Abstract
Collecting duct (CD) endothelin-1 (ET-1) is an important autocrine inhibitor of CD Na+ reabsorption. Salt loading is thought to increase CD ET-1 production; however, definitive evidence of this, as well as understanding of the mechanisms transducing this effect, is lacking. Tubule fluid flow increases in response to Na+ loading; hence, we studied flow modulation of CD ET-1 production. Three days of a high-salt diet increased mouse and rat inner medullary CD (IMCD) ET-1 mRNA expression. Acute furosemide infusion increased urinary ET-1 excretion in anesthetized rats. Primary cultures of mouse or rat IMCD detached in response to flow using a closed perfusion chamber, consequently a CD cell line (mpkCCDcl4) was examined. Flow increased ET-1 mRNA at shear stress rates exceeding 1 dyne/cm2, with the maximal effect seen between 2 and 10 dyne/cm2. Induction of ET-1 mRNA was first evident after 1 h, and most apparent after 2 h, of flow. Inhibition of calmodulin or dihydropyridine-sensitive Ca2+ channels did not alter the flow response; however, chelation of intracellular Ca2+ or removal of extracellular Ca2+ largely prevented flow-stimulated ET-1 mRNA accumulation. Downregulation of protein kinase C (PKC) using phorbol 12-myristate 13-acetate, or PKC inhibition with calphostin C, markedly reduced flow-stimulated ET-1 mRNA levels. Flow-stimulated ET-1 mRNA accumulation was abolished by inhibition of phospholipase C (PLC). Taken together, these data indicate that flow increases CD ET-1 production and this is dependent on extracellular and intracellular Ca2+, PKC, and PLC. These studies suggest a novel pathway for coupling alterations in extracellular fluid volume to CD ET-1 production and ultimately control of CD Na+ reabsorption.
Keywords: tubule, PKC, calcium, PLC, shear stress
collecting duct (CD)-derived endothelin-1 (ET-1) is an important regulator of urinary Na+ excretion and arterial pressure. The CD is the major renal site of ET-1 production (9, 21, 44). ET-1 inhibits epithelial Na+ channel (ENaC)- and Na+-K+-ATPase-mediated Na+ reabsorption in the CD (7, 38, 58). CD-specific knockout of ET-1 causes marked hypertension and reduced urinary Na+ excretion (2). Thus, CD-derived ET-1 exerts a natriuretic and hypotensive effect that is highly physiologically relevant.
CD ET-1 production appears to be increased by elevated Na+ intake, an apparently appropriate response to promote a natriuresis. A positive correlation exists between urinary Na+ and ET-1 excretion in experimental animals and humans (10, 11, 19, 27, 33). Na+ loading increases medullary ET-1 mRNA in rats and increases urinary ET-1 excretion associated with a natriuresis (2). CD-specific ET-1 knockout mice have markedly reduced renal ET-1 excretion compared with controls and show an attenuated increase in response to a high-sodium diet (2). Together, these data support the hypothesis that CD ET-1 production is increased by Na+ loading.
How Na+ loading stimulates CD ET-1 production is not understood. Several factors can modify CD ET-1 synthesis; however, these are not clearly associated with salt balance. Aldosterone augments ET-1 production by inner medullary CD (IMCD) (17); this may provide negative feedback for the Na+-retaining effects of aldosterone, but it does not explain Na+ loading induction of CD ET-1 production. High-Na+ intake can increase medullary osmolality; hypertonic media increased thick ascending limb (18) or Madin-Darby canine kidney (MDCK) cell (24) ET-1 production. However, hypertonicity decreases CD ET-1 synthesis by IMCD (22) or cortical CD (52). Increased tubule flow, as occurs during Na+ loading, is an intriguing possibility for regulating CD ET-1 release. Shear stress can increase (13, 35, 55) or decrease (28, 29) ET-1 release from endothelial or mesothelial cells. Low level shear stress in endothelial cells increased ET-1 release via activation of protein kinase C (PKC), increased [Ca2+]i, or activation of lung Kruppel-like factor (14, 25, 34, 37); higher shear stress inhibited ET-1 release via nitric oxide (NO), cGMP, and possibly reactive oxygen species (25, 29, 34). Thus, it is apparent that flow can regulate ET-1 production, although such regulation is complex and potentially bidirectional. Consequently, the current study examined flow regulation of ET-1 biosynthesis in the CD and began to explore signaling mechanisms for how such regulation might occur.
MATERIALS AND METHODS
Materials.
W-7, phorbol 12-myristate 13-acetate (PMA), calphostin C, triiodothyronine, and U73122 were obtained from Calbiochem (San Diego, CA). NG-nitro-l-arginine-methyl ester (l-NAME) was obtained from Enzo Life Sciences (Plymouth Meeting, PA). All other reagents were obtained from Sigma (St. Louis, MO) unless specifically stated otherwise.
Furosemide studies in rat.
All studies involving rats used in the furosemide experiments were reviewed and approved by the Medical College of Georgia Institutional Animal Care and Use Committee (IACUC) requirements. Male Sprague-Dawley rats (350–350 g; Harlan Laboratories, Indianapolis, IN) were maintained in controlled light and temperature conditions. Rats were anesthetized with thiobutabarbitone (100 mg/kg ip) before being surgically catheterized for intravenous fluid administration and arterial pressure measurement as previously described (5). Urine was collected through a catheter placed directly in the bladder. After equilibration for 60–90 min, a 30-min baseline urine collection period was obtained. In experimental rats, furosemide was then infused at a dose of 0.50 mg/kg at 0.6 ml/h for 1 h, while urine was collected for two additional 30-min periods. Control rats received 0.9% NaCl infusion at 0.6 ml/h during the urine collection periods. Electrolyte concentration was analyzed by ion-selective electrodes.
Acutely isolated IMCD studies.
All rats and mice used in the acutely isolated IMCD experiments were handled in accordance with University of Utah IACUC requirements. Mice (C57BL6; 25 g) or rats (Sprague-Dawley; 200–250 g) were fed a normal (0.25%)- or high (3.1%)-Na+ diet (Harlan Teklad, Indianapolis, IN) for 3 days and given free access to drinking water. IMCD cells were then obtained using a modification of previously described procedures (49, 50). Renal inner medullas were minced and incubated at 37°C in 0.1% collagenase (Type I; Worthington, Freehold, NJ) containing 0.01% DNase (type I) in HBSS supplemented with 15 mM HEPES (pH 7.4). When a suspension containing predominantly single cells and individual tubules was obtained (∼45 min), the digest was filtered through a 74-μm mesh screen to remove any residual tissue. The tubule suspension was centrifuged at 1,500 rpm for 5 min, and the cell pellet was resuspended in 10% bovine serum albumin in HBSS, followed by an additional 2 centrifuge/washes with HBSS. The final pellet containing primarily tubules was used for RNA analysis. This procedure has previously been shown to yield predominantly CDs (49, 50).
Cell culture.
Acutely isolated rat and mouse IMCD fragments were suspended in renal epithelial growth media (Cambex, Watersville, MD) and plated on 10-cm plastic culture plates. Cells were grown at 37°C in 5% CO2 until confluence. In some cases, after confluent for no more than a day, cells were growth arrested in DMEM/F12 without phenol red + 1,000 U/ml penicillin, 1,000 μg/ml streptomycin, and 0.292 mg/ml glutamine for 24 h.
The cortical CD cell line, mpkCCDc14, was generously provided by Prof. A. Vandewalle at Institut National de la Santé et de la Recherche Médicale, France (4). Cells were grown to confluence at 37°C in 5% CO2 in 50:50 DMEM:F12 containing 2 μg/ml dexamethasone, insulin, transferrin, selenium, 400 nM triiodothyronine, 1 μg/ml EGF, 2 mM glutamine, 1 mg/ml penicillin, 1 mg/ml streptomycin, and 2% fetal bovine serum.
Flow studies.
Confluent primary cultures of rat or mouse IMCD cells, or mpkCCDc14 cells, grown on 10-cm dishes, were rinsed with HBSS and chambers were attached to the plates. A rectangular parallel plate flow chamber (Cat. no. 31–010; Glycotech, Gaithersburg, MD) was vacuum sealed onto a portion of the 10-cm dish using a silastic gasket that runs around the periphery of the chamber. The channel depth was 0.25 mm, the width was 1 cm, and the length was 5.9 cm. The surface area of cells exposed to flow was 5.9 cm2. The polycarbonate plate has two manifolds through which medium enters and exits the channel. A pump (Ismatec, Glattbrugg, Switzerland) drives the recirculating perfusate (∼3 ml). Flow was set at different rates to administer various shear stress (0–20 dyne/cm2). Perfusion fluid was HBSS + 10 mM HEPES (pH 7.4). All experiments were conducted at 37°C. In initial studies, the perfusion fluid was removed after varying time periods of flow to determine ET-1 release. In subsequent studies, the cells outside the gasket (not within the perfusion chamber) were scraped off and discarded. RNA was isolated from the cells exposed to perfusion as described below.
ET-1 release.
Perfusate or cell lysates from flow studies were vacuum concentrated to dryness. Urinary, cell, and perfusate ET-1 content were determined using a QuantiGlo ET-1 enzyme immunoassay (R&D Systems, Minneapolis, MN). Luminescence was measured using a Molecular Devices LMaxII Luminometer and Softmax Pro 4.7 data analysis program. Cells were dissolved in 0.1 N NaOH and protein content of cultured cells was determined using the Bradford assay.
ET-1 and ET receptor mRNA.
RNA from acutely isolated and/or cultured cells was extracted using phenol/chloroform, reverse transcribed, and cDNA levels for ET-1, the ETA receptor, the ETB receptor, and GAPDH was determined using real-time PCR (StepOnePlus, Applied Biosystems, Foster City, CA). PCR was performed according to the manufacturer's instructions using the Taqman Gene Expression Assay (Applied Biosystems) with ET-1 (Cat. no. Mm00438656_m1), ETA receptor (Mm01243722_m1), ETB receptor (Mm00432989_m1), and GAPDH (Cat. no. Mm03302249_g1) primers.
Statistics.
Data are presented as means ± SE. Differences between groups were determined using two-way ANOVA, while time course and dose-response data were analyzed by one-way ANOVA. Bonferroni post hoc tests were used to compare individual means. P < 0.05 was taken as significant.
RESULTS
Na+ loading regulation of IMCD ET-1 production.
To definitively determine the effect of Na+ loading on CD ET-1 synthesis, rats and mice were given 3 days of a 3.1 or a 0.25% Na+ diet, followed by determination of ET-1 mRNA in acutely isolated IMCD fragments. Initially, we attempted to measure ET-1 release into the media; however, we were unable to detect ET-1 released into the media using the ELISA method despite 4 h of incubation of IMCD suspensions in 200 μl of HBSS; IMCD began to die (increased trypan blue uptake) after longer periods of incubation. Na+ loading was associated with a 20 ± 5 and 54 ± 13% increase in ET-1 mRNA in mouse and rat IMCD, respectively, compared with IMCD from animals on normal-Na+ intake (P < 0.05 vs. control in both mouse and rat, n = 6 each condition).
Effect of furosemide on ET-1 production.
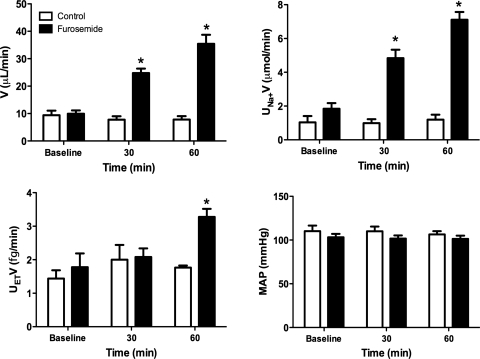
To begin to evaluate whether tubule fluid flow may modulate distal nephron ET-1 production in vivo, the effect of acutely increasing distal nephron flow rate, without altering systemic biology, was evaluated. In anesthetized rats, intravenous furosemide increased urinary Na+ and water excretion after 30 and 60 min of drug administration (Fig. 1). At 60, but not 30, min, there was a marked increase in urinary ET-1 excretion. At no point was there a change in mean arterial pressure. Since urinary ET-1 derives entirely from the kidney (1) and in large part from the CD (2), this experiment suggests that furosemide increases distal nephron ET-1 production and supports the possibility that increased tubule fluid flow may stimulate ET-1 release. One limitation, however, is that the study was done under anesthesia, which can reduce sympathetic drive and affect other factors, thereby potentially influencing the response to furosemide.
Fig. 1.
Urine flow rate (V), Na+ excretion (UNaV), ET-1 excretion (UETV), and mean arterial pressure (MAP) in control or furosemide-infused rats; n = 5 for control and n = 6 for furosemide. *P < 0.05 vs. control at same time point.
Determination of flow conditions.
Initial studies attempted to examine the effect of flow on ET-1 production by primary cultures of rat and mouse IMCD. Despite the media composition (with or without serum), culture surface (glass slides or plastic dishes of varying polymer composition), or the attachment substrate (surface alone, gelatin, collagen, fibronectin, or laminin), IMCD cells detached during even low shear stress (as low as 1 dyne/cm2). Consequently, it was not possible to study primary CD cell cultures (it is not possible to get sufficient cortical or outer medullary CD cells).
Several CD cell lines are available, including K2, mIMCD3, M1, and mpkCCDc14. In preliminary studies, we determined that mpkCCDc14 cells regulated ET-1 production, insofar as we could determine, by identical signaling pathways as did primary cultures of IMCD cells (50). Furthermore, mpkCCDc14 cells released comparable amounts of ET-1 as did the other CD cell lines. Hence, mpkCCDc14 cells were chosen for all subsequent analysis. These cells remained attached under flow when grown on plastic, but not glass. To maximize the surface area of cells exposed to flow, cells were grown on 10-cm dishes. The flow chamber fit snugly into these dishes and could be tightly adhered to them with vacuum sealing and a thin gasket. The flow system was designed to use as little perfusate volume as possible to maximize chances of measuring released ET-1 (as well as to reduce the cost of the various agents added to the perfusate). The flow studies were conducted under conditions designed to mimic physiologic tubular fluid flow rates. These rates were based on best estimates as previously reported in cultured IMCD (8) and perfused CCD (26) and ranged between 1 and 20 dyne/cm2.
In initial studies, ET-1 release into the perfusate was measured under varying shear stress. However, even after measuring ET-1 from all 3 ml of perfusate (after concentrating), the levels of ET-1 barely reached the level of detection using a highly sensitive ET-1 assay (regardless of the flow conditions). This was so even after 4 h of exposure of perfusate to cells; longer periods resulted in cells starting to detach. Adding small amounts of albumin to the system (to reduce any possible ET-1 binding to plastic) did not help. In additional studies, cells were lysed with water, the lysate was centrifuged, and the supernatant was measured for cellular ET-1 content. As for perfusate, no immunologically detectable ET-1 was present; such findings are not surprising given the relatively small numbers of cells in the perfusion chambers. Consequently, all subsequent studies involved measuring ET-1 mRNA. Since ET-1 release mirrors ET-1 mRNA levels in virtually every condition under which this has been studied (20, 50), ET-1 mRNA was taken as a valid index of ET-1 production. This supposition is further supported by the finding that the 3-UTR of ET-1 mRNA contains AUUUA sequences that destabilize the message, conferring a very short half-life (∼15 min); this is highly characteristic of gene products that are largely regulated at the transcriptional level (31, 32). GAPDH was used to normalize mRNA amount; GAPDH amounts (per 3 μg RNA) did not significantly vary under any of the conditions studied (varying flow and/or added agents; data not shown), indicating that GAPDH is a valid marker of total RNA in the sample.
Effect of flow on CD ET-1 production.
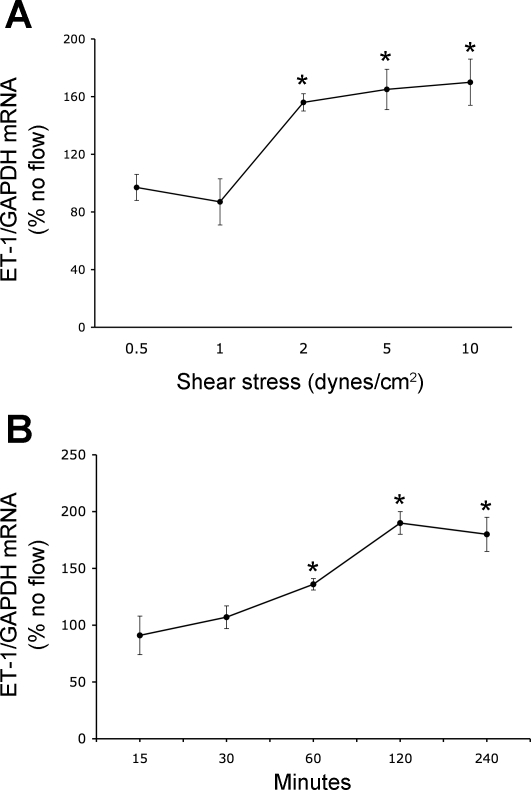
With the use of an empirically chosen 2-h period of exposure, mpkCCDc14 cells were exposed to shear stress of 0–20 dyne/cm2. The no flow condition consisted of setting up cells in the flow chamber in an identical manner as cells exposed to flow, but leaving the pump off. No flow controls were performed simultaneously with each experimental condition. That is, for every shear stress rate, different times of shear stress exposure, or experiment agent added, no flow controls were performed. The no flow control values varied enough between experiments, probably due to small variances that occur between cultured cells from day to day, that all the experimental data were compared with the paired no flow controls done simultaneously with the particular experimental condition. Flow stimulated ET-1 mRNA content at shear stress of 2 dyne/cm2 and higher (Fig. 2A). There was a trend toward greater stimulation of ET-1 mRNA at flow rates up to 10 dyne/cm2; however, this was not statistically significant. At 20 dyne/cm2, some cells began to detach, so these data were not used. To determine the time course of flow-stimulated ET-1 mRNA levels, cells were exposed to 2 dyne/cm2 for 15–240 min (Fig. 2B). A stimulatory effect was first evident after 1 h of flow and was maximally apparent after 2 h of flow; more prolonged flow did not further enhance ET-1 mRNA content. Based on these studies, all subsequent analyses of a flow effect were done at a shear stress of 2 dyne/cm2 for 2 h.
Fig. 2.
Dose response (A) and time course (B) of shear stress-induced alterations in ET-1 mRNA content in mpkCCDc14 cell. For the dose response, cells were exposed to varying flow for 2 h, while for the time course, cells were exposed to a shear stress of 2 dyne/cm2 for varying times; n = 6–14 each data point. *P < 0.05 vs. cells not exposed to flow.
Mechanism of flow-stimulated ET-1 mRNA content.
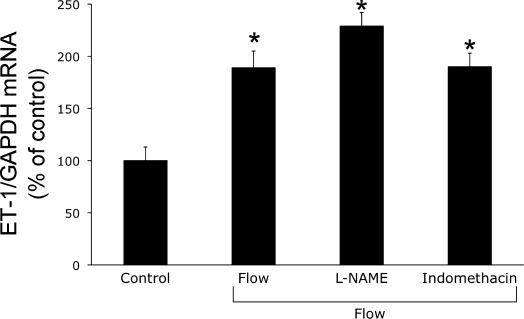
Since NO and PGE2 are 1) produced by the CD (6, 39), 2) can modulate ET-1 production (20, 39), and 3) are increased by shear stress in renal tubular epithelial cells (8, 47), we examined the effect of cyclooxygenase and NO synthase inhibition on flow-stimulated ET-1 mRNA levels. As shown in Fig. 3, neither l-NAME nor indomethacin significantly altered flow-stimulated ET-1 mRNA, suggesting that NO and cycooxygenase metabolites are not involved in the ET-1 response to flow.
Fig. 3.
Effect of inhibition of cyclooxygenase or nitric oxide synthase on flow-stimulated ET-1 mRNA content in mpkCCDc14 cells. Cells were preincubated with vehicle, 1 mM NG-nitro-l-arginine methyl ester (l-NAME), or 1 μM indomethacin for 30 min, followed by exposure to shear stress at 2 dyne/cm2 for 2 h in the presence of the same agents and then determination of ET-1/GAPDH mRNA levels; n = 6–8 each data point. *P < 0.05 vs. cells treated identically with the same agents, but not exposed to flow (control).
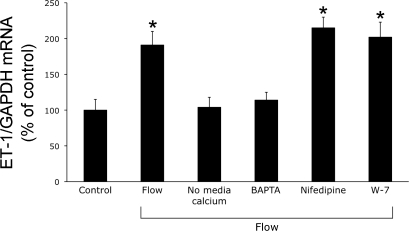
Since shear stress can increase intracellular Ca2+ concentration ([Ca2+]i) in CD cells (41) and Ca2+-regulated signaling can affect CD ET-1 production (50), the effect of modulating Ca2+ pathways on flow-regulated ET-1 mRNA was examined. Inhibition of dihydropyridine-sensitive Ca2+ channels or CaM did not affect flow-stimulated ET-1 production (Fig. 4). However, removal of extracellular Ca2+ or chelation of intracellular Ca2+ (BAPTA) virtually abolished the flow response. Thus, flow-enhanced ET-1 mRNA depends on both extracellular and intracellular Ca2+.
Fig. 4.
Effect of modulation of Ca2+ pathways on flow-stimulated ET-1 mRNA content in mpkCCDc14 cells. Cells were preincubated with vehicle, HBSS without Ca2+, 50 μM BAPTA (to chelate intracellular Ca2+), 100 μM nifedipine, or 10 μM W-7 (CaM inhibition) for 30 min, followed by exposure to shear stress at 2 dyne/cm2 for 2 h in the presence of the same agents/media and then determination of ET-1/GAPDH mRNA levels; n = 6–8 each data point. *P < 0.05 vs. cells treated identically with the same agents, but not exposed to control.
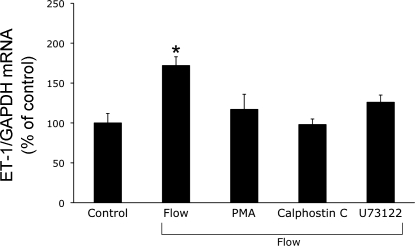
Since shear stress can modulate ET-1 in endothelial cells through activation of PKC (25, 34, 37), the effect of modulating PKC activity on the ET-1 flow response was assessed. Inhibition of diacylglycerol-dependent isoforms of PKC (calphostin C) or downregulation of these isoforms (prolonged exposure to PMA) prevented flow-stimulated ET-1 mRNA accumulation (Fig. 5). Since phospholipase C (PLC) activates PKC via generation of diacylglycerol and increases [Ca2+]i via inositol triphosphate, we examined the effect of PLC inhibition. Blockade of PLC (U73122) also abrogated flow-stimulated ET-1 mRNA augmentation (Fig. 5).
Fig. 5.
Effect of modulation of protein kinase C (PKC) and phospholipase C (PLC) on flow-stimulated ET-1 mRNA content in mpkCCDc14 cells. For phorbol 12-myristate 13-acetate (PMA) studies, cells were preincubated with vehicle or 50 ng/ml PMA for 2 h. Otherwise, cells were incubated with vehicle, 100 nM calphostin C (PKC inhibitor), or 2 μM U73122 (PLC inhibitor) for 30 min. After preincubations, cells were exposed to shear stress at 2 dyne/cm2 for 2 h in the presence of the same agents and then determination of ET-1/GAPDH mRNA levels; n = 6–8 each data point. *P < 0.05 vs. cells treated identically with the same agents, but not exposed to flow (control).
Involvement of ET receptors in the flow response.
Although it seemed unlikely that 2 h of flow would be long enough to change ET receptor expression, we examined whether these were involved in the flow response. Shear stress under the same conditions as used for ET-1 mRNA levels (2 dyne/cm2 for 2 h) did not change ETA or ETB receptor mRNA content. Western and immunostaining were not sufficiently sensitive to detect ETA or ETB receptors. Since ETB receptors are the predominant ET receptor isoform in the CD, the effect of blocking this receptor was examined. Pretreatment of cells with 1 μM BQ788, an ETB-selective antagonist, had no effect on the ET-1 mRNA response to 2 dyne/cm2 for 2 h (145 ± 5% increase in ET-1 mRNA in response to flow in the presence of BQ788; P < 0.05 vs. no flow, n = 6).
DISCUSSION
In the current study, we 1) definitively demonstrate that Na+ loading increases CD ET-1 mRNA in vivo, 2) show that in vivo furosemide-induced increases in tubule fluid flow are associated with increased urinary ET-1 excretion, 3) demonstrate that flow directly stimulates CD ET-1 mRNA accumulation, and 4) determine that Ca2+, PKC, and PLC are involved in the flow response. Viewed individually, the in vivo and cell culture studies have potential weaknesses. The Na+ loading and furosemide studies do not directly demonstrate that flow per se increases CD ET-1 synthesis. Furthermore, the furosemide experiments measured urinary ET-1 excretion, not CD ET-1 production (problematic under the study conditions), and were conducted under anesthesia. Finally, the in vitro studies are obviously subject to whatever influences are introduced by using a particular cell line and the given culture conditions. However, when taken together, our findings provide substantial evidence in support of a novel mechanism for coupling Na+ intake to renal Na+ excretion. In this scenario, increased Na+ intake augments tubule fluid flow that stimulates CD ET-1 production, leading to enhanced autocrine ET-1 inhibition of Na+ reabsorption in the CD, promoting a natriuresis, and maintaining Na+ balance. Such a system could operate independently of circulating hormones, the nervous system, or other systemic physiologic parameters. Notably, this concept is consistent with several previous observations showing that urine flow directly correlates with urinary ET-1 excretion (27, 30, 56, 59).
One might ask why such an apparently positive feedback system would exist, i.e., increased Na+ and fluid delivery to the CD promote a further reduction in renal Na+ reabsorption. A potential explanation could be related to the observation that increased flow in the CD, as occurs in a natriuretic state, can, in of itself, stimulate Na+ absorption through activation of ENaC (36). If this process were to proceed unabated, then it is conceivable that the desired natriuresis would be substantially reduced. However, if flow increases CD ET-1, then this could act to mitigate flow-stimulated ENaC activity. Notably, the increase in ET-1 appears to take about an hour to occur; hence, it may be that this system is primarily operative when a prolonged natriuresis is needed.
Shear stress has also been shown to increase NO production by mIMCD3 cells (8). Since NO can inhibit CD Na+ transport (48), it is conceivable that flow-stimulated NO in the CD could serve a similar function as ET-1. One interesting possibility is that flow stimulation of NO is related, at least in part, to ET-1. Schneider et al. (45) determined that CD-derived ET-1 regulation of arterial pressure and natriuresis was partly mediated by NO. This raises the possibility that flow-enhanced NO production in the CD is ET-1 dependent. However, flow-stimulated NO production occurred within 15–30 min at comparable shear stress rates to the current study (8), while we found that flow stimulation of ET-1 required 1 h. Thus, while it is possible that ET-1 plays a role in flow-stimulated NO production in the CD, it likely does not fully account for this effect. Finally, it is possible that the results observed in mIMCD3 and mpkCCDc14 cells could be due, at least in part, to inherent differences in the cell lines. Ultimately, it will be important to characterize flow regulation of NO and ET-1 in the same cells.
The current study shows that shear stress stimulation of CD ET-1 mRNA content is mediated by PKC. As discussed previously, shear stress in endothelial cells increases ET-1 release via activation of PKC (25, 34, 37). It is notable that shear stress increased ET-1 production in endothelium via an AP-1 site within the proximal 156 bp of the ET-1 promoter, suggestive of a PKC-regulated mechanism (12). Our studies indicate that diacyglycerol-regulated PKC isoforms are involved in the ET-1 response to flow. Not surprisingly, we also found that PLC activation, which generates diacylglycerol, was necessary for the flow response. Ultimately, the specific diacyglycerol-regulated PKC isoforms involved in the flow response [those in the conventional (α, β1, β2, γ) and novel (δ, ε, η, θ, μ) families] will need to be determined.
The CD ET-1 response to flow was dependent on intracellular and extracellular Ca2+. Several possible mechanisms may be involved in flow-stimulated Ca2+ entry and increases in [Ca2+]i. One possibility is that flow-induced bending of primary cilia leads to Ca2+ entry and increases in [Ca2+]i. Particularly attractive candidates for flow sensing in the CD are polycystin-1 (PC1) and -2 (PC2). PC2 is a nonselective cation channel with high permeability to Ca2+ and is associated with PC1 at the base of primary cilia (15). Antibodies to PC1 or PC2 markedly inhibit the Ca2+ response to flow in kidney epithelial cells, while PC1 knockdown prevents flow-induced increases in [Ca2+]i (3, 23). In addition, bending primary cilium on MDCK cells increases [Ca2+]i (43), while removal of the primary cilium abolishes flow-induced [Ca2+]i increases (42). Another possibility is that TRPV4 may be involved since this channel may function as a flow sensor in regulation of mouse cortical CD K+ secretion (51, 57). Multiple other possible mechanisms exist for sensing including caveolin-associated proteins, ENaC, receptor tyrosine kinases, and mechanosensitive ion channels (shear-responsive K channel and stretch-activated Ca2+ channels) (46, 53, 54). Clearly, further studies are needed to identify the proximal flow-sensing mechanism involved in stimulation of CD ET-1 production.
It is conceivable that flow also regulates ET receptors. Our study was not optimized to detect such regulation since flow experiments were typically conducted for only 2 h, a time period likely too brief to affect ET receptor expression due to alterations in receptor synthesis. Indeed, no effect of flow on ETA or ETB receptor mRNA content was observed. It is possible that ET receptor expression was altered through posttranscriptional mechanisms; however, current techniques (Western, immunostaining) using anti-ET receptor antibodies are insufficiently sensitive for such analysis in the small numbers of cells utilized. It is also possible that changes in ET receptor expression could affect ET-1 synthesis or clearance. With regard to ET-1 synthesis, ETB receptor inhibition did not affect the ET-1 flow response. We could not assess flow modulation of ET-1 clearance by the CD (via the ETB receptor); however, whether CD or renal ETB blockade affects ET-1 clearance under any conditions is unclear (16, 40).
In summary, the current study demonstrates that flow, via Ca2+, PKC, and PLC, stimulates CD ET-1 mRNA accumulation and suggests that this system is operative during Na+ loading. Given that the CD ET-1 system is a potent anti-hypertensive system by virtue of inhibition of renal Na+ transport and that Na+ loading enhances CD ET-1 production, the current studies support the notion that flow-stimulated CD ET-1 production serves a potentially physiologically important role for coupling extracellular fluid volume status to renal Na excretion.
GRANTS
This research was supported by the Medical College of Georgia Cardiovascular Discovery Institute and National Institutes of Health Grants R01-DK-96392 (to D. E. Kohan) and PO1-HL-95499 (D. M. Pollock, J. S. Pollock, and D. E. Kohan).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
ACKNOWLEDGMENTS
The authors thank Prof. A. Vandewalle for generously providing the mpkCCDc14 cells.
REFERENCES
- 1. Abassi ZA, Klein H, Golomb E, Keiser HR. Regulation of the urinary excretion of endothelin in the rat. Am J Hypertens 6: 453–457, 1993 [DOI] [PubMed] [Google Scholar]
- 2. Ahn D, Ge Y, Stricklett PK, Gill P, Taylor D, Hughes AK, Yanagisawa M, Miller L, Nelson RD, Kohan DE. Collecting duct-specific knockout of endothelin-1 causes hypertension and sodium retention. J Clin Invest 114: 504–511, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Anyatonwu GI, Ehrlich BE. Calcium signaling and polycystin-2. Biochem Biophys Res Commun 322: 1364–1373, 2004 [DOI] [PubMed] [Google Scholar]
- 4. Bens M, Vallet V, Cluzeaud F, Pascual-Letallec L, Kahn A, Rafestin-Oblin ME, Rossier BC, Vandewalle A. Corticosteroid-dependent sodium transport in a novel immortalized mouse collecting duct principal cell line. J Am Soc Nephrol 10: 923–934, 1999 [DOI] [PubMed] [Google Scholar]
- 5. Boesen EI, Pollock DM. Acute increases of renal medullary osmolality stimulate endothelin release from the kidney. Am J Physiol Renal Physiol 292: F185–F191, 2007 [DOI] [PubMed] [Google Scholar]
- 6. Breyer MD, Breyer RM. G protein-coupled prostanoid receptors and the kidney. Annu Rev Physiol 63: 579–605, 2001 [DOI] [PubMed] [Google Scholar]
- 7. Bugaj V, Pochynyuk O, Mironova E, Vandewalle A, Medina JL, Stockand JD. Regulation of the epithelial Na+ channel by endothelin-1 in rat collecting duct. Am J Physiol Renal Physiol 295: F1063–F1070, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Cai Z, Xin J, Pollock DM, Pollock JS. Shear stress-mediated NO production in inner medullary collecting duct cells. Am J Physiol Renal Physiol 279: F270–F274, 2000 [DOI] [PubMed] [Google Scholar]
- 9. Chen M, Todd-Turla K, Wang WH, Cao X, Smart A, Brosius FC, Killen PD, Keiser JA, Briggs JP, Schnermann J. Endothlein-1 mRNa in glomerular and epithelial cells of kidney. Am J Physiol Renal Fluid Electrolyte Physiol 265: F542–F550, 1993 [DOI] [PubMed] [Google Scholar]
- 10. Chu T, Wu MS, Hsieh BS. Urinary endothelin-1 in patients with renal disease. J Formos Med Assoc 97: 667–672, 1998 [PubMed] [Google Scholar]
- 11. Cuzzola F, Mallamaci F, Tripepi G, Parlongo S, Cutrupi S, Cataliotti A, Stancanelli B, Malatino LS, Bellanuova I, Ferri C, Galletti F, Filigheddu F, Glorioso N, Strazzullo P, Zoccali C. Urinary adrenomedullin is related to ET-1 and salt intake in patients with mild essential hypertension. Am J Hypertens 14: 224–230, 2001 [DOI] [PubMed] [Google Scholar]
- 12. Da Silva RF, Chambaz C, Stergiopulos N, Hayoz D, Silacci P. Transcriptional and post-transcriptional regulation of preproendothelin-1 by plaque-prone hemodynamics. Atherosclerosis 194: 383–390, 2007 [DOI] [PubMed] [Google Scholar]
- 13. Dancu MB, Berardi DE, Vanden Heuvel JP, Tarbell JM. Asynchronous shear stress and circumferential strain reduces endothelial NO synthase and cyclooxygenase-2 but induces endothelin-1 gene expression in endothelial cells. Arterioscler Thromb Vasc Biol 24: 2088–2094, 2004 [DOI] [PubMed] [Google Scholar]
- 14. Dekker RJ, van Thienen JV, Rohlena J, de Jager SC, Elderkamp YW, Seppen J, de Vries CJ, Biessen EA, van Berkel TJ, Pannekoek H, Horrevoets AJ. Endothelial KLF2 links local arterial shear stress levels to the expression of vascular tone-regulating genes. Am J Pathol 167: 609–618, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Gallagher AR, Germino GG, Somlo S. Molecular advances in autosomal dominant polycystic kidney disease. Adv Chronic Kidney Dis 17: 118–130, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Goddard J, Johnston NR, Cumming AD, Webb DJ. Fractional urinary excretion of endothelin-1 is reduced by acute ETB receptor blockade. Am J Physiol Renal Physiol 293: F1433–F1438, 2007 [DOI] [PubMed] [Google Scholar]
- 17. Gumz ML, Popp MP, Wingo CS, Cain BD. Early transcriptional effects of aldosterone in a mouse inner medullary collecting duct cell line. Am J Physiol Renal Physiol 285: F664–F673, 2003 [DOI] [PubMed] [Google Scholar]
- 18. Herrera M, Garvin J. A high-salt diet stimulates thick ascending limb eNOS expression by raising medullary osmolality and increasing release of endothelin-1. Am J Physiol Renal Physiol 288: F58–F64, 2005 [DOI] [PubMed] [Google Scholar]
- 19. Hwang YS, Hsieh TJ, Lee YJ, Tsai JH. Circadian rhythm of urinary endothelin-1 excretion in mild hypertensive patients. Am J Hypertens 11: 1344–1351, 1998 [DOI] [PubMed] [Google Scholar]
- 20. Kohan D. Endothelins in the normal and diseased kidney. Am J Kidney Dis 29: 2–26, 1997 [DOI] [PubMed] [Google Scholar]
- 21. Kohan DE. Endothelin synthesis by rabbit renal tubule cells. Am J Physiol Renal Fluid Electrolyte Physiol 261: F221–F226, 1991 [DOI] [PubMed] [Google Scholar]
- 22. Kohan DE, Padilla E. Osmolar regulation of endothelin-1 production by rat inner medullary collecting duct. J Clin Invest 91: 1235–1240, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kottgen M. TRPP2 and autosomal dominant polycystic kidney disease. Biochim Biophys Acta 1772: 836–850, 2007 [DOI] [PubMed] [Google Scholar]
- 24. Kramer H, Hashemi T, Backer A, Bokemeyer D. Hyperosmolality induced by betaine or urea stimulates endothelin synthesis by differential activation of ERK and p38 MAP kinase in MDCK cells. Kidney Blood Press Res 25: 65–70, 2002 [DOI] [PubMed] [Google Scholar]
- 25. Kuchan MJ, Frangos JA. Shear stress regulates endothelin-1 release via protein kinase C and cGMP in cultured endothelial cells. Am J Physiol Heart Circ Physiol 264: H150–H156, 1993 [DOI] [PubMed] [Google Scholar]
- 26. Liu W, Xu S, Woda C, Kim P, Weinbaum S, Satlin LM. Effect of flow and stretch on the [Ca2+]i response of principal and intercalated cells in cortical collecting duct. Am J Physiol Renal Physiol 285: F998–F1012, 2003 [DOI] [PubMed] [Google Scholar]
- 27. Malatino LS, Bellanuova I, Cataliotti A, Cuzzola F, Mallamaci F, Tripepi G, Parlongo S, Cutrupi S, Mangiafico RA, Ferri C, Galletti F, Gloriso N, Strazzullo P, Zoccali C. Renal endothelin-1 is linked to changes in urinary salt and volume in essential hypertension. J Nephrol 13: 178–184, 2000 [PubMed] [Google Scholar]
- 28. Malek AM, Greene AL, Izumo S. Regulation of endothelin 1 gene by fluid shear stress is transcriptionally mediated and independent of protein kinase C and cAMP. Proc Natl Acad Sci USA 90: 5999–6003, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Masatsugu K, Itoh H, Chun T, Saito T, Yamashita J, Doi K, Inoue M, Sawada N, Fukunaga Y, Sakaguchi S, Sone M, Yamahara K, Yurugi T, Nakao K. Shear stress attenuates endothelin and endothelin-converting enzyme expression through oxidative stress. Regul Pept 111: 13–19, 2003 [DOI] [PubMed] [Google Scholar]
- 30. Mattyus I, Zimmerhack LB, Schwarz A, Hentschel M, Brandis M, Milteny M, Tulassay T. Renal excretion of endothelin in children is influenced by age and diuresis. Acta Paediatr 83: 468–472, 1994 [DOI] [PubMed] [Google Scholar]
- 31. Mawji IA, Marsden PA. Perturbations in paracrine control of the circulation: role of the endothelial-derived vasomediators, endothelin-1 and nitric oxide. Microsc Res Tech 60: 46–58, 2003 [DOI] [PubMed] [Google Scholar]
- 32. Mawji IA, Robb GB, Tai SC, Marsden PA. Role of the 3′-untranslated region of human endothelin-1 in vascular endothelial cells. Contribution to transcript lability and the cellular heat shock response. J Biol Chem 279: 8655–8667, 2004 [DOI] [PubMed] [Google Scholar]
- 33. Modesti PA, Cecioni I, Costoli A, Poggesi L, Galanti G, Serneri GG. Renal endothelin in heart failure and its relation to sodium excretion. Am Heart J 140: 617–622, 2000 [DOI] [PubMed] [Google Scholar]
- 34. Morawietz H, Talanow R, Szibor M, Rueckschloss U, Schubert A, Bartling B, Darmer D, Holtz J. Regulation of the endothelin system by shear stress in human endothelial cells. J Physiol 525: 761–770, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Morgera S, Schlenstedt J, Hambach P, Giessing M, Deger S, Hocher B, Neumayer H. Combined ETA/ETB receptor blockade of human peritoneal mesothelial cells inhibits collagen I RNA synthesis. Kidney Int 64: 2033–2040, 2003 [DOI] [PubMed] [Google Scholar]
- 36. Morimoto T, Liu W, Woda C, Carattino MD, Wei Y, Hughey RP, Apodaca G, Satlin LM, Kleyman TR. Mechanism underlying flow stimulation of sodium absorption in the mammalian collecting duct. Am J Physiol Renal Physiol 291: F663–F669, 2006 [DOI] [PubMed] [Google Scholar]
- 37. Morita T, Kurihara H, Maemura K, Yoshizumi M, Nagai R, Yazaki Y. Role of Ca2+ and protein kinase C in shear stress-induced actin depolymerization and endothelin 1 gene expression. Circ Res 75: 630–636, 1994 [DOI] [PubMed] [Google Scholar]
- 38. Pavlov TS, Chahdi A, Ilatovskaya DV, Levchenko V, Vandewalle A, Pochynyuk O, Sorokin A, Staruschenko A. Endothelin-1 inhibits the epithelial Na+ channel through betaPix/14–3-3/Nedd4–2. J Am Soc Nephrol 21: 833–843, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Plato C, Garvin J. Nitric oxide, endothelin and nephron transport: potential interactions. Clin Exp Pharmacol Physiol 26: 262–268, 1999 [DOI] [PubMed] [Google Scholar]
- 40. Pollock DM, Pollock JS. Evidence for endothelin involvement in the response to high salt. Am J Physiol Renal Physiol 281: F144–F150, 2001 [DOI] [PubMed] [Google Scholar]
- 41. Praetorius H, Spring K. A physiological view of the primary cilium. Annu Rev Physiol 67: 515–529, 2005 [DOI] [PubMed] [Google Scholar]
- 42. Praetorius H, Spring K. The renal cell primary cilium functions as a flow sensor. Curr Opin Nephrol Hypertens 12: 517–520, 2003 [DOI] [PubMed] [Google Scholar]
- 43. Praetorius HA, Spring KR. Bending the MDCK cell primary cilium increases intracellular calcium. J Membr Biol 184: 71–79, 2001 [DOI] [PubMed] [Google Scholar]
- 44. Pupilli C, Brunori M, Misciglia N, Selli C, Ianni L, Yanagisawa M, Mannelli M, Serio M. Presence and distribution of endothelin-1 gene expression in human kidney. Am J Physiol Renal Fluid Electrolyte Physiol 267: F679–F687, 1994 [DOI] [PubMed] [Google Scholar]
- 45. Schneider MP, Ge Y, Pollock DM, Pollock JS, Kohan DE. Collecting duct-derived endothelin regulates arterial pressure and Na excretion via nitric oxide. Hypertension 51: 1605–1610, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Siasos G, Tousoulis D, Siasou Z, Stefanadis C, Papavassiliou AG. Shear stress, protein kinases and atherosclerosis. Curr Med Chem 14: 1567–1572, 2007 [DOI] [PubMed] [Google Scholar]
- 47. Srivastava T, McCarthy ET, Sharma R, Cudmore PA, Sharma M, Johnson ML, Bonewald LF. Prostaglandin E(2) is crucial in the response of podocytes to fluid flow shear stress. J Cell Commun Signal 4: 79–90, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Stoos BA, Garcia NH, Garvin JL. Nitric oxide inhibits sodium reabsorption in the isolated perfused cortical collecting duct. J Am Soc Nephrol 6: 89–94, 1995 [DOI] [PubMed] [Google Scholar]
- 49. Strait KA, Stricklett PK, Kohan DE. Altered collecting duct adenylyl cyclase content in collecting duct endothelin-1 knockout mice. BMC Nephrol 8: 8, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Strait KA, Stricklett PK, Kohan JL, Miller MB, Kohan DE. Calcium regulation of endothelin-1 synthesis in rat inner medullary collecting duct. Am J Physiol Renal Physiol 293: F601–F606, 2007 [DOI] [PubMed] [Google Scholar]
- 51. Taniguchi J, Tsuruoka S, Mizuno A, Sato J, Fujimura A, Suzuki M. TRPV4 as a flow sensor in flow-dependent K+ secretion from the cortical collecting duct. Am J Physiol Renal Physiol 292: F667–F673, 2007 [DOI] [PubMed] [Google Scholar]
- 52. Todd-Turla KM, Zhu XL, Shu X, Chen M, Yu T, Smart A, Killen PD, Fejes-Toth G, Briggs JP, Schnermann JB. Synthesis and secretion of endothelin in a cortical collecting duct cell line. Am J Physiol Renal Fluid Electrolyte Physiol 271: F330–F339, 1996 [DOI] [PubMed] [Google Scholar]
- 53. Traub O, Berk BC. Laminar shear stress: mechanisms by which endothelial cells transduce an atheroprotective force. Arterioscler Thromb Vasc Biol 18: 677–685, 1998 [DOI] [PubMed] [Google Scholar]
- 54. Tzima E. Role of small GTPases in endothelial cytoskeletal dynamics and the shear stress response. Circ Res 98: 176–185, 2006 [DOI] [PubMed] [Google Scholar]
- 55. Walshe T, Ferguson G, Connell P, O'Brien C, Cahill P. Pulsatile flow increases the expression of eNOS, ET-1, and prostacyclin in a novel in vitro cocultrue model of the retinal vasculature. Invest Opthalmol Vis Sci 46: 375–382, 2005 [DOI] [PubMed] [Google Scholar]
- 56. Worgall S, Manz F, Kleschin K, Feth F, Rascher W. Elevated urinary excretion of endothelin-like immunoreactivity in children with renal disease is related to urine flow rate. Clin Nephrol 41: 331–337, 1994 [PubMed] [Google Scholar]
- 57. Wu L, Gao X, Brown RC, Heller S, O'Neil RG. Dual role of the TRPV4 channel as a sensor of flow and osmolality in renal epithelial cells. Am J Physiol Renal Physiol 293: F1699–F1713, 2007 [DOI] [PubMed] [Google Scholar]
- 58. Zeidel ML, Brady HR, Kone BC, Gullans SR, Brenner BM. Endothelin, a peptide inhibitor of Na+-K+-ATPase in intact tubular epithelial cells. Am J Physiol Cell Physiol 257: C1101–C1107, 1989 [DOI] [PubMed] [Google Scholar]
- 59. Zeiler M, Löffler BM, Bock HA, Thiel G, Basel K. Water diuresis increases endothelin-1 excretion in humans. J Am Soc Nephrol 6: 751, 1995 [Google Scholar]