Abstract
Alterations in the balance between ANG II/ACE and ANG 1–7/ACE2 in ANG II-dependent hypertension could reduce the generation of ANG 1–7 and contribute further to increased intrarenal ANG II. Upregulation of collecting duct (CD) renin may lead to increased ANG II formation during ANG II-dependent hypertension, thus contributing to this imbalance. We measured ANG I, ANG II, and ANG 1–7 contents, angiotensin-converting enzyme (ACE) and ACE2 gene expression, and renin activity in the renal cortex and medulla in the clipped kidneys (CK) and nonclipped kidneys (NCK) of 2K1C rats. After 3 wk of unilateral renal clipping, systolic blood pressure and plasma renin activity increased in 2K1C rats (n = 11) compared with sham rats (n = 9). Renal medullary angiotensin peptide levels were increased in 2K1C rats [ANG I: (CK = 171 ± 4; NCK = 251 ± 8 vs. sham = 55 ± 3 pg/g protein; P < 0.05); ANG II: (CK = 558 ± 79; NCK = 328 ± 18 vs. sham = 94 ± 7 pg/g protein; P < 0.001)]; and ANG 1–7 levels decreased (CK = 18 ± 2; NCK = 19 ± 2 pg/g vs. sham = 63 ± 10 pg/g; P < 0.001). In renal medullas of both kidneys of 2K1C rats, ACE mRNA levels and activity increased but ACE2 decreased. In further studies, we compared renal ACE and ACE2 mRNA levels and their activities from chronic ANG II-infused (n = 6) and sham-operated rats (n = 5). Although the ACE mRNA levels did not differ between ANG II rats and sham rats, the ANG II rats exhibited greater ACE activity and reduced ACE2 mRNA levels and activity. Renal medullary renin activity was similar in the CK and NCK of 2K1C rats but higher compared with sham. Thus, the differential regulation of ACE and ACE2 along with the upregulation of CD renin in both the CK and NCK in 2K1C hypertensive rats indicates that they are independent of perfusion pressure and contribute to the altered content of intrarenal ANG II and ANG 1–7.
Keywords: ANG II-dependent hypertension, angiotensin peptides, angiotensin-converting enzyme, qRT-PCR, immunohistochemistry
in angiotensin ii (ANG II)-dependent hypertension, increased circulating ANG II levels lead to progressive augmentation of intrarenal ANG II content (35, 57, 61). The increased intrarenal ANG II content results from AT1 receptor (AT1R)-mediated uptake of circulating ANG II, as well as de novo intrarenal ANG II generation (52). Increased local ANG II formation has been associated with augmentation of intrarenal angiotensinogen (AGT) synthesis and secretion by proximal tubule (PT) cells (27, 29, 59) leading to augmented urinary AGT excretion indicating that proximally secreted AGT traverses the distal nephron segments (27, 30).
Renin, primarily synthesized and released by juxtaglomerular (JG) cells, is also produced by principal cells of connecting tubules and collecting duct (CD) cells of rat, mouse, and human kidneys (22, 42, 48). Previous studies suggest that tubular renin is differentially regulated from renin in JG cells (20, 37, 42, 43, 47). In response to chronic ANG II infusions, renin mRNA and protein levels increase in principal cells from connecting tubules and CD, indicating stimulation of tubular renin during ANG II-dependent hypertension (42). This stimulatory effect of CD renin is prevented by treatment with AT1R blockers suggesting that it is mediated by an AT1R mechanism (43). Studies showing augmentation of CD renin in both clipped and unclipped kidneys of Goldblatt hypertensive rats indicate that the enhanced CD renin occurs independently of blood pressure (44). Thus, enhanced CD renin acting on AGT delivered from proximal nephron segments may contribute to increased ANG I generation that can then be converted to ANG II by the substantial angiotensin-converting enzyme (ACE) activity present in the CD (4, 5).
ACE2, the homolog of ACE, is abundantly expressed in the kidney and metabolizes ANG II to generate the heptapeptide ANG 1–7 (10, 51, 53). ANG 1–7 may antagonize the actions of ANG II especially in situations of an overactive renin-angiotensin system (RAS) (12, 13, 15, 51). Recent studies showed decreased ACE2 mRNA expression in spontaneous hypertensive rats and mRen2 hypertensive rats (7, 58). The decreased ACE2 expression is associated with the onset and severity of hypertension (53). ANG II has been shown to downregulate ACE2 expression in astrocytes, heart, and kidney, suggesting that during high-ANG II states, the generation of ANG 1–7 might be reduced (17–19). Alternatively, the reduced ACE2 expression might be due to the increased renal perfusion pressure observed in these models. Goldblatt hypertensive rats exhibit high intrarenal ANG II content in both kidneys, as well as upregulation of renin in distal nephron segments that may lead to regional differences in the content of intrarenal angiotensin peptides, as well as changes in the expression of ACE and ACE2 within the kidneys. Accordingly, the present study was performed to determine the regional changes in ANG II/ACE and ANG 1–7/ACE2 in ANG II-dependent hypertension by using Goldblatt hypertensive rats and examining the levels of ANG I, ANG II, and ANG 1–7, as well as ACE and ACE2 gene expression separately in renal cortical and medullary tissues from both kidneys of 2K1C hypertensive rats after 3 wk of unilateral renal artery clipping. Additional studies were performed in rats infused chronically with ANG II (80 ng/min) for 2 wk. We hypothesized that, in 2K1C hypertensive rats, the intrarenal contents of ANG 1–7 and ACE2 transcript levels are decreased in both clipped and unclipped kidneys. These results indicate that there is differential regulation of ACE/ACE2 in renal tissues of 2K1C rats, which may help to explain the reciprocal changes in ANG II and ANG 1–7 observed in the kidneys of Goldblatt hypertensive rats and ANG II-infused rats.
MATERIALS AND METHODS
Experimental animals, sample collections, and tissue preparation.
All protocols were approved by the Tulane University Health Sciences Center Animal Care and Use Committee. Male Sprague-Dawley rats (150 to 175 g; Charles River Laboratories, Wilmington, MA) were anesthetized and a u-shaped silver clip (lumen diameter of 0.25 mm) was placed on the left renal artery (n = 11), or the rats were just subjected to a sham operation (n = 9). Following a training period, the systolic blood pressures (SBP) were measured by tail-cuff plethysmography (Visitech, BP-2000, Apex, NC) 1 day before placement of the silver clip (lumen diameter of 0.25 mm) on the left renal artery, and at the end of the first, second, and third weeks of the study. On day 25 after unilateral renal clipping, rats were euthanized by conscious decapitation and trunk blood samples and kidneys were harvested; the cortex and inner medulla were separated for measurements of tissue renin activity, ANG I, ANG II, and ANG 1–7 contents, and ACE and ACE2 gene expression studies, respectively. Additional kidney tissues from rats chronically infused with ANG II via SC minipumps (80 ng/min for 14 days; n = 6) and sham-operated (n = 5) were used to examine ACE and ACE2 gene expression and functional enzymatic activities.
Angiotensin peptides kidney content.
Renal cortical and medullary levels of ANG I, ANG II, and ANG 1–7 were extracted from kidney sample homogenates, purified in Sep-Pak-C18 columns (Waters, Milford, MA), and measured by HPLC as previously described (1). Peptides were identified according to retention time. The identity of eluted ANG I, ANG II, and ANG 1–7 was confirmed by direct sequencing (protein sequencer PPSQ-23; Shimadzu, Tokyo, Japan). For concentration determination, commercially available peptides were employed to develop a standard curve. Peptide levels were normalized according to total protein concentration and results were expressed as picograms per gram of protein.
ACE and ACE2 renal expression and functional activity.
Twenty nanograms per well of total RNA extracted from rat kidney cortex and inner medulla samples were quantified by real-time qRT-PCR for ACE and ACE2 mRNA levels, using protocols previously described (28, 42). Data of quantitative qRT-PCR were normalized by β-actin mRNA expression. The sequences of primers and probes of ACE, ACE2, and β-actin are provided in the methods expanded section (the online version of this article contains supplemental data). For ACE2 immunoexpression, affinity-purified primary antibody against ACE2 (Dr. C. M. Ferrario; Wake Forest University, NC) was assessed by the immunoperoxidase technique as previously described (44) at a dilution of 1:200 for ACE2. The ACE and ACE2 catalytic activities were determined fluorimetrically by two different laboratories using either the benzyloxycarbonyl-l-phenylalanyl-l-histidyl-l-leucine as a substrate (5) with values reported in nanomoles per milligram of protein; or the Fluorogenic Peptide Substrate VI [FPSVI, 7Mca-Y-V-A-D-A-P-K(Dnp)-OH; R&D Systems, Minneapolis, MN] as previously described (11) with values reported in reverse light units per milligram of protein. Details of these protocols are provided in the methods expanded section.
Total renin, renin, and (pro)renin activities in kidney cortical and medullary tissues.
Active renin was determined as previously described (56) by measuring the amount of ANG I generated per hour from the renal tissue homogenates incubated at 37°C over a period of 24 h with the renin substrate tetradecapeptide (Sigma, St. Louis, MO) and PMSF, as described in detail in the methods expanded section. Trypsinized tissue samples were also subjected to the above protocol to determine total renin activity. (Pro)renin activity levels were calculated as the difference between the total renin and the active renin. The results are corrected by intracellular protein and expressed as specific activity micrograms per microgram.
Statistical analyses.
Results are presented as means ± SE. Data were evaluated by Grubb's test (25) using GraphPad Software (San Diego, CA) and analyzed when appropriate by unpaired Student's t-test or by one-way ANOVA with Dunnett's Multiple Comparison tests using GraphPad Prism Software, Version 5.0 for Windows (GraphPad Software). A value of P < 0.05 was regarded as significant.
RESULTS
Table 1 shows the average body weights (BW), SBP, and plasma renin activities (PRA) of rats subjected to sham operation or clipping of the left renal artery during the 25 days. In 2K1C rats, the BW was slightly lower than in sham rats after 7 days of surgery (251 ± 5 vs. 236 ± 5 g) and this trend continued during the following 2 wk of the study (2K1C: 253 ± 6 g vs. sham: 280 ± 4 g, after 14 days; and 2K1C: 291 ± 8 g vs. sham: 320 ± 3 g, after 25 days; P < 0.05). SBP values were similar in both groups of rats at the beginning of the study (2K1C: 126 ± 2 vs. sham: 118 ± 3 mmHg); however, after 1 wk following placement of the clip, the SBP was increased in 2K1C rats relative to sham-operated rats (2K1C: 154 ± 5 vs. 129 ± 4 mmHg) and continued to increase during the second and third week following clipping (day 14 = 179 ± 6 vs. 126 ± 4 mmHg; day 25 = 183 ± 6 vs. 125 ± 4 mmHg; P < 0.001). At 25 days, PRA was elevated in 2K1C rats compared with sham-operated rats (13.0 ± 2 vs. 5.6 ± 1 ng ANG I·ml−1·h−1; P < 0.05).
Table 1.
Body wt, SBP, and PRA after 25 days of unilateral renal artery clipping
| Body wt, g |
SBP, mmHg |
|||||
|---|---|---|---|---|---|---|
| Exp. group | n | Start | End | Baseline on day −1 | 25 days | PRA, ng ANG I•ml−1•h−1 |
| 2K1C | 11 | 186 6 | 291 8* | 126 2 | 183 6* | 13.0 2* |
| Sham | 9 | 164 12 | 322 3 | 118 3 | 125 4 | 5.6 1 |
Values are means ± SE. Comparisons of body wt, systolic blood pressure (SBP), and plasma renin activity (PRA) in sham-operated rats (n = 9) and two-kidney one-clip Goldblatt hypertensive (2K1C) rats (n = 11).
P < 0.05 Goldblatt rats vs. sham rats.
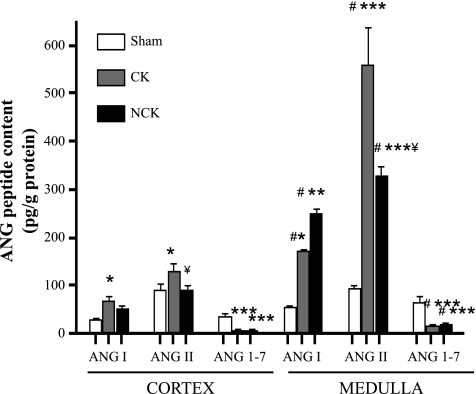
The concentrations of ANG I, ANG II, and ANG 1–7 measured in renal cortical and medullary homogenates after 3 wk of unilateral renal clipping from two independent studies are shown in Fig. 1. Contents of all angiotensin peptides were higher in the renal medullary tissues than in the renal cortexes of 2K1C rats. In the cortex, only the CK showed higher ANG I and ANG II content than the sham kidney [ANG I (CK: 68 ± 10; NCK: 51 ± 8 vs. sham: 29 ± 2 pg/g; P < 0.05 CK vs. sham); ANG II (CK: 129 ± 17; NCK: 90 ± 11 vs. sham 91 ± 15 pg/g; P < 0.05 CK vs. sham)]. However, in the medulla, the levels of ANG I and ANG II of both the CK and NCK kidneys of 2K1C rats were significantly higher than in the sham kidneys [ANG I (CK = 171 ± 4; NCK = 251 ± 8 vs. sham = 55 ± 3 pg/g protein; P < 0.05 CK vs. sham, P < 0.01 NCK vs. sham); ANG II (CK = 558 ± 79; NCK = 328 ± 18 vs. sham = 94 ± 7 pg/g protein); P < 0.001 CK and NCK vs. sham]. In contrast, the content of ANG 1–7 was substantially lower in both the cortex and medulla of CK and NCK of 2K1C rats [cortex: (CK = 7 ± 1; NCK = 7 ± 2 pg/g); medulla: (CK = 18 ± 2; NCK = 19 ± 2 pg/g); P < 0.05 kidney cortex vs. kidney medulla] compared with sham rats [cortex: (36 ± 6 pg/g); medulla (63 ± 10 pg/g); P < 0.001 vs. sham].
Fig. 1.
Angiotensin peptide contents in rat kidney cortex and medulla. The levels of ANG I, ANG II, and ANG 1–7 were measured by HPLC in kidney cortex and medulla samples from Goldblatt (n = 11) rats and sham (n = 9) rats. Values are expressed in pg/g of protein. Values are means ± SE. *P < 0.05, **P < 0.01, ***P < 0.001 vs. sham. #P < 0.05 kidney cortex vs. kidney medulla. #P < 0.05 clipped (CK) vs. nonclipped (NCK).
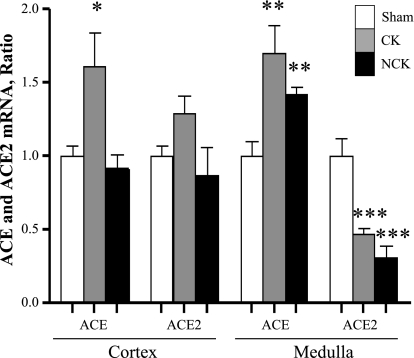
In the renal cortex of 2K1C rats, ACE mRNA levels were increased in the CK but not in the NCK [ACE (CK: 1.6 ± 0.4; NCK: 1.0 ± 0 vs. 1.0 ± 0.0 AU; P < 0.05)]; however, ACE2 transcript levels were similar between the CK and the NCK [ACE2 (CK: 1.1 ± 0.2; NCK: 0.7 ± 0.2 vs. 1.0 ± 0.0 AU)]. In contrast, in the renal medulla, ACE mRNA levels were significantly increased in the CK and NCK of 2K1C rats compared with sham rats (CK: 1.9 ± 0.2; NCK: 1.6 ± 0.1 vs. 1.0 ± 0.1 AU; P < 0.001), while the levels of ACE2 mRNA levels were decreased (CK = 0.5 ± 0.1; NCK = 0.3 ± 0.1 vs. 1.0 ± 0.1 AU; P < 0.0001; Fig. 2). In addition, CK and NCK compared with sham kidneys showed activities significantly higher for ACE (CK: 7.5 ± 1.0; NCK: 6.8 ± 0.4 vs. 4.3 ± 0.2 nmol/mg protein; P < 0.05) but decreased for ACE2 (CK: 0.9 ± 0.2; NCK: 0.8 ± 0.1 vs. 1.5 ± 0.1 nmol/mg protein; P < 0.05).
Fig. 2.
Comparison of angiotensin-converting enzyme (ACE) and ACE2 mRNA expression levels in rat renal cortex and medulla tissues. Quantification of mRNA levels was measured by real-time qRT-PCR in CK and NCK kidneys of Goldblatt (n = 11) rats and compared with sham-operated (n = 9) rats. The mRNA levels for the target genes were determined in each sample per triplicate and expressed as a ratio relative to β-actin. Values are means ± SE. *P < 0.05, **P < 0.001, ***P < 0.0001 vs. sham kidney.
To further validate the reciprocal changes in ACE and ACE2, we examined the mRNA abundance of ACE and ACE2, as well as their functional activities in kidney tissues from chronic ANG II-infused (n = 6) rats and sham-operated rats (n = 5). In these rats, although the ACE mRNA levels were not significantly different between the two groups, the ANG II rats exhibited substantially greater ACE activity in the kidneys than the control rats (ANG II = 2,712 ± 334 vs. sham = 487 ± 51 RLU/mg; P < 0.05). In addition, the ACE2 transcript levels were reduced in chronic ANG II-infused rats (ANG II = 0.6 ± 0.1 vs. sham = 1.0 ± 0.0 AU; P < 0.05) but we did find any difference in its activity (ANG II = 2,356 ± 786 vs. sham = 1,290 ± 225 RLU/mg) compared with sham rats.
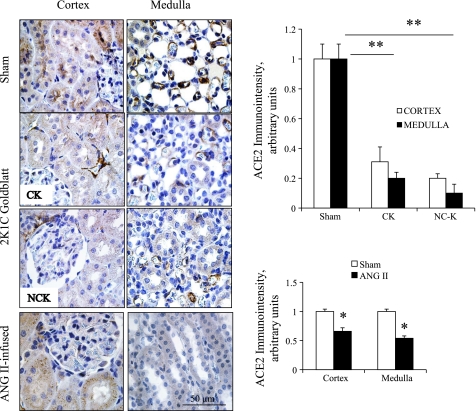
Figure 3 depicts the specific immunoreactivity and semiquantitation for ACE2 immunostaining by using the immunoperoxidase technique on a kidney section from sham-operated (top panels); clipped and nonclipped kidneys of 2K1C rats (middle panels) and ANG II-infused rats (bottom panels). ACE2 immunoreactivity was predominantly observed in the cytoplasm of epithelial cells of PTs in the cortex and inner cortex (left panels) and to a lesser extent in the CD cells of the inner medulla.
Fig. 3.
Immunohistochemistry of ACE2 using immunoperoxidase technique on 3-μm paraffin-embedded cortex and medulla kidney sections from sham, 2K1C, and chronic ANG II-infused rats. Panels show, respectively, cortical and medullary regions with specific ACE2 immunostaining in sham rats (top panels), 2K1C Goldblatt (CK and NCK; middle panels), and ANG II-infused rats (bottom panels). *P < 0.05 and **P < 0.001 vs. sham.
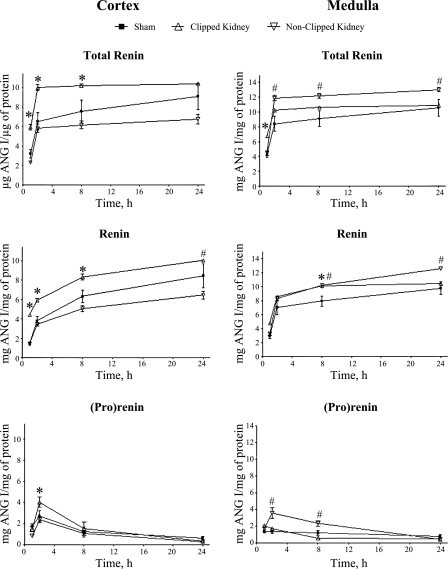
Figure 4 reflects the specific renin activity measured as ANG I generated per hour over a period of 24 h from the time the tetradecapeptide was added and corrected by total protein in the tissue kidney lysates. Between 2 and 8 h of incubation with the tetradecapeptide, the specific activity of renin was significantly higher in the cortex of CK than in the cortex of NCK or sham kidneys. However, in the renal medullary tissues during the same period, the renin activities in both the CK and NCK were higher than in sham kidneys. By 12–14 h later, renin activities became comparable between the CK and the sham kidney. In addition, the incubation with the tetradecapeptide in the presence of trypsin showed that in the renal cortex, the maximum (pro)renin activity observed at 2 h was significantly higher in the CK, while in the renal medulla, the NCK exhibited greater (pro)renin activity.
Fig. 4.
Curves of specific enzymatic activity of total renin, free renin, and (pro)renin in cortical and medullary tissues of both kidneys from 2K1C rats. Enzymatic activity was measured in the presence and absence of trypsin over a period of 24-h incubation with excess of renin substrate. Values were determined from the amount of ANG I (μg) product measured by HPLC per μg of tissue protein and expressed as means ± SE. *P < 0.05 sham kidneys vs. clipped kidneys. #P < 0.05 sham kidneys vs. nonclipped kidneys.
DISCUSSION
The present study demonstrates that both kidneys of 2K1C hypertensive rats exhibit reciprocal changes in ANG II/ACE and ANG 1–7/ACE2 in association with an enhancement of CD renin activity. This observation provides the basis for an additional mechanism that may contribute to explain high intrarenal ANG II content in the kidneys during Goldblatt hypertension. In a previous study, we demonstrated that in 2K1C hypertensive rats evaluated after 25 days of unilateral renal artery clipping, there is an increased renin immunostaining in cortical and medullary CD cells accompanied by augmented renin mRNA and protein levels in the renal medullary tissues (44). The present study extends those observations by confirming that in the renal medullary regions of both kidneys of 2K1C rats, most of the renin present is active. In contrast, in the renal cortical region only the CK exhibits higher renin activity (Fig. 4). Thus, it is likely that the enhancement of renin activity in the renal medulla of 2K1C rats may contribute to increased intrarenal ANG I and ultimately ANG II content regardless of the suppression of JG renin such as occurs in the NCK of this experimental model of hypertension.
Compared with other organs, the kidney expresses relatively high levels of ACE2. In the rat kidney, mRNA for ACE2 has been detected in all nephron segments including PTs, CDs, and vasa recta (3, 33, 54). Although ACE2 was first shown to cleave ANG I to ANG 1–9 (10), subsequent studies revealed that this enzyme exhibits a greater catalytic activity for ANG II than ANG I (55). The consequences of intrarenal ACE2-mediated formation of ANG 1–7 from ANG II remain unclear. Evidence suggests that ANG 1–7 exerts a countering influence against ANG II, which could partially protect against the development of hypertension (2, 14, 16, 23, 34, 46). Recent studies demonstrated that ANG II downregulates ACE2 mRNA expression in the heart, kidney, and astrocytes (17–19). Furthermore, it has been suggested that ANG II downregulates ACE2 via the AT1R-ERK/p38 MAP kinase pathway (31). Chappell and Ferrario (7) reported pronounced increases in ACE2 transcript levels within the kidney of the normotensive Lewis and hypertensive mRen2.Lewis rats following ARB treatment with losartan. In the present study, we show that both the CK and the NCK medullary tissues exhibit decreased ACE2 immunoreactivity, activity, and transcript levels compared with kidneys from sham-operated rats. Reduced levels of ACE2 gene expression and activity are consistent also with our finding of decreased levels of ANG 1–7 content in the renal medulla of CK and NCK of 2K1C hypertensive rats. The observation of increased ANG I and ANG II contents in the renal medulla of both kidneys of 2K1C rats, along with the previous demonstration that levels of renin mRNA and protein as well as its activity are augmented in these tissues (44), suggests that the upregulation of CD renin may contribute to the increases in intrarenal ANG II content.
In the 2K1C Goldblatt hypertension model during the initial phase of hypertension, there are ANG II-dependent alterations in tubular reabsorption of the NCK due to the elevations in circulating and intrarenal ANG II levels (9, 21, 41). Accumulated evidence indicates that there is enough ACE activity in the CDs (4, 45) that may lead to intraluminal conversion of ANG I to ANG II in this part of the nephron (32) and contribute to the ANG II stimulation of epithelial sodium channel activity at the surface of the CD cells (40). In the present study, we found that in the renal cortical regions, ACE transcript levels are augmented only in the CK while in the renal medulla, ACE mRNA levels and its activity are augmented in both kidneys of 2K1C hypertensive rats. Guan et al. (21) suggested that the de novo formation of intrarenal ANG II in the NCK of 2K1C rats is partially due to augmented intrarenal ACE activity (21) and our current data support this notion. In the chronic ANG II-infused rat model, it has also been reported that augmentation of intrarenal ANG II content is likely related to an increase in intrarenal ACE protein since Harrison-Bernard et al. (24) observed an increase in ACE immunostaining in PTs; and Sadjadi et al. (50) reported increased renal ACE activity despite no changes in plasma ACE activity. Using the chronic ANG II hypertensive rat model, we found that, although renal ACE mRNA levels did not differ from those in sham rats, the ACE activity was also significantly augmented in the ANG II-infused rats. This is consistent with the report showing that changes in enzymatic activity may occur without transcriptional and/or posttranslational alterations; i.e., by changing the open structure of the specific protein conformation (26).
It has been suggested that ACE2 is the major enzyme involved in ANG 1–7 formation from ANG II in tissues; thus contributing to ANG II degradation (8, 60). Our data demonstrating reduced ACE2 mRNA levels and activity in both kidneys of Goldblatt hypertensive rats as well as decreased specific immunoreactivity of ACE2 in the kidneys of chronic ANG II-infused rats support a functional role for ACE2 to contribute to the increases of intrarenal ANG II content by reducing the ACE2-mediated degradation of ANG II. The collective effect of increased ACE leading to increased ANG II formation, coupled with decreased ACE2, diminishing ANG II degradation to ANG 1–7, would be to increase prevailing levels of ANG II, as well as reduce ANG 1–7 levels. These observations do not mean that this is the only mechanism responsible for the reciprocal changes in intrarenal ANG II/ANG 1–7 contents during ANG II-dependent hypertension. In this regard, it has been reported that augmentation of chymase activity in the ischemic kidney of 2K1C rats (49) and in diabetic renal vascular disease (39) is also a mechanism responsible for ACE-independent intrarenal ANG II production. The augmentation of renin activity in distal nephron segments along with increases in medullary ANG I content of both kidneys of 2K1C rats support the notion of increased intrarenal ANG II formation, which ultimately may stimulate sodium reabsorption in the CD and may explain the attenuation of the pressure natriuretic response to elevations in arterial blood pressure and the development and maintenance of hypertension in Goldblatt hypertensive rats. Therefore, the synergistic actions of distal nephron renin-mediated increases of ANG II, along with the diminished counteracting influence of ANG 1–7 associated with the direct effects of ANG II on distal nephron transport mechanisms could provide a powerful sodium-retaining stimulus in Goldblatt hypertension (2, 36, 38), thereby allowing for a greater influence in the progression of high blood pressure in the 2K1C hypertensive rats.
In summary, the present findings indicate that the upregulation of CD renin in the renal medulla of both CK and NCK of 2K1C rats is associated with reciprocal changes in the ANG II and ANG 1–7 contents in the medullary regions of the kidneys of these rats. During conditions of RAS activation, subsequent to clipping one of the renal arteries or during chronic ANG II infusion, there are increases in CD renin activity coupled with substantial decreases of ANG 1–7 content and ACE2 and increases in ANG II and ACE; which may contribute to the progression of high blood pressure during ANG II-dependent hypertension.
GRANTS
This work was supported by National Heart, Lung, and Blood Institute Grant HL-26731 and COBRE Grant P20-RR-017659 from National Center for Research Resources. M. C. Prieto is a Building Interdisciplinary Research Careers in Women's Health scholar supported by the Eunice Kennedy Shriver National Institute of Child Health & Human Development (K12HD043451) and the American Heart Association (09BGIA2280440). L. S. Lara is a recipient of a Coordinacion de Apoyo de Personas de Educacion Superior PostDoctoral Fellowship from Brazil.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
ACKNOWLEDGMENTS
We thank Dr. C. M. Ferrario (Wake Forest Univ., NC) for generously providing the rat anti-ACE2 and anti-ANG 1–7 antibodies, Dr. E. Lazartigues (Louisiana Health Science Center, LA) for providing the facilities for the measurements of ACE and ACE2 activities in the chronic ANG II hypertensive rat model, and Dr. L. Bazzano for providing advice for the data statistical analyses. Digital images of histological specimens were obtained at the Imaging Core Facility of Hypertension and Renal Center of Excellence at Tulane University Health Sciences Center.
REFERENCES
- 1. Almeida WS, Maciel TT, Di Marco GS, Casarini DE, Campos AH, Schor N. Escherichia coli lipopolysaccharide inhibits renin activity in human mesangial cells. Kidney Int 69: 974–980, 2006 [DOI] [PubMed] [Google Scholar]
- 2. Burgelova M, Kramer HJ, Teplan V, Thumova M, Cervenka L. Effects of angiotensin-(1–7) blockade on renal function in rats with enhanced intrarenal ANG II activity. Kidney Int 67: 1453–1461, 2005 [DOI] [PubMed] [Google Scholar]
- 3. Cao C, Lee-Kwon W, Silldorff EP, Pallone TL. KATP channel conductance of descending vasa recta pericytes. Am J Physiol Renal Physiol 289: F1235–F1245, 2005 [DOI] [PubMed] [Google Scholar]
- 4. Casarini DE, Boim MA, Stella RCR, Krieger-Azzolini MH, Krieger JE, Schor N. Angiotensin I-converting enzyme activity in tubular fluid along the rat nephron. Am J Physiol Renal Physiol 272: F405–F409, 1997 [DOI] [PubMed] [Google Scholar]
- 5. Casarini DE, Plavinik FL, Zanella MT, Marson O, Krieger JE, Hirata IY, Stella RC. Angiotensin converting enzymes from human urine of mild hypertensive untreated patients resemble the N-terminal fragment of human angiotensin I-converting enzyme. Int J Biochem Cell Biol 33: 75–85, 2001 [DOI] [PubMed] [Google Scholar]
- 7. Chappell M, Ferrario C. Angiotensin-(1–7) in hypertension. Curr Opin Nephrol Hypertens 88: 231–235, 1999 [Google Scholar]
- 8. Crackower MA, Sarao R, Oudit GY, Yagil C, Kozieradzki I, Scanga SE, Oliveira-dos-Santos AJ, da Costa J, Zhang L, Pei Y, Scholey J, Ferrario CM, Manoukian AS, Chappell MC, Backx PH, Yagil Y, Penninger JM. Angiotensin-converting enzyme 2 is an essential regulator of heart function. Nature 417: 822–828, 2002 [DOI] [PubMed] [Google Scholar]
- 9. DeForrest J, Knappenberger R, Antonaccio M, Ferrone R, Creekmore J. Angiotensin II is a necessary component for the development of hypertension in the two kidney, one clip rat. Am J Cardiol 49: 1515–1517, 1982 [DOI] [PubMed] [Google Scholar]
- 10. Donoghue M, Hsieh F, Baronas E, Godbout K, Gosselin M, Stagliano N, Donovan M, Woolf B, Robison K, Jeyaseelan R, Breitbart RE, Acton S. A novel angiotensin-converting enzyme-related carboxypeptidase (ACE2) converts angiotensin I to angiotensin 1–9. Circ Res 87: E1–E9, 2000 [DOI] [PubMed] [Google Scholar]
- 11. Feng Y, Xia H, Cai Y, Halabi CM, Becker LK, Santos RAS, Speth RC, Sigmund CD, Lazartigues E. Brain-selective overexpression of human angiotensin-converting enzyme type 2 attenuates neurogenic hypertension. Circ Res 106: 373–382, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ferrario CM. Angiotension-(1–7) and antihypertensive mechanisms. J Nephrol 11: 278–283, 1998 [PubMed] [Google Scholar]
- 13. Ferrario CM. Contribution of Angiotensin-(1–7) to cardiovascular physiology and pathology. Curr Hypertens Rep 5: 129–134, 2003 [DOI] [PubMed] [Google Scholar]
- 14. Ferrario CM. Angiotensin-converting enzyme 2 and angiotensin-(1–7): an evolving story in cardiovascular regulation. Hypertension 47: 515–521, 2006 [DOI] [PubMed] [Google Scholar]
- 15. Ferrario CM, Chappell MC, Dean RH, Iyer SN. Novel angiotensin peptides regulate blood pressure, endothelial function, and natriuresis. J Am Soc Nephrol 9: 1716–1722, 1998 [DOI] [PubMed] [Google Scholar]
- 16. Ferrario CM, Chappell MC, Tallant EA, Brosnihan KB, Diz DI. Counterregulatory actions of angiotensin-(1–7). Hypertension 30: 535–541, 1997 [DOI] [PubMed] [Google Scholar]
- 17. Ferrario CM, Jessup J, Chappell MC, Averill DB, Brosnihan KB, Tallant EA, Diz DI, Gallagher PE. Effect of angiotensin-converting enzyme inhibition and angiotensin II receptor blockers on cardiac angiotensin-converting enzyme 2. Circulation 111: 2605–2610, 2005 [DOI] [PubMed] [Google Scholar]
- 18. Ferrario CM, Jessup J, Gallagher PE, Averill DB, Brosnihan KB, Ann TE, Smith RD, Chappell MC. Effects of renin-angiotensin system blockade on renal angiotensin-(1–7) forming enzymes and receptors. Kidney Int 68: 2189–2196, 2005 [DOI] [PubMed] [Google Scholar]
- 19. Gallagher PE, Chappell MC, Ferrario CM, Tallant EA. Distinct roles for ANG II and ANG-(1–7) in the regulation of angiotensin-converting enzyme 2 in rat astrocytes. Am J Physiol Cell Physiol 290: C420–C426, 2006 [DOI] [PubMed] [Google Scholar]
- 20. Gilbert RE, Wu LL, Kelly DJ, Cox A, Wilkinson-Berka JL, Johnston CI, Cooper ME. Pathological expression of renin and angiotensin II in the renal tubule after subtotal nephrectomy. Implications for the pathogenesis of tubulointerstitial fibrosis. Am J Pathol 155: 429–440, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Guan S, Fox J, Mitchell KD, Navar LG. Angiotensin and angiotensin converting enzyme tissue levels in two-kidney, one clip hypertensive rats. Hypertension 20: 763–767, 1992 [DOI] [PubMed] [Google Scholar]
- 22. Hackenthal E, Paul M, Ganten D, Taugner R. Morphology, physiology, and molecular biology of renin secretion. Physiol Rev 70: 1067–1116, 1990 [DOI] [PubMed] [Google Scholar]
- 23. Handa RK, Ferrario CM, Strandhoy JW. Renal actions of angiotensin-(1–7): in vivo and in vitro studies. Am J Physiol Renal Fluid Electrolyte Physiol 270: F141–F147, 1996 [DOI] [PubMed] [Google Scholar]
- 24. Harrison-Bernard LM, Zhuo J, Kobori H, Ohishi M, Navar LG. Intrarenal AT1 receptor and ACE binding in angiotensin II-induced hypertensive rats. Am J Physiol Renal Physiol 281: F19–F25, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Hayes K, Kinsella A, Coffey N. A note on the use of outlier criteria in Ontario laboratory quality control schemes. Clin Biochem 40: 147–152, 2007 [DOI] [PubMed] [Google Scholar]
- 26. Hernandez Prada JA, Ferreira AJ, Katovich MJ, Shenoy V, Qi Y, Santos RAS, Castellano RK, Lampkins AJ, Gubala V, Ostrov DA, Raizada MK. Structure-based identification of small-molecule angiotensin-converting enzyme 2 activators as novel antihypertensive agents. Hypertension 51: 1312–1317, 2008 [DOI] [PubMed] [Google Scholar]
- 27. Kobori H, Harrison-Bernard LM, Navar LG. Urinary excretion of angiotensinogen reflects intrarenal angiotensinogen production. Kidney Int 61: 579–585, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kobori H, Harrison-Bernard LM, Navar LG. Expression of angiotensinogen mRNA and protein in angiotensin II-dependent hypertension. J Am Soc Nephrol 12: 431–439, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kobori H, Harrison-Bernard LM, Navar LG. Enhancement of angiotensinogen expression in angiotensin II-dependent hypertension. Hypertension 37: 1329–1335, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kobori H, Nishiyama A, Abe Y, Navar LG. Enhancement of intrarenal angiotensinogen in Dahl salt-sensitive rats on high salt diet. Hypertension 41: 592–597, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Koka V, Huang XR, Chung ACK, Wang W, Truong LD, Lan HY. Angiotensin II upregulates angiotensin I-converting enzyme (ACE), but downregulates ACE2 via the AT1-ERK/p38 MAP kinase pathway. Am J Pathol 172: 1174–1183, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Komlosi P, Fuson AL, Fintha A, Peti-Peterdi J, Rosivall L, Warnock DG, Bell PD. Angiotensin I conversion to angiotensin II stimulates cortical collecting duct sodium transport. Hypertension 42: 195–199, 2003 [DOI] [PubMed] [Google Scholar]
- 33. Li N, Zimpelmann J, Cheng K, Wilkins JA, Burns KD. The role of angiotensin converting enzyme 2 in the generation of angiotensin 1–7 by rat proximal tubules. Am J Physiol Renal Physiol 288: F353–F362, 2005 [DOI] [PubMed] [Google Scholar]
- 34. Mendes AC, Ferreira AJ, Pinheiro SVB, Santos RAS. Chronic infusion of angiotensin-(1–7) reduces heart angiotensin II levels in rats. Regul Pept 125: 29–34, 2005 [DOI] [PubMed] [Google Scholar]
- 35. Mitchell KD, Jacinto SM, Mullins JJ. Proximal tubular fluid, kidney, and plasma levels of angiotensin II in hypertensive ren-2 transgenic rats. Am J Physiol Renal Physiol 273: F246–F253, 1997 [DOI] [PubMed] [Google Scholar]
- 36. Mitchell KD, Navar LG. Intrarenal actions of angiotensin II in the pathogenesis of experimental hypertension. In: Hypertension: Pathophysiology, Diagnosis, and Management, edited by Laragh JH, Brenner BM.New York: Raven, 1995, p. 1437–1450 [Google Scholar]
- 37. Moe OW, Ujiie K, Star RA, Miller RT, Widell J, Alpern RJ, Henrich WL. Renin expression in renal proximal tubule. J Clin Invest 91: 774–779, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Navar LG, Prieto-Carrasquero MC, Kobori H. Renin renin-angiotensin system. In: Handbook of Biologically Active Peptides, edited by Kastin AJ.London: Academic, 2006, p. 1235–1242 [Google Scholar]
- 39. Park S, Bivona BJ, Kobori H, Seth DM, Chappell MC, Lazartigues E, Harrison-Bernard LM. Major role for ACE-independent intrarenal ANG II formation in type II diabetes. Am J Physiol Renal Physiol 298: F37–F48, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Peti-Peterdi J, Warnock DG, Bell PD. Angiotensin II directly stimulates ENaC activity in the cortical collecting duct via AT(1) receptors. J Am Soc Nephrol 13: 1131–1135, 2002 [DOI] [PubMed] [Google Scholar]
- 41. Ploth DW, Roy RN. Renin-angiotensin influences on tubuloglomerular feedback activity in the rat. Kidney Int 22, Suppl 12: S114–S121, 1982 [PubMed] [Google Scholar]
- 42. Prieto-Carrasquero MC, Harrison-Bernard LM, Kobori H, Ozawa Y, Hering-Smith KS, Hamm LL, Navar LG. Enhancement of collecting duct renin in angiotensin II-dependent hypertensive rats. Hypertension 44: 223–229, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Prieto-Carrasquero MC, Kobori H, Ozawa Y, Gutierrez A, Seth D, Navar LG. AT1 receptor-mediated enhancement of collecting duct renin in angiotensin II-dependent hypertensive rats. Am J Physiol Renal Physiol 289: F632–F637, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Prieto-Carrasquero MC, Botros FT, Pagan J, Kobori H, Seth DM, Casarini DE, Navar LG. Collecting duct renin is upregulated in both kidneys of 2-kidney, 1-clip Goldblatt hypertensive rats. Hypertension 51: 1590–1596, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Redublo Quinto BM, Camargo de Andrade MC, Ronchi FA, Santos EL, ves Correa SA, Shimuta SI, Pesquero JB, Mortara RA, Casarini DE. Expression of angiotensin I-converting enzymes and bradykinin B2 receptors in mouse inner medullary collecting duct cells. Int Immunopharmacol 8: 254–260, 2008 [DOI] [PubMed] [Google Scholar]
- 46. Ren Y, Garvin JL, Carretero OA. Vasodilator action of angiotensin-(1–7) on isolated rabbit afferent arterioles. Hypertension 39: 799–802, 2002 [DOI] [PubMed] [Google Scholar]
- 47. Rohrwasser A, Ishigami T, Gociman B, Lantelme P, Morgan T, Cheng T, Hillas E, Zhang S, Ward K, Bloch-Faure M, Meneton P, Lalouel JM. Renin and kallikrein in connecting tubule of mouse. Kidney Int 64: 2155–2162, 2003 [DOI] [PubMed] [Google Scholar]
- 48. Rohrwasser A, Morgan T, Dillon HF, Zhao L, Callaway CW, Hillas E, Zhang S, Cheng T, Inagami T, Ward K, Terreros DA, Lalouel JM. Elements of a paracrine tubular renin-angiotensin system along the entire nephron. Hypertension 34: 1265–1274, 1999 [DOI] [PubMed] [Google Scholar]
- 49. Sadjadi J, Kramer GL, Yu CH, Welborn MB, III, Chappell MC, Modrall JG. Angiotensin converting enzyme-independent angiotensin II production by chymase is upregulated in the ischemic kidney in renovascular hypertension. J Surg Res 127: 65–69, 2005 [DOI] [PubMed] [Google Scholar]
- 50. Sadjadi J, Kramer GL, Yu CH, Welborn MB, III, Modrall JG. Angiotensin II exerts positive feedback on the intrarenal renin-angiotensin system by an angiotensin converting enzyme-dependent mechanism. J Surg Res 129: 272–277, 2005 [DOI] [PubMed] [Google Scholar]
- 51. Santos RAS, Campagnole-Santos MJ, Andrade SP. Angiotensin (1–7): an update. Regul Pept 91: 45–62, 2000 [DOI] [PubMed] [Google Scholar]
- 52. Shao W, Seth DM, Navar LG. Augmentation of endogenous intrarenal angiotensin II levels in Val5-ANG II-infused rats. Am J Physiol Renal Physiol 296: F1067–F1071, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Tikellis C, Cooper ME, Bialkowski K, Johnston CI, Burns WC, Lew RA, Smith AI, Thomas MC. Developmental expression of ACE2 in the SHR kidney: a role in hypertension? Kidney Int 70: 34–41, 2006 [DOI] [PubMed] [Google Scholar]
- 54. Tikellis C, Johnston CI, Forbes JM, Burns WC, Burrell LM, Risvanis J, Cooper ME. Characterization of renal angiotensin-converting enzyme 2 in diabetic nephropathy. Hypertension 41: 392–397, 2003 [DOI] [PubMed] [Google Scholar]
- 55. Vickers C, Hales P, Kaushik V, Dick L, Gavin J, Tang J, Godbout K, Parsons T, Baronas E, Hsieh F, Acton S, Patane M, Nichols A, Tummino P. Hydrolysis of biological peptides by human angiotensin-converting enzyme-related carboxypeptidase. J Biol Chem 277: 14838–14843, 2002 [DOI] [PubMed] [Google Scholar]
- 56. Vidotti DB, Casarini DE, Cristovam PC, Leite A, Schor N, Boim MA. High glucose concentration stimulates intracellular renin activity and angiotensin II generation in rat mesangial cells. Am J Physiol Renal Physiol 286: F1039–F1045, 2004 [DOI] [PubMed] [Google Scholar]
- 57. Von Thun AM, Vari RC, El-Dahr SS, Navar LG. Augmentation of intrarenal angiotensin II levels by chronic angiotensin II infusion. Am J Physiol Renal Fluid Electrolyte Physiol 266: F120–F128, 1994 [DOI] [PubMed] [Google Scholar]
- 58. Zhong JC, Huang DY, Yang YM, Li YF, Liu GF, Song XH, Du K. Upregulation of angiotensin-converting enzyme 2 by all-trans retinoic acid in spontaneously hypertensive rats. Hypertension 44: 907–912, 2004 [DOI] [PubMed] [Google Scholar]
- 59. Zhuo JL, Imig JD, Hammond TG, Orengo S, Benes E, Navar LG. ANG II accumulation in rat renal endosomes during ANG II-induced hypertension: role of AT(1) receptor. Hypertension 39: 116–121, 2002 [DOI] [PubMed] [Google Scholar]
- 60. Zisman LS, Keller RS, Weaver B, Lin Q, Speth R, Bristow MR, Canver CC. Increased angiotensin-(1–7)-forming activity in failing human heart ventricles: evidence for upregulation of the angiotensin-converting enzyme homologue ACE2. Circulation 108: 1707–1712, 2003 [DOI] [PubMed] [Google Scholar]
- 61. Zou L, Imig JD, Von Thun AM, Hymel A, Ono H, Navar LG. Receptor-mediated intrarenal ANG II augmentation in ANG II-infused rats. Hypertension 28: 669–677, 1996 [DOI] [PubMed] [Google Scholar]