Abstract
Establishment of epithelial apicobasal polarity is crucial for proper kidney development and function. In recent years, there have been important advances in our understanding of the factors that mediate the initiation of apicobasal polarization. Key among these are the polarity complexes that are evolutionarily conserved from simple organisms to humans. Three of these complexes are discussed in this review: the Crumbs complex, the Par complex, and the Scribble complex. The apical Crumbs complex consists of three proteins, Crumbs, PALS1, and PATJ, whereas the apical Par complex consists of Par-3, Par-6, and atypical protein kinase C. The lateral Scribble complex consists of Scribble, discs large, and lethal giant larvae. These complexes modulate kinase and small G protein activity such that the apical and basolateral complexes signal antagonistically, leading to the segregation of the apical and basolateral membranes. The polarity complexes also serve as scaffolds to direct and retain proteins at the apical membrane, the basolateral membrane, or the intervening tight junction. There is plasticity in apicobasal polarity, and this is best seen in the processes of epithelial-to-mesenchymal transition and the converse mesenchymal-to-epithelial transition. These transitions are important in kidney disease as well as kidney development, and modulation of the polarity complexes are critical for these transitions.
Keywords: Crumbs, apical, basolateral, Scribble, Par proteins
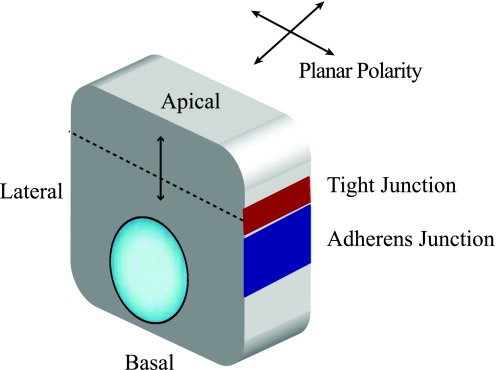
epithelial polarization refers to the asymmetric distribution of biomolecules that allow epithelia to form organized membrane subdomains for specialized functions, including secretion, filtration, absorption, and sensory function. Apicobasal polarity is established by an intricate framework combining protein-protein interactions, control of transcription, and ion flux (119, 139). In the kidney, like many organs, polarized epithelia form tubular structures, with the apical membrane facing the lumen and the basolateral membrane formed by cell-cell (lateral) and cell-matrix interactions (basal) (Fig. 1) (123). Cell-cell interactions are mediated by transmembrane proteins such as cadherins and nectins, while cell-matrix interactions are mediated by proteins such as integrins and dystroglycans (119, 123, 152). In between the apical and basolateral membranes sits the tight junction that limits movements of molecules between the cells and separates the apical from the basolateral membranes. Tight junctions represent the fusion of membranes from two adjacent cells that is primarily mediated by the transmembrane claudin proteins (35, 143). This review will focus on proteins that mediate apicobasal polarity. In addition to apical basal polarity, planar polarity also exists perpendicular to the apical basal axis, and this has been reviewed elsewhere (Fig. 1) (105).
Fig. 1.
Basic model of epithelial cells. Epithelial cells arrange themselves in monolayers and are connected by junctions. Tight junctions (red) delineate apical vs. basolateral surfaces, while adherens junctions (blue) adhere cells to each other. Planar polarity is found perpendicular to the plane of apicobasal polarity.
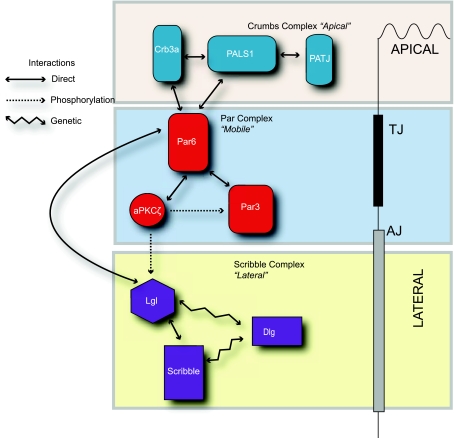
Although many proteins participate in apicobasal polarity, three major polarity complexes serve as the core proteins initiating this process. The complexes are the apical Crumbs and Par complexes along with the basolateral Scribble complex (Fig. 2). In general, it is the mutual antagonism of the apical vs. the basolateral complexes as mediated by protein-protein interactions and protein phosphorylation that defines the apical-basolateral boundary that forms at the tight junction. This review will focus its discussion on the composition and function of these polarity complexes.
Fig. 2.
Interactions of conserved polarity complexes. The Crumbs, Par, and Scribble polarity complexes interact with each other to modulate the formation of junctions and epithelial polarity. The Crumbs complex regulates the formation of the apical surface, the Scribble complex regulates formation of the lateral surface, and the Par complex modulates the balance between apical and lateral surfaces via multiple interactions. See text for definitions or terms.
The Crumbs Complex
Crumbs.
In renal epithelia, the Crumbs complex consists of three major proteins and multiple associated proteins. The core complex consists of the proteins Crumbs (Crb), protein associated with Lin-7 1 (PALS1), and PALS1-associated tight junction protein (PATJ), all of which are highly conserved from invertebrates to vertebrates (Fig. 2) (139). Crumbs was first identified in Drosophila as a regulator of epithelial polarity and its mutation led to a speckled cuticle abnormality (156). There are three human homologs of the Crb gene, termed Crb1, Crb2, and Crb3. Crb1 is primarily localized to the retinal pigmented epithelium of the eye, and mutations in the gene have been linked to retinitis pigmentosa (24). Crb2 gene expression has been detected in brain, testis, uterus, eye, and embryonic tissue, but its full function remains to be elucidated. Recent work in zebrafish has pointed to an important role for a Crb2 isoform in podocytes (29). The Crb3 gene is highly expressed in the majority of mammalian epithelia and in the case of the kidney is the predominant isoform (98). Since Crb3 is highly expressed in kidney epithelia, it can be readily studied in cultured renal cells, such as Madin-Darby canine kidney (MDCK) cells.
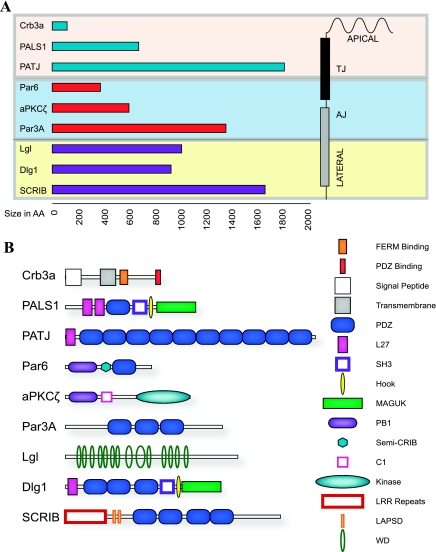
Each member of the Crb family encodes a single span transmembrane protein with an extracellular amino terminus and conserved cytoplasmic carboxy terminus, the latter of which contains a FERM (F for 4.1 protein, E for ezrin, R for radixin, and M for moesin) binding domain and a PDZ [P for postsynaptic density-95, D for discs large, and Z for zonula occludens (ZO) 1] binding domain (Fig. 3) (98). Crb1 and Crb2 share sequence homology in their EGF repeat-rich extracellular domains, while the extracellular domain of Crb3 is markedly shorter and lacks any identifiable domain structure (98). Additionally, Crb3 exists in two variants due to alternate splicing of the final exon of the Crb3 gene. Alternate splicing results in a 22-amino acid difference in the carboxy terminus of each isoform (32), altering the sequence of the PDZ binding domain. Crb3a is termed ERLI for the carboxy-terminal glutamic acid-arginine-leucine-isoleucine (E-R-L-I in single letter code) amino acids which comprise its PDZ binding motif. Crb3b is known as the CLPI isoform, named for the four terminal cysteine-leucine-proline-isoleucine (C-L-P-I in single letter code) sequence comprising its carboxy-terminal residues.
Fig. 3.
Core components of polarity complexes. A: location of protein complexes and their size in amino acids. B: domain structure of polarity proteins. See text for details on each protein and its domains as well as definitions of terms.
The difference in PDZ binding domains of Crb3 has been shown to alter the function of the Crb3 isoforms. Both isoforms localize to the apical membrane and the primary cilium of polarized MDCK cells (32). However, Crb3b (CLPI) also localizes to the spindle pole of mitotic MDCK cells, and specific knockdown of Crb3b (CLPI) disrupts cell division and cytokinesis, with cells demonstrating a multinuclear phenotype (32). The Crb3a (ERLI) isoform knockdown does not impair cell division, but rather affects the polarization and the formation of tight junctions in MDCK cells (32). The knockdown phenotypes indicate that both isoforms are essential for epithelial formation and function; however, the mechanisms regulating the abundance of each splice variant are not yet determined.
The intracellular domains of Crb3a have been shown to be essential for Crb3 function in epithelial cells (34). Crb3a binds to the conserved polarity proteins, PALS1 and Par6, via its PDZ binding carboxy-terminal sequence, ERLI (Figs. 2 and 3) (57, 87). This interaction functionally links the Crumbs and Par polarity complexes and is essential for Crb3a effector function (87, 131). Insights into the binding partners for the FERM binding domain of Crb3a are just emerging. The protein Yurt was shown to bind the FERM binding domain of Drosophila Crb and function as a negative regulator of Crb (81). Studies have also indicated the mammalian orthologs of Yurt, YMO1, EHM2, and EPB41L5 have a similar function in mammalian cells and form a novel complex with Crb3 and PALS1 (43, 81, 82). Similarly, the ortholog for Yurt in zebrafish, known as mosaic eyes (moe), also codes for a FERM protein, and knockouts of this gene give rise to a phenotype similar to that of a PALS1 knockdown (known as Nagie Oko in zebrafish nomenclature) (68). Recent work in Drosophila has indicated that the Crb FERM binding domain may interact with the Expanded protein (46, 91, 130). Expanded is a FERM domain protein that has been shown to regulate the function of the growth-controlling Hippo pathway (178). Overexpression of Crb in Drosophila leads to loss of Expanded and activation of the Hippo-Warts-Yorkie pathway, leading to cell overgrowth (46, 91, 130). While the role of Crb in mammalian epithelial growth is still unclear, it has been suggested that loss of Crb3 contributes to tumor progression in a mouse model (74).
Although the function of Crb3 in growth control is uncertain, there is no doubt that modulation of Crb3 protein levels has major effects on cell polarity and tight junction formation. Overexpression of Crb3 leads to an expansion of the apical surface, an extended tight junction, and a reduced basolateral membrane (131). Knockdown of Crb3 in mammalian epithelia leads to an extensive loss of the tight junction best seen in three-dimensional culture (136, 159). Additionally, forced expression of high levels of the Crb3 gene in normally unpolarized epithelia can lead to the establishment of tight junctions, including the sequestering of tight junction structural proteins including those of the ZO, claudin, and occludin families (34).
Many groups including ours have been interested in the very early events that lead to lumen formation (123, 135). This would be highly relevant to epithelial lumen that forms when mesenchyme is converted to epithelia in early stages of kidney development. Our group and others have found that single epithelial cells grown in three-dimensional culture can develop a lumen during the first division (67, 136). These studies theorize that lumen formation is closely connected to cytokinetic mechanisms that occur in the first cell division and provide a possible mechanism for how lumens are initially formed in developing renal epithelia (135).
PALS1.
PALS1, also referred to as MPP5, is a multidomain adapter protein associated with the conserved Crumbs complex in mammalian epithelia (131, 133). In fully polarized epithelia, the PALS1 protein is localized to the tight junction (Fig. 2) (72). PALS1 contains at least six distinct protein interacting domains, including a pair of L27 domains (named L27N and L27C, respectively), a PDZ domain, a Src homology 3 (SH3) domain, a Hook domain, and a guanylate kinase (GUK) domain (Fig. 3) (72). The presence of a GUK domain makes PALS1 a member of the membrane-associated guanylate kinase (MAGUK) family of modular adaptor proteins (17). The GUK domain of PALS1, like other MAGUK proteins, lacks any discernable kinase activity and likely is involved in protein-protein interactions (16, 103, 154). PALS1 has important homologs in lower organisms, including Stardust in Drosophila and nagie Oko in zebrafish. Stardust functions similarly to mammalian PALS1 binding Crumbs (8, 51), and mutations in Stardust lead to speckled cuticle defects similar to Crumbs (155). Nagie oko and Stardust have crucial roles in retinal development through interactions with Crumbs proteins (16, 169). In addition, a role for nagie oko in zebrafish heart development is well documented (13, 134).
PALS1 not only binds Crumbs proteins, it also interacts with PATJ (Fig. 2) (131, 133). PALS1 interacts with Crumbs proteins via its PDZ domain and binds PATJ with its L27N domain (88). Domain deletion analysis has illustrated that the interactions with both Crb3 and PATJ are essential for cell polarity in mammals (88, 145). PALS1 also links the Crumbs complex to the Par complex via the binding of the PDZ domain of Par6 to the N terminus of PALS1 (127, 167). Knockdown studies of PALS1 indicate that it is essential for cell polarity, affecting levels of PATJ and the ability of atypical PKC (aPKC) to target to the tight junction (145).
The regulation of PALS1 protein in mammalian epithelia is maintained by its association and binding to other proteins, illustrating the complex nature of the tight junction. Mammalian PALS1 was identified in a screen for proteins associated with Lin-7, a small PDZ domain containing protein necessary for proper development in C. elegans (72). Lin-7 stabilizes PALS1 protein in MDCK cells by binding to the L27C domain found in PALS1 (15, 124, 144). Loss of Lin-7 destabilizes PALS1, leading to tight junction defects (144). The Lin-7c knockout mouse displays a variety of renal defects, including cystic kidneys (124). In this regard, it has been reported that PALS1 can interact with nephrocystins, genes involved in the pediatric cystic kidney disease nephronophthisis (23). Additionally, PALS1 is destabilized by the lack of Crb3 in MDCK cells. Snail-induced epithelial-to-mesenchymal transition (EMT) results in decreased PALS1 protein levels with only a modest decrease in transcription of the gene (171).
It has been demonstrated that PALS1 regulates the architecture of not only the tight junction but also the lateral membrane. Wang et al. (166) illustrated that PALS1 knockdown affected the trafficking of E-cadherin to the lateral surface of MDCK cells through the exocyst complex. This is also found in Drosophila where loss of Stardust or Crumbs affects adherens junctions (44). This reflects the close interplay between polarity proteins, formation of the lateral membrane, and cell adhesion. A knockout of PALS1 was found to be early embryonically lethal, and selective depletion of PALS1 from the cortical progenitors of the medial cortex and hippocampus led to mice with essentially no cerebral cortex (76). This was ascribed in part to defects in mammalian target of rapamycin (mTOR) signaling, as mTOR components have previously been shown to interact with the Crumbs complex (101).
PATJ and multi-PDZ protein-1.
PATJ and multi-PDZ protein-1 (MUPP1) are two highly related multiple PDZ-domain-containing proteins found in mammalian epithelia. The presence of multiple PDZ domains on both PATJ and MUPP1 gives rise to a multitude of functions for both proteins in mammalian epithelial cells. Both PATJ and MUPP1 contain N-terminal L27 domains (Fig. 3), which have been shown to bind to PALS1 (133), and MUPP1 has also been shown to directly interact with MPP4 (also known as Dlg6) via this L27 domain (Fig. 2) (164). PATJ and MUPP1 both contain multiple PDZ domains, making them molecular scaffolds at the tight junction. PATJ contains 10 PDZ domains, while MUPP1 contains 13. PDZ domains 6 and 8 of PATJ directly bind to ZO-3 and claudin-1, respectively (132). The binding of the ZO and claudin families to PATJ directly links conserved polarity complexes to tight junction structural proteins. Similarly, MUPP1 is also linked to tight junction structural proteins, including those of the claudin and junctional adhesion molecule family (47).
However, while PATJ has been demonstrated to be important for the maintenance and establishment of epithelial integrity, MUPP1 nay not be essential for tight junction formation (1). PATJ has been shown to interact with angiomotin, and this interaction may provide clues into the role PATJ plays as a signaling molecule in polarized epithelia (147, 170). Angiomotin binds PDZ 2 and 3 of PATJ and has a large number of interactions that may shed light on how the epithelial barrier is maintained. In addition to binding PATJ, angiomotin also associates with Rich1, a Cdc42 GAP, thus directly linking PATJ to the Rho-family of GTPases and the Par polarity complex through Cdc42 (170). Since both proteins are associated with the tight junction and found within close proximity of the cell membrane, PATJ and MUPP1 can act as anchors of transmembrane receptors localized at or near the tight junction and adherens junction (22, 89). These transmembrane receptors may represent key upstream signaling molecules responsible for maintenance and function of the epithelial monolayer.
The Par Complex
The mammalian Par complex comprises the proteins partitioning defective-3 (Par3), partitioning defective-6 (Par6), and aPKC (42). The Par complex is a cornerstone of apicobasal cell polarity, first identified in the C. elegans zygote (97). Genetic studies in C. elegans provided important insights into the mechanisms of asymmetric distribution of proteins, as removal of the Par proteins led to a failure of the zygote to correctly segregate proteins needed for proper development (31).
Par3.
Deletion of the Par3 gene leads to the defective partitioning of proteins during the first cell division in the C. elegans zygote (75). In mammalian epithelia, Par3 has been shown to be necessary for tight junction formation and the localization of other members of the Par complex (38, 78, 151). Par3 is a multidomain scaffold protein, with three PDZ domains that can interact with Par6 and aPKC. (Figs. 2 and 3) (55, 66, 69) The first PDZ domain of Par3 interacts with Par6, and this interaction along with the Par3-aPKC interaction are indispensable for the development of cell polarity (52, 69, 90). It has been demonstrated that Par3 can homodimerize via its N terminus, and this interaction is important for the apical localization of Par3 (107). In the same study, the authors showed that the extreme N terminus of Par3 is necessary for proper localization of the other Par complex and tight junction proteins (107).
In mammals, there are two Par3 proteins, Par3A and Par3B (38), and although expression of these proteins overlap in renal epithelia, Par3B does not interact with aPKC (38). This exclusion is not fully understood, as Par3B can still bind Par6 and overexpression of the N terminus of Par3B can block tight junction formation similarly to overexpression of Par3A (38). There are also three different Par3A protein isoforms due to alternate splicing in mammalian epithelia, and only two of these can bind aPKC (38). Each Par3A isoform is distinguishable due to significant differences in the molecular masses of the translated sequence (180, 150, and 100 kDa, respectively), but they still possess a similar domain structure. The 180- and 150-kDa isoforms of Par3A bind aPKC on their C terminus, which is absent in the 100-kDa isoform (90).
The identity of the Par polarity complex is regulated, at least in part, by the ability of aPKC to interact with and phosphorylate Par3A (49, 66, 109). Phosphorylation of Par3 at S827 by aPKC is required for proper tight junction localization of Par3A (49, 111, 150). Overexpression of either a nonphosphorylatable mutant of Par3A or a dominant negative aPKC results in a similar phenotype, expanded lateral and reduced apical surfaces (111). In Drosophila, the effects of Par3 phosphorylation have been recently studied. Phosphorylation of Par3 by aPKC dissociates Par3 from aPKC and removes Par3 from the apical complex and sends it to the lateral zonula adherens. At the same time, the phosphorylation of Par3 frees Par6 to bind to Crumbs in the apical membrane (109, 165). It has also been suggested that Par3 binds directly to Stardust/PALS1 and that phosphorylation of Par3 releases aPKC and Stardust to move to the apical membrane (80). These results, taken together, lead to the same general finding as in mammalian cells that phosphorylation of Par3 promotes definition of the apical complexes. The main difference is that while Par3 is functional for zonula adherens development in Drosophila, in mammalian cells it is more important for ZO/tight junction formation. The necessity of the Par3A-aPKC interaction has been further demonstrated as essential by knockdown experiments in both two- and three-dimensional mammalian tissue culture models. In a Par3A knockdown system, MDCK cells formed intercellular lumens in monolayer culture and exhibited a multiple lumen phenotype in three dimensions (52). In complementation assays, these Par3A knockdown phenotypes were only rescued by introduction of a Par3A construct that could specifically interact with aPKC (52).
In addition to phosphorylation by aPKC, phosphorylation of Par3A by other kinases also regulates the function of the protein in polarized epithelia. Phosphorylation of S144 and S885 of Par3A by the polarity kinase Par1 (also known as EMK1/MARK2) leads to the binding of protein 14-3-3 (also known as Par5) to Par3A in both canine and murine models (9). Disruption of Par3A S144 phosphorylation prevents a Par3A-14-3-3 interaction, resulting in polarity defects in three-dimensional culture (56). In MDCK cells, Y1127 of Par3A is phosphorylated by c-Src or c-Yes through an epidermal growth factor (EGF)-dependent mechanism, and the phosphorylation event is necessary for EGF-induced tight junction formation (168). In addition, dephosphorylation of serines in Par3A can be regulated. Par3 dephosphorylation is mediated, in part, by protein phophastase 1-α (PP1α), and repression of PP1α expression in MDCK cells causes delays in tight junction formation (160).
Par3 regulates and is regulated by Ras family GTPases (59). Ras family GTPases such as Rho and Rac are known for regulating a multitude of cellular functions, including modulating cytoskeleton and epithelial architecture through cytoskeleton dynamics. Tiam1/2 is a Rac1 guanine nucleotide exchange factor (GEF) that binds the C terminus of Par3A. The interaction of Tiam1/2 to Par3A spatially regulates activation of Rac1 at the cell periphery, leading to a stabilization of epithelial junctions (21). Another Ras family member, RhoA, is a potent antagonist of Rac1 activity. RhoA activates Rho kinase (ROCK), leading to phosphorylation of yet another site on Par3A, T833 (115). The phosphorylation of Par3 at T833 has been recently demonstrated to prevent formation of the Par polarity complex, leading to the inactivation of Rac1, presumably by impairing the function of Tiam1/2 bound to Par3A (115). It has been demonstrated that a functional Par polarity complex (consisting of Par3A, Par6, and aPKC) enhances Rac1 activity and is antagonized by Rho activity (115).
Par3 also interacts with several additional proteins that regulate cell polarity. Par3A has been shown to interact with cytoplasmic keratin via the keratin binding protein Albatross. The association of Par3A with Albatross enforces the identity of the basolateral surfaces (148). In an Albatross knockdown cell, lateral membrane components are mislocalized in the cell (148). The second and third PDZ domains of Par3A have been suggested to anchor the Par complex to the cell membrane and to be associated with phosphoinositide signaling. PDZ2 of Par3A binds phospholipids found in the inner leaflet of the plasma membrane, while PDZ3 of Par3A has been shown to bind the phosphoinositide phosphatase, phosphatase and tensin homolog (PTEN) (33, 172). Par3A also directly interacts with junction adhesion molecule (JAM) via PDZ domains 1 and 3 (30, 64). The binding of JAMs to Par3A occurs at the tight junction, suggesting that JAMs are an anchor for the Par polarity complex.
Par6.
In mammals, there are three identified Par6 genes, Par6A (also known as Par6C and Par6α), Par6B, and Par6G (also known as Par6D or Par6γ), and all appear to be ubiquitously expressed, albeit with slightly different temporal-spatial and subcellular localizations (37). Regardless of the isoform, it appears that Par6 is a multifunctional protein in epithelial cells, being a key adapter protein that allows the Par complex to interact with both the Crumbs and Scribble polarity complexes (57, 87, 167). Par6 contains an N-terminal Phox and Bem1 (PB1) domain, followed by a semi-Cdc42/Rac interactive binding (semi-CRIB) domain, and a C-terminal PDZ domain (Fig. 3). Par6A directly interacts with Crumb3 via Par6A's PDZ domain, and this binding is important for proper tight junction formation (Fig. 2) (57, 87). The PDZ domain of Par6 also binds PALS1 with the PDZ domain of Par6B, binding PALS1 with highest affinity (37). Par6B binding to PALS1 can interrupt the binding of PALS1 to PATJ (167). The difference in binding preference between Par6A and Par6B may be explained by the conformation of the protein when bound with another binding partner, Cdc42 (37).
The Par6 isoforms interact with members of the aPKC family in a multitude of tissues (151). The function of Par6 binding to aPKC is to link Par6 and Par3 into a complex that may be dependent on the phosphorylation status of Par3 and to control the activity of the complex. The semi-CRIB domain and the PDZ domain of Par6 binds the N terminus of Par3 forming a stable tripartite complex that promotes junction formation (69). Par6 is thought to activate aPKC through direct interaction, allowing aPKC to phosphorylate Par3 (176). Par6 interacts with Cdc42 and Rac1 via the semi-CRIB domain (69, 70). The interaction of Par6 with Cdc42 renders a conformational change in Par6, altering the binding of Par6 to PALS1 and Crb3 (37, 40). When activated, GTP-bound Cdc42 binds Par6; the PDZ domain of Par6 most likely becomes hidden through conformational change, resulting in the masking of the PDZ domain of Par6, leaving Par6 with weakened interactions with PALS1 and possibly Crb3 (37).
Par6 also plays a pivotal role in polarity by binding members of the lateral polarity complex (Fig. 2). Par6 binds Lgl, and the binding of Lgl to Par6 excludes the binding of Par3 and PALS1 to Par6 (128, 175). The binding of Lgl by Par6 also mediates phosphorylation of Lgl by aPKC (more details below). With the binding between Par3 and Lgl to Par6 being mutually exclusive, Par6 is likely a regulator of the delicate balance between the formations of distinct apical vs. basolateral domains in polarized epithelial cells. In this case, Par6 reinforces both basolateral and apical identities through binding to both apical and basolateral polarity complexes. It is not surprising then that overexpression of Par6 in MDCK cells inhibits tight junction formation, as the dosage of Par6 is critical in maintaining proper protein interactions and subsequent polarity (36). On the other hand, Par6 overexpression has also been linked to cell hyperproliferation without loss of apicobasal polarity, suggesting that Par6 effects may be Par6 isoform as well as tissue specific (122).
aPKC.
There are two major forms of aPKC, PKCζ and PKCλ/ι. Members of the aPKC family lack most of the C1 and all of the C2 domains, distinguishing themselves from typical PKCs (Fig. 3) (4). aPKCs directly bind to Par6 via an N-terminal PB1 domain and to Par3 via interactions involving the aPKC kinase domain (Fig. 2) (66, 69, 90). While aPKC has many functions in cells, in polarized epithelia it acts as a molecular switch controlling the identity of apical and lateral domains. This is accomplished by the selective phosphorylation of known targets of aPKC in mammals, including the previously mentioned Par3A and Lgl (49, 66, 90, 128, 175). The scaffold Par6 is critical in regulating aPKC and placing polarity substrates in close proximity.
aPKC phosphorylation of Par3 and Lgl is believed to modulate the identity of the Par complex in mammalian cells, where the phosphorylated species of either Par3A or Lgl are excluded or included in a particular complex based on conformational change. As discussed above, phosphorylation of Par3A by aPKC results in Par3-Par6 dissociation, beginning a cascade resulting in the stabilization of tight junctions and the development of apical-basal polarity (49, 109, 111, 165). In a similar way, aPKC phosphorylates Lgl, inducing Lgl dissociation from Par6 and the inclusion of Lgl in the Scribble complex (128, 174, 175). The dissociation of Lgl from Par6 leads to Par complex reformation and results in cytoskeletal rearrangements that reinforce surface identity through Ras family GTPases (see above). The Par complex with aPKC also regulates the basolateral membrane by promoting endocytosis of E-cadherin. This process, observed in Drosophila, also requires CDC42 and Par6 as well as the actin cytoskeleton (41, 85). Similar mechanisms may function in mammalian cells to mold adherens junctions and are important in early polarity and junctional remodeling (138). Similar endocytic regulation may also have relevance for apical membrane regulation (140).
Recent studies have examined mice with knockouts of aPKC. Knockout mice of PKCλ are lethal at an early embryonic stage (141), while PKCζ knockouts have primarily immunological defects (86). In neuronal development, selective loss of PKCλ leads to a defect in adherens junctions but ultimately neurogenesis is not affected (62). Interestingly, tissue-specific knockout of PKCλ has been examined in podocytes by two groups (50, 54). The loss of aPKC leads to polarity and slit diaphragm defects in podocytes, resulting in severe proteinuria. Recent work indicates that the crucial slit diaphragm proteins nephrin and neph1 interact with the Par3/Par6/aPKC complex (48).
The Scribble Complex
The mammalian Scribble complex comprises the genetically linked conserved polarity proteins Scribble (SCRIB), discs large (Dlg), and lethal giant larvae (Lgl). Although the evidence for physical interaction between these proteins is somewhat limited, studies in lower organisms suggest that they function in the same genetic pathway. The Scribble complex localizes to the lateral membrane in polarized epithelia (Fig. 2), a location consistent with a role in defining the lateral vs. the apical surface of these cells.
Scribble.
SCRIB is a large cytoplasmic scaffold protein associated with the lateral membrane in polarized renal epithelial cells (25, 114). Originally described in Drosophila, SCRIB's name is derived from the disorganized phenotype described in the developing embryo, imaginal wing discs, and follicles of the fly (11, 12). SCRIB is a member of the leucine-rich repeat (LRR) and PDZ domain (LAP) family of proteins with 16 LRRs in the N terminus and 4 PDZ domains in the C terminus (Fig. 3) (10).
The N-terminal 16 LRRs of SCRIB are necessary for binding to Lgl2 and targeting SCRIB to the lateral membrane in polarized renal epithelia (71, 117). The association of Scribble with the lateral membrane, however, appears to be cell type specific. In intestinal epithelia, Scribble associates with the tight junction structural protein ZO-1 and regulates tight junction stability, a phenomenon not seen in renal epithelia (65). The lateral targeting of SCRIB depends on the presence of E-cadherin, and in MCF10A cells lacking E-cadherin SCRIB is no longer associated with the membrane (117). The association of SCRIB with the intracellular domain of E-cadherin at the lateral membrane of polarized renal epithelia is necessary for proper cell-cell adhesion, as SCRIB knockdown alters adherens junction stability(129). In this study, knockdown of SCRIB in MDCK cells was shown to lead to increased motility and reduced adhesion, a phenotype similar to that seen with knockdown of E-cadherin. However, it is interesting to note that overall cell polarity was not affected. The PDZ domains of SCRIB bind βPix, a Rac/Cdc42 GEF involved in exocytosis (7). SCRIB has been shown to regulate directed migration and wound healing in association with Rac1, Cdc42, and presumably the Par complex (27, 121),. Also, PDZ domains 3 and 4 of SCRIB bind the tight junction structural protein ZO-2 in contact naive epithelial cells, but not fully polarized epithelia, further suggesting a role for SCRIB in cell migration (106). Similarly, mice displaying mutated variants of SCRIB display impaired directed epithelial migration phenotypes (27).
PDZ domains 3 and 4 of SCRIB bind to the planar cell polarity factor and noncanonical Wnt-signaling molecule Vangl2, and this interaction is responsible for proper orientation of polarized structures in mammals (71, 108). Abrogation of Vangl2 in mouse reproductive tract epithelium, in turn, resulted in a mislocalization of SCRIB and defects in apical membrane formation (163). A recent study suggested that the tumor-suppressor function of SCRIB is related to its ability to regulate the Ras-MAPK pathway, and overexpressed SCRIB was able to effectively suppress oncogenic Ras-associated invasiveness (26). Drosophila models of tumor formation confirm that oncogenic Ras and SCRIB mutants cooperate to form metastatic tumors similar to those found in human cancers (173). The tumor suppressor adenomatous polyposis coli (APC) also binds SCRIB through PDZ1 and PDZ4, suggesting that SCRIB regulates the cell cycle, cell growth, and cell morphology (153). In addition, the SCRIB protein is a target of oncogenic viruses. High-risk human papilloma virus oncoprotein E6-E6AP is a ubiquitin ligase complex that tags SCRIB with a ubiquitin signature, causing proteosomal degradation of the SCRIB protein (114).
Lgl.
Mammals contain multiple Lgl genes, termed Lgl1–4. Lgl is characterized by a series of WD-40 repeats that are thought to facilitate binding to SCRIB (Fig. 3) (71). Mammalian Lgl localizes to basolateral membranes in renal epithelia upon cell-to-cell contacts and is localized to the cytoplasm in contact naive cells (108). Mammalian Lgl binds Par6/aPKC, and this binding complex lacks Par3 (128, 175). As expected, knockdown of Lgl increases the amount of Par3 that interacts with Par6, and knockdown or overexpression of Lgl perturbs polarity (174, 175). As mentioned previously, mLgl is phosphorlyated by aPKC, and the phosphorylation of Lgl restricts its localization to the basolateral membrane, as a nonphosphorylatable mutant of mLgl localizes with apical markers (110, 128). In human cancers, overexpression of aPKC at the membrane results in a cytosolic accumulation of Lgl, suggesting that membrane-bound Lgl is necessary for tissue homeostasis (45, 92). In terms of vertebrate development, it has been demonstrated that Lgl gives identity to the basolateral membrane and the interaction between Lgl and aPKC results in definition of apical vs. basolateral surfaces (20). Similarly, in depolarization models, knockdown of Lgl prevented the dissociation of the Par polarity complex, resulting in cellular overgrowth (174). Knockout of Lgl1 in mice leads to a central nervous system hyperproliferation phenotype possibly due to defects in asymmetric cell division (77). These data suggest that there exists a coordinated series of events where the action of Lgl and aPKC function to define the apical and basolateral surfaces by mutual inhibition and scaffolding.
Lgl has also been demonstrated to interact with trafficking machinery. Lgl interacts with myosin II heavy chain and the basolateral targeted syntaxin 4, suggesting it plays a role in the trafficking of components to the lateral membrane (110). More studies are needed on this phenomenon in mammalian systems; however, work in Drosophila has illustrated that Lgl may be involved with the regulated endocytosis and exocytosis of Crumbs (14, 95). In particular, disrupting exocytosis of Crumbs resulted in an expanded lateral membrane, but suppression of Lgl in this system resulted in a rescue of Crumbs presentation on the apical surface (14).
Dlg.
Dlg is the mammalian homolog of the Drosophila gene Discs large, of which there are five mammalian family members (Dlg1–5), with Dlg1 being the most thoroughly studied in the kidney (104). Dlg1 (also known as SAP97) localizes to the lateral membrane in polarized epithelia (96). Dlg1 contains, in order, from N terminus to C terminus, an L27 domain, three PDZ domains, and SH3 domain, a 4.1 binding domain, and a MAGUK domain (Fig. 3) (96).
The Scribble complex consisting of SCRIB, Lgl, and Dlg1 have a strong genetic interaction; however, physical interaction between Dlg1 and the other members of the complex in mammalian cells has remained elusive. However, multiple other binding partners for Dlg1 have been identified. Proteins that bind the L27 domain of Dlg1 include membrane palmitolyated protein (MPP) family members MPP2, MPP3, and MPP7 (73). The tripartite complex consisting of Dlg1, MPP7, and Lin-7 is important for tight junction formation and stability of Dlg1 (15, 146). Dlg1 also interacts with Lin2/CASK(120), another MAGUK polarity protein, via an L27-L27 domain dimerization interaction (84), and this interaction is important for Dlg1 recruitment to the lateral membrane (84, 94). This interaction may be most important in the nervous system, where Lin-2/CASK has a role in synaptic function and gene expression (6, 53). Lin-2/CASK is mutated in a human disorder characterized by microcephaly and mental retardation (113).
In addition, Dlg1 interacts with APC via its PDZ domains (102). APC is a tumor suppressor that binds β-catenin in polarized epithelial cells and negatively regulates Wnt signaling, suggesting that Dlg1 may play a role in planar cell polarity (79). The interaction of Dlg1 and APC has been shown to affect cell cycle progression, as overexpressed Dlg1 leads to overproliferation most likely due to an increase in Wnt signaling (63). Adey and colleagues (2) also identified Dlg1 as a binding partner for the tumor suppressor PTEN via a yeast two-hybrid screen and demonstrated that unphosphorylated PTEN interacts with the second PDZ domain of Dlg1. Although the functional significance of this lipid phosphatase binding to Dlg1 is not known at this time, it is possible that Dlg1 acts to stabilize PTEN. Dlg1 would then facilitate PTEN phosphorylation, affecting PTEN interactions with other PDZ domain-containing proteins including Par3 (162).
Posttranslational modifications to Dlg1 protein have also given insight into the role of Dlg1 in epithelial polarity. Dlg1 is phosphorylated in epithelial cells, and phosphorylation appears to have a significant role in maintaining the structural integrity of the adherens junction (83). Additionally, the phosphorylation of Dlg1 may confer its subcellular localization and stability during the cell cycle and be regulated by cyclin-dependent kinases (CDK) 1 and 2 (116). The stability and half-life of Dlg1 in epithelial cells is regulated by ubiquitination (99, 100). Ubiquitination of Dlg1 can be a result of viral infection, where oncogenic viruses coding for ubiquitin ligases selectively target PDZ polarity proteins for degradation. Virally mediated ubiquitination of polarity proteins like Dlg1 or SCRIB results in a breakdown of the polarity program (39, 100, 158). A Dlg1 knockout mouse has been generated, and the phenotype includes hypoplastic kidneys associated with the lack of epithelial development (60). The C-terminal SH3, Hook, and GUK domains alone of Dlg1 are known to be important in Dlg1 function. A gene trap mouse that leads to a C-terminal truncation of Dlg1 displays an array of developmental phenotypes, including defects in nephrogenesis and cleft palate (18, 112).
An additional comment is required when the function of the lateral complex proteins including Dlg is considered. Lgl proteins are known to have a role in polarity due to their interaction with Par6, but the role of Dlg and SCRIB in apicobasal polarity is less clear. In fact, these proteins appear to play a larger role in planar polarity and cancer. Also missing from this complex are effectors such as kinases although SCRIB interacts with βPix to control small G proteins (7). Prominently absent from this discussion of the SCRIB complex is the Par1 kinase that was first identified in C. elegans as a critical effector in zygote polarity (42). Par1 has the ability to phosphorylate Par3 and in this fashion antagonize the apical complexes (9, 56). Reciprocally, Par1 is phosphorylated by aPKC, and this induces 14-3-3 binding to Par1 and reduces Par1 membrane targeting (58, 149). However, the factors that target Par1 to the lateral membrane are poorly understood (161) and do not involve the SCRIB complex although Par1 can phosphorylate Dlg (177). In general, our knowledge of the function of apical polarity complexes exceeds our knowledge of the lateral complexes in mammalian apicobasal polarity.
Polarity Proteins and Mesenchymal-Epithelial Transitions
This review catalogues the polarity complexes and their multiple interactions. Together via multiple interactions and protein modifications, these complexes work to define the apical and basolateral surfaces. Modulation of these polarity complexes and their function in physiology is best demonstrated in EMT and the converse mesenchymal-to-epithelial transition (MET) (19, 157). EMT is characterized by the loss of epithelial morphology where cells first lose junctional architecture and then move toward a fibroblastic morphology. To exhibit such a morphological change, the cell undergoes massive alterations in its transcription and translation networks, along with a vast reorganization of its cytoskeletal network. The EMT process is a normal part of development during gastrulation where cells need to move away from the epithelial sheet and migrate in the embryo. In contrast to EMT, renal tubules are initially formed by the reverse process of MET (19). During MET in kidney development, the ureteric bud induces the metanephric mesenchyme to become tubular epithelia. (28). In the process of MET when mesenchyme is converted to epithelia, expression of polarity complexes and tight junction proteins is increased (137). This is due to a reversal of transcriptional pathways that are discussed below. Our work and the work of others have described how these polarity proteins once induced in MET can lead to primordial tubular structures (135).
Regulation of EMT or MET primarily rests in transcriptional regulation. A series of transcriptional repressors including the Snail family, the ZEBs, Twist, E47, and other transcription factors induce EMT by directly or indirectly repressing epithelial gene expression and inducing mesenchymal genes (126). Many of the EMT-inducing transcription factors bind E-boxes in the promoter region of E-cadherin, downregulating cell-cell adhesion and promoting cell migration. Another major target of Snail and related proteins are tight junction proteins such as occludin and claudins (61). Most relevant to this review is the effect of EMT on polarity complexes. If epithelial cells are to move to the nonpolarized mesenchymal phenotype, then the main polarity complexes must be neutralized. The major effects are seen with the Crumbs3 protein with Snail and ZEB transcriptional repressors binding directly to the promoter of Crumbs3 (3, 171). Other members of the Crumbs complex are also repressed but mainly at a protein level (171). This could be due to instability secondary to repression of Crumbs3 expression. Expression of lateral polarity proteins such as Lgl2 are also repressed in EMT likely through direct transcriptional repression by ZEB1 (142).
EMT is induced by a variety of upstream factors including transforming growth factor (TGF)-β, Wnts, growth factors, and hypoxia (157). Many of these factors can lead to the upregulation of Snail and promote EMT via transcriptional repression of polarity proteins. However, there are other nontranscriptional mechanisms to modulate polarity proteins. One of the best described is the effect of TGF-β on the Par polarity complex. The TGF-β receptor interacts with Par6 and, upon stimulation with TGF-β, Par6 is phosphorylated. This phosphorylation serves to destabilize tight junction proteins (125). A similar effect has been seen with the growth factor receptor ErbB2, which interacts with Par6 and disrupts the Par6/Par3/aPKC complex (5). Taken together, these upstream signaling pathways can perturb polarity and tight junctions by immediate disruption of polarity complexes as well as by long-term transcriptional repression of component proteins. In pathophysiology, the effects of TGF-β and other immune modulators during renal inflammation is of interest. One school of thought is that renal fibrosis is due to EMT with epithelia transforming to fibroblasts (118). However, this is not completely accepted (93). In contrast, incomplete EMT may be common when tight junctions are loosened but complete mesenchymal transformation does not occur. This incomplete EMT is likely to occur in renal inflammation and would lead to worsening of inflammation due to edema and increased ingress of inflammatory cells. Thus therapies that maintain epithelial polarity and tight junctions may have some efficacy in inflammatory and fibrotic disorders.
GRANTS
This work is supported by National Institute of Diabetes and Digestive and Kidney Diseases Grants DK69605, DK58208, and DK084725.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
ACKNOWLEDGMENTS
We regret that we could not reference all work in this area due to space limitations.
REFERENCES
- 1. Adachi M, Hamazaki Y, Kobayashi Y, Itoh M, Tsukita S, Furuse M. Similar and distinct properties of MUPP1 and Patj, two homologous PDZ domain-containing tight-junction proteins. Mol Cell Biol 29: 2372–2389, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Adey NB, Huang L, Ormonde PA, Baumgard ML, Pero R, Byreddy DV, Tavtigian SV, Bartel PL. Threonine phosphorylation of the MMAC1/PTEN PDZ binding domain both inhibits and stimulates PDZ binding. Cancer Res 60: 35–37, 2000 [PubMed] [Google Scholar]
- 3. Aigner K, Dampier B, Descovich L, Mikula M, Sultan A, Schreiber M, Mikulits W, Brabletz T, Strand D, Obrist P, Sommergruber W, Schweifer N, Wernitznig A, Beug H, Foisner R, Eger A. The transcription factor ZEB1 (deltaEF1) promotes tumour cell dedifferentiation by repressing master regulators of epithelial polarity. Oncogene 26: 6979–6988, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Akimoto K, Mizuno K, Osada S, Hirai S, Tanuma S, Suzuki K, Ohno S. A new member of the third class in the protein kinase C family, PKC lambda, expressed dominantly in an undifferentiated mouse embryonal carcinoma cell line and also in many tissues and cells. J Biol Chem 269: 12677–12683, 1994 [PubMed] [Google Scholar]
- 5. Aranda V, Haire T, Nolan M, Calarco J, Rosenberg A, Fawcett J, Pawson T, Muthuswamy S. Par6-aPKC uncouples ErbB2 induced disruption of polarized epithelial organization from proliferation control. Nat Cell Biol 8: 1235–1245, 2006 [DOI] [PubMed] [Google Scholar]
- 6. Atasoy D, Schoch S, Ho A, Nadasy K, Liu X, Zhang W. Deletion of CASK in mice is lethal and impairs synaptic function. Proc Natl Acad Sci USA 104: 2525–2530, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Audebert S, Navarro C, Nourry C, Chasserot-Golaz S, Lecine P, Bellaiche Y, Dupont JL, Premont RT, Sempere C, Strub JM, Van Dorsselaer A, Vitale N, Borg JP. Mammalian Scribble forms a tight complex with the betaPIX exchange factor. Curr Biol 14: 987–995, 2004 [DOI] [PubMed] [Google Scholar]
- 8. Bachmann A, Schneider M, Theilenberg E, Grawe F, Knust E. Drosophila Stardust is a partner of Crumbs in the control of epithelial cell polarity. Nature 414: 638–643, 2001 [DOI] [PubMed] [Google Scholar]
- 9. Benton R, St Johnston D. Drosophila PAR-1 and 14–3-3 inhibit Bazooka/PAR-3 to establish complementary cortical domains in polarized cells. Cell 115: 691–704, 2003 [DOI] [PubMed] [Google Scholar]
- 10. Bilder D, Birnbaum D, Borg JP, Bryant P, Huigbretse J, Jansen E, Kennedy MB, Labouesse M, Legouis R, Mechler B, Perrimon N, Petit M, Sinha P. Collective nomenclature for LAP proteins. Nat Cell Biol 2: E114, 2000 [DOI] [PubMed] [Google Scholar]
- 11. Bilder D, Li M, Perrimon N. Cooperative regulation of cell polarity and growth by Drosophila tumor suppressors. Science 289: 113–116, 2000 [DOI] [PubMed] [Google Scholar]
- 12. Bilder D, Perrimon N. Localization of apical epithelial determinants by the basolateral PDZ protein Scribble. Nature 403: 676–680, 2000 [DOI] [PubMed] [Google Scholar]
- 13. Bit-Avragim N, Hellwig N, Rudolph F, Munson C, Stainier DYS, Abdelilah-Seyfried S. Divergent polarization mechanisms during vertebrate epithelial development mediated by the Crumbs complex protein nagie oko. J Cell Sci 121: 2503–2510, 2008 [DOI] [PubMed] [Google Scholar]
- 14. Blankenship JT, Fuller MT, Zallen JA. The Drosophila homolog of the Exo84 exocyst subunit promotes apical epithelial identity. J Cell Sci 120: 3099–3110, 2007 [DOI] [PubMed] [Google Scholar]
- 15. Bohl J, Brimer N, Lyons C, Vande Pol SB. The stardust family protein MPP7 forms a tripartite complex with LIN7 and DLG1 that regulates the stability and localization of DLG1 to cell junctions. J Biol Chem 282: 9392–9400, 2007 [DOI] [PubMed] [Google Scholar]
- 16. Bulgakova NA, Kempkens O, Knust E. Multiple domains of Stardust differentially mediate localisation of the Crumbs-Stardust complex during photoreceptor development in Drosophila. J Cell Sci 121: 2018–2026, 2008 [DOI] [PubMed] [Google Scholar]
- 17. Caruana G. Genetic studies define MAGUK proteins as regulators of epithelial cell polarity. Int J Dev Biol 46: 511–518, 2002 [PubMed] [Google Scholar]
- 18. Caruana G, Bernstein A. Craniofacial dysmorphogenesis including cleft palate in mice with an insertional mutation in the discs large gene. Mol Cell Biol 21: 1475–1483, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Chaffer CL, Thompson EW, Williams ED. Mesenchymal to epithelial transition in development and disease. Cells Tissues Organs 185: 7–19, 2007 [DOI] [PubMed] [Google Scholar]
- 20. Chalmers AD, Pambos M, Mason J, Lang S, Wylie C, Papalopulu N. aPKC, Crumbs3 and Lgl2 control apicobasal polarity in early vertebrate development. Development 132: 977–986, 2005 [DOI] [PubMed] [Google Scholar]
- 21. Chen X, Macara IG. Par-3 controls tight junction assembly through the Rac exchange factor Tiam1. Nat Cell Biol 7: 262–269, 2005 [DOI] [PubMed] [Google Scholar]
- 22. Chen Z, Leibiger I, Katz A, Bertorello A. Pals-associated tight junction protein functionally links dopamine and angiotensin II to the regulation of sodium transport in renal epithelial cells. Br J Pharmacol 158: 486–493, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Delous M, Hellman NE, Gaude HM, Silbermann F, Le Bivic A, Salomon R, Antignac C, Saunier S. Nephrocystin-1 and nephrocystin-4 are required for epithelial morphogenesis and associate with PALS1/PATJ and Par6. Hum Mol Genet 18: 4711–4723, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. den Hollander AI, ten Brink JB, de Kok YJ, van Soest S, van den Born LI, van Driel MA, van de Pol DJ, Payne AM, Bhattacharya SS, Kellner U, Hoyng CB, Westerveld A, Brunner HG, Bleeker-Wagemakers EM, Deutman AF, Heckenlively JR, Cremers FP, Bergen AA. Mutations in a human homologue of Drosophila crumbs cause retinitis pigmentosa (RP12). Nat Genet 23: 217–221, 1999 [DOI] [PubMed] [Google Scholar]
- 25. Dow LE, Brumby AM, Muratore R, Coombe ML, Sedelies KA, Trapani JA, Russell SM, Richardson HE, Humbert PO. hScrib is a functional homologue of the Drosophila tumour suppressor Scribble. Oncogene 22: 9225–9230, 2003 [DOI] [PubMed] [Google Scholar]
- 26. Dow LE, Elsum IA, King CL, Kinross KM, Richardson HE, Humbert PO. Loss of human Scribble cooperates with H-Ras to promote cell invasion through deregulation of MAPK signalling. Oncogene 27: 5988–6001, 2008 [DOI] [PubMed] [Google Scholar]
- 27. Dow LE, Kauffman JS, Caddy J, Zarbalis K, Peterson AS, Jane SM, Russell SM, Humbert PO. The tumour-suppressor Scribble dictates cell polarity during directed epithelial migration: regulation of Rho GTPase recruitment to the leading edge. Oncogene 26: 2272–2282, 2007 [DOI] [PubMed] [Google Scholar]
- 28. Dressler GR. The cellular basis of kidney development. Annu Rev Cell Dev Biol 22: 509–529, 2006 [DOI] [PubMed] [Google Scholar]
- 29. Ebarasi L, He L, Hultenby K, Takemoto M, Betsholtz C, Tryggvason K, Majumdar A. A reverse genetic screen in the zebrafish identifies crb2b as a regulator of the glomerular filtration barrier. Dev Biol, 2009 [DOI] [PubMed] [Google Scholar]
- 30. Ebnet K, Suzuki A, Horikoshi Y, Hirose T, Meyer Zu Brickwedde MK, Ohno S, Vestweber D. The cell polarity protein ASIP/PAR-3 directly associates with junctional adhesion molecule (JAM). EMBO J 20: 3738–3748, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Etemad-Moghadam B, Guo S, Kemphues KJ. Asymmetrically distributed PAR-3 protein contributes to cell polarity and spindle alignment in early C. elegans embryos. Cell 83: 743–752, 1995 [DOI] [PubMed] [Google Scholar]
- 32. Fan S, Fogg V, Wang Q, Chen XW, Liu CJ, Margolis B. A novel Crumbs3 isoform regulates cell division and ciliogenesis via importin beta interactions. J Cell Biol 178: 387–398, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Feng W, Wu H, Chan LN, Zhang M. Par-3-mediated junctional localization of the lipid phosphatase PTEN is required for cell polarity establishment. J Biol Chem 283: 23440–23449, 2008 [DOI] [PubMed] [Google Scholar]
- 34. Fogg VC, Liu CJ, Margolis B. Multiple regions of Crumbs3 are required for tight junction formation in MCF10A cells. J Cell Sci 118: 2859–2869, 2005 [DOI] [PubMed] [Google Scholar]
- 35. Furuse M. Molecular basis of the core structure of tight junctions. Cold Spring Harb Perspect Biol 2: a002907–a002907, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Gao L, Joberty G, Macara IG. Assembly of epithelial tight junctions is negatively regulated by Par6. Curr Biol 12: 221–225, 2002 [DOI] [PubMed] [Google Scholar]
- 37. Gao L, Macara IG. Isoforms of the polarity protein par6 have distinct functions. J Biol Chem 279: 41557–41562, 2004 [DOI] [PubMed] [Google Scholar]
- 38. Gao L, Macara IG, Joberty G. Multiple splice variants of Par3 and of a novel related gene, Par3L, produce proteins with different binding properties. Gene 294: 99–107, 2002 [DOI] [PubMed] [Google Scholar]
- 39. Gardiol D, Kuhne C, Glaunsinger B, Lee SS, Javier R, Banks L. Oncogenic human papillomavirus E6 proteins target the discs large tumour suppressor for proteasome-mediated degradation. Oncogene 18: 5487–5496, 1999 [DOI] [PubMed] [Google Scholar]
- 40. Garrard SM, Capaldo CT, Gao L, Rosen MK, Macara IG, Tomchick DR. Structure of Cdc42 in a complex with the GTPase-binding domain of the cell polarity protein, Par6. EMBO J 22: 1125–1133, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Georgiou M, Marinari E, Burden J, Baum B. Cdc42, Par6, and aPKC regulate Arp2/3-mediated endocytosis to control local adherens junction stability. Curr Biol 18: 1631–1638, 2008 [DOI] [PubMed] [Google Scholar]
- 42. Goldstein B, Macara I. The PAR proteins: fundamental players in animal cell polarization. Dev Cell 13: 609–622, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Gosens I, Sessa A, den Hollander AI, Letteboer SJ, Belloni V, Arends ML, Le Bivic A, Cremers FP, Broccoli V, Roepman R. FERM protein EPB41L5 is a novel member of the mammalian CRB-MPP5 polarity complex. Exp Cell Res 313: 3959–3970, 2007 [DOI] [PubMed] [Google Scholar]
- 44. Grawe F, Wodarz A, Lee B, Knust E, Skaer H. The Drosophila genes crumbs and stardust are involved in the biogenesis of adherens junctions. Development 122: 951–959, 1996 [DOI] [PubMed] [Google Scholar]
- 45. Grifoni D, Garoia F, Bellosta P, Parisi F, De Biase D, Collina G, Strand D, Cavicchi S, Pession A. aPKCzeta cortical loading is associated with Lgl cytoplasmic release and tumor growth in Drosophila and human epithelia. Oncogene 26: 5960–5965, 2007 [DOI] [PubMed] [Google Scholar]
- 46. Grzeschik N, Parsons L, Allott M, Harvey K, Richardson H. Lgl, aPKC, and Crumbs regulate the Salvador/Warts/Hippo pathway through two distinct mechanisms. Curr Biol 20: 573–581, 2010 [DOI] [PubMed] [Google Scholar]
- 47. Hamazaki Y, Itoh M, Sasaki H, Furuse M, Tsukita S. Multi-PDZ domain protein 1 (MUPP1) is concentrated at tight junctions through its possible interaction with claudin-1 and junctional adhesion molecule. J Biol Chem 277: 455–461, 2002 [DOI] [PubMed] [Google Scholar]
- 48. Hartleben B, Schweizer H, Lbben P, Bartram M, Mller C, Herr R, Wei C, Neumann-Haefelin E, Schermer B, Zentgraf H, Kerjaschki D, Reiser J, Walz G, Benzing T, Huber T. Neph-Nephrin proteins bind the Par3-Par6-atypical protein kinase C (aPKC) complex to regulate podocyte cell polarity. J Biol Chem 283: 23033–23038, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Hirose T, Izumi Y, Nagashima Y, Tamai-Nagai Y, Kurihara H, Sakai T, Suzuki Y, Yamanaka T, Suzuki A, Mizuno K, Ohno S. Involvement of ASIP/PAR-3 in the promotion of epithelial tight junction formation. J Cell Sci 115: 2485–2495, 2002 [DOI] [PubMed] [Google Scholar]
- 50. Hirose T, Satoh D, Kurihara H, Kusaka C, Hirose H, Akimoto K, Matsusaka T, Ichikawa I, Noda T, Ohno S. An essential role of the universal polarity protein, aPKClambda, on the maintenance of podocyte slit diaphragms. PloS one 4: e4194–e4194, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Hong Y, Stronach B, Perrimon N, Jan LY, Jan YN. Drosophila Stardust interacts with Crumbs to control polarity of epithelia but not neuroblasts. Nature 414: 634–638, 2001 [DOI] [PubMed] [Google Scholar]
- 52. Horikoshi Y, Suzuki A, Yamanaka T, Sasaki K, Mizuno K, Sawada H, Yonemura S, Ohno S. Interaction between PAR-3 and the aPKC-PAR-6 complex is indispensable for apical domain development of epithelial cells. J Cell Sci 122: 1595–1606, 2009 [DOI] [PubMed] [Google Scholar]
- 53. Hsueh Y, Wang T, Yang F, Sheng M. Nuclear translocation and transcription regulation by the membrane-associated guanylate kinase CASK/LIN-2. Nature 404: 298–302, 2000 [DOI] [PubMed] [Google Scholar]
- 54. Huber T, Hartleben B, Winkelmann K, Schneider L, Becker J, Leitges M, Walz G, Haller H, Schiffer M. Loss of podocyte aPKClambda/iota causes polarity defects and nephrotic syndrome. J Am Soc Nephrol 20: 798–806, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Hung TJ, Kemphues KJ. PAR-6 is a conserved PDZ domain-containing protein that colocalizes with PAR-3 in Caenorhabditis elegans embryos. Development 126: 127–135, 1999 [DOI] [PubMed] [Google Scholar]
- 56. Hurd TW, Fan S, Liu CJ, Kweon HK, Hakansson K, Margolis B. Phosphorylation-dependent binding of 14–3-3 to the polarity protein Par3 regulates cell polarity in mammalian epithelia. Curr Biol 13: 2082–2090, 2003 [DOI] [PubMed] [Google Scholar]
- 57. Hurd TW, Gao L, Roh MH, Macara IG, Margolis B. Direct interaction of two polarity complexes implicated in epithelial tight junction assembly. Nat Cell Biol 5: 137–142, 2003 [DOI] [PubMed] [Google Scholar]
- 58. Hurov J, Watkins J, Piwnica-Worms H. Atypical PKC phosphorylates PAR-1 kinases to regulate localization and activity. Curr Biol 14: 736–741, 2004 [DOI] [PubMed] [Google Scholar]
- 59. Iden S, Collard J. Crosstalk between small GTPases and polarity proteins in cell polarization. Nat Rev Mol Cell Biol 9: 846–859, 2008 [DOI] [PubMed] [Google Scholar]
- 60. Iizuka-Kogo A, Ishidao T, Akiyama T, Senda T. Abnormal development of urogenital organs in Dlgh1-deficient mice. Development 134: 1799–1807, 2007 [DOI] [PubMed] [Google Scholar]
- 61. Ikenouchi J, Matsuda M, Furuse M, Tsukita S. Regulation of tight junctions during the epithelium-mesenchyme transition: direct repression of the gene expression of claudins/occludin by Snail. J Cell Sci 116: 1959–1967, 2003 [DOI] [PubMed] [Google Scholar]
- 62. Imai F, Hirai Si Akimoto K, Koyama H, Miyata T, Ogawa M, Noguchi S, Sasaoka T, Noda T, Ohno S. Inactivation of aPKClambda results in the loss of adherens junctions in neuroepithelial cells without affecting neurogenesis in mouse neocortex. Development 133: 1735–1744, 2006 [DOI] [PubMed] [Google Scholar]
- 63. Ishidate T, Matsumine A, Toyoshima K, Akiyama T. The APC-hDLG complex negatively regulates cell cycle progression from the G0/G1 to S phase. Oncogene 19: 365–372, 2000 [DOI] [PubMed] [Google Scholar]
- 64. Itoh M, Sasaki H, Furuse M, Ozaki H, Kita T, Tsukita S. Junctional adhesion molecule (JAM) binds to PAR-3: a possible mechanism for the recruitment of PAR-3 to tight junctions. J Cell Biol 154: 491–497, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Ivanov AI, Young C, Den Beste K, Capaldo CT, Humbert PO, Brennwald P, Parkos CA, Nusrat A. Tumor suppressor scribble regulates assembly of tight junctions in the intestinal epithelium. Am J Pathol 176: 134–145, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Izumi Y, Hirose T, Tamai Y, Hirai S, Nagashima Y, Fujimoto T, Tabuse Y, Kemphues KJ, Ohno S. An atypical PKC directly associates and colocalizes at the epithelial tight junction with ASIP, a mammalian homologue of Caenorhabditis elegans polarity protein PAR-3. J Cell Biol 143: 95–106, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Jaffe A, Kaji N, Durgan J, Hall A. Cdc42 controls spindle orientation to position the apical surface during epithelial morphogenesis. J Cell Biol 183: 625–633, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Jensen AM, Westerfield M. Zebrafish mosaic eyes is a novel FERM protein required for retinal lamination and retinal pigmented epithelial tight junction formation. Curr Biol 14: 711–717, 2004 [DOI] [PubMed] [Google Scholar]
- 69. Joberty G, Petersen C, Gao L, Macara IG. The cell-polarity protein Par6 links Par3 and atypical protein kinase C to Cdc42. Nat Cell Biol 2: 531–539, 2000 [DOI] [PubMed] [Google Scholar]
- 70. Johansson A, Driessens M, Aspenstrom P. The mammalian homologue of the Caenorhabditis elegans polarity protein PAR-6 is a binding partner for the Rho GTPases Cdc42 and Rac1. J Cell Sci 113: 3267–3275, 2000 [DOI] [PubMed] [Google Scholar]
- 71. Kallay LM, McNickle A, Brennwald PJ, Hubbard AL, Braiterman LT. Scribble associates with two polarity proteins, Lgl2 and Vangl2, via distinct molecular domains. J Cell Biochem 99: 647–664, 2006 [DOI] [PubMed] [Google Scholar]
- 72. Kamberov E, Makarova O, Roh M, Liu A, Karnak D, Straight S, Margolis B. Molecular cloning and characterization of Pals, proteins associated with mLin-7. J Biol Chem 275: 11425–11431, 2000 [DOI] [PubMed] [Google Scholar]
- 73. Karnak D, Lee S, Margolis B. Identification of multiple binding partners for the amino-terminal domain of synapse-associated protein 97. J Biol Chem 277: 46730–46735, 2002 [DOI] [PubMed] [Google Scholar]
- 74. Karp C, Tan T, Mathew R, Nelson D, Mukherjee C, Degenhardt K, Karantza-Wadsworth V, White E. Role of the polarity determinant crumbs in suppressing mammalian epithelial tumor progression. Cancer Res 68: 4105–4115, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Kemphues KJ, Priess JR, Morton DG, Cheng NS. Identification of genes required for cytoplasmic localization in early C. elegans embryos. Cell 52: 311–320, 1988 [DOI] [PubMed] [Google Scholar]
- 76. Kim S, Lehtinen M, Sessa A, Zappaterra M, Cho SH, Gonzalez D, Boggan B, Austin C, Wijnholds J, Gambello M, Malicki J, LaMantia A, Broccoli V, Walsh C. The apical complex couples cell fate and cell survival to cerebral cortical development. Neuron 66: 69–84, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Klezovitch O, Fernandez T, Tapscott S, Vasioukhin V. Loss of cell polarity causes severe brain dysplasia in Lgl1 knockout mice. Genes Dev 18: 559–571, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Kohjima M, Noda Y, Takeya R, Saito N, Takeuchi K, Sumimoto H. PAR3beta, a novel homologue of the cell polarity protein PAR3, localizes to tight junctions. Biochem Biophys Res Commun 299: 641–646, 2002 [DOI] [PubMed] [Google Scholar]
- 79. Korinek V, Barker N, Morin PJ, van Wichen D, de Weger R, Kinzler KW, Vogelstein B, Clevers H. Constitutive transcriptional activation by a beta-catenin-Tcf complex in APC-/- colon carcinoma. Science 275: 1784–1787, 1997 [DOI] [PubMed] [Google Scholar]
- 80. Krahn M, Bckers J, Kastrup L, Wodarz A. Formation of a Bazooka-Stardust complex is essential for plasma membrane polarity in epithelia. J Cell Biol 190: 751–760, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Laprise P, Beronja S, Silva-Gagliardi NF, Pellikka M, Jensen AM, McGlade CJ, Tepass U. The FERM protein Yurt is a negative regulatory component of the Crumbs complex that controls epithelial polarity and apical membrane size. Dev Cell 11: 363–374, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Laprise P, Lau KM, Harris KP, Silva-Gagliardi NF, Paul SM, Beronja S, Beitel GJ, McGlade CJ, Tepass U. Yurt, Coracle, Neurexin IV and the Na+,K+-ATPase form a novel group of epithelial polarity proteins. Nature 459: 1141–1145, 2009 [DOI] [PubMed] [Google Scholar]
- 83. Laprise P, Viel A, Rivard N. Human homolog of disc-large is required for adherens junction assembly and differentiation of human intestinal epithelial cells. J Biol Chem 279: 10157–10166, 2004 [DOI] [PubMed] [Google Scholar]
- 84. Lee S, Fan S, Makarova O, Straight S, Margolis B. A novel and conserved protein-protein interaction domain of mammalian Lin-2/CASK binds and recruits SAP97 to the lateral surface of epithelia. Mol Cell Biol 22: 1778–1791, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Leibfried A, Fricke R, Morgan M, Bogdan S, Bellaiche Y. Drosophila Cip4 and WASp define a branch of the Cdc42-Par6-aPKC pathway regulating E-cadherin endocytosis. Curr Biol 18: 1639–1648, 2008 [DOI] [PubMed] [Google Scholar]
- 86. Leitges M, Sanz L, Martin P, Duran A, Braun U, Garcia J. Targeted disruption of the zeta PKC gene results in the impairment of the NF-kappa B pathway. Mol Cell 8: 771–780, 2001 [DOI] [PubMed] [Google Scholar]
- 87. Lemmers C, Michel D, Lane-Guermonprez L, Delgrossi MH, Medina E, Arsanto JP, Le Bivic A. CRB3 binds directly to Par6 and regulates the morphogenesis of the tight junctions in mammalian epithelial cells. Mol Biol Cell 15: 1324–1333, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Li Y, Karnak D, Demeler B, Margolis B, Lavie A. Structural basis for L27 domain-mediated assembly of signaling and cell polarity complexes. EMBO J 23: 2723–2733, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Liew CW, Vockel M, Glassmeier G, Brandner JM, Fernandez-Ballester GJ, Schwarz JR, Schulz S, Buck F, Serrano L, Richter D, Kreienkamp HJ. Interaction of the human somatostatin receptor 3 with the multiple PDZ domain protein MUPP1 enables somatostatin to control permeability of epithelial tight junctions. FEBS Lett 583: 49–54, 2009 [DOI] [PubMed] [Google Scholar]
- 90. Lin D, Edwards AS, Fawcett JP, Mbamalu G, Scott JD, Pawson T. A mammalian PAR-3-PAR-6 complex implicated in Cdc42/Rac1 and aPKC signalling and cell polarity. Nat Cell Biol 2: 540–547, 2000 [DOI] [PubMed] [Google Scholar]
- 91. Ling C, Zheng Y, Yin F, Yu J, Huang J, Hong Y, Wu S, Pan D. The apical transmembrane protein Crumbs functions as a tumor suppressor that regulates Hippo signaling by binding to Expanded. Proc Natl Acad Sci USA 107: 10532–10537, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Lisovsky M, Dresser K, Baker S, Fisher A, Woda B, Banner B, Lauwers GY. Cell polarity protein Lgl2 is lost or aberrantly localized in gastric dysplasia and adenocarcinoma: an immunohistochemical study. Mod Pathol 22: 977–984, 2009 [DOI] [PubMed] [Google Scholar]
- 93. Liu Y. New insights into epithelial-mesenchymal transition in kidney fibrosis. J Am Soc Nephrol 21: 212–222, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Lozovatsky L, Abayasekara N, Piawah S, Walther Z. CASK deletion in intestinal epithelia causes mislocalization of LIN7C and the DLG1/Scrib polarity complex without affecting cell polarity. Mol Biol Cell 20: 4489–4499, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Lu H, Bilder D. Endocytic control of epithelial polarity and proliferation in Drosophila. Nat Cell Biol 7: 1232–1239, 2005 [DOI] [PubMed] [Google Scholar]
- 96. Lue RA, Marfatia SM, Branton D, Chishti AH. Cloning and characterization of hdlg: the human homologue of the Drosophila discs large tumor suppressor binds to protein 4.1. Proc Natl Acad Sci USA 91: 9818–9822, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Lynch AM, Hardin J. The assembly and maintenance of epithelial junctions in C. elegans. Front Biosci 14: 1414–1432, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Makarova O, Roh MH, Liu CJ, Laurinec S, Margolis B. Mammalian Crumbs3 is a small transmembrane protein linked to protein associated with Lin-7 (Pals1). Gene 302: 21–29, 2003 [DOI] [PubMed] [Google Scholar]
- 99. Mantovani F, Banks L. Regulation of the discs large tumor suppressor by a phosphorylation-dependent interaction with the beta-TrCP ubiquitin ligase receptor. J Biol Chem 278: 42477–42486, 2003 [DOI] [PubMed] [Google Scholar]
- 100. Mantovani F, Massimi P, Banks L. Proteasome-mediated regulation of the hDlg tumour suppressor protein. J Cell Sci 114: 4285–4292, 2001 [DOI] [PubMed] [Google Scholar]
- 101. Massey-Harroche D, Delgrossi MH, Lane-Guermonprez L, Arsanto JP, Borg JP, Billaud M, Le Bivic A. Evidence for a molecular link between the tuberous sclerosis complex and the Crumbs complex. Hum Mol Genet 16: 529–536, 2007 [DOI] [PubMed] [Google Scholar]
- 102. Matsumine A, Ogai A, Senda T, Okumura N, Satoh K, Baeg GH, Kawahara T, Kobayashi S, Okada M, Toyoshima K, Akiyama T. Binding of APC to the human homolog of the Drosophila discs large tumor suppressor protein. Science 272: 1020–1023, 1996 [DOI] [PubMed] [Google Scholar]
- 103. McGee AW, Dakoji SR, Olsen O, Bredt DS, Lim WA, Prehoda KE. Structure of the SH3-guanylate kinase module from PSD-95 suggests a mechanism for regulated assembly of MAGUK scaffolding proteins. Mol Cell 8: 1291–1301, 2001 [DOI] [PubMed] [Google Scholar]
- 104. McLaughlin M, Hale R, Ellston D, Gaudet S, Lue RA, Viel A. The distribution and function of alternatively spliced insertions in hDlg. J Biol Chem 277: 6406–6412, 2002 [DOI] [PubMed] [Google Scholar]
- 105. McNeill H. Planar cell polarity and the kidney. J Am Soc Nephrol 20: 2104–2111, 2009 [DOI] [PubMed] [Google Scholar]
- 106. Metais JY, Navarro C, Santoni MJ, Audebert S, Borg JP. hScrib interacts with ZO-2 at the cell-cell junctions of epithelial cells. FEBS Lett 579: 3725–3730, 2005 [DOI] [PubMed] [Google Scholar]
- 107. Mizuno K, Suzuki A, Hirose T, Kitamura K, Kutsuzawa K, Futaki M, Amano Y, Ohno S. Self-association of PAR-3-mediated by the conserved N-terminal domain contributes to the development of epithelial tight junctions. J Biol Chem 278: 31240–31250, 2003 [DOI] [PubMed] [Google Scholar]
- 108. Montcouquiol M, Sans N, Huss D, Kach J, Dickman JD, Forge A, Rachel RA, Copeland NG, Jenkins NA, Bogani D, Murdoch J, Warchol ME, Wenthold RJ, Kelley MW. Asymmetric localization of Vangl2 and Fz3 indicate novel mechanisms for planar cell polarity in mammals. J Neurosci 26: 5265–5275, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Morais-de-Sa E, Mirouse V, St. Johnston D. aPKC phosphorylation of Bazooka defines the apical/lateral border in Drosophila epithelial cells. Cell 141: 509–523, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Musch A, Cohen D, Yeaman C, Nelson WJ, Rodriguez-Boulan E, Brennwald PJ. Mammalian homolog of Drosophila tumor suppressor lethal (2) giant larvae interacts with basolateral exocytic machinery in Madin-Darby canine kidney cells. Mol Biol Cell 13: 158–168, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Nagai-Tamai Y, Mizuno K, Hirose T, Suzuki A, Ohno S. Regulated protein-protein interaction between aPKC and PAR-3 plays an essential role in the polarization of epithelial cells. Genes Cells 7: 1161–1171, 2002 [DOI] [PubMed] [Google Scholar]
- 112. Naim E, Bernstein A, Bertram JF, Caruana G. Mutagenesis of the epithelial polarity gene, discs large 1, perturbs nephrogenesis in the developing mouse kidney. Kidney Int 68: 955–965, 2005 [DOI] [PubMed] [Google Scholar]
- 113. Najm J, Horn D, Wimplinger I, Golden J, Chizhikov V, Sudi J, Christian S, Ullmann R, Kuechler A, Haas C, Flubacher A, Charnas L, Uyanik G, Frank U, Klopocki E, Dobyns W, Kutsche K. Mutations of CASK cause an X-linked brain malformation phenotype with microcephaly and hypoplasia of the brainstem and cerebellum. Nat Genet 40: 1065–1067, 2008 [DOI] [PubMed] [Google Scholar]
- 114. Nakagawa S, Huibregtse JM. Human scribble (Vartul) is targeted for ubiquitin-mediated degradation by the high-risk papillomavirus E6 proteins and the E6AP ubiquitin-protein ligase. Mol Cell Biol 20: 8244–8253, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Nakayama M, Goto TM, Sugimoto M, Nishimura T, Shinagawa T, Ohno S, Amano M, Kaibuchi K. Rho-kinase phosphorylates PAR-3 and disrupts PAR complex formation. Dev Cell 14: 205–215, 2008 [DOI] [PubMed] [Google Scholar]
- 116. Narayan N, Massimi P, Banks L. CDK phosphorylation of the discs large tumour suppressor controls its localisation and stability. J Cell Sci 122: 65–74, 2009 [DOI] [PubMed] [Google Scholar]
- 117. Navarro C, Nola S, Audebert S, Santoni MJ, Arsanto JP, Ginestier C, Marchetto S, Jacquemier J, Isnardon D, Le Bivic A, Birnbaum D, Borg JP. Junctional recruitment of mammalian Scribble relies on E-cadherin engagement. Oncogene 24: 4330–4339, 2005 [DOI] [PubMed] [Google Scholar]
- 118. Neilson EG. Mechanisms of disease: fibroblasts—a new look at an old problem. Nat Clin Pract Nephrol 2: 101–108, 2006 [DOI] [PubMed] [Google Scholar]
- 119. Nelson WJ. Adaptation of core mechanisms to generate cell polarity. Nature 422: 766–774, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Nix SL, Chishti AH, Anderson JM, Walther Z. hCASK and hDlg associate in epithelia, and their src homology 3 and guanylate kinase domains participate in both intramolecular and intermolecular interactions. J Biol Chem 275: 41192–41200, 2000 [DOI] [PubMed] [Google Scholar]
- 121. Nola S, Sebbagh M, Marchetto S, Osmani N, Nourry C, Audebert S, Navarro C, Rachel R, Montcouquiol M, Sans N, Etienne-Manneville S, Borg JP, Santoni MJ. Scrib regulates PAK activity during the cell migration process. Hum Mol Genet 17: 3552–3565, 2008 [DOI] [PubMed] [Google Scholar]
- 122. Nolan ME, Aranda V, Lee S, Lakshmi B, Basu S, Allred DC, Muthuswamy SK. The polarity protein Par6 induces cell proliferation and is overexpressed in breast cancer. Cancer Res 68: 8201–8209, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. O'Brien L, Zegers MMP, Mostov K. Opinion. Building epithelial architecture: insights from three-dimensional culture models. Nat Rev Mol Cell Biol 3: 531–537, 2002 [DOI] [PubMed] [Google Scholar]
- 124. Olsen O, Funke L, Long JF, Fukata M, Kazuta T, Trinidad JC, Moore KA, Misawa H, Welling PA, Burlingame AL, Zhang M, Bredt DS. Renal defects associated with improper polarization of the CRB and DLG polarity complexes in MALS-3 knockout mice. J Cell Biol 179: 151–164, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Ozdamar B, Bose R, Barrios-Rodiles M, Wang HR, Zhang Y, Wrana J. Regulation of the polarity protein Par6 by TGFbeta receptors controls epithelial cell plasticity. Science 307: 1603–1609, 2005 [DOI] [PubMed] [Google Scholar]
- 126. Peinado H, Olmeda D, Cano A. Snail, Zeb and bHLH factors in tumour progression: an alliance against the epithelial phenotype? Nat Rev Cancer 7: 415–428, 2007 [DOI] [PubMed] [Google Scholar]
- 127. Penkert RR, DiVittorio HM, Prehoda KE. Internal recognition through PDZ domain plasticity in the Par-6-Pals1 complex. Nat Struct Mol Biol 11: 1122–1127, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Plant PJ, Fawcett JP, Lin DC, Holdorf AD, Binns K, Kulkarni S, Pawson T. A polarity complex of mPar-6 and atypical PKC binds, phosphorylates and regulates mammalian Lgl. Nat Cell Biol 5: 301–308, 2003 [DOI] [PubMed] [Google Scholar]
- 129. Qin Y, Capaldo C, Gumbiner BM, Macara IG. The mammalian Scribble polarity protein regulates epithelial cell adhesion and migration through E-cadherin. J Cell Biol 171: 1061–1071, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Robinson B, Huang J, Hong Y, Moberg K. Crumbs regulates Salvador/Warts/Hippo signaling in Drosophila via the FERM-domain protein Expanded. Curr Biol 20: 582–590, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Roh MH, Fan S, Liu CJ, Margolis B. The Crumbs3-Pals1 complex participates in the establishment of polarity in mammalian epithelial cells. J Cell Sci 116: 2895–2906, 2003 [DOI] [PubMed] [Google Scholar]
- 132. Roh MH, Liu CJ, Laurinec S, Margolis B. The carboxyl terminus of zona occludens-3 binds and recruits a mammalian homologue of discs lost to tight junctions. J Biol Chem 277: 27501–27509, 2002 [DOI] [PubMed] [Google Scholar]
- 133. Roh MH, Makarova O, Liu CJ, Shin K, Lee S, Laurinec S, Goyal M, Wiggins R, Margolis B. The Maguk protein, Pals1, functions as an adapter, linking mammalian homologues of Crumbs and Discs Lost. J Cell Biol 157: 161–172, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Rohr S, Bit-Avragim N, Abdelilah-Seyfried S. Heart and soul/PRKCi and Nagie Oko/Mpp5 regulate myocardial coherence and remodeling during cardiac morphogenesis. Development 133: 107–115, 2006 [DOI] [PubMed] [Google Scholar]
- 135. Schluter MA, Margolis B. Apical lumen formation in renal epithelia. J Am Soc Nephrol 20: 1444–1452, 2009 [DOI] [PubMed] [Google Scholar]
- 136. Schluter MA, Pfarr CS, Pieczynski J, Whiteman EL, Hurd TW, Fan S, Liu CJ, Margolis B. Trafficking of Crumbs3 during cytokinesis is crucial for lumen formation. Mol Biol Cell 20: 4652–4663, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Schmidt-Ott K, Yang J, Chen X, Wang H, Paragas N, Mori K, Li JY, Lu B, Costantini F, Schiffer M, Bottinger E, Barasch J. Novel regulators of kidney development from the tips of the ureteric bud. J Am Soc Nephrol 16: 1993–2002, 2005 [DOI] [PubMed] [Google Scholar]
- 138. Shen L, Turner J. Intercellular junctions: actin the PARt. Curr Biol 18: R1014–R1017, 2008 [DOI] [PubMed] [Google Scholar]
- 139. Shin K, Fogg V, Margolis B. Tight junctions and cell polarity. Annu Rev Cell Dev Biol 22: 207–235, 2006 [DOI] [PubMed] [Google Scholar]
- 140. Shivas J, Morrison H, Bilder D, Skop A. Polarity and endocytosis: reciprocal regulation. Trends Cell Biol 20: 445–452, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Soloff RS. Targeted deletion of protein kinase C λ reveals a distribution of functions between the two atypical protein kinase C isoforms. J Immunol 173: 3250–3260, 2004 [DOI] [PubMed] [Google Scholar]
- 142. Spaderna S, Schmalhofer O, Wahlbuhl M, Dimmler A, Bauer K, Sultan A, Hlubek F, Jung A, Strand D, Eger A, Kirchner T, Behrens J, Brabletz T. The transcriptional repressor ZEB1 promotes metastasis and loss of cell polarity in cancer. Cancer Res 68: 537–544, 2008 [DOI] [PubMed] [Google Scholar]
- 143. Steed E, Balda M, Matter K. Dynamics and functions of tight junctions. Trends Cell Biol 20: 142–149, 2010 [DOI] [PubMed] [Google Scholar]
- 144. Straight SW, Pieczynski JN, Whiteman EL, Liu CJ, Margolis B. Mammalian lin-7 stabilizes polarity protein complexes. J Biol Chem 281: 37738–37747, 2006 [DOI] [PubMed] [Google Scholar]
- 145. Straight SW, Shin K, Fogg VC, Fan S, Liu CJ, Roh M, Margolis B. Loss of PALS1 expression leads to tight junction and polarity defects. Mol Biol Cell 15: 1981–1990, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Stucke VM, Timmerman E, Vandekerckhove J, Gevaert K, Hall A. The MAGUK protein MPP7 binds to the polarity protein hDlg1 and facilitates epithelial tight junction formation. Mol Biol Cell 18: 1744–1755, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Sugihara-Mizuno Y, Adachi M, Kobayashi Y, Hamazaki Y, Nishimura M, Imai T, Furuse M, Tsukita S. Molecular characterization of angiomotin/JEAP family proteins: interaction with MUPP1/Patj and their endogenous properties. Genes Cells 12: 473–486, 2007 [DOI] [PubMed] [Google Scholar]
- 148. Sugimoto M, Inoko A, Shiromizu T, Nakayama M, Zou P, Yonemura S, Hayashi Y, Izawa I, Sasoh M, Uji Y, Kaibuchi K, Kiyono T, Inagaki M. The keratin-binding protein Albatross regulates polarization of epithelial cells. J Cell Biol 183: 19–28, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Suzuki A, Hirata M, Kamimura K, Maniwa R, Yamanaka T, Mizuno K, Kishikawa M, Hirose H, Amano Y, Izumi N, Miwa Y, Ohno S. aPKC acts upstream of PAR-1b in both the establishment and maintenance of mammalian epithelial polarity. Curr Biol 14: 1425–1435, 2004 [DOI] [PubMed] [Google Scholar]
- 150. Suzuki A, Ishiyama C, Hashiba K, Shimizu M, Ebnet K, Ohno S. aPKC kinase activity is required for the asymmetric differentiation of the premature junctional complex during epithelial cell polarization. J Cell Sci 115: 3565–3573, 2002 [DOI] [PubMed] [Google Scholar]
- 151. Suzuki A, Yamanaka T, Hirose T, Manabe N, Mizuno K, Shimizu M, Akimoto K, Izumi Y, Ohnishi T, Ohno S. Atypical protein kinase C is involved in the evolutionarily conserved par protein complex and plays a critical role in establishing epithelia-specific junctional structures. J Cell Biol 152: 1183–1196, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Takai Y, Miyoshi J, Ikeda W, Ogita H. Nectins and nectin-like molecules: roles in contact inhibition of cell movement and proliferation. Nat Rev Mol Cell Biol 9: 603–615, 2008 [DOI] [PubMed] [Google Scholar]
- 153. Takizawa S, Nagasaka K, Nakagawa S, Yano T, Nakagawa K, Yasugi T, Takeuchi T, Kanda T, Huibregtse JM, Akiyama T, Taketani Y. Human scribble, a novel tumor suppressor identified as a target of high-risk HPV E6 for ubiquitin-mediated degradation, interacts with adenomatous polyposis coli. Genes Cells 11: 453–464, 2006 [DOI] [PubMed] [Google Scholar]
- 154. Tavares GA, Panepucci EH, Brunger AT. Structural characterization of the intramolecular interaction between the SH3 and guanylate kinase domains of PSD-95. Mol Cell 8: 1313–1325, 2001 [DOI] [PubMed] [Google Scholar]
- 155. Tepass U, Knust E. Crumbs and stardust act in a genetic pathway that controls the organization of epithelia in Drosophila melanogaster. Dev Biol 159: 311–326, 1993 [DOI] [PubMed] [Google Scholar]
- 156. Tepass U, Theres C, Knust E. Crumbs encodes an EGF-like protein expressed on apical membranes of Drosophila epithelial cells and required for organization of epithelia. Cell 61: 787–799, 1990 [DOI] [PubMed] [Google Scholar]
- 157. Thiery J, Acloque H, Huang RYJ, Nieto MA. Epithelial-mesenchymal transitions in development and disease. Cell 139: 871–890, 2009 [DOI] [PubMed] [Google Scholar]
- 158. Tomaic V, Gardiol D, Massimi P, Ozbun M, Myers M, Banks L. Human and primate tumour viruses use PDZ binding as an evolutionarily conserved mechanism of targeting cell polarity regulators. Oncogene 28: 1–8, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159. Torkko JM, Manninen A, Schuck S, Simons K. Depletion of apical transport proteins perturbs epithelial cyst formation and ciliogenesis. J Cell Sci 121: 1193–1203, 2008 [DOI] [PubMed] [Google Scholar]
- 160. Traweger A, Wiggin G, Taylor L, Tate SA, Metalnikov P, Pawson T. Protein phosphatase 1 regulates the phosphorylation state of the polarity scaffold Par-3. Proc Natl Acad Sci USA 105: 10402–10407, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161. Vaccari T, Rabouille C, Ephrussi A. The Drosophila PAR-1 spacer domain is required for lateral membrane association and for polarization of follicular epithelial cells. Curr Biol 15: 255–261, 2005 [DOI] [PubMed] [Google Scholar]
- 162. Valiente M, Andres-Pons A, Gomar B, Torres J, Gil A, Tapparel C, Antonarakis SE, Pulido R. Binding of PTEN to specific PDZ domains contributes to PTEN protein stability and phosphorylation by microtubule-associated serine/threonine kinases. J Biol Chem 280: 28936–28943, 2005 [DOI] [PubMed] [Google Scholar]
- 163. Vandenberg AL, Sassoon DA. Non-canonical Wnt signaling regulates cell polarity in female reproductive tract development via Van Gogh-like 2. Development 136: 1559–1570, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164. van de Pavert SA, Kantardzhieva A, Malysheva A, Meuleman J, Versteeg I, Levelt C, Klooster J, Geiger S, Seeliger MW, Rashbass P, Le Bivic A, Wijnholds J. Crumbs homologue 1 is required for maintenance of photoreceptor cell polarization and adhesion during light exposure. J Cell Sci 117: 4169–4177, 2004 [DOI] [PubMed] [Google Scholar]
- 165. Walther RF, Pichaud F. Crumbs/DaPKC-dependent apical exclusion of Bazooka promotes photoreceptor polarity remodeling. Curr Biol 20: 1065–1074, 2010 [DOI] [PubMed] [Google Scholar]
- 166. Wang Q, Chen XW, Margolis B. PALS1 regulates E-cadherin trafficking in mammalian epithelial cells. Mol Biol Cell 18: 874–885, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167. Wang Q, Hurd TW, Margolis B. Tight junction protein Par6 interacts with an evolutionarily conserved region in the amino terminus of PALS1/stardust. J Biol Chem 279: 30715–30721, 2004 [DOI] [PubMed] [Google Scholar]
- 168. Wang Y, Du D, Fang L, Yang G, Zhang C, Zeng R, Ullrich A, Lottspeich F, Chen Z. Tyrosine phosphorylated Par3 regulates epithelial tight junction assembly promoted by EGFR signaling. EMBO J 25: 5058–5070, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169. Wei X, Malicki J. Nagie Oko, encoding a MAGUK-family protein, is essential for cellular patterning of the retina. Nat Genet 31: 150–157, 2002 [DOI] [PubMed] [Google Scholar]
- 170. Wells CD, Fawcett JP, Traweger A, Yamanaka Y, Goudreault M, Elder K, Kulkarni S, Gish G, Virag C, Lim C, Colwill K, Starostine A, Metalnikov P, Pawson T. A Rich1/Amot complex regulates the Cdc42 GTPase and apical-polarity proteins in epithelial cells. Cell 125: 535–548, 2006 [DOI] [PubMed] [Google Scholar]
- 171. Whiteman EL, Liu CJ, Fearon ER, Margolis B. The transcription factor snail represses Crumbs3 expression and disrupts apico-basal polarity complexes. Oncogene 27: 3875–3879, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172. Wu H, Feng W, Chen J, Chan LN, Huang S, Zhang M. PDZ domains of Par-3 as potential phosphoinositide signaling integrators. Mol Cell 28: 886–898, 2007 [DOI] [PubMed] [Google Scholar]
- 173. Wu M, Pastor-Pareja JC, Xu T. Interaction between Ras(V12) and scribbled clones induces tumour growth and invasion. Nature 463: 545–548, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174. Yamanaka T, Horikoshi Y, Izumi N, Suzuki A, Mizuno K, Ohno S. Lgl mediates apical domain disassembly by suppressing the PAR-3-aPKC-PAR-6 complex to orient apical membrane polarity. J Cell Sci 119: 2107–2118, 2006 [DOI] [PubMed] [Google Scholar]
- 175. Yamanaka T, Horikoshi Y, Sugiyama Y, Ishiyama C, Suzuki A, Hirose T, Iwamatsu A, Shinohara A, Ohno S. Mammalian Lgl forms a protein complex with PAR-6 and aPKC independently of PAR-3 to regulate epithelial cell polarity. Curr Biol 13: 734–743, 2003 [DOI] [PubMed] [Google Scholar]
- 176. Yamanaka T, Horikoshi Y, Suzuki A, Sugiyama Y, Kitamura K, Maniwa R, Nagai Y, Yamashita A, Hirose T, Ishikawa H, Ohno S. PAR-6 regulates aPKC activity in a novel way and mediates cell-cell contact-induced formation of the epithelial junctional complex. Genes Cells 6: 721–731, 2001 [DOI] [PubMed] [Google Scholar]
- 177. Zhang Y, Guo H, Kwan H, Wang JW, Kosek J, Lu B. PAR-1 kinase phosphorylates dlg and regulates its postsynaptic targeting at the Drosophila neuromuscular junction. Neuron 53: 201–215, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178. Zhao B, Lei QY, Guan KL. The Hippo-YAP pathway: new connections between regulation of organ size and cancer. Curr Opin Cell Biol 20: 638–646, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]