Abstract
Dystroglycan (DG or DAG1) is considered a critical link between the basement membrane and the cytoskeleton in multiple tissues. DG consists of two subunits, an extracellular α-subunit that binds laminin and other basement membrane components, and a transmembrane β-subunit. DG-null mouse embryos die during early embryogenesis because DG is required for Reichert's membrane formation. DG also forms an integral part of the dystrophin-glycoprotein complex in muscle. Although no human DG mutations have been reported, multiple forms of muscular dystrophy have been linked to DG glycosylation defects, and targeted deletion of muscle DG causes muscular dystrophy in mice. Moreover, DG is widely distributed in endothelial and epithelial cells, including those in the kidney. There has therefore been significant interest in DG's role in the kidney, especially in podocytes. Previous reports suggested that DG's disturbance in podocytes might cause glomerular filtration barrier abnormalities. To fully understand DG's contribution to nephrogenesis and kidney function, we used a conditional DG allele and a variety of Cre mice to systematically delete DG from podocytes, ureteric bud, metanephric mesenchyme, and then from the whole kidney. Surprisingly, none of these conditional deletions resulted in significant morphological or functional abnormalities in the kidney. Furthermore, DG-deficient podocytes did not show increased susceptibility to injury, and DG-deficient kidneys did not show delayed recovery. Integrins are therefore likely the primary extracellular matrix receptors in renal epithelia.
Keywords: basement membrane, integrin, podocyte, kidney injury
dystroglycan (DG or DAG1) is an integral component of the dystrophin glycoprotein complex (DGC) in skeletal muscle (1, 33). The DGC plays a central role in stabilizing the skeletal muscle cell membrane and its contractile elements. Mutation or absence of individual DGC components renders the complex dysfunctional and results in muscular dystrophy (MD). DG forms a bridge from laminin, perlecan, and agrin in the basement membrane (BM) outside the cell to the actin cytoskeleton inside the cell via cytoplasmic components of the DGC, such as dystrophin in muscle and utrophin in epithelial cells (33). Mammalian DG consists of two subunits, a heavily glycosylated extracellular α-subunit and a transmembrane β-subunit (19). Both are the product of a single gene (Dag1) and a single mRNA and are derived from cleavage of a single precursor polypeptide.
Although there are no reported human DAG1 mutations, multiple human diseases have been linked to DG glycosylation defects resulting from mutations in enzymes that modify DG. These include Fukuyama congenital MD, muscle-eye-brain disease, Walker-Warburg syndrome, and some forms of limb-girdle MD (1, 3, 8, 15, 16, 32, 33). The glycosylation defects reduce the affinity of DG for laminin and thereby impair DGC function (24, 57).
Some important insights into DG's function have come from animal studies. Dag1 knockout mice die during early embryogenesis due to failure of extraembryonic Reichert's membrane formation, although the embryonic BM forms (56). To circumvent this early lethality, a conditional Dag1 mutant allele was generated. Neural mutation recapitulates some of the abnormalities of congenital MD with mental retardation (38). Deletions of Dag1 in skeletal muscle result in MD of varying severities, depending on the spatiotemporal properties of Cre expression (5, 21). Schwann cell deletion results in myelination defects (6).
DG is widely expressed in nonneuromuscular tissues (9), where it interacts primarily with the utrophin glycoprotein complex (UGC), which is analogous to the DGC of muscle. DG expression is prominent in branched epithelia of kidney, lung, and salivary gland. However, its importance and function in epithelia have not been fully explored. C. elegans studies suggest that DG is important for normal epithelial development (23), as its mutation results in a phenotype similar to those observed in laminin mutants (17) but dissimilar to other DGC mutant phenotypes. Dystroglycan depletion in Xenopus laevis larvae causes reduced pronephric tubulogenesis and renal agenesis, depending on the degree of depletion (2). Additional studies implicated DG in mediating polarization of and signal transduction in mammary epithelial cells (29, 54) and Drosophila follicular epithelium (7, 49), in repairing airway epithelium (55), and in cancer (50).
In developing kidney, DG function has been implicated in branching of the ureteric bud (UB). Culture of embryonic day 12.5 kidneys in the presence of DG-blocking antibodies caused a reduction in UB branching and resulted in small kidneys (10). Studies using an analogous design with cultured embryonic lung and salivary gland resulted in similar findings (11). DG has also been linked to maintaining the glomerular filtration barrier by influencing podocyte and foot process architecture. Podocyte DG has been suggested to be as important for podocyte adhesion to glomerular BM (GBM) laminin as is integrin α3β1 (52). Normal DG expression and basal distribution are thought to be crucial for a normal filtration barrier. Podocyte DG expression is reduced or mislocalized in minimal change disease, but not in focal segmental glomerulosclerosis (13, 46). Protamine sulfate perfusion of isolated kidneys results in redistribution of podocyte DG from the soles of foot processes to a diffuse pattern, accompanied by podocyte foot process effacement (25, 26). In vitro studies showed that reactive oxygen species might cause deglycosylation of α-DG and reduce its affinity for ligand (51, 57). Finally, DG clustering by fibronectin or biglycan results in increased cytosolic Ca that may alter the podocyte cytoskeleton and cause foot process effacement (52).
While many of the aforementioned studies assigned a function for DG in renal cells, there is significant evidence against an important role for DG in the kidney. There are no reported kidney function abnormalities in either mouse or human mutants with impaired DG function due to glycosylation defects, although a recent report showed GBM thickening and podocyte foot process widening, but without albuminuria, in chimeric mice generated with fukutin-null embryonic stem cells (27). Furthermore, the utrophin knockout mouse, even when combined with the dystrophin knockout, has normal kidneys (45). This unexpected finding suggests that 1) other DGC/UGC proteins compensate for the absence of utrophin and dystrophin, 2) epithelial DG functions independently of UGC proteins, or 3) the UGC is dispensable for kidney formation and function.
Here, we directly studied DG's contribution to kidney formation and function by specific deletion of DG from podocytes, UB, metanephric mesenchyme (MM) derivatives, and from all renal epithelial cells using the Cre-lox system. Surprisingly, any mode of DG deletion from kidney yielded only mild or no phenotypes. A mild increase in urinary protein in males, but without an increase in urinary albumin, was the most prominent feature. Ultrastructural analysis showed only mild GBM thickening associated with the loss of DG from podocytes. Furthermore, DG-null podocytes and tubular cells did not show increased susceptibility to injury.
MATERIALS AND METHODS
Animals
Dag1loxp/loxp animals (38) were obtained from Dr. K. Campbell. Podocin-Cre (2.5PCre) mice (37) were obtained from Dr. L. Holzman. HoxB7-Cre mice (58) were obtained from Dr. A. McMahon. Pax2-Cre mice (40) were obtained from Dr. A. Groves. Pax3-Cre transgenic (P3Pro-Cre) mice (30) were obtained from Dr. J. Epstein. Itga3 mutant mice have been described (28). Mice were maintained on a primarily C57BL/6J-CBA/J mixed strain background. Mice with either two loxP alleles or with one null and one loxP allele in the presence of Cre were used as mutants in the studies and gave similar results. Littermates with at least one wild-type allele were used as normal controls in all studies.
All mice were housed in a specific pathogen-free facility. The Animal Studies Committee of Washington University School of Medicine approved the experiments.
Immunofluorescence, Histology, and Electron Microscopy
The antibodies used are listed in Table 1. For immunofluorescence studies, fresh kidneys were embedded in optimal cutting compound. Seven-micrometer fresh frozen sections were stained according to published methods (20). Detection of β-DG in kidney with antibody 7D11 required urea-glycine treatment (36).
Table 1.
List of antibodies used in this study
| Antibody | Species | Dilution | Source and/or Reference |
|---|---|---|---|
| α-DG (IIH6) | Mouse anti-rabbit | 1/50 | Millipore |
| β-DG (7D11) | Mouse anti-human | 1/100 | Developmental Studies Hybridoma Bank |
| Laminin α1 (8B3) | Rat anti-mouse | 1/1,000 | Dale Abrahamson (Univ. of Kansas Medical Center, Kansas City, KS) |
| Laminin α2 (4H8-2) | Rat anti-mouse | 1/1,000 | Enzo Life Sciences |
| Laminin α5 (8498) | Rabbit anti-mouse | 1/800 | (35) |
| Laminin β1 | Rabbit anti-mouse | 1/1,000 | (48) |
| Laminin β2 | Rabbit anti-mouse | 1/2,000 | (48) |
| Integrin α3 | Rabbit anti-chicken | 1/200 | C. Mike Dipersio (Albany Medical College, Albany, NY) |
| Integrin α6 (GoH3) | Rat anti-human | 1/200 | Abcam |
| Integrin β1 (MAB1997) | Rat anti-mouse | 1/200 | Millipore |
| Nephrin | Rabbit anti-mouse | 1/200 | Sigma |
| Podocin | Rabbit anti-human | 1/200 | Sigma |
| Synaptopodin | Mouse anti-rat | 1/300 | (39) |
| Agrin (LG) | Rabbit anti-mouse | 1/20,000 | Takako Sasaki (Univ. of Erlangen-Nuernberg, Germany) |
| WT1 | Rabbit anti-human | 1/200 | Santa Cruz Biotechnology |
| Entactin/Nidogen (MAB1946) | Rat anti-mouse | 1/300 | Millipore |
For histology, tissues were fixed in 10% buffered formalin, followed by embedding in paraffin. Four-micrometer sections were stained with hematoxylin and eosin, periodic acid Schiff, and trichrome according to published methods. For electron microscopy, small pieces of kidney cortex were fixed in 2% paraformaldehyde, 2% glutaraldehyde in 0.1 M sodium cacodylate buffer. Tissues were dehydrated, embedded in plastic, and ultrathin sections were viewed by transmission electron microscopy as described (20).
Renal Chemistries
Urine and serum were assayed using an automated analyzer (Roche Cobas Mira Plus). For urine SDS-PAGE, equal amounts of urine corrected for creatinine were run on 4–20% gels. Gels were stained with Coomassie blue.
Injury Models
The LPS (purchased from Invivogen, San Diego, CA) (53) and adriamycin (purchased from Sigma, St. Louis, MO) (4) injury models have been described. For anti-glomerular antibody-mediated injury (41), we injected a total 1.25 μg antibody/g body wt, divided on 2 consecutive days, into 5 control males, 7 mutant males, 3 control females, and 4 mutant females. Urine was collected before antibody injection and twice weekly afterwards. All mice were killed at the conclusion of 3 wk. Serum samples were obtained before the mice were killed.
RESULTS
Mice with DG Deletion from Kidney Epithelial Cell Subsets Are Viable
DG deletion from podocytes.
Considering the proposed importance of DG for podocyte function, we used the well-characterized podocin-Cre transgene to delete DG from podocytes. Immunostaining for either α- or β-DG showed complete absence of both from mutant podocytes as early as 2 wk of age (Fig. 1A and data not shown). 2.5PCre mutant mice exhibited normal life spans.
Fig. 1.
Analysis of kidney dystroglycan (DG) expression in mice of various genotypes confirmed efficient deletion of DG consistent with the pattern of Cre recombinase expression. A: podocin- (2.5P-) Cre mutant. DG is absent from podocytes but preserved in mesangial cells and in parietal and tubular epithelial cells. (Compare with control glomeruli in B.) B–E: double staining with anti-DG (red) and LTL (proximal tubule marker; green) in control (B, D) and Pax3-Cre mutant kidney (C, E) shows efficient deletion of DG by Pax3-Cre from glomeruli and all tubules. F, G: DG was preserved in collecting duct cells in Pax3-Cre mutant kidney (+ in F and G), identified by aquaporin-2 (AQP2) staining (G, green). H–K: costaining for DG (red) and AQP2 (I, K; green) in control (H and I) and HoxB7-Cre mutant kidney (J, K) reveals the absence of DG from collecting ducts (+ in H–K) in the latter. L–O: analysis of DG distribution in control (L, M) and Pax2-Cre mutant (N, O) cortex (L, N) and medulla (M, O) shows the disappearance of DG from all kidney epithelia, but with preservation of smooth muscle DG (“X” in N) in Pax2-Cre mutant kidney.
DG deletion from MM derivatives.
We deleted DG from both the nephron and stromal cell lineages at early time points using the Pax3-Cre transgene, which is expressed in the MM, as well as in several extrarenal tissues (21, 30), but not in the UB. Immunostaining of kidneys from Pax3-Cre mutant mice showed efficient deletion of DG from the entire nephron (Fig. 1B–E), whereas UB derivatives [i.e., collecting ducts (CDs)] retained their normal DG distribution. This was confirmed by colocalization of DG with CD markers such as aquaporin-2 and cytokeratin-8 (Fig. 1F and G, and data not shown). Pax3-Cre mutant mice did not show significant early mortality, and some lived more than 15 mo. However, mutants weighed 20–30% less than their control sex-matched littermates due to severe caudally restricted MD (21).
DG deletion from UB.
Considering the proposed role for DG in UB branching (10), we utilized the HoxB7-Cre transgene to delete DG from the UB. HoxB7-Cre mutant mice were viable and had apparently normal kidneys. DG staining and colocalization with aquaporin-2 confirmed the lack of DG in CDs (Fig. 1H–K). HoxB7-Cre mutant kidneys did not show any significant abnormalities. Kidney size and morphology were normal. The life span of HoxB7-Cre mutant mice was normal.
DG deletion from all renal epithelial cells.
We utilized the Pax2-Cre transgene to delete DG from both the UB and from nephron progenitors. Pax2-Cre is also expressed in several extrarenal tissues (40). Pax2-Cre mutant mice were viable for several months. There was a complete absence of DG from all kidney epithelial cells, with preservation of DG staining in vascular smooth muscle cells (Fig. 1L–O). Pax2-Cre mutants were 30–50% smaller than their control littermates and died between 3–4 mo of age because of severe MD that involved all muscle groups, including the diaphragm. Further investigation showed expression of Pax2-Cre in all skeletal muscles (data not shown). Pax2-Cre mutant kidneys were small compared with control kidneys, but kidney/body weight ratios for Pax2-Cre mutants were higher than controls due to the severe MD.
Kidney Morphology and Glomerular Function
Surprisingly, mutant mice lacking DG in podocytes (due to deletion by Podocin-Cre, Pax3-Cre, or Pax2-Cre) showed no increase in albumin excretion compared with sex-matched control littermates. However, we did detect higher total protein/creatinine ratios in young male Pax3-Cre mutant mice between 2–6 mo of age (Fig. 2). This difference decreased as the mice aged, and by 12 mo the protein/creatinine ratios approached the values observed in control males. Albumin excretion was also variable, with some mutant and control mice showing very low levels of albuminuria, as revealed by SDS-PAGE (data not shown). Blood urea nitrogen and creatinine levels in all mutants were similar to those in sex-matched littermate controls. Furthermore, conventional light microscopy revealed that all mutant kidneys exhibited normal overall architecture (Fig. 3A and B). Pax2-Cre and Pax3-Cre mutant kidney/body weight ratios were higher than controls (data not shown), which likely reflects the associated MD-induced weight loss.
Fig. 2.
Urinary protein/creatinine ratios (P/C) from Pax3-Cre mutants (Mut) and control littermates (WT), segregated by gender. A: females. B: males. Age was estimated to the nearest month. Average ± SD P/C (mg/mg) were for all mice: controls = 20.75 ± 9.2, mutants = 28.5 ± 16.2; P = 0.0048; for females: controls = 13.7 ± 3.8, mutants = 16.4 ± 4.4; P = 0.015; for males: controls = 27.9 ± 7.14, mutants = 46 ± 9.6; P = 2.4 × 10−6.
Fig. 3.
Histological analysis of control and mutant kidneys. A–D: periodic acid Schiff (PAS) staining of kidney sections from control (A, C) and Pax3-Cre mutant (B, D) mice. Note the absence of any glomerular or tubular pathology. E–H: transmission electron microscopic images of glomerular capillary walls from control (E, G) and Pax3-Cre mutant (F, H) kidneys at ×6,400 (E, F) and ×14,000 (G, H) magnification. Podocyte foot processes and slit diaphragms appeared normal. The glomerular basement membrane (GBM) appeared slightly thicker in the mutant.
Glomerular/Podocyte Phenotype
Kidneys with DG-deficient podocytes (using any of the relevant Cre transgenes) did not show any significant glomerular pathology (Fig. 3C and D). Electron microscopy did not show significant foot process effacement, slit diaphragm loss, or defective podocyte adhesion to the GBM; the only detectable abnormality was a mild increase in GBM thickness without electron-dense deposits or lamination (Fig. 3E–H).
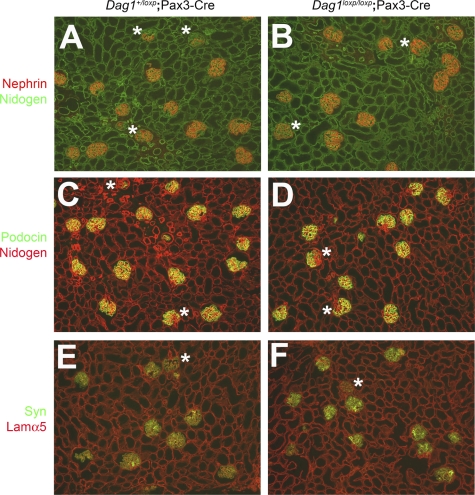
Immunofluorescence studies showed normal intensity and distribution of podocyte-specific markers such as podocin, nephrin, and synaptopodin in mutant glomeruli (Fig. 4A and B). Podocyte cell number appeared to be normal by WT1 staining (Fig. 4C and D). GBM composition was normal, as indicated by the presence of the normal GBM laminin-521 (Fig. 5). There was no significant accumulation of ectopic laminins (Fig. 5A–H, laminin staining) or reduction in GBM agrin staining (data not shown). Furthermore, α3β1 integrin staining using individual antibodies against the α3 and β1 subunits showed normal distribution and intensity in glomeruli (Fig. 5I, J, M, and N).
Fig. 4.
Immunohistochemical analysis of podocyte marker expression. Frozen sections from adult control (A, C) and Pax3-Cre mutant (B, D) kidneys were stained with antibodies to nephrin (A, B) and to WT1 (green) and nidogen (red; C, D). There were no significant differences in the intensity or distribution of nephrin staining and no change in podocyte number by WT1 staining. Anti-nidogen was used to label BMs.
Fig. 5.
Detection of BM components (A–H) and integrins (I–P) in control (A–D and I–L) and Pax3-Cre mutant (E–H and M–P) kidney sections. A, E: laminin β1. B, F: laminin β2. C, G: laminin α1. D, H: laminin α5. I, M: integrin α3. J, N: integrin β1, with focus on glomerular staining. K, O: integrin β1, with focus on tubular staining. L, P: integrin α6. There were no detectable differences in the laminin composition of GBM or tubular BM or in the intensity or localization of integrins.
Podocyte DG/Integrin α3 Double Mutant Phenotype
Integrin α3β1 is highly expressed in podocytes and is the most important laminin receptor in these cells (28, 44). It is possible that DG also plays a role-perhaps a minor one-in mediating podocyte interactions with the GBM that is masked by the presence of integrin α3β1 in the mutants discussed above. We therefore attempted to uncover a role for DG in podocytes by studying podocyte DG's contribution to glomerular phenotype in the absence of integrin α3. Itga3−/− mice die at birth or shortly thereafter and exhibit a severely thickened and laminated GBM and impaired foot process formation. Itga3−/−; Dag1loxp/loxp;2.5PCre mice lack integrin α3β1 in all cells and also lack DG specifically in podocytes. Double mutants exhibited the same GBM thickening and lamination observed in Itga3−/− mice (Fig. 6). Furthermore, both Itga3−/− and Itga3−/−; Dag1loxp/loxp;2.5PCre podocytes showed similar foot process abnormalities (Fig. 6). Because the overall glomerular phenotype of Itga3−/−; Dag1loxp/loxp;2.5PCre mice was indistinguishable from that of Itga3−/− mice (28), we conclude that DG does not play a significant role in podocytes, even in the absence of the major GBM-binding receptor.
Fig. 6.
Lack of DG in podocytes does not exacerbate the integrin α3-null glomerular phenotype. Shown are representative electron micrographs of newborn kidneys from control (A), Dag1loxp/loxp;2.5PCre (B), Itga3−/− (C), and Itga3−/−; Dag1loxp/loxp;2.5PCre (D). The phenotype of podocytes that lack both DG and integrin α3β1 was not different from the phenotype of integrin α3-null podocytes. Both show foot process abnormalities, as well as a disorganized GBM with thickening and extensive lamination. Original magnification ×12,000.
Tubule Phenotype
Pax3-Cre and Pax2-Cre mutant kidneys, which lack DG in all MM derivatives and from all kidney epithelial cells, respectively, did not show any histopathology in the tubulointerstitial compartment (Fig. 3A and B, and data not shown). Tubular BM composition and localization of the tubular cell integrins tested (integrins β1 and α6) were also unchanged (Fig. 5).
Absence of DG and Susceptibility to Renal Injury
Despite the lack of pathology associated with DG deletion from podocytes and from tubular epithelial cells, we hypothesized that DG might still play an important role under pathologic conditions. To study a potential role for renal DG in the setting of injury/repair, we induced glomerular and/or tubular injury in Dag1 mutants using LPS (12, 31, 42, 53), adriamycin (4, 47), and antiglomerular antibody (41, 43). We used Pax3-Cre mutant mice, because the lack of DG in tubules (in addition to podocytes) might have unmasked a role for tubular DG in some aspect of kidney disease. Neither DG mutant podocytes nor tubular epithelial cells showed increased susceptibility to LPS-induced injury (data not shown). Moreover, the absence of DG from podocytes did not render them susceptible to adriamycin-induced injury on the normally resistant mixed C57BL/6J × CBA/J mixed strain background (data not shown).
Next, we induced injury using anti-glomerular antibodies given as two intraperitoneal injections on 2 consecutive days. We treated a total of 19 mice (see materials and methods). Treated mice became proteinuric within 24 h after the first antibody dose. Proteinuria peaked at 7–10 days, with precipitous improvement afterwards in both mutant and control mice. When we considered all mice, proteinuria was not different between mutants and controls throughout the experimental period. However, we observed two different courses of proteinuria depending on the gender, but not on the genotype. Females showed a rapid improvement after the first week, and urinary protein/creatinine ratios returned to baseline after 14 days. In contrast, males showed a higher peak proteinuria that persisted longer (Fig. 7). Furthermore, there was a trend toward higher protein/creatinine ratios in mutant males between days 10 and 14. By the conclusion of the study period (21 days), protein/creatinine ratios were still higher than baseline, but were nearly identical in controls and mutants. Blood urea nitrogen and creatinine levels were similar in mutants and controls at the conclusion of the study.
Fig. 7.
Spot urine total P/C (mg/mg) in control (Cont) and Pax3-Cre mutant (Mut) mice before (day 0) and at different time points (in days) after anti-glomerular antibody injury in all mice (A) and males only (B). There was a trend toward higher P/C in male mutant mice between 10 and 14 days. Graphs show total P/C from spot urine expressed as average ± SD. Mutant males displayed higher P/C at 10–14 days. The P value for the 10-day time point was 0.066 and for the 14-day time point was 0.0046. At the conclusion of 3 wk, P/C was not significantly different in males or females.
There were two mortalities in the group of seven injected male mutants; one occurred shortly after the first antibody injection, and the second at 2 days after the second dose. The cause of these deaths was unclear. All surviving mice were killed at 3 wk postinjections.
Glomerular phenotypes within each kidney varied widely, from normal to globally sclerosed (Fig. 8). We also observed variable degrees of segmental glomerulosclerosis in 10–15% of glomeruli (Fig. 8). We noted the presence of proteinaceous tubular casts and mild tubular dilation. However, there were no significant differences between mutant and control kidneys, even in males. The extent of glomerulosclerosis (global and segmental) and tubulointerstitial injury was comparable for the mutants and controls. Expression of podocin, nephrin, and synaptopodin was not significantly different between mutants and controls; the vast majority of glomeruli showed a normal distribution of these podocyte markers, with few showing segmental and occasionally global reduction (Fig. 9). Ultrastructural analysis revealed an expanded mesangium and segmental areas of foot process effacement in both mutant and control mice (data not shown).
Fig. 8.
PAS staining of kidney sections from male control (A, C, E) and Pax3-Cre mutant (B, D, F) mice 21 days after anti-glomerular antibody injury shows variable glomerular phenotypes. A, B: relatively normal glomerular morphology. C, D: segmental sclerosis, which was observed in 10–15% of glomeruli in control and mutant mice. E, F: near total glomerulosclerosis, which was observed in <5% of glomeruli in control and mutant mice.
Fig. 9.
Immunostaining for podocyte markers in antibody-injured glomeruli. Double staining of kidney sections for nephrin (red) and nidogen (green; A and B), podocin (green) and nidogen (red; C and D), and synaptopodin (Syn; green) and laminin α5 (red; E and F). Sections were from control (A, C, E) and Pax3-Cre mutant (B, D, F) mice 21 days after anti-glomerular antibody injection. *Glomeruli with abnormal intensity or distribution of the podocyte marker. Nidogen and laminin α5 antibodies were used to label BMs.
DISCUSSION
DG's role in epithelial cells appears to have diminished through evolution from invertebrates, in which loss of DG causes clear epithelial phenotypes, toward mammals. In mammals, DG plays an important role in formation of Reichert's membrane, and the null phenotype is reminiscent of embryos lacking LM-111 (34). However, after early embryogenesis, DG dysfunction causes primarily neuromuscular abnormalities. This likely stems in part from the increased complexity of extracellular matrix (ECM) proteins and their receptors (primarily integrins) that are used to mediate cell-matrix interactions in vertebrates (18, 22).
In this study, we investigated DG's contribution to kidney formation and function. We expected to validate much of the published in vitro cell and organ culture data, injury model data, and observations in human proteinuric kidney diseases. To our surprise, DG deletion from the kidney epithelium throughout nephrogenesis seems to be without consequences. Kidney formation and function proceeded normally in the absence of DG, even when deleted from the UB and from MM derivatives early during development using Hoxb7-Cre, Pax2-Cre, and Pax3-Cre. DG deletion did not result in significant changes in BM laminin, one of its main extracellular matrix ligands. Furthermore, we were unable to detect compensatory changes in kidney epithelial cell integrins. To attempt to uncover a role for DG in GBM organization, we studied the effect of DG deletion in the absence of integrin α3β1, the primary integrin in podocytes. Surprisingly, the glomerular phenotype in mice lacking both DG and integrin α3β1 from podocytes was not different from the phenotype in integrin α3-null mice, suggesting that DG does not contribute to podocyte adhesion and that integrin α3β1 is the main laminin receptor/BM organizer in podocytes.
These results are in stark contrast to previous reports obtained from cultured embryonic kidneys treated with DG blocking antibodies (10). Our results also did not reveal any role for DG in podocyte differentiation and function, in contrast to conclusions drawn from animal injury models and from studying biopsies from proteinuric human kidney diseases. The glomerular filtration barrier developed normally in the absence of podocyte DG. DG-null podocytes had normal morphology without evidence of foot process effacement. DG-null podocytes showed normal immunostaining for podocyte markers such as podocin, nephrin, and synaptopodin and did not show increased susceptibility to three different types of injury.
Our results contradict published studies obtained using accepted scientific methods and reinforce the importance of validating both in vitro and observational data in a living organism. The culture of embryonic kidneys to study UB branching and patterning has been an important tool for studying kidney development and has produced important results that have shaped our current understanding of nephrogenesis. However, the use of blocking antibodies in organ culture may have consequences beyond the desired inhibitory activity. In addition, the ex vivo environment is harsh and does not replicate all aspects of normal development, so it is important to view it as a screening tool and to validate any results in vivo. Furthermore, conclusions drawn from human biopsies, while important, cannot differentiate between primary (causative) abnormalities and secondary changes.
Our negative results validate multiple observations from human and animal studies, which suggest the limited importance of DG and the UGC in epithelial cells under physiologic conditions, despite the near ubiquitous expression of multiple UGC proteins in epithelia. Furthermore, there is a fair amount of evidence showing that integrins are crucial ECM receptors in renal epithelial cells (28, 44). DG was not sufficient to compensate and was not upregulated in association with podocyte-specific deletion of integrin α3β1 (44).
Some potential problems with our approaches should be mentioned. The Podocin-Cre transgene may not have been expressed early enough to remove all DG during podocyte development, as our recent data suggest (14). However, both Pax2-Cre and Pax3-Cre are active in metanephric mesenchyme, so DG should be mutated in nephron precursors well before podocyte differentiation begins. Another issue is that Pax2-Cre and Pax3-Cre transgenes are widely expressed outside the kidney. Most surprising was the widespread expression of Pax2-Cre in skeletal muscles, which had not been previously reported. In that regard, the resulting MD and weight loss in Pax2-Cre and Pax3-Cre mutants might have clouded the picture, either by rendering kidney/body weight ratios less meaningful or by altering urinary protein excretion. Moreover, we have no clear explanation for the two Pax3-Cre mutant deaths after anti-glomerular antibody injection. We suspect at least one was the result of a technical problem, as it occurred immediately after the first injection. Although it is easy to speculate that the mortalities were the result of a more severe kidney injury due to the absence of DG, we have not been able to find evidence supporting a worse outcome in the surviving mutant animals. Nevertheless, it is possible that DG-null podocytes and tubular cells may still be more (or perhaps less) susceptible to other types of kidney injury.
GRANTS
This work was supported by grants from the National Institutes of Health (R01GM060432, R21DK074613, and R01DK078314 to J. H. Miner; O'Brien Center Pilot and Feasibility Grant P30DK079333 to G. Jarad) and by a grant from the Alaska Kidney Foundation-American Society of Nephrology (to G. Jarad).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
ACKNOWLEDGMENTS
We thank generous colleagues for providing antibodies and transgenic mice; J. Richardson for mouse genotyping; the Washington University Mouse Genetics Core for animal husbandry; the Pulmonary Morphology Core [National Institutes of Health (NIH) P01HL029594] for paraffin histology; and the Renal Disease Models Core of the Washington University Center for Kidney Disease Research O'Brien Center (NIH P30DK079333) for assistance with electron microsocopy, renal chemistries, and imaging.
REFERENCES
- 1. Barresi R, Campbell KP. Dystroglycan: from biosynthesis to pathogenesis of human disease. J Cell Sci 119: 199–207, 2006 [DOI] [PubMed] [Google Scholar]
- 2. Bello V, Sirour C, Moreau N, Denker E, Darribere T. A function for dystroglycan in pronephros development in Xenopus laevis. Dev Biol 317: 106–120, 2008 [DOI] [PubMed] [Google Scholar]
- 3. Brockington M, Yuva Y, Prandini P, Brown SC, Torelli S, Benson MA, Herrmann R, Anderson LV, Bashir R, Burgunder JM, Fallet S, Romero N, Fardeau M, Straub V, Storey G, Pollitt C, Richard I, Sewry CA, Bushby K, Voit T, Blake DJ, Muntoni F. Mutations in the fukutin-related protein gene (FKRP) identify limb girdle muscular dystrophy 2I as a milder allelic variant of congenital muscular dystrophy MDC1C. Hum Mol Genet 10: 2851–2859, 2001 [DOI] [PubMed] [Google Scholar]
- 4. Chen X, Moeckel G, Morrow JD, Cosgrove D, Harris RC, Fogo AB, Zent R, Pozzi A. Lack of integrin alpha1beta1 leads to severe glomerulosclerosis after glomerular injury. Am J Pathol 165: 617–630, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Cohn RD, Henry MD, Michele DE, Barresi R, Saito F, Moore SA, Flanagan JD, Skwarchuk MW, Robbins ME, Mendell JR, Williamson RA, Campbell KP. Disruption of DAG1 in differentiated skeletal muscle reveals a role for dystroglycan in muscle regeneration. Cell 110: 639–648, 2002 [DOI] [PubMed] [Google Scholar]
- 6. Court FA, Hewitt JE, Davies K, Patton BL, Uncini A, Wrabetz L, Feltri ML. A laminin-2, dystroglycan, utrophin axis is required for compartmentalization and elongation of myelin segments. J Neurosci 29: 3908–3919, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Deng WM, Schneider M, Frock R, Castillejo-Lopez C, Gaman EA, Baumgartner S, Ruohola-Baker H. Dystroglycan is required for polarizing the epithelial cells and the oocyte in Drosophila. Development 130: 173–184, 2003 [DOI] [PubMed] [Google Scholar]
- 8. Durbeej M, Campbell KP. Muscular dystrophies involving the dystrophin-glycoprotein complex: an overview of current mouse models. Curr Opin Genet Dev 12: 349–361, 2002 [DOI] [PubMed] [Google Scholar]
- 9. Durbeej M, Henry MD, Ferletta M, Campbell KP, Ekblom P. Distribution of dystroglycan in normal adult mouse tissues.J Histochem Cytochem 46: 449–457, 1998 [DOI] [PubMed] [Google Scholar]
- 10. Durbeej M, Larsson E, Ibraghimov-Beskrovnaya O, Roberds SL, Campbell KP, Ekblom P. Nonmuscle alpha-dystroglycan is involved in epithelial development. J Cell Biol 130: 79–91, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Durbeej M, Talts JF, Henry MD, Yurchenco PD, Campbell KP, Ekblom P. Dystroglycan binding to laminin alpha1LG4 module influences epithelial morphogenesis of salivary gland and lung in vitro. Differentiation 69: 121–134, 2001 [DOI] [PubMed] [Google Scholar]
- 12. Faul C, Donnelly M, Merscher-Gomez S, Chang YH, Franz S, Delfgaauw J, Chang JM, Choi HY, Campbell KN, Kim K, Reiser J, Mundel P. The actin cytoskeleton of kidney podocytes is a direct target of the antiproteinuric effect of cyclosporine A. Nat Med 14: 931–938, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Giannico G, Yang H, Neilson EG, Fogo AB. Dystroglycan in the diagnosis of FSGS. Clin J Am Soc Nephrol 4: 1747–1753, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Goldberg S, Adair-Kirk TL, Senior RM, Miner JH. Maintenance of glomerular filtration barrier integrity requires laminin alpha5. J Am Soc Nephrol 21: 579–586, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Haliloglu G, Gross C, Senbil N, Talim B, Hehr U, Uyanik G, Winkler J, Topaloglu H. Clinical spectrum of muscle-eye-brain disease: from the typical presentation to severe autistic features. Acta Myol 23: 137–139, 2004 [PubMed] [Google Scholar]
- 16. Haliloglu G, Topaloglu H. Glycosylation defects in muscular dystrophies. Curr Opin Neurol 17: 521–527, 2004 [DOI] [PubMed] [Google Scholar]
- 17. Huang CC, Hall DH, Hedgecock EM, Kao G, Karantza V, Vogel BE, Hutter H, Chisholm AD, Yurchenco PD, Wadsworth WG. Laminin alpha subunits and their role in C. elegans development. Development 130: 3343–3358, 2003 [DOI] [PubMed] [Google Scholar]
- 18. Hutter H, Vogel BE, Plenefisch JD, Norris CR, Proenca RB, Spieth J, Guo C, Mastwal S, Zhu X, Scheel J, Hedgecock EM. Conservation and novelty in the evolution of cell adhesion and extracellular matrix genes. Science 287: 989–994, 2000 [DOI] [PubMed] [Google Scholar]
- 19. Ibraghimov-Beskrovnaya O, Ervasti JM, Leveille CJ, Slaughter CA, Sernett SW, Campbell KP. Primary structure of dystrophin-associated glycoproteins linking dystrophin to the extracellular matrix. Nature 355: 696–702, 1992 [DOI] [PubMed] [Google Scholar]
- 20. Jarad G, Cunningham J, Shaw AS, Miner JH. Proteinuria precedes podocyte abnormalities in Lamb2−/− mice, implicating the glomerular basement membrane as an albumin barrier. J Clin Invest 116: 2272–2279, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Jarad G, Miner JH. The Pax3-Cre transgene exhibits a rostrocaudal gradient of expression in the skeletal muscle lineage. Genesis 47: 1–6, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Johnson MS, Lu N, Denessiouk K, Heino J, Gullberg D. Integrins during evolution: evolutionary trees and model organisms. Biochim Biophys Acta 1788: 779–789, 2009 [DOI] [PubMed] [Google Scholar]
- 23. Johnson RP, Kang SH, Kramer JMC. elegans dystroglycan DGN-1 functions in epithelia and neurons, but not muscle, and independently of dystrophin. Development 133: 1911–1921, 2006 [DOI] [PubMed] [Google Scholar]
- 24. Kanagawa M, Nishimoto A, Chiyonobu T, Takeda S, Miyagoe-Suzuki Y, Wang F, Fujikake N, Taniguchi M, Lu Z, Tachikawa M, Nagai Y, Tashiro F, Miyazaki J, Tajima Y, Endo T, Kobayashi K, Campbell KP, Toda T. Residual laminin-binding activity and enhanced dystroglycan glycosylation by LARGE in novel model mice to dystroglycanopathy. Hum Mol Genet 18: 621–631, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kojima K, Davidovits A, Poczewski H, Langer B, Uchida S, Nagy-Bojarski K, Hovorka A, Sedivy R, Kerjaschki D. Podocyte flattening and disorder of glomerular basement membrane are associated with splitting of dystroglycan-matrix interaction. J Am Soc Nephrol 15: 2079–2089, 2004 [DOI] [PubMed] [Google Scholar]
- 26. Kojima K, Kerjaschki D. Is podocyte shape controlled by the dystroglycan complex? Nephrol Dial Transplant 17, Suppl 9: 23–24, 2002 [DOI] [PubMed] [Google Scholar]
- 27. Kojima K, Nosaka H, Kishimoto Y, Nishiyama Y, Fukuda S, Shimada M, Kodaka K, Saito F, Matsumura K, Shimizu T, Toda T, Takeda S, Kawachi H, Uchida S. Defective glycosylation of alpha-dystroglycan contributes to podocyte flattening. Kidney Int 79: 311–316, 2011 [DOI] [PubMed] [Google Scholar]
- 28. Kreidberg JA, Donovan MJ, Goldstein SL, Rennke H, Shepherd K, Jones RC, Jaenisch R. Alpha 3 beta 1 integrin has a crucial role in kidney and lung organogenesis. Development 122: 3537–3547, 1996 [DOI] [PubMed] [Google Scholar]
- 29. Leonoudakis D, Singh M, Mohajer R, Mohajer P, Fata JE, Campbell KP, Muschler JL. Dystroglycan controls signaling of multiple hormones through modulation of STAT5 activity. J Cell Sci 123: 3683–3692, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Li J, Chen F, Epstein JA. Neural crest expression of Cre recombinase directed by the proximal Pax3 promoter in transgenic mice. Genesis 26: 162–164, 2000 [DOI] [PubMed] [Google Scholar]
- 31. Meyer-Schwesinger C, Dehde S, von Ruffer C, Gatzemeier S, Klug P, Wenzel UO, Stahl RA, Thaiss F, Meyer TN. Rho kinase inhibition attenuates LPS-induced renal failure in mice in part by attenuation of NF-κB p65 signaling. Am J Physiol Renal Physiol 296: F1088–F1099, 2009 [DOI] [PubMed] [Google Scholar]
- 32. Michele DE, Barresi R, Kanagawa M, Saito F, Cohn RD, Satz JS, Dollar J, Nishino I, Kelley RI, Somer H, Straub V, Mathews KD, Moore SA, Campbell KP. Posttranslational disruption of dystroglycan-ligand interactions in congenital muscular dystrophies. Nature 418: 417–422, 2002 [DOI] [PubMed] [Google Scholar]
- 33. Michele DE, Campbell KP. Dystrophin-glycoprotein complex: posttranslational processing and dystroglycan function. J Biol Chem 278: 15457–15460, 2003 [DOI] [PubMed] [Google Scholar]
- 34. Miner JH, Li C, Mudd JL, Go G, Sutherland AE. Compositional and structural requirements for laminin and basement membranes during mouse embryo implantation and gastrulation. Development 131: 2247–2256, 2004 [DOI] [PubMed] [Google Scholar]
- 35. Miner JH, Patton BL, Lentz SI, Gilbert DJ, Snider WD, Jenkins NA, Copeland NG, Sanes JR. The laminin alpha chains: expression, developmental transitions, and chromosomal locations of alpha1–5, identification of heterotrimeric laminins 8–11, and cloning of a novel alpha3 isoform. J Cell Biol 137: 685–701, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Miner JH, Sanes JR. Collagen IV alpha 3, alpha 4, and alpha 5 chains in rodent basal laminae: sequence, distribution, association with laminins, and developmental switches. J Cell Biol 127: 879–891, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Moeller MJ, Sanden SK, Soofi A, Wiggins RC, Holzman LB. Podocyte-specific expression of cre recombinase in transgenic mice. Genesis 35: 39–42, 2003 [DOI] [PubMed] [Google Scholar]
- 38. Moore SA, Saito F, Chen J, Michele DE, Henry MD, Messing A, Cohn RD, Ross-Barta SE, Westra S, Williamson RA, Hoshi T, Campbell KP. Deletion of brain dystroglycan recapitulates aspects of congenital muscular dystrophy. Nature 418: 422–425, 2002 [DOI] [PubMed] [Google Scholar]
- 39. Mundel P, Gilbert P, Kriz W. Podocytes in glomerulus of rat kidney express a characteristic 44 KD protein. J Histochem Cytochem 39: 1047–1056, 1991 [DOI] [PubMed] [Google Scholar]
- 40. Ohyama T, Groves AK. Generation of Pax2-Cre mice by modification of a Pax2 bacterial artificial chromosome. Genesis 38: 195–199, 2004 [DOI] [PubMed] [Google Scholar]
- 41. Ophascharoensuk V, Pippin JW, Gordon KL, Shankland SJ, Couser WG, Johnson RJ. Role of intrinsic renal cells versus infiltrating cells in glomerular crescent formation. Kidney Int 54: 416–425, 1998 [DOI] [PubMed] [Google Scholar]
- 42. Pawar RD, Castrezana-Lopez L, Allam R, Kulkarni OP, Segerer S, Radomska E, Meyer TN, Schwesinger CM, Akis N, Grone HJ, Anders HJ. Bacterial lipopeptide triggers massive albuminuria in murine lupus nephritis by activating Toll-like receptor 2 at the glomerular filtration barrier. Immunology 128: e206–e221, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Pippin JW, Brinkkoetter PT, Cormack-Aboud FC, Durvasula RV, Hauser PV, Kowalewska J, Krofft RD, Logar CM, Marshall CB, Ohse T, Shankland SJ. Inducible rodent models of acquired podocyte diseases. Am J Physiol Renal Physiol 296: F213–F229, 2009 [DOI] [PubMed] [Google Scholar]
- 44. Pozzi A, Jarad G, Moeckel GW, Coffa S, Zhang X, Gewin L, Eremina V, Hudson BG, Borza DB, Harris RC, Holzman LB, Phillips CL, Fassler R, Quaggin SE, Miner JH, Zent R. Beta1 integrin expression by podocytes is required to maintain glomerular structural integrity. Dev Biol 316: 288–301, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Rafael JA, Trickett JI, Potter AC, Davies KE. Dystrophin and utrophin do not play crucial roles in nonmuscle tissues in mice. Muscle Nerve 22: 517–519, 1999 [DOI] [PubMed] [Google Scholar]
- 46. Regele HM, Fillipovic E, Langer B, Poczewki H, Kraxberger I, Bittner RE, Kerjaschki D. Glomerular expression of dystroglycans is reduced in minimal change nephrosis but not in focal segmental glomerulosclerosis. J Am Soc Nephrol 11: 403–412, 2000 [DOI] [PubMed] [Google Scholar]
- 47. Sakamaki Y, Sasamura H, Hayashi K, Ishiguro K, Takaishi H, Okada Y, D'Armiento JM, Saruta T, Itoh H. Absence of gelatinase (MMP-9) or collagenase (MMP-13) attenuates adriamycin-induced albuminuria and glomerulosclerosis. Nephron Exp Nephrol 115: e22–e32, 2010 [DOI] [PubMed] [Google Scholar]
- 48. Sasaki T, Mann K, Miner JH, Miosge N, Timpl R. Domain IV of mouse laminin β1 and β2 chains: structure, glycosaminoglycan modification and immunochemical analysis of tissue contents. Eur J Biochem 269: 431–442, 2002 [DOI] [PubMed] [Google Scholar]
- 49. Schneider M, Khalil AA, Poulton J, Castillejo-Lopez C, Egger-Adam D, Wodarz A, Deng WM, Baumgartner S. Perlecan and Dystroglycan act at the basal side of the Drosophila follicular epithelium to maintain epithelial organization. Development 133: 3805–3815, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Sgambato A, Brancaccio A. The dystroglycan complex: from biology to cancer. J Cell Physiol 205: 163–169, 2005 [DOI] [PubMed] [Google Scholar]
- 51. Vogtlander NP, Tamboer WP, Bakker MA, Campbell KP, van der Vlag J, Berden JH. Reactive oxygen species deglycosilate glomerular alpha-dystroglycan. Kidney Int 69: 1526–1534, 2006 [DOI] [PubMed] [Google Scholar]
- 52. Vogtlander NP, Visch HJ, Bakker MA, Berden JH, van der Vlag J. Ligation of alpha-dystroglycan on podocytes induces intracellular signaling: a new mechanism for podocyte effacement? PLoS One 4: e5979, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Wei C, Moller CC, Altintas MM, Li J, Schwarz K, Zacchigna S, Xie L, Henger A, Schmid H, Rastaldi MP, Cowan P, Kretzler M, Parrilla R, Bendayan M, Gupta V, Nikolic B, Kalluri R, Carmeliet P, Mundel P, Reiser J. Modification of kidney barrier function by the urokinase receptor. Nat Med 14: 55–63, 2008 [DOI] [PubMed] [Google Scholar]
- 54. Weir ML, Oppizzi ML, Henry MD, Onishi A, Campbell KP, Bissell MJ, Muschler JL. Dystroglycan loss disrupts polarity and beta-casein induction in mammary epithelial cells by perturbing laminin anchoring. J Cell Sci 119: 4047–4058, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. White SR, Wojcik KR, Gruenert D, Sun S, Dorscheid DR. Airway epithelial cell wound repair mediated by alpha-dystroglycan. Am J Respir Cell Mol Biol 24: 179–186, 2001 [DOI] [PubMed] [Google Scholar]
- 56. Williamson RA, Henry MD, Daniels KJ, Hrstka RF, Lee JC, Sunada Y, Ibraghimov-Beskrovnaya O, Campbell KP. Dystroglycan is essential for early embryonic development: disruption of Reichert's membrane in Dag1-null mice. Hum Mol Genet 6: 831–841, 1997 [DOI] [PubMed] [Google Scholar]
- 57. Yoshida-Moriguchi T, Yu L, Stalnaker SH, Davis S, Kunz S, Madson M, Oldstone MB, Schachter H, Wells L, Campbell KP. O-mannosyl phosphorylation of alpha-dystroglycan is required for laminin binding. Science 327: 88–92, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Yu J, Carroll TJ, McMahon AP. Sonic hedgehog regulates proliferation and differentiation of mesenchymal cells in the mouse metanephric kidney. Development 129: 5301–5312, 2002 [DOI] [PubMed] [Google Scholar]