Abstract
Normal body homeostasis of biotin is critically dependent on its renal recovery by kidney proximal tubular epithelial cells, a process that is mediated by the sodium-dependent multivitamin transporter (SMVT; a product of the SLC5A6 gene). Chronic ethanol consumption interferes with the renal reabsorption process of a variety of nutrients, including water-soluble vitamins. To date, however, there is nothing known about the effect of chronic alcohol feeding on physiological and molecular parameters of the renal biotin reabsorption process. We addressed these issues using rats and transgenic mice carrying the human SLC5A6 (P1P2) 5′-regulatory region as an in vivo model systems of alcohol exposure, and cultured human renal proximal tubular epithelial HK-2 cells chronically exposed to alcohol as an in vitro model of alcohol exposure. The [3H]biotin uptake results showed that chronic ethanol feeding in rats leads to a significant inhibition in carrier-mediated biotin transport across both renal brush border and basolateral membrane domains. This inhibition was associated with a marked reduction in the level of expression of SMVT protein, mRNA, and heterogenous nuclear RNA (hnRNA). Furthermore, studies with transgenic mice carrying the SLC5A6 5′-regulatory region showed that chronic alcohol feeding leads to a significant decrease in promoter activity. Studies with HK-2 cells chronically exposed to alcohol again showed a marked reduction in carrier-mediated biotin uptake, which was associated with a significant reduction in promoter activity of the human SLC5A6 5′-regulatory region. These findings demonstrate for the first time that chronic ethanol feeding inhibits renal biotin transport and that this effect is, at least in part, being exerted at the transcriptional level.
Keywords: SMVT, SLC5A6, uptake, transporter, kidney
the water-soluble vitamin biotin is required for normal cellular metabolism and growth in humans owing to its participation in many critical metabolic reactions (8, 44). Biotin deficiency leads to a variety of conditions including, neurological and dermatological disorders (17, 46). Suboptimal biotin levels and biotin deficiency have been observed in a variety of conditions including chronic alcoholism, in patients with inborn errors of biotin metabolism, those on long-term therapy with anticonvulsant agents, or parentreal nutrition, in patients with Crohn's disease, and in infants with seborrhoeic dermatitis and Leiner's disease (1, 7, 15–17, 20–22, 27, 28, 45).
Mammals, including humans, have lost the ability for de novo biosynthesis of biotin and therefore must obtain the vitamin from exogenous sources via intestinal absorption. Circulating biotin undergoes filtration in the renal glomeruli and is salvaged via reabsorption by renal proximal tubular epithelial cells. Thus the kidneys also play an important role in maintaining and regulating normal body homeostasis of biotin. Studies from our laboratory and others have examined the transport process of biotin in renal epithelia using a variety of human and animal kidney preparations (3–5, 29, 30, 39, 40). The results showed that the entry of biotin into the polarized renal epithelial cells across the brush-border membrane (BBM) is via a specific, carrier-mediated, and Na+-dependent process that is mediated by the sodium-dependent multivitamin transporter (SMVT) (4, 5, 29, 30). On the other hand, exits of biotin across the basolateral membrane domain (BLM) of renal epithelial cells are via a specialized carrier-mediated system that is Na+ independent in nature (30). Exclusive expression of SMVT at the apical membrane domain of renal reabsorptive epithelia has also been confirmed by immunological and confocal imaging studies (29, 39). Other studies have examined cellular and molecular aspects of the SMVT system that have been cloned and characterized the 5′-regulatory region of SLC5A6 gene (which encodes SMVT) from different species (10, 11, 13, 31, 32, 34). Furthermore, the renal biotin uptake process has been shown to be adaptively upregulated during biotin deficiency via a transcriptional regulatory mechanism(s) involving the SMVT system (3).
In humans, chronic alcohol use is associated with a marked reduction in plasma biotin levels (7, 15). Chronic alcohol feeding in rats was also found to be associated with a significant reduction in plasma biotin level and with an inhibition in intestinal absorption of the vitamin (37, 42). Whether chronic alcohol feeding also affects the renal reabsorption process of biotin is not known and was examined in this study. We used rat and transgenic mice carrying the full-length human SLC5A6 5′-regulatory region as an in vivo model of chronic alcohol exposure, and human-derived proximal tubular kidney epithelial HK-2 cells chronically exposed to ethanol in culture as an in vitro model system of alcohol exposure in our investigations. The results showed, for the first time, that chronic alcohol feeding of rats inhibits the entry and the exit steps of biotin in the polarized renal reabsorptive epithelia, i.e., transport across the BBM and BLM domains, respectively; it was also associated with a significant reduction in the level of expression of SMVT protein, mRNA, and heterogenous nuclear RNA (hnRNA). Also, chronic alcohol feeding of transgenic mice carrying the human SLC5A6 5′-regulatory region led to a significant reduction in promoter activity of the region in the kidney. Finally, chronic alcohol exposure of HK-2 cells was found to lead to a significant inhibition of biotin uptake which was associated with a reduction in promoter activity of the SLC5A6 5′-regulatory region.
MATERIALS AND METHODS
Materials
[3H]biotin (specific activity 30 Ci/mmol; radiochemical purity >98%) was obtained from American Radiolabel (ARC, St. Louis, MO). Cellulose nitrate filters (0.45-μm pore size) were purchased from Fisher Scientific. Unlabeled biotin and other chemicals including molecular biology reagents were obtained from commercial vendors and were of analytic grade.
Ethanol Feeding of Rats and Transgenic Mice
Male Wistar rats (Charles River, Wilmington, MA) weighing ∼120 g (∼14 wk old) were housed at the Animal Core of the NIAA-funded Southern California Research Center for Alcohol Liver and Pancreatic Diseases (ALPD) and Cirrhosis at the University of Southern California (Los Angeles, CA). The animal use committee of both the University of Southern California and the Long Beach Veterans Affairs Medical Center approved the experimental protocols. Rats were fed the Lieber-DeCarli alcohol liquid diet (ethanol provided 36% of total ingested calories; BioServe, Frenchtown, NJ) (24, 25) for 2 and 4 wk. Control rats were pair-fed with the same liquid diet but without ethanol (maltose-dextrin isocalorically replaced ethanol). Two alcohol-fed rats and their two pair-fed controls were euthanized at the time of the study, and their kidney cortices were removed and pooled and processed immediately for isolation of renal BBM vesicles (BBMV) or BLM vesicles (BLMV). For RNA expression studies, part of the fresh kidney tissue from the ethanol-fed and control rats was removed and stored at −80°C in TRIzol (Invitrogen, Carlsbad, CA) for RNA isolation.
Transgenic mice carrying the hSMVT [SLC5A6 (P1P2)] 5′-regulatory region generated and characterized before (34) were used in this investigation. Briefly, we cloned the human SLC5A6 5′-regulatory region of 2,167 bp (P1P2; −5846 to −3679 using the A in the initiator ATG sequence as position 1) and fused the fragment to the luciferase reporter gene (13). We then generated a mouse line that carries the human SLC5A6 5′-regulatory region (P1P2) fused to luciferase reporter gene (using the University of California-Irvine transgenic mouse facility) and determined promoter activity in various tissues (34). The transgenic mice were fed the Lieber-DeCarli ethanol liquid diet (ethanol provided 25% of total ingested calories and was introduced gradually; calories were increased by 5% every day until we achieved 25%; Dyets, Bethlehem, PA) (25) for 4 wk. Control littermates (sex matched that had similar basal firefly luciferase mRNA expression) were pair-fed with the same liquid diet but without ethanol (maltose-dextrin isocalorically replaced ethanol). The weight of the mice was monitored every week, and there was no significant difference in weight noticed between alcohol-fed mice and their pair-fed controls. To determine the firefly luciferase activity in renal tissue of mice, animals were euthanized, and kidneys were removed and placed in ice-cold passive lysis buffer (Promega). The kidneys were then homogenized using a hand blender, and the firefly luciferase activity was measured as described previously (34). Luciferase assays were normalized to total protein for each sample measured using a protein assay kit (Bio-Rad). The total RNA was also isolated from fresh kidneys for measuring mouse endogenous SMVT mRNA expression level by real-time PCR (33) using mSMVT (forward: 5′-CGTAGGAACTTTGGTAGCCCTGG-3′; reverse: 5′-CTTAGGTGTGATGGGTCTCTCC-3′) and mouse β-actin (forward: 5′-CATCCTGCGTCTGGACCT-3′; reverse: 5′-TGATGTCACGCACGATTTCC-3′) gene-specific primers as described below. Both Long Beach Veterans Affairs and University of California, Irvine institutional animal care and use committees approved the experimental procedures used in this investigation.
Isolation of Rat Renal BBMV and BLMV and Transport Investigations
Isolated rat renal BBMV and BLMV were freshly prepared using the divalent (Mg2+) cation chelation method and Percoll-gradient differential centrifugation method, respectively, as described previously (6, 29, 36, 38, 41). The purified BBMV and BLMV preparations were preloaded with a buffer of 280 mM mannitol and 20 mM Tris-HEPES, pH 7.4, and incubation was performed in a buffer containing 100 mM NaCl, 80 mM mannitol, and 20 mM Tris-HEPES, pH 7.4 in the presence of 0.25 μM [3H]biotin. Uptake studies were performed using BBMV and BLMV at 10 s (initial rate) (5) at 37°C using a rapid-filtration procedure (18).
Quantitative Real-Time PCR Analysis
Quantitative real-time PCR was performed using a Bio-Rad iCycler (Hercules, CA) and their SYBR green PCR mix. Total RNA (5 μg) was isolated from the kidneys of ethanol-fed rats and their pair-fed controls; this was primed with oligo-dT primers to synthesize first-strand cDNA (Superscript First Strand Synthesis RT-PCR kit, Invitrogen). To amplify the coding region of rat SMVT and β-actin, we used gene-specific primers for rat SMVT (forward: 5′-GAGGATGACTGTGGCGAGCAC-3′; reverse: 5′-CAGCTCACCAACAGTATGGC-3′) and β-actin (forward: 5′-GTCAGGTCACTATCGGC-3′; reverse: 5′-CATGGATGCCACAGGATTCC-3′). Real-time PCR was performed as described previously (33) using a Bio-Rad CFX96 real-time PCR machine. Data were normalized to β-actin and then quantified using a relative relationship method supplied by the iCycler's manufacturer (Bio-Rad) and as described before (26, 33).
Western Blot Analysis
To determine specific protein abundance in purified BBM, we performed Western blot analysis using purified BBM prepared from the kidneys of ethanol-fed rats and their pair-fed controls as described earlier (29, 41). BBM proteins (60 μg) were resolved onto premade 4–12% Bis-Tris minigels (Invitrogen) as described before (39, 41). After protein separation, proteins were electroblotted onto polyvinylidene fluoride membrane (Bio-Rad) and then blocked with a PBS-Tween 20 solution containing 5% nonfat dry milk (Bio-Rad) for 1 h at room temperature. The membrane blot was then incubated with rat SMVT-specific polyclonal antibodies that were raised in rabbits against the LYHACRGWGRHTVGELLMADRK peptide, which corresponds to amino acids 44–65 of the rat SMVT sequence (Alpha Diagnostic, San Antonio, TX). Immunodetection of the specific bands was performed by incubating the membrane with secondary antibodies [goat anti-rabbit conjugated to horseradish peroxidase (HRP); Santa Cruz Biotechnology, Santa Cruz, CA] and with an enhanced chemiluminescent (ECL) substrate (Amersham, Arlington Heights, IL) as described before (39, 41). The appropriate membranes were stripped using a reblotting stripping solution (Chemicon, Temecula, CA) and incubated with β-actin antibodies raised in goat (Santa Cruz Biotechnology) and then incubated with bovine anti-goat HRP-conjugated secondary antibodies (Santa Cruz Biotechnology). The immunoreactive bands were developed using an ECL substrate as described previously (41, 43) then quantitated (as unitless measurements) using the UNSCAN-IT gel automated digitizing system, version 6.1 (Silk Scientific).
hnRNA Analysis
To examine the effect of chronic alcohol feeding on the level of expression of hnRNA of SMVT, total RNA [treated with DNase I (Invitrogen), 1 μg RNA/unit to exclude genomic DNA contamination during PCR amplification] was isolated from the kidneys cortex of ethanol-fed rats and their pair-fed controls as described previously (2, 14, 19, 41, 43). DNase I-treated RNA was then reverse transcribed as described above with the random hexamer (Invitrogen). To ensure amplification of hnRNA, the forward and reverse primers were designed as described before (14). Semiquantitative RT-PCR was performed using the rat SMVT- and β-actin gene-specific primers [SMVT (forward: 5′-CTAAGGAAGATGCCTGATG-3′; reverse: 5′-CTCTGGGAAAAAGAGTCAG-3′)] and β-actin (forward: 5′-CTGCTCTTTCCCAGATGAG-3′; reverse: 5′-CTCATAGATGGGCACAGTG -3′) and conditions as described previously (41, 43). RNA treated with DNase I (without conversion into cDNA) was used for PCR amplification with rat SMVT-specific primers as a negative control (data not shown). The PCR amplified products were run on 1% agarose gels, the image was captured using Gel-doc (Bio-Rad), and specific bands were quantified (as unitless measurements) using UNSCAN-IT software (Silk Scientific).
Cell Culture, Alcohol Exposure, and Uptake Assay
The human-derived renal proximal tubule epithelial HK-2 cells (passages 12–20, ATCC, Manassas, VA) were grown on 12-well cell culture plates (Corning) in K-SFM medium containing epidermal growth factor (5 ng/ml), bovine pituitary extract (40 μg/ml), 10% fetal bovine serum, and penicillin and streptomycin as we described before (3, 23). Fifty millimolar ethanol (a concentration comparable to a blood alcohol level of chronic alcoholic subjects) (12)-containing medium was added to cells on the following day, and cells were grown in an ethanol-saturated 5% CO2 incubator, maintained at 37°C, for 96 h with a media change every 12 h to minimize changes in ethanol concentration (43, 47). An uptake assay was performed at 37°C in Krebs-Ringer buffer as described before (3, 39, 40). The protein content of digested samples was determined using a Bio-Rad protein assay kit.
Cell Transfection and Promoter Assay
HK-2 cells were cotransfected with human full-length SLC5A6 (P1P2) 5′-regulatory region constructs which we generated and characterized previously (13) along with the pRL-TK (Renilla luciferase-thymidine kinase, Promega) in the presence of Lipofectamine 2000 (Invitrogen). After 24 h of transfection, cells were exposed to 50 mM ethanol-containing growth medium and kept at 37°C in an ethanol-saturated incubator for 96 h with media changed for every 12 h. Ninety-six hours later, chronic ethanol-exposed cells were lysed and both firefly and Renilla luciferase activity were measured using a 20/20 luminometer as described previously (13, 34).
Statistical Analysis
Data on carrier-mediated biotin uptake in renal BBMV and BLMV were the result of multiple separate determinations from multiple rats and are expressed as means ± SE. Similarly, carrier-mediated biotin uptake by human proximal tubular epithelial HK-2 cells is means ± SE from multiple separate determinations from different cell batches. Uptake of [3H]biotin by the carrier-mediated process was determined by subtracting the uptake in the presence of 1 mM unlabeled biotin from the uptake in its absence. Uptake data are expressed as the percentage relative to simultaneously performed controls because of observed variability in the absolute amount of biotin uptake. Differences between control and alcohol-fed animals or alcohol-exposed HK-2 cells were tested for significance level at P < 0.05 using Student's t-test. Western blot, real-time PCR, hnRNA analysis, and luciferase activity were performed with at least three separate samples prepared on different occasions.
RESULTS
Effect of Chronic Alcohol Feeding on Physiological and Molecular Parameters of Biotin Uptake Process in Rat Kidney Cortex and in Transgenic Mice Carrying SLC5A6 5′-Regulatory Region
Effect of chronic alcohol consumption by rats on carrier-mediated biotin transport across rat renal BBM and BLM domains of polarized renal epithelia.
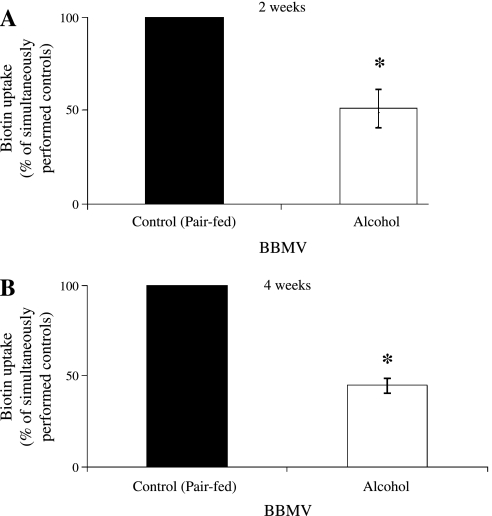
The effect of chronic alcohol use on biotin entry into rat renal epithelial cells, i.e., transport across the BBM, was examined using purified BBMV isolated from the kidney cortex of alcohol-fed rats and their pair-fed controls. The biotin uptake assay was performed as described in materials and methods. Results showed a significant (P < 0.01) inhibition of biotin (0.25 μM) uptake in rats fed alcohol for 2 and 4 wk compared with their pair-fed controls (Fig. 1, A and B, respectively).
Fig. 1.
Effect of chronic alcohol feeding on [3H]biotin uptake by rat renal brush-border membrane vesicles (BBMV). A and B: carrier-mediated uptake of [3H]biotin (0.25 μM) by kidney BBMV of rats fed an alcohol liquid diet for 2 and 4 wk, respectively, and their pair-fed controls as described in materials and methods was examined. Values are means ± SE of 6–9 separate uptake determinations from multiple sets of rats. *P < 0.01.
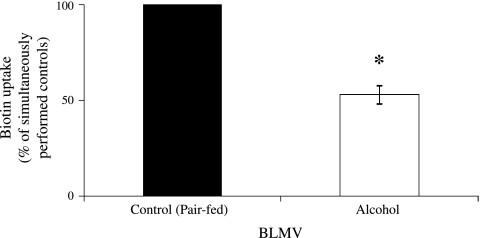
In another study, we examined the effect of chronic alcohol feeding (for 4 wk) on the exit of biotin from polarized renal epithelial cells, i.e., transport across the BLM, using purified BLMV isolated from the kidney cortex of alcohol-fed rats and their pair-fed controls. The results showed a significant (P < 0.01) inhibition of carrier-mediated biotin (0.25 μM) uptake by renal BLMV from alcohol-fed rats compared with their pair-fed controls (Fig. 2).
Fig. 2.
Effect of chronic alcohol feeding on [3H]biotin uptake by rat renal basolateral membrane vesicles (BLMV). Carrier-mediated uptake of [3H]biotin (0.25 μM) by kidney BLMV of rats fed an alcohol liquid diet for 4 wk and their pair-fed controls was examined, as described in materials and methods. Values are means ± SE of 6–8 separate uptake determinations from multiple sets of rats. *P < 0.01.
Effect of chronic alcohol feeding on molecular parameters of biotin transport in rat kidney cortex.
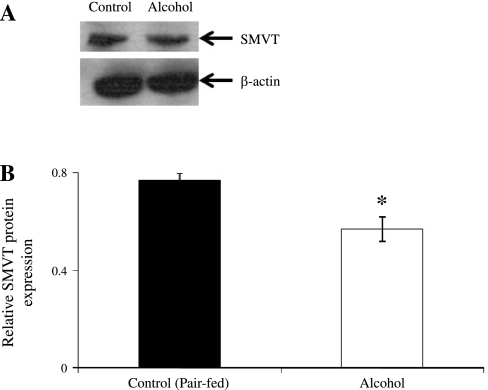
We utilized Western blotting techniques to investigate the effect of chronic alcohol feeding on the level of expression of the rat SMVT protein at the renal BBM domain. Rat SMVT polyclonal antibodies used in this study have been previously characterized (29, 39). Western blot analysis showed a significant (P < 0.01) reduction in the level of expression of SMVT protein in BBM preparations isolated from kidneys of alcohol-fed rats compared with those isolated from their pair-fed controls (Fig. 3).
Fig. 3.
Effects of chronic alcohol feeding of rats on level of expression of sodium-dependent multivitamin transporter (SMVT) protein in the kidney brush-border membrane (BBM). A: Western blot analysis was performed in kidney BBM (60 μg) proteins isolated from alcohol-fed rats (4 wk) and their pair-fed controls and was resolved onto premade 4–12% Bis-Tris minigels, as described in materials and methods. Blots were incubated with rabbit polyclonal anti-rSMVT antibodies, and proteins were visualized by incubating with goat anti-rabbit horseradish peroxidase-conjugated secondary antibody, as described in materials and methods. Respective blots were stripped and reprobed with β-actin antibodies to normalize equal loading in each wells (bottom). Immunoreactive bands were detected using an enhanced chemiluminescence substrate, as described in materials and methods. B: densitometric values of the immunoreactive bands were quantified, as described in materials and methods. Values are means ± SE of 4 separate samples from multiple rats. *P < 0.01.
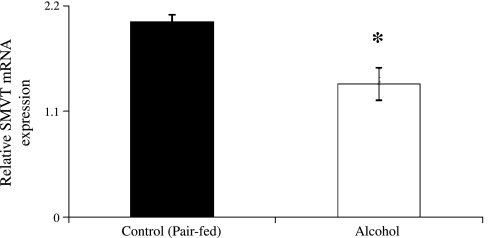
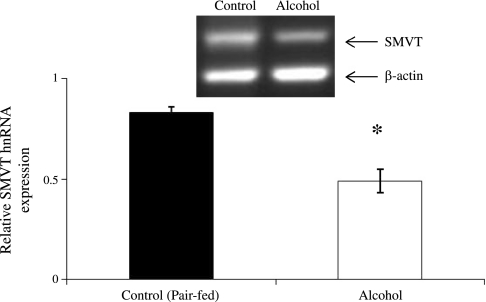
In another study, we examined the effect of chronic alcohol feeding (4 wk) on steady-state mRNA level of expression of SMVT in rat kidney cortex using quantitative real-time PCR. We performed PCR using gene-specific primers designed from the open reading frame of rat SMVT on mRNA isolated from alcohol-fed rats and their pair-fed controls (materials and methods). The real-time PCR analysis (normalized relative to β-actin) showed a significant (P < 0.01) decrease in the level of expression of SMVT mRNA in the kidneys of alcohol-fed rats compared with pair-fed controls (Fig. 4). These studies suggest that decrease in mRNA level in chronic alcohol feeding may be (in part) due to a decrease in the rate of transcription of the Slc5a6 gene (which encodes SMVT). To address this issue, we employed two approaches. In the first approach, we determined the level of expression of SMVT hnRNA (level of hnRNA of given gene can be used as an indicator of the transcriptional rate of that gene) (2, 9, 14, 19, 35, 41, 43) in kidney cortex of rats fed alcohol chronically (4 wk) and compared the findings with the level in pair-fed controls. The results showed a significant (P < 0.05) decrease in the level of expression of SMVT hnRNA in the kidneys of alcohol-fed rats compared with their pair-fed controls (Fig. 5).
Fig. 4.
Effect of chronic alcohol feeding of rats on level of expression of SMVT mRNA in kidney cortex of alcohol-fed rats and their pair-fed controls. Real-time PCR was performed using gene-specific primers for rSMVT and β-actin, as described in materials and methods on total RNA isolated from alcohol-fed (4 wk) rats and their pair-fed controls. Values are means ± SE of 5 separate samples from 5 rats. *P < 0.01.
Fig. 5.
Semiquantitative RT-PCR analysis of SMVT (SLC5A6) hnRNA expression levels in kidney cortex of alcohol-fed rats and their pair-fed controls. Semiquantitative RT-PCR products for SMVT (SLC5A6) in the kidney cortex of alcohol-fed (4 wk) and their pair-fed controls were performed using gene-specific primers for SLC5A6 and rat β-actin, as described in materials and methods. Data were normalized to rat β-actin. Values are means ± SE of 4 separate samples from 4 rats. *P < 0.05.
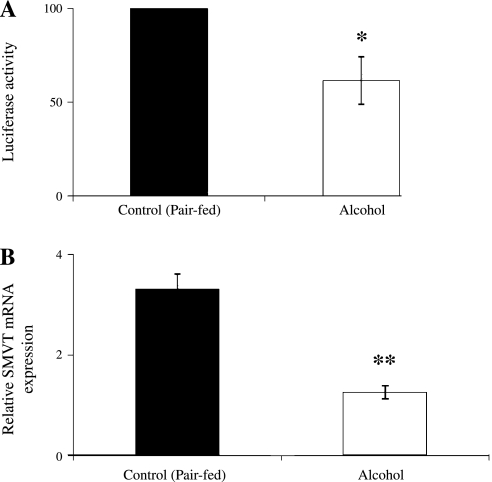
In the second approach, we took advantage of the availability in our laboratory of transgenic mice carrying the human SLC5A6 5′-regulatory region to examine whether chronic alcohol feeding affects SMVT transcription rate. In these studies, transgenic mice carrying the hSMVT (SLC5A6) 5′-regulatory region (−5846 to −3679), which we generated and characterized before (34), were fed an alcohol liquid diet for 4 wk; sex-matched littermates were fed an alcohol-free diet. At the end of alcohol feeding, mice were euthanized and the kidneys were removed to examine the activity of the SLC5A6 5′-regulatory region (i.e., luciferase activity). We also examined the effect of chronic alcohol feeding on the level of expression of mouse endogenous SMVT mRNA as a control. The results showed a marked reduction in the activity of the SLC5A6 5′-regulatory region (P < 0.05) (Fig. 6A) as well as in the level of expression of the endogenous SMVT mRNA (P < 0.01) (Fig. 6B) in alcohol-fed transgenic mice compared with their pair-fed control transgenic animals.
Fig. 6.
Effect of chronic alcohol feeding on activity of the SLC5A6 5′-regulatory region in kidneys of transgenic mice carrying full-length promoter construct. A: SLC5A6 5′-regulatory region activity in kidneys of alcohol-fed transgenic mice and their pair-fed controls kidney as determined by a luminometer and presented in luciferase light units. Mice carrying the human SLC5A6 5′-regulatory region (P1P2) were used, as described in materials and methods. B: real-time PCR was performed on RNA isolated from alcohol-fed and pair-fed control mice kidney cortex using mouse SMVT gene-specific primers. Values are means ± SE of 3 separate samples from 3 mice. *P < 0.05, **P < 0.01.
Effect of Chronic Alcohol Exposure on Physiological and Molecular Parameters of Biotin Uptake Process in Human Kidney Epithelial HK-2 Cells
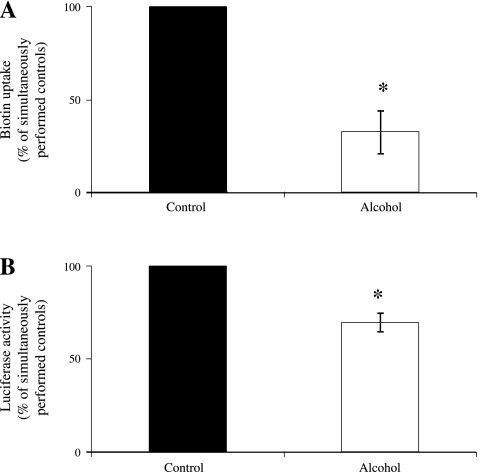
We used HK-2 cells as an in vitro model to examine the effect of chronic alcohol exposure on physiological and molecular parameters of biotin uptake by this human renal epithelial cell line. Our previous studies have established the suitability of HK-2 cells for studying the renal biotin uptake process (3). The HK-2 cells were chronically exposed (96 h) to 50 mM alcohol, and biotin uptake was examined as described in materials and methods. The results showed a significant (P < 0.01) inhibition of carrier-mediated biotin uptake in cells chronically exposed to alcohol compared with simultaneously performed control cells (Fig. 7A).
Fig. 7.
Effect of chronic alcohol exposure of HK-2 cells on carrier-mediated biotin uptake and on promoter activity of the 5′-regulatory region of the SLC5A6 gene. A: HK-2 cells were exposed to 50 mM alcohol for 96 h, and carrier-mediated biotin (25 nM) uptake was carried out and compared with simultaneously performed controls, as described in materials and methods. Values are means ± SE of 3 separate uptake determinations performed using different passages of cells on 4 different days. *P < 0.01. B: the P1P2 (full-length SLC5A6 promoter region) in pGL3 was transfected into HK-2 cells, and the cells were then exposed to 50 mM alcohol for 96 h, as described in materials and methods. The firefly luciferase activity was measured and normalized to Renilla luciferase activity, as described in materials and methods. Values are means ± SE of 3 separate experiments performed on different batches of cells on 3 different days. *P < 0.01.
In parallel, we transiently transfected HK-2 cells with full-length human SLC5A6 5′-regulatory region (−5846 to −3679 bp) and chronically (96 h) exposed the cells to alcohol (50 mM) as described in materials and methods, then determined promoter activity (with the luciferase reporter). Our aims here were to confirm the observed finding on the effect of chronic alcohol exposure in transgenic mice carrying the human SLC5A6 5′-regulatory region and to extend to the situation of human renal epithelial cells. The results showed that chronic alcohol exposure significantly (P < 0.01) inhibited the activity of the full-length SLC5A6 5′-regulatory region compared with its activity in control HK-2 cells (Fig. 7B).
DISCUSSION
Chronic alcohol use leads to a marked decrease in plasma biotin levels in humans (7, 15). While the recently reported inhibition of intestinal biotin absorption process by chronic alcohol feeding/exposure (42) may contribute toward this reduction in plasma biotin levels, a decrease in renal reabsorption of biotin may be an additional contributing factor. We investigated this possibility using an in vivo model of chronic alcohol exposure (rats and transgenic mice carrying the human full-length SLC5A6 5′-regulatory region) and an in vitro model of chronic alcohol exposure (human-derived proximal tubular kidney epithelial HK-2 cells).
Chronic alcohol feeding of rats was found to cause a significant inhibition of both the entry step (transport across the BBM domain) and the exit step (transport across the BLM domain) of biotin in the polarized renal reabsorptive epithelia. Previous studies have shown that biotin transport across the BBM of the renal reabsorptive epithelial cells to occur via a carrier-mediated, Na- dependent, electroneutral (4, 5), while exit of biotin from the renal epithelial cells across the BLM is via a carrier-mediated, Na-independent, and electrogenic mechanism (30). It is worth mentioning here that while ample evidence exists regarding the molecular identity of the biotin transport process across epithelial BBM, little is known about molecular identity of the biotin transport process across epithelial BLM. Our studies have also shown that the level of expression of the SMVT protein and mRNA were significantly decreased in rats fed alcohol chronically compared with their pair-fed controls. This reduction in the level of expression of SMVT mRNA in chronic alcohol-fed rats suggests that the inhibition may be exerted, at least in part, at the transcriptional level of the Slc5a6 gene. Support for this possibility came from the observation that chronic alcohol feeding leads to significant inhibition of the level of expression of SMVT hnRNA in kidney cortex of rats fed alcohol chronically. Further validation came from the observation in transgenic mice carrying the full-length human SLC5A6 5′-regulatory region where chronic alcohol feeding led to a significant inhibition of transcriptional activity of the region in the kidney. As with rats, chronic alcohol feeding led to a significant reduction in the level of expression of mouse endogenous SMVT mRNA in the kidneys of the transgenic mice fed alcohol chronically compared with its level of expression in pair-fed transgenic littermates. Obviously, other mechanisms (e.g., changes in SMVT RNA and/or protein stability) may also be involved in causing the inhibition of renal biotin transport and further investigations are required to address these issues.
We also extended our studies on the effect of chronic alcohol feeding of animals on renal biotin transport to the situation of human renal epithelial cells. This was done by examining the effect of chronic alcohol exposure of HK-2 cells on physiological and molecular parameters of biotin uptake. Again, chronic alcohol exposure of HK-2 cells led to an observed significant inhibition of carrier-mediated biotin uptake. This inhibition was again associated with a significant reduction in promoter activity of the SLC5A6 (P1P2) 5′-regulatory region in alcohol-exposed cells compared with controls. These findings demonstrate that the biotin uptake by human kidney epithelial HK-2 cells is similarly affected by chronic alcohol exposure and that the inhibitory effect is being exerted, at least in part, at the transcriptional level of the SLC5A6 gene.
If the above-described findings using animals and cultured human renal cellular preparation can be extended to the human situation in vivo, it would then suggest that impairment in the renal biotin reabsorption process is another important contributing factor in the decrease in plasma biotin level observed in chronic alcoholic subjects. However, further studies are needed to confirm this possibility. In summary, our present studies show, for the first time, that chronic alcohol feeding/exposure inhibits physiological and molecular parameters of the renal biotin reabsorption process and that the effect is (at least in part) being exerted at the transcriptional level of the SLC5A6 gene.
GRANTS
This work was supported by grants from the Department of Veterans Affairs and the National Institutes of Health (DK58057 and AA18071 to HMS and DK84094 to VSS).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
REFERENCES
- 1. Aronson PJ, Malick F. Towards rational treatment of severe psoriasis in alcoholics: report of two cases. J Drugs Dermatol 9: 405–408, 2010 [PubMed] [Google Scholar]
- 2. Aydemir F, Jenkitkasemwong S, Gulec S, Knutson MD. Iron loading increases ferroportin heterogeneous nuclear RNA and mRNA levels in murine J774 macrophages. J Nutr 139: 434–438, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Balamurugan K, Vaziri ND, Said HM. Biotin uptake by human proximal tubular epithelial cells: cellular and molecular aspects. Am J Physiol Renal Physiol 288: F823–F831, 2005 [DOI] [PubMed] [Google Scholar]
- 4. Baur B, Baumgartner ER. Na+-dependent biotin transport into brush-border membrane vesicles from human kidney cortex. Pflügers Arch 422: 499–505, 1993 [DOI] [PubMed] [Google Scholar]
- 5. Baur B, Wick H, Baumgartner ER. Na+-dependent biotin transport into brush-border membrane vesicles from rat kidney. Am J Physiol Renal Fluid Electrolyte Physiol 258: F840–F847, 1990 [DOI] [PubMed] [Google Scholar]
- 6. Biber J, Stieger B, Haase W, Murer H. A high yield preparation for rat kidney brush border membranes. Different behaviour of lysosomal markers. Biochim Biophys Acta 647: 169–176, 1981 [DOI] [PubMed] [Google Scholar]
- 7. Bonjour JP. Vitamins and alcoholism. V. Riboflavin, VI. Niacin, VII. Pantothenic acid, and VIII. Biotin. Int J Vitam Nutr Res 50: 425–440, 1980 [PubMed] [Google Scholar]
- 8. Bonjour JP, Bausch J, Suormala T, Baumgartner ER. Detection of biocytin in urine of children with congenital biotinidase deficiency. Int J Vitam Nutr Res 54: 223–231, 1984 [PubMed] [Google Scholar]
- 9. Buttice G, Kurkinen M. A polyomavirus enhancer A-binding protein-3 site and Ets-2 protein have a major role in the 12-O-tetradecanoylphorbol-13-acetate response of the human stromelysin gene. J Biol Chem 268: 7196–7204, 1993 [PubMed] [Google Scholar]
- 10. Chatterjee NS, Kumar CK, Ortiz A, Rubin SA, Said HM. Molecular mechanism of the intestinal biotin transport process. Am J Physiol Cell Physiol 277: C605–C613, 1999 [DOI] [PubMed] [Google Scholar]
- 11. Chatterjee NS, Rubin SA, Said HM. Molecular characterization of the 5′ regulatory region of rat sodium-dependent multivitamin transporter gene. Am J Physiol Cell Physiol 280: C548–C555, 2001 [DOI] [PubMed] [Google Scholar]
- 12. Devenyi P, Kapur BM, Roy JH. High-density lipoprotein response to alcohol consumption and abstinence as an indicator of liver function in alcoholic patients. Can Med Assoc J 130: 1445–1447, 1984 [PMC free article] [PubMed] [Google Scholar]
- 13. Dey S, Subramanian VS, Chatterjee NS, Rubin SA, Said HM. Characterization of the 5′ regulatory region of the human sodium-dependent multivitamin transporter, hSMVT. Biochim Biophys Acta 1574: 187–192, 2002 [DOI] [PubMed] [Google Scholar]
- 14. Elferink CJ, Reiners JJ., Jr Quantitative RT-PCR on CYP1A1 heterogeneous nuclear RNA: a surrogate for the in vitro transcription run-on assay. Biotechniques 20: 470–477, 1996 [DOI] [PubMed] [Google Scholar]
- 15. Fennelly J, Frank O, Baker H, Leevy CM. Peripheral neuropathy of the alcoholic: I. Aetiological role of aneurin and other B-complex vitamins. Br Med J 2: 1290–1292, 1964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Fernandez-Banares F, Abad-Lacruz A, Xiol X, Gine JJ, Dolz C, Cabre E, Esteve M, Gonzalez-Huix F, Gassull MA. Vitamin status in patients with inflammatory bowel disease. Am J Gastroenterol 84: 744–748, 1989 [PubMed] [Google Scholar]
- 17. Gehrig KA, Dinulos JG. Acrodermatitis due to nutritional deficiency. Curr Opin Pediatr 22: 107–112, 2010 [DOI] [PubMed] [Google Scholar]
- 18. Hopfer U, Nelson K, Perrotto J, Isselbacher KJ. Glucose transport in isolated brush border membrane from rat small intestine. J Biol Chem 248: 25–32, 1973 [PubMed] [Google Scholar]
- 19. Kohler CU, Roos PH. Focus on the intermediate state: immature mRNA of cytochromes P450—methods and insights. Anal Bioanal Chem 392: 1109–1122, 2008 [DOI] [PubMed] [Google Scholar]
- 20. Kostovic K, Lipozencic J. Skin diseases in alcoholics. Acta Dermatovenerol Croat 12: 181–190, 2004 [PubMed] [Google Scholar]
- 21. Krause KH, Berlit P, Bonjour JP. Impaired biotin status in anticonvulsant therapy. Ann Neurol 12: 485–486, 1982 [DOI] [PubMed] [Google Scholar]
- 22. Krause KH, Bonjour JP, Berlit P, Kochen W. Biotin status of epileptics. Ann NY Acad Sci 447: 297–313, 1985 [DOI] [PubMed] [Google Scholar]
- 23. Kumar CK, Yanagawa N, Ortiz A, Said HM. Mechanism and regulation of riboflavin uptake by human renal proximal tubule epithelial cell line HK-2. Am J Physiol Renal Physiol 274: F104–F110, 1998 [DOI] [PubMed] [Google Scholar]
- 24. Lieber CS, DeCarli LM. The feeding of ethanol in liquid diets. Alcohol Clin Exp Res 10: 550–553, 1986 [DOI] [PubMed] [Google Scholar]
- 25. Lieber CS, DeCarli LM. Liquid diet technique of ethanol administration: 1989 update. Alcohol Alcohol 24: 197–211, 1989 [PubMed] [Google Scholar]
- 26. Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2[-delta delta C(T)] method. Methods 25: 402–408, 2001 [DOI] [PubMed] [Google Scholar]
- 27. Messaritakis J, Kattamis C, Karabula C, Matsaniotis N. Generalized seborrhoeic dermatitis. Clinical and therapeutic data of 25 patients. Arch Dis Child 50: 871–874, 1975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Mock DM, Stadler DD, Stratton SL, Mock NI. Biotin status assessed longitudinally in pregnant women. J Nutr 127: 710–716, 1997 [DOI] [PubMed] [Google Scholar]
- 29. Nabokina SM, Subramanian VS, Said HM. Comparative analysis of ontogenic changes in renal and intestinal biotin transport in the rat. Am J Physiol Renal Physiol 284: F737–F742, 2003 [DOI] [PubMed] [Google Scholar]
- 30. Podevin RA, Barbarat B. Biotin uptake mechanisms in brush-border and basolateral membrane vesicles isolated from rabbit kidney cortex. Biochim Biophys Acta 856: 471–481, 1986 [DOI] [PubMed] [Google Scholar]
- 31. Prasad PD, Wang H, Huang W, Fei YJ, Leibach FH, Devoe LD, Ganapathy V. Molecular and functional characterization of the intestinal Na+-dependent multivitamin transporter. Arch Biochem Biophys 366: 95–106, 1999 [DOI] [PubMed] [Google Scholar]
- 32. Prasad PD, Wang H, Kekuda R, Fujita T, Fei YJ, Devoe LD, Leibach FH, Ganapathy V. Cloning and functional expression of a cDNA encoding a mammalian sodium-dependent vitamin transporter mediating the uptake of pantothenate, biotin, and lipoate. J Biol Chem 273: 7501–7506, 1998 [DOI] [PubMed] [Google Scholar]
- 33. Reidling JC, Nabokina SM, Balamurugan K, Said HM. Developmental maturation of intestinal and renal thiamin uptake: studies in wild-type and transgenic mice carrying human THTR-1 and 2 promoters. J Cell Physiol 206: 371–377, 2006 [DOI] [PubMed] [Google Scholar]
- 34. Reidling JC, Said HM. Regulation of the human biotin transporter hSMVT promoter by KLF-4 and AP-2: confirmation of promoter activity in vivo. Am J Physiol Cell Physiol 292: C1305–C1312, 2007 [DOI] [PubMed] [Google Scholar]
- 35. Ryu DY, Levi PE, Fernandez-Salguero P, Gonzalez FJ, Hodgson E. Piperonyl butoxide and acenaphthylene induce cytochrome P450 1A2 and 1B1 mRNA in aromatic hydrocarbon-responsive receptor knock-out mouse liver. Mol Pharmacol 50: 443–446, 1996 [PubMed] [Google Scholar]
- 36. Said HM, Redha R. A carrier-mediated transport for folate in basolateral membrane vesicles of rat small intestine. Biochem J 247: 141–146, 1987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Said HM, Sharifian A, Bagherzadeh A, Mock D. Chronic ethanol feeding and acute ethanol exposure in vitro: effect on intestinal transport of biotin. Am J Clin Nutr 52: 1083–1086, 1990 [DOI] [PubMed] [Google Scholar]
- 38. Scalera V, Storelli C, Storelli-Joss C, Haase W, Murer H. A simple and fast method for the isolation of basolateral plasma membranes from rat small-intestinal epithelial cells. Biochem J 186: 177–181, 1980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Subramanian VS, Marchant JS, Boulware MJ, Ma TY, Said HM. Membrane targeting and intracellular trafficking of the human sodium-dependent multivitamin transporter in polarized epithelial cells. Am J Physiol Cell Physiol 296: C663–C671, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Subramanian VS, Marchant JS, Said HM. Biotin-responsive basal ganglia disease-linked mutations inhibit thiamine transport via hTHTR2: biotin is not a substrate for hTHTR2. Am J Physiol Cell Physiol 291: C851–C859, 2006 [DOI] [PubMed] [Google Scholar]
- 41. Subramanian VS, Subramanya SB, Tsukamoto H, Said HM. Effect of chronic alcohol feeding on physiological and molecular parameters of renal thiamin transport. Am J Physiol Renal Physiol 299: F28–F34, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Subramanya SB, Subramanian VS, Kumar SJ, Hoiness R, Said HM. Inhibition of intestinal biotin absorption by chronic alcohol feeding: cellular and molecular mechanisms. Am J Physiol Gastrointest Liver Physiol. (First published December 9, 2010). doi:10/1152.ajpgi.00564.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Subramanya SB, Subramanian VS, Said HM. Chronic alcohol consumption and intestinal thiamin absorption: effects on physiological and molecular parameters of the uptake process. Am J Physiol Gastrointest Liver Physiol 299: G23–G31, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Sweetman L, Nyhan WL. Inheritable biotin-treatable disorders and associated phenomena. Annu Rev Nutr 6: 317–343, 1986 [DOI] [PubMed] [Google Scholar]
- 45. Urabe K. [Decreased plasma biotin levels in patients with Crohn's disease]. Nippon Shokakibyo Gakkai Zasshi 83: 697, 1986 [PubMed] [Google Scholar]
- 46. Wolf B. Disorders of biotin metabolism. In: The Metabolic and Molecular Bases of Inherited Disease, edited by Scriver CR, Beaudet AL, Aly WS, Valle D, Childs B, Kinzler KW, Vogelstein B. New York: McGraw-Hill; 2001, p. 3935–3960 [Google Scholar]
- 47. You M, Liang X, Ajmo JM, Ness GC. Involvement of mammalian sirtuin 1 in the action of ethanol in the liver. Am J Physiol Gastrointest Liver Physiol 294: G892–G898, 2008 [DOI] [PubMed] [Google Scholar]