colorectal mechanotransduction. However, the magnitude of colorectal hypersensitivity in GFRα3 KO mice was significantly attenuated relative to C57BL/6 mice after intracolonic instillation of the inflammogen TNBS. In support, stretch-responsive muscular and muscular-mucosal pelvic nerve afferent endings recorded from colorectums of GFRα3 KO mice failed to sensitize when exposed to an IS. Together, these findings reveal a role for GFRα3 in modulating the excitability of colorectal afferents, suggesting that artemin and GFRα3 normally contribute to processes of sensitization of the colorectal innervation.
Previous reports have documented the expression of GFRα3 in rodent and human DRG sensory neurons, implicating GFRα3 in nociceptive processing (19, 28). We found that ∼55–60% of thoracolumbar and lumbosacral colorectal DRG neurons expressed GFRα3, a proportion that increased significantly in both pathways of colorectal innervation 2 days after TNBS treatment, a time when colorectal inflammation is maximal in this model. We did not find a corresponding significant increase in colorectal artemin, although artemin tended to increase modestly after TNBS treatment. In a related study (25), artemin mRNA in mouse colorectum was significantly increased twofold after TNBS treatment, consistent with the trend seen here. In addition, artemin mRNA significantly increased in inflamed skin produced by injection of Freund's complete adjuvant. This increase corresponded with the period of complete Freund's adjuvant-induced behavioral hyperalgesia and with an increase in GFRα3 mRNA in DRG neurons (23). In clinical studies, artemin and GFRα3 are significantly overexpressed in chronic pancreatitis, correlating with pain severity (9). The augmentation of GFRα3 expression in the present study was early and transient after intracolonic TNBS treatment, consistent with the development but not maintenance of colorectal inflammation and hypersensitivity. TNBS-produced colorectal hypersensitivity was absent in GFRα3 KO mice on day 2 after treatment and significantly attenuated thereafter compared with C57BL/6 mice. This time course of events mirrors the changes in GFRα3 in C57BL/6 mice after TNBS treatment, suggesting that the inability of GFRα3 KO mice to develop colorectal hypersensitivity is related to the absence of the receptor and not associated with colorectal inflammation, which did not differ between the two genotypes. We thus suggest that GFRα3 upregulation, associated perhaps with a tissue increase in its endogenous ligand artemin, is important for the development, but not maintenance, of TNBS-induced colorectal hypersensitivity.
The present findings add to growing evidence that GFRα3 signaling in sensory neurons is an important modulator of nociceptive transduction. Artemin has been shown to potentiate capsaicin-evoked Ca2+ influx and calcitonin gene-related peptide release in isolated DRG neurons (30) and to sensitize thermal responses of nociceptors in an ex vivo preparation (23). In addition, artemin overexpression in skin increases the expression of TRPV1 in DRG neurons, suggesting modification of TRPV1 function by GFRα3 signaling (13). Therefore, we investigated a functional contribution of elevated GFRα3 signaling in primary afferents to visceral hypersensitivity electrophysiologically using a colorectal-pelvic nerve preparation. As reported previously (14), IS produced robust sensitization of pelvic nerve colorectal afferents encoding circumferential stretch in C57BL/6 mice (muscular and muscular-mucosal receptive endings), whereas this sensitization was absent in GFRα3 KO mice. In this context, we previously demonstrated that sensitization of pelvic nerve colon afferents was attenuated in TRPV1 null mice in which muscular-mucosal but not muscular afferents sensitized to IS (17). Given the alignment of these two proteins, the present finding suggests an important contribution of GFRα3 to inflammation-related visceral hypersensitivity, partly by modulating TRPV1 function in colorectal primary afferents.
In summary, we noted significant attenuation of visceral hypersensitivity in GFRα3 KO mice after intracolonic TNBS relative to C57BL/6 mice. In addition, C57BL/6 mice exhibited an increase in GFRα3 protein expression in colon DRG neurons 2 days after intracolonic TNBS. In complementary electrophysiological studies, we observed no sensitization in stretch-sensitive colorectal pelvic nerve afferents following IS application in GFRα3 KO mice. We conclude that enhanced GFRα3 signaling in visceral afferents may contribute to development of long-lasting visceral hypersensitivity, providing a novel therapeutic strategy for refractory abdominal pain in IBS.
GRANTS
This work was supported by National Institute of Neurological and Communicative Disorders and Stroke Grant NS-19912.
DISCLOSURES
No conflicts of interest are declared by the authors.
ACKNOWLEDGMENTS
We thank Dr. Erica Schwartz for instruction and invaluable advice related to tissue preparation and analysis of myeloperoxidase activity and Michael Burcham for preparation of the graphics.
Current address of M. Shinoda: Dept. of Physiology, Nihon University School of Dentistry, 1-8-13 Kandasurugadai, Chiyoda-ku, Tokyo 101-8310, Japan.
REFERENCES
- 1. Azpiroz F, Bouin M, Camilleri M, Mayer EA, Poitras P, Serra J, Spiller RC. Mechanisms of hypersensitivity in IBS and functional disorders. Neurogastroenterol Motil 19: 62–88, 2007 [DOI] [PubMed] [Google Scholar]
- 2. Bielefeldt K, Ozaki N, Gebhart GF. Role of nerve growth factor in modulation of gastric afferent neurons in the rat. Am J Physiol Gastrointest Liver Physiol 284: G499–G507, 2003 [DOI] [PubMed] [Google Scholar]
- 3. Brierley SM, Jones RC, 3rd, Gebhart GF, Blackshaw LA. Splanchnic and pelvic mechanosensory afferents signal different qualities of colonic stimuli in mice. Gastroenterology 127: 166–178, 2004 [DOI] [PubMed] [Google Scholar]
- 4. Brierley SM, Page AJ, Hughes PA, Adam B, Liebregts T, Cooper NJ, Holtmann G, Liedtke W, Blackshaw LA. Selective role for TRPV4 ion channels in visceral sensory pathways. Gastroenterology 134: 2059–2069, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Brierley SM, Hughes PA, Page AJ, Kwan KY, Martin CM, O'Donnell TA, Cooper NJ, Harrington AM, Adam B, Liebregts T, Holtmann G, Corey DP, Rychkov GY, Blackshaw LA. The ion channel TRPA1 is required for normal mechanosensation and is modulated by algesic stimuli. Gastroenterology 137: 2084–2095, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Camilleri M. Drug development and IBS drugs: experience from the past, current challenges, and proposal for the future. Curr Opin Pharmacol 8: 671–676, 2008 [DOI] [PubMed] [Google Scholar]
- 7. Cattaruzza F, Spreadbury I, Miranda-Morales M, Grady EF, Vanner SJ, Bunnett NW. Transient receptor potential ankyrin-1 has a major role in mediating visceral pain in mice. Am J Physiol Gastrointest Liver Physiol 298: G81–G91, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Cenac N, Altier C, Chapman K, Liedtke W, Zamponi G, Vergnolle N. Transient receptor potential vanilloid-4 has a major role in visceral hypersensitivity symptoms. Gastroenterology 135: 937–946, 2008 [DOI] [PubMed] [Google Scholar]
- 9. Ceyhan GO, Bergmann F, Kadihasanoglu M, Erkan M, Park W, Hinz U, Giese T, Müller MW, Büchler MW, Giese NA, Friess H. The neurotrophic factor artemin influences the extent of neural damage and growth in chronic pancreatitis. Gut 56: 534–544, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Christianson JA, Gebhart GF. Assessment of colon sensitivity by luminal distension in mice. Nat Protoc 2: 2624–2631, 2007 [DOI] [PubMed] [Google Scholar]
- 11. Delafoy L, Raymond F, Doherty AM, Eschalier A, Diop L. Role of nerve growth factor in the trinitrobenzene sulfonic acid-induced colonic hypersensitivity. Pain 105: 489–497, 2003 [DOI] [PubMed] [Google Scholar]
- 12. Dorn SD, Palsson OS, Thiwan SI, Kanazawa M, Clark WC, van Tilburg MA, Drossman DA, Scarlett Y, Levy RL, Ringel Y, Crowell MD, Olden KW, Whitehead WE. Increased colonic pain sensitivity in irritable bowel syndrome is the result of an increased tendency to report pain rather than increased neurosensory sensitivity. Gut 56: 1202–1209, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Elitt CM, McIlwrath SL, Lawson JJ, Malin SA, Molliver DC, Cornuet PK, Koerber HR, Davis BM, Albers KM. Artemin overexpression in skin enhances expression of TRPV1 and TRPA1 in cutaneous sensory neurons and leads to behavioral sensitivity to heat and cold. J Neurosci 26: 8578–8587, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Feng B, Brumovsky PR, Gebhart GF. Differential roles of stretch-sensitive pelvic nerve afferents innervating mouse distal colon and rectum. Am J Physiol Gastrointest Liver Physiol 298: G402–G409, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Honma Y, Araki T, Gianino S, Bruce A, Heuckeroth R, Johnson E, Milbrandt J. Artemin is a vascular-derived neurotropic factor for developing sympathetic neurons. Neuron 35: 267–282, 2002 [DOI] [PubMed] [Google Scholar]
- 16. Hughes PA, Brierley SM, Martin CM, Brookes SJ, Linden DR, Blackshaw LA. Post-inflammatory colonic afferent sensitisation: different subtypes, different pathways and different time courses. Gut 58: 1333–1341, 2009 [DOI] [PubMed] [Google Scholar]
- 17. Jones RC, 3rd, Xu L, Gebhart GF. The mechanosensitivity of mouse colon afferent fibers and their sensitization by inflammatory mediators require transient receptor potential vanilloid 1 and acid-sensing ion channel 3. J Neurosci 25: 10981–10989, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Jones RC, 3rd, Otsuka E, Wagstrom E, Jensen CS, Price MP, Gebhart GF. Short-term sensitization of colon mechanoreceptors is associated with long-term hypersensitivity to colon distention in the mouse. Gastroenterology 133: 184–194, 2007 [DOI] [PubMed] [Google Scholar]
- 19. Josephson A, Widenfalk J, Trifunovski A, Widmer HR, Olson L, Spenger C. GDNF and NGF family members and receptors in human fetal and adult spinal cord and dorsal root ganglia. J Comp Neurol 440: 204–217, 2001 [DOI] [PubMed] [Google Scholar]
- 20. Kamp EH, Jones RC, 3rd, Tillman SR, Gebhart GF. Quantitative assessment and characterization of visceral nociception and hyperalgesia in mice. Am J Physiol Gastrointest Liver Physiol 284: G434–G444, 2003 [DOI] [PubMed] [Google Scholar]
- 21. Lamb K, Kang YM, Gebhart GF, Bielefeldt K. Nerve growth factor and gastric hyperalgesia in the rat. Neurogastroenterol Motil 15: 355–361, 2003 [DOI] [PubMed] [Google Scholar]
- 22. Lewin GR, Mendell LM. Nerve growth factor and nociception. Trends Neurosci 16: 353–359, 1993 [DOI] [PubMed] [Google Scholar]
- 23. Malin SA, Molliver DC, Koerber HR, Cornuet P, Frye R, Albers KM, Davis BM. Glial cell line-derived neurotrophic factor family members sensitize nociceptors in vitro and produce thermal hyperalgesia in vivo. J Neurosci 26: 8588–8599, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Malin SA, Christianson JA, Bielefeldt K, Davis BM. TPRV1 expression defines functionally distinct pelvic colon afferents. J Neurosci 29: 743–752, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Malin SA, Molliver DC, Christianson JA, Albers KM, Davis BM. TRPV1 and TRPA1 function and modulation are target tissue-dependent. J Neurosci In press [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. McMahon SB. NGF as a mediator of inflammatory pain. Philos Trans R Soc Lond B Biol Sci 351: 431–440, 1996 [DOI] [PubMed] [Google Scholar]
- 27. Mendell LM, Albers KM, Davis BM. Neurotrophins, nociceptors, pain. Microsc Res Tech 45: 252–261, 1999 [DOI] [PubMed] [Google Scholar]
- 28. Orozco OE, Walus L, Sah DW, Pepinsky RB, Sanicola M. GFRalpha3 is expressed predominantly in nociceptive sensory neurons. Eur J Neurosci 13: 2177–2182, 2001 [DOI] [PubMed] [Google Scholar]
- 29. Ritchie J. Pain from distension of the pelvic colon by inflating a balloon in the irritable colon syndrome. Gut 14: 125–132, 1973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Schmutzler BS, Roy S, Hingtgen CM. Glial cell line-derived neurotrophic factor family ligands enhance capsaicin-stimulated release of calcitonin gene-related peptide from sensory neurons. Neuroscience 161: 148–156, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Shinoda M, Feng B, Gebhart GF. Peripheral and central P2X receptor contributions to colon mechanosensitivity and hypersensitivity in the mouse. Gastroenterology 137: 2096–2104, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Verne GN, Robinson ME, Vase L, Price DD. Reversal of visceral and cutaneous hyperalgesia by local rectal anesthesia in irritable bowel syndrome (IBS) patients. Pain 105: 223–230, 2003 [DOI] [PubMed] [Google Scholar]
- 33. Wynn G, Rong W, Xiang Z, Burnstock G. Purinergic mechanisms contribute to mechanosensory transduction in the rat colorectum. Gastroenterology 125: 1398–1409, 2003 [DOI] [PubMed] [Google Scholar]