Abstract
The present studies examined the role and mechanism of action of infiltrating T lymphocytes in the kidney during salt-sensitive hypertension. Infiltrating T lymphocytes in the Dahl salt-sensitive (SS) kidney significantly increased from 7.2 ± 1.8 × 105 cells/2 kidneys to 18.2 ± 3.9 × 105 cells/2 kidneys (n = 6/group) when dietary NaCl was increased from 0.4 to 4.0%. Furthermore, the expression of immunoreactive p67phox, gp91phox, and p47phox subunits of NADPH oxidase was increased in T cells isolated from the kidneys of rats fed 4.0% NaCl. The urinary excretion of thiobarbituric acid-reactive substances (TBARS; an index of oxidative stress) also increased from 367 ± 49 to 688 ± 92 nmol/day (n = 8/group) when NaCl intake was increased in Dahl SS rats. Studies were then performed on rats treated with a daily injection of vehicle (5% dextrose) or tacrolimus (0.25 mg·kg−1·day−1 ip), a calcineurin inhibitor that suppresses immune function, during the period of high-NaCl intake (n = 5/group). In contrast to the immune cell infiltration, increased NADPH oxidase expression, and elevated urine TBARS excretion in vehicle-treated Dahl SS fed high salt, these parameters were unaltered as NaCl intake was increased in Dahl SS rats administered tacrolimus. Moreover, tacrolimus treatment blunted high-salt mean arterial blood pressure and albumin excretion rate (152 ± 3 mmHg and 20 ± 9 mg/day, respectively) compared with values in dextrose-treated Dahl SS rats (171 ± 8 mmHg and 74 ± 28 mg/day). These experiments indicate that blockade of infiltrating immune cells is associated with decreased oxidative stress, an attenuation of hypertension, and a reduction of renal damage in Dahl SS rats fed high salt.
Keywords: reactive oxygen species generation, chronic renal insufficiency
oxidative stress, defined as a persistent imbalance between the production of highly reactive molecular species (mainly oxygen and nitrogen) and antioxidant defenses, has been implicated in pathophysiological conditions that affect the cardiovascular system (12, 28, 35). Increased levels of oxidative stress have been described in experimental models of hypertension (2, 6, 20) and hypertensive patients (22, 35).
A number of studies in animal models of hypertension demonstrated elevations of blood pressure by stimulation of reactive oxygen species (ROS) generation (24, 44, 47). Moreover, treatment with a variety of antioxidants reduces blood pressure in several genetic and experimental models of hypertension (4, 7, 30, 40, 48). One of the most important biological mechanisms for the production of ROS results from the generation of superoxide (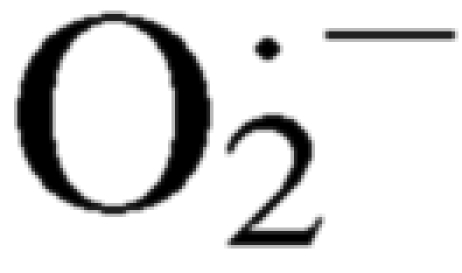 ) from O2 by the enzyme NADPH oxidase (14). Stimulation of NADPH oxidase appears to be the primary source of oxidants in systemic arterial vessels in renovascular hypertension (16), ANG II-induced hypertension (8, 34), DOCA-salt hypertension (42, 49), chronic renal insufficiency (47), and the spontaneously hypertensive rat (49). In humans, NADPH oxidase is the principal source of
) from O2 by the enzyme NADPH oxidase (14). Stimulation of NADPH oxidase appears to be the primary source of oxidants in systemic arterial vessels in renovascular hypertension (16), ANG II-induced hypertension (8, 34), DOCA-salt hypertension (42, 49), chronic renal insufficiency (47), and the spontaneously hypertensive rat (49). In humans, NADPH oxidase is the principal source of 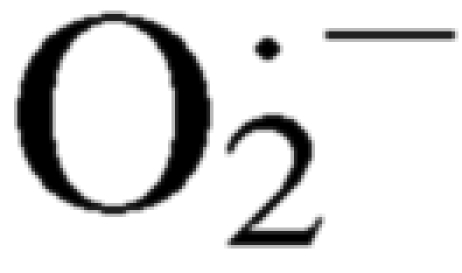 in vascular smooth muscle cells (1), and levels of NADPH oxidase are increased in patients with essential hypertension (45).
in vascular smooth muscle cells (1), and levels of NADPH oxidase are increased in patients with essential hypertension (45).
Infiltration of immune cells into the kidney has been documented in many animal models of nonimmune hypertension (15, 27, 29, 33); however, the exact mechanism by which this infiltration may lead to an elevation of mean arterial pressure (MAP) and/or renal damage has not yet been elucidated. Chronic inhibition of the immune system has been shown to attenuate hypertension and renal damage in different animal models of hypertension (10, 13, 25, 27, 31, 33) and human patients (17, 41), further suggesting a relationship between the immune system and the disease. Interestingly, elevated intrarenal oxidative state is often correlated to immune cell infiltration, and it has been proposed that both processes participate in a vicious positive feedback cycle (13), where ROS activate the synthesis of several proinflammatory chemokines and cytokines (11, 32, 39), that in turn stimulate the renal infiltration of immune cells. Moreover, different immune cell types contain the necessary machinery for the production of ROS (5, 19, 23), making them capable of further increasing the intrarenal levels of free radicals.
Although some studies demonstrated a relationship between infiltrating immune cells and oxidative stress in other models of salt-sensitive hypertension (3, 8, 38), little is known about this interconnection in the Dahl salt-sensitive rat. Our laboratory previously demonstrated that immunosuppressive treatment with mycophenolate mofetil ameliorates the sodium-sensitive hypertension and renal damage that occur in this rat strain (10, 25). Studies described in this manuscript were designed to test the hypothesis that renal infiltration of immune cells following an increase in the sodium intake mediates further development of hypertension and renal damage by increasing the intrarenal levels of free radicals.
METHODS
Experimental Animals
Experiments were performed on male Dahl salt-sensitive (SS) rats (SS/JrHSDMcwi) obtained from a colony maintained at the Medical College of Wisconsin. Some experiments were performed on outbred Sprague-Dawley (SD) rats obtained from Harlan Laboratories (Madison, WI). All experiments were performed with age-matched (littermates when possible) animals serving as control and experimental subjects. This experimental design minimized the impact of group-to-group variation on the experimental results. The Dahl SS breeders and weanlings were maintained on purified AIN-76A rodent diet (Dyets, Bethelem, PA) containing 0.4% NaCl. At 9 wk of age, the salt content of the diet was increased to 4.0% NaCl and maintained for the remaining 3 wk of the study; all experimental measurements were made during the final week of the study (i.e., during the third week of the 4.0% NaCl diet). Other Dahl SS rats, as described below, were maintained on the 0.4% NaCl diet for the final 3 wk of the study; experimental measurements in these rats were also made at 12 wk of age. The SD rats, which served as a normotensive control strain in this experiment, were fed the 0.4% NaCl diet from the time they were received at Medical College of Wisconsin; the rats were then fed 4.0% NaCl for the 3-wk experimental period and experimental variables were measured after 3 wk of the high-salt diet. All the animals were provided water and food ad libitum, and the protocols were approved by the Medical College of Wisconsin Institutional Animal Care and Use Committee.
Experimental Design
Group 1.
The initial studies were performed in two subgroups of Dahl SS rats. When the animals were 9 wk old, half of the animals were switched from 0.4% NaCl to 4.0% NaCl for the final 3 wk of the study. The remaining animals were maintained on 0.4% NaCl. Overnight urine samples were collected on the last night of the study for measurement of creatinine, protein, and excretion of thiobarbituric acid-reactive substances (TBARS). On the last day of the study, the animals were deeply anesthetized with pentobarbital sodium (50 mg/kg ip) and blood and kidneys were collected for T lymphocyte isolation and TBARS measurement in renal tissue.
Group 2.
Dahl SS rats were maintained on 0.4% NaCl until 9 wk of age when the dietary NaCl content was increased to 4.0%; at the time of the increase in salt intake, the animals were randomly assigned to receive daily injections of the immunosuppressant agent tacrolimus (0.25 mg·kg−1·day−1 ip) or vehicle (dextrose). The daily injections started on the same day as the diet was changed and lasted for the final 3 wk of the protocol. When the rats were 10 wk old, polyvinyl catheters were placed in the femoral artery, the animals were permitted to recover, and daily blood pressure recordings were made at 12 wk of age. Daily blood pressure measurements were made during a 3- to 4-h period with solid state pressure transducers and a general purpose amplifier. The output of the amplifiers was directed into an A/D convertor and a personal computer; the data were collected at 100 Hz and minute averages of MAP were calculated on line. The mean of three daily MAP averages was used as the blood pressure value for each rat.
Overnight urine samples were collected on the final night of the study for measurement of creatinine, protein, and TBARS excretion. On the last day of the study, the animals were deeply anesthetized with pentobarbital sodium (50 mg/kg ip) and blood and kidneys were collected for T lymphocyte isolation and TBARS measurement in renal tissue.
Group 3.
To test for the effects of the immunosuppressive agent in normal rats, a group of SD rats were fed 4.0% NaCl chow and randomly assigned to receive daily injections of tacrolimus (0.25 mg·kg−1·day−1 ip) or vehicle (dextrose). The daily injections started on the same day as the diet was changed and lasted for 3 wk. As described above, the rats were instrumented and daily blood pressure recordings were made at 12 wk of age. Overnight urine samples were collected on the final night of the study.
Group 4.
Dahl SS rats were maintained on 0.4% NaCl until 9 wk of age when the dietary NaCl content was increased to 4.0%; at the time of the increase in salt intake, the animals were randomly assigned to receive normal water or water containing the antioxidant Tempol (10 mM; Sigma, St. Louis, MO). The treatment started on the same day as the sodium content of the diet was increased and lasted for 3 wk. MAP in these rats was determined after 3 wk on high-salt diet with implanted telemetry transmitters. Daily blood pressure measurements were made during a 3- to 4-h period; the mean of three daily MAP averages was used as the blood pressure value for each rat. In addition, an overnight urine sample was obtained to quantify urinary albumin excretion rate.
Surgical Preparation
Rats were deeply anesthetized with a mixture of ketamine (75 mg/kg ip), xylazine (10 mg/kg ip), and acepromazine (2.5 mg/kg ip), with supplemental anesthesia administered as needed. With the use of aseptic techniques, polyvinyl catheters were placed in the femoral artery for measurement of arterial pressure. Catheters were tunneled subcutaneously and exteriorized at the back of the neck. In some rats, a telemetry transmitter (Data Sciences International) for measuring arterial blood pressure was aseptically implanted in the carotid artery, with the body of the transmitter implanted subcutaneously in the back of the animal. Animals were maintained on warming trays during and following surgery. Analgesics and antibiotics were administered after surgery to control pain and infection. Rats were allowed to recover for 5–7 days before the experimental protocol started.
T Lymphocyte Isolation
Infiltrating immune cells were isolated from the kidney using methods we previously described (10). Briefly, the rats were anesthetized with pentobarbital sodium (50 mg/kg ip), the kidneys were flushed with heparinized saline, and the kidneys were minced and incubated in a phosphate-buffered solution containing collagenase. The digested renal tissue was centrifuged on Histopaque to isolate mononuclear cells; the mononuclear cells were counted by hemacytometry and incubated with rat Pan T cell antibody coupled to magnetic microbeads (MACS Rat Pan T Cells Microbeads, Miltenyi Biotec); the T lymphocytes were then isolated with a magnetic column (MACS Separation Columns, Miltenyi Biotec) and counted. Circulating T cells were isolated from the blood using a protocol similar to the one described for the kidneys.
Histological and Immunohistochemical Analysis
Kidneys were obtained for histological and immunohistochemical analysis using methods we previously described (10, 25). The kidneys were fixed in a 10% formaldehyde solution; and the tissue was paraffin embedded (Microm HMP 300), cut in 3-μm sections (Microm HM355S), mounted, and stained with Gomori's One-Step Trichrome. For immunohistochemistry, slides were deparaffinized and incubated with Proteinase K for antigen retrieval. The primary monoclonal antibody used to detect T cells was anti-CD43 (Abcam). A biotinylated horse anti-mouse secondary antibody was used for development with avidin-biotinylated horseradish peroxidase complex (Vectastain ABC kits; Vector Laboratory, Burlingame, CA). The slides were lightly counterstained with aniline blue dye and photographed.
Urine and Renal Tissue TBARS Analysis
Overnight urine samples and renal protein homogenates were used to assess TBARS. At the end of the studies, the animals were deeply anesthetized with pentobarbital sodium (50 mg/kg ip) and their kidneys were collected, snap-frozen, and stored at −80°C. Before analysis, the kidneys were thawed, homogenized, and sonicated over ice in RIPA buffer. TBARS were measured using a TBARS assay kit (Cayman Chemical, Ann Arbor, MI). Decomposition of the unstable peroxides derived from polyunsaturated fatty acids results in the formation of malondialdehyde, which is quantified fluorometrically (excitation wavelength: 530 nm; emission wavelength: 550 nm) following its controlled reaction with thiobarbituric acid. Measurement of TBARS is a well-established method for screening and monitoring lipid peroxidation (50), a sign of oxidative stress.
Western Blot Analysis
Blotting experiments were performed with 100 μg of protein obtained from a renal tissue homogenate or protein from 2.5 × 105 T lymphocytes. Proteins were separated on an 8–16% SDS-PAGE gel, transferred to a nitrocellulose membrane (Bio-Rad), blocked for 1 h at room temperature, and incubated overnight with primary antibody at 4°C. The primary antibodies used against the different subunits of NADPH oxidase were gp91phox (BD), p67phox (Upstate), p47phox (Santa Cruz Biotechnology), and p22phox (Santa Cruz Biotechnology). Bound primary antibodies were detected with a horseradish peroxidase-labeled secondary antibody and visualized by chemiluminescence. Band intensities were quantified using densitometry (UN-SCAN-IT Gel Automated Digitizing System, Silk Scientific) and normalized to β-actin expression.
Statistical Analysis
Data are presented as means ± SE. For comparisons within a group, data were analyzed using a one-way ANOVA for repeated measures with a Tukey's post hoc test. For comparisons between groups, the data were analyzed using a two-way ANOVA for repeated measures with a Holm-Sidak post hoc test. A probability value of P < 0.05 was considered significant.
RESULTS
Effects of Elevated Sodium Intake on Oxidative State, Expression of NADPH Oxidase, and Immune Cell Infiltration in SS Rats
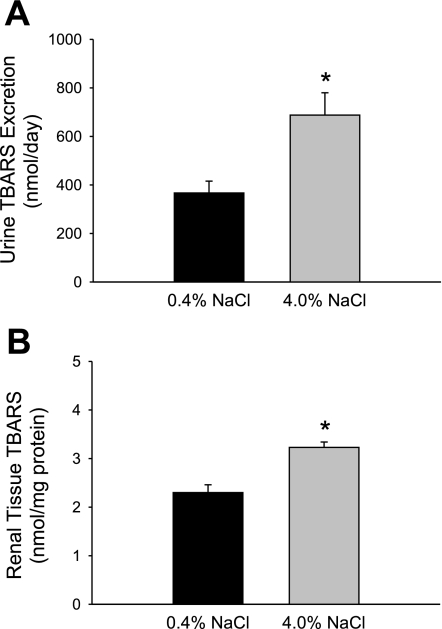
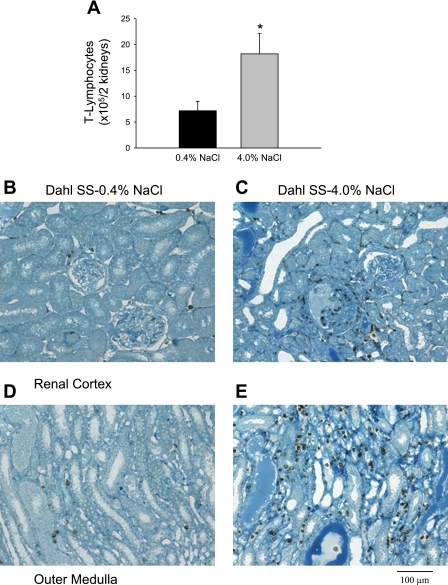
Elevated sodium consumption in Dahl SS rats was associated with an increased oxidative state in the kidneys. After 3 wk of high-salt diet, the urinary excretion of TBARS was significantly greater than the levels presented by the same animals during the low-salt period (688 ± 92 vs. 367 ± 49 nmol/day, respectively; n = 7/group), indicating that the systemic oxidative stress of these animals had increased with the elevated sodium intake (Fig. 1A). Renal tissue TBARS levels were also significantly increased in the animals that were fed the high-salt diet for 3 wk, with values that were ∼1.3 times greater than those in the low salt-fed animals (3.2 ± 0.1 vs. 2.3 ± 0.1 nmol/mg of tissue protein; n = 7/group; Fig. 1B). The elevated sodium intake was associated with a significantly increased infiltration of T lymphocytes (18.2 ± 3.9 vs. 7.2 ± 1.8 × 105 cells/2 kidneys; n = 6/group), as indicated in Fig. 2A. An immunohistochemical analysis localized the increased number of T cells to areas surrounding glomeruli in the renal cortex (Fig. 2, B and C) and damaged tubules in the renal outer medulla (Fig. 2, D and E) of the Dahl SS rats fed the high-salt diet.
Fig. 1.
Thiobarbituric acid-reactive substances (TBARS) in urine (A) and in renal tissue (B) of Dahl salt-sensitive (SS) rats maintained on 0.4 or 4.0% NaCl diet for 3 wk. *P < 0.05 vs. 0.4% NaCl-fed animals.
Fig. 2.
A: total number of T lymphocytes isolated from both kidneys of Dahl SS rats maintained on 0.4 or 4.0% NaCl diet for 3 wk. Immunohistochemical localization of T cells in the renal cortex (B and C) and medulla (D and E) of Dahl SS rats fed 0.4% (B and D) or 4.0% NaCl chow (C and E). T cells were localized in the renal tissue with antibodies directed against the cell surface marker CD43 (original magnification ×20). *P < 0.05 vs. 0.4% NaCl-fed animals.
Effects of Chronic Administration of Tacrolimus on MAP, Albumin Excretion Rate, and Renal Damage
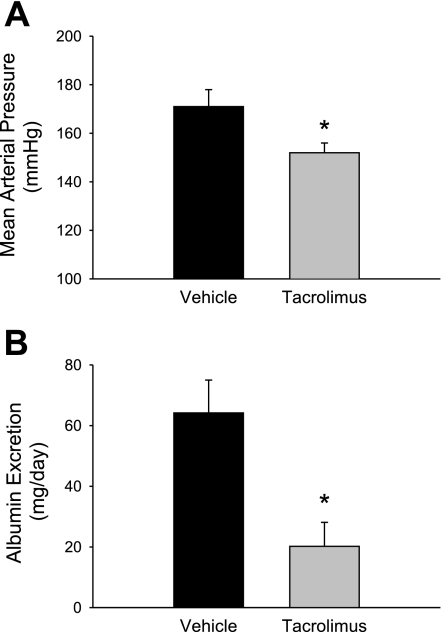
Figure 3 illustrates the effects of chronic administration of the immunosuppressant agent tacrolimus on MAP (Fig. 3A) and albumin excretion rate (Fig. 3B). Dahl SS rats were administered daily intraperitoneal injections of vehicle (dextrose) or tacrolimus (0.25 mg·kg−1·day−1) during the 3-wk period that they were fed 4.0% NaCl. Chronic treatment with tacrolimus significantly attenuated the development of hypertension and albumin excretion rate. MAP values for vehicle-treated animals averaged 171 ± 7 mmHg, while tacrolimus-treated animals presented average values of 152 ± 2 mmHg (n = 5/group). Treatment with tacrolimus also decreased the albumin excretion rate from 64 ± 11 to 20 ± 8 mg/day (n = 5/group).
Fig. 3.
Influence of chronic treatment with the immunosuppressant agent tacrolimus on mean arterial blood pressure (A) and albumin excretion rate (B) in Dahl SS rats fed 4.0% NaCl for a period of 3 wk. *P < 0.05 vs. vehicle-treated animals.
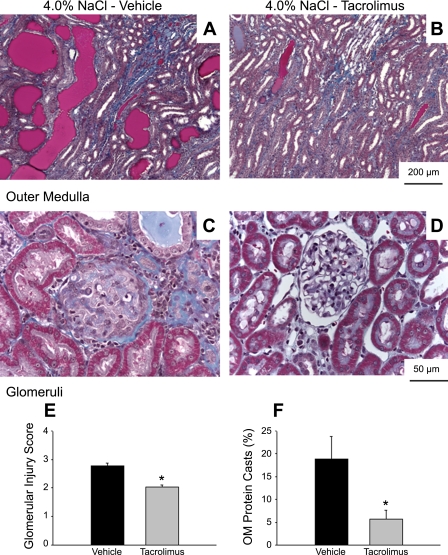
Daily administration of tacrolimus ameliorated the development of renal damage (Fig. 4, A–D). The vehicle-treated Dahl SS rats fed 4.0% NaCl for 3 wk had both glomerular (blue-stained fibrotic tissue and collapsed capillary structure) and tubular damage (red protein deposition casts indicating blocked tubules in the outer medulla). Visibly less glomerular and tubular injury was observed in the kidneys of the tacrolimus-treated rats. Renal damage, evaluated by the glomerular injury index (Fig. 4E) and the percentage of renal medullary area consisting of protein casts (Fig. 4F), was significantly decreased in rats treated with tacrolimus (n = 5/group). The renal damage in tacrolimus-treated rats was similar to that we previously described in Dahl SS rats maintained on a 0.4% NaCl diet (10).
Fig. 4.
Light microscopy images of trichrome-stained sections of renal outer medulla (×10 original magnification; A-B) and renal cortex (×40 original magnification; C–D) of Dahl SS rats receiving daily injections of vehicle (A and C) or tacrolimus (B and D) during the 3-wk period they were fed 4.0% NaCl diet. Bottom: calculated glomerular injury score (E) and the percentage of renal outer medulla (OM) consisting of protein casts (F). *P < 0.05 vs. vehicle-treated animals.
To test for nonspecific effects of the immunosuppressive agent, additional studies were performed in SD rats fed the 4.0% NaCl chow and administered vehicle or tacrolimus for 3 wk (n = 4–5/group). At the end of the 3-wk period, MAP was significantly elevated (133 ± 4 mmHg) in the tacrolimus-treated rats compared with the vehicle-treated group (120 ± 3 mmHg). Albumin excretion rate was unaltered between the vehicle-treated (5.1 ± 2.3 mg/day) and tacrolimus-treated rats (9.1 ± 7.5 mg/day).
Influence of Tacrolimus on Oxidative State and Renal Immune Infiltration
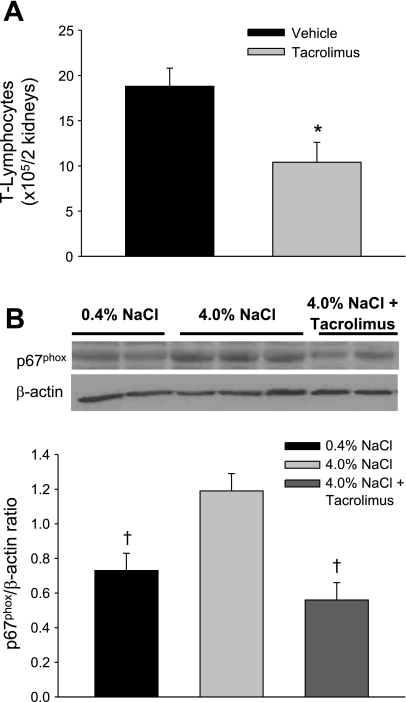
In contrast to the increase in oxidative stress observed in untreated Dahl SS rats fed 4.0% NaCl chow (n = 12/group), there was no increase in TBARS excretion in tacrolimus-treated SS rats fed 4.0% NaCl (510 ± 208 nmol/day) compared with TBARS excretion rate (551 ± 80 nmol/day) during the low-salt period. Chronic treatment with tacrolimus during the high-salt period significantly decreased the absolute numbers of infiltrating T lymphocytes in the kidneys (18.8 ± 2 × 105 cells/2 kidneys in vehicle-treated animals vs. 10.4 ± 2.2 × 105 cells/2 kidneys in tacrolimus-treated animals; n = 6/group; Fig. 5A). Although tacrolimus administration tended to decrease blood T lymphocytes in the rats, this decrease was not significant (4.7 ± 1.1 × 106 vs. 8.3 ± 1.9 × 106 cells/ml; n = 5/group).
Fig. 5.
Effects of chronic treatment with the immunosuppressant tacrolimus during the high-salt period on the absolute numbers of infiltrating T lymphocytes (A) and on the expression of NADPH subunit p67phox (B) in the renal tissue of Dahl SS rats. *P < 0.05 vs. vehicle-treated animals. †P < 0.05 vs. 0.4% NaCl-fed animals.
Immunoblotting protocols were performed to study differences in expression of NADPH oxidase in renal tissue when the Dahl SS rats were fed 0.4 or 4.0% NaCl or fed 4.0% NaCl and receiving tacrolimus treatment. As indicated in Fig. 5B, the elevation of the sodium content of the diet was accompanied by a significant increase of the renal expression of p67phox, one of the subunits that form NADPH oxidase. When the expression of p67phox was normalized to β-actin, Dahl SS rats fed 4.0% NaCl presented 1.6 times greater p67phox than the animals that were fed 0.4% NaCl (1.19 ± 0.1 vs. 0.73 ± 0.1 arbitrary units; n = 4–6/group). Expression of p67phox in renal tissue was significantly decreased in the animals treated with tacrolimus during the high-salt period (0.56 ± 0.1 arbitrary units). Tacrolimus-treated animals presented levels of p67phox expression in the renal tissue that were not different than the low salt-fed animal levels, indicating that the enhanced oxidative state in the kidney of Dahl SS rats fed 4.0% NaCl was attenuated by this immunosuppressive treatment.
NADPH Oxidase Expression in Isolated T Lymphocytes
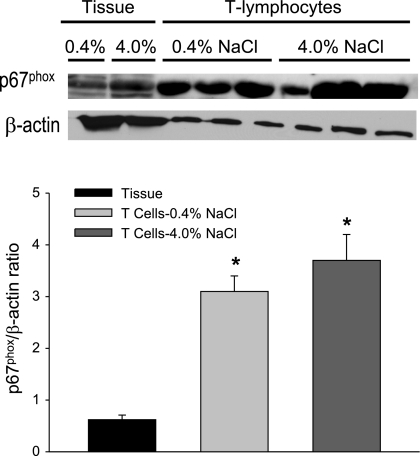
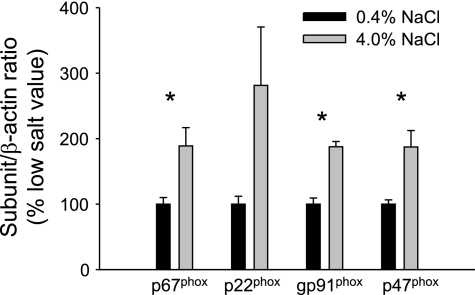
Additional experiments were then performed to determine whether T lymphocytes isolated from kidneys of Dahl SS rats are capable of participating in free radical formation. As illustrated in Fig. 6, both renal tissue and isolated T cells express the NADPH oxidase subunit p67phox. Moreover, when normalized to β-actin, the expression was significantly greater in the T cells compared with renal tissue. In addition, gp91phox, p22phox, and p47phox subunits of NADPH oxidase were also identified in T cells, indicating that the infiltrating cells have the molecular machinery needed for the production of ROS. Of the different subunits, significantly greater expression of p67phox and gp91phox was observed in T cells compared with tissue. Figure 7 illustrates the calculated NADPH oxidase expression contributed by infiltrating T lymphocytes in kidneys of Dahl SS rats fed low- or high-salt diet for 3 wk (n = 6/group). The significant increase in infiltrating T cells contributes to a significantly greater expression of p67phox, gp91phox, and p47phox in the kidneys of Dahl SS rats.
Fig. 6.
Expression of p67phox in renal tissue and renal T lymphocytes obtained from Dahl SS rats fed 0.4 or 4.0% NaCl diet for 3 wk. *P < 0.05 vs. expression in tissue; 100 μg of protein or 2.5 × 105 cells were loaded per well.
Fig. 7.
Calculated NADPH oxidase subunit expression, normalized to low-salt diet, contributed by infiltrating T lymphocytes in kidneys of rats fed 0.4 or 4.0% NaCl for 3 wk. *P < 0.05 vs. expression of same subunit in 0.4% NaCl-fed animals.
Effects of Tempol on MAP and Albumin Excretion Rate
A final set of studies was performed to assess the effects of the antioxidant tempol on MAP (Fig. 8A) and albumin excretion rate (Fig. 8B) in Dahl SS rats fed high salt. The salt-sensitive increase in blood pressure was significantly ameliorated in rats that were treated with tempol (10 mmol/l; Sigma) during the high-salt period (126 ± 1 vs. 146 ± 2 mmHg in the control animals; n = 6/group). In addition, chronic administration of tempol to Dahl SS rats fed the high-salt diet significantly reduced the urinary albumin excretion rate by 46% (121 ± 19 vs. 223 ± 36 mg/day; n = 6/group), indicating that the renal damage in these animals was diminished by treatment with this SOD mimetic.
Fig. 8.
Effect of chronic administration of the antioxidant tempol on mean arterial blood pressure (A) and albumin excretion rate (B) in Dahl SS rats fed 4.0% NaCl for a period of 3 wk. *P < 0.05 vs. vehicle control animals.
DISCUSSION
The present studies demonstrate that T lymphocytes that infiltrate the kidney can participate in the development of SS hypertension and renal disease in Dahl SS rats by increasing the intrarenal level of free radicals. These experiments demonstrate an association between increased renal infiltration of T cells, increased oxidative stress, and increased expression of NADPH oxidase in the renal tissue of Dahl SS rats fed a high-salt diet. Moreover, chronic administration of the immunosuppressant agent tacrolimus during the period of high-salt intake decreased T lymphocyte infiltration, reduced the oxidative state and renal expression of the NADPH oxidase subunit p67phox, and ameliorated hypertension and renal disease. The attenuation of SS hypertension and albumininura observed with the administration of the antioxidant tempol also highlights the importance of ROS production by the infiltrating T lymphocytes. Taken together, these data indicate that infiltrating T lymphocytes are capable of participating in the progression of hypertension and renal damage by elevating intrarenal oxidative stress in the Dahl SS rat.
Oxidative stress has been implicated in pathophysiological conditions that affect the cardiovascular system. Increased levels of oxidative stress have been described in experimental models of hypertension (2, 6, 20) and hypertensive patients (22, 35). Treatment with a variety of antioxidants has been shown to decrease arterial blood pressure in several experimental and genetic models of hypertension (4, 7, 30, 40, 48). Several groups also demonstrated the association between elevated levels of intrarenal ROS and renal damage (44), and the deleterious effects that deficiencies in catalase (21, 24) or superoxide dismutase (26, 37) have on renal function.
Renal infiltration of immune cells has been reported in many experimental models of nonimmune hypertension (15, 27, 29, 31, 33); however, the exact mechanisms by which these infiltrating cells participate in the development or progression of hypertension and renal disease are still unclear. It has been proposed that the infiltrating immune cells could be participating in hypertension and renal damage by producing vasoactive factors such as ANG II and ROS (13, 36, 38). Interestingly, renal infiltration of immune cells is often correlated to elevated intrarenal oxidative stress, and a positive feedback loop has been suggested between ROS and infiltrating immune cells. ROS have been proven to stimulate the synthesis of a variety of proinflammatory cytokines (11, 32, 39), that, in turn, stimulate the infiltration of immune cells into the renal tissue. Different immune cells, such as macrophages (5), monocytes (23), and T lymphocytes (19), have been shown to contain the necessary machinery for the production of ROS; therefore, it has been proposed that they may contribute to the generation of free radicals, leading to a further increase of the oxidative stress.
Immunoblotting and immunohistochemical studies demonstrated that infiltrating immune cells stain positive for superoxide (13, 38) in the kidneys of hypertensive rats. Our present data are consistent with these observations since treatment with the immunosuppressant agent tacrolimus decreased the infiltration of T lymphocytes and the elevated intrarenal levels of ROS that are associated with an increased sodium intake in Dahl SS rats. Given the similar levels of expression of NADPH subunit p67phox for equal numbers of T cells isolated from rats on 0.4 or 4.0% NaCl chow, the present data indicate that the increase in T cell infiltration in the kidneys of the high-salt rats is the mechanism leading to increased free radical production in the kidney. Interestingly, our data also show a greater expression of NADPH oxidase in the infiltrating T cells than in the whole kidney, indicating that these cells are enriched in this enzyme.
Although our data describe a significant inhibition of renal T cell infiltration with administration of the immunosuppressant agent tacrolimus, we cannot ensure that these effects are limited to this immune cell type. Tacrolimus is an inhibitor of calcineurin, a widely distributed enzyme; therefore, it is possible that the effects of administration of this drug are not limited to T lymphocytes and could affect other immune cell types or even resident cells within the kidney. Recent studies by Guzik et al. (15), however, demonstrated the importance of T lymphocytes in hypertension. They demonstrated that RAG1−/− mice, which lack both T and B lymphocytes, fail to develop ANG II-induced hypertension, and only become hypertensive after an adoptive transfer of T cells. In addition, the importance of NADPH oxidase within the T cells to maintain the hypertensive phenotype was also highlighted by those studies, by showing that transfer of T cells lacking p47phox subunit only partially recovered the hypertensive phenotype in mice (15). These studies, together with our present data, implicate T lymphocytes in the elevation of the oxidative state during hypertensive conditions.
Treatment with the antioxidant tempol has been proven to decrease hypertension and renal damage in Dahl SS rats (18, 26). In the present set of experiments, tempol treatment during the high-salt period ameliorated hypertension and albuminuria without affecting the infiltration of T cells into the kidney, which remained elevated. Tempol (4-hydroxy-2,2,6,6-tetramethyl-piperidine-1-oxyl) is a small cell-permeable cyclic nitroxide with SOD activity (46); hence, it is capable of catalyzing the conversion of superoxide to hydrogen peroxide, scavenging in this way the 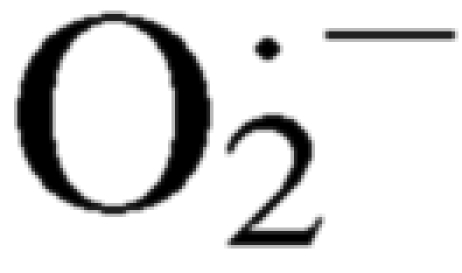 being produced intracellularly. Therefore, it seems probable that tempol ameliorates hypertension and albuminuria by scavenging the
being produced intracellularly. Therefore, it seems probable that tempol ameliorates hypertension and albuminuria by scavenging the 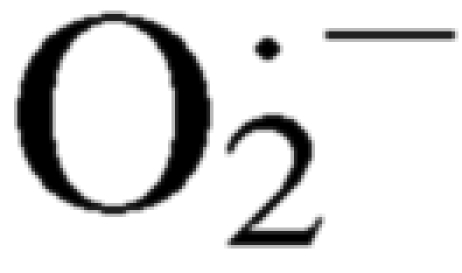 that the infiltrating T lymphocytes produce within the kidney. It is possible, however, that the effects of free radical scavenging in the heart, aorta, brain, or other organs and tissues important in blood pressure regulation could also be involved in the antihypertensive responses observed in the present study.
that the infiltrating T lymphocytes produce within the kidney. It is possible, however, that the effects of free radical scavenging in the heart, aorta, brain, or other organs and tissues important in blood pressure regulation could also be involved in the antihypertensive responses observed in the present study.
An interesting observation in the present study is the anti-hypertensive effect of tacrolimus in the Dahl SS rats fed the high-salt diet but the prohypertensive effects of tacrolimus in normal SD rats fed the same diet. The observation in the SD rats is consistent with previous reports (43) and is likely a result of effects of this agent to decrease nitric oxide production by calcineurin-independent effects (9). In contrast to the prohypertensive effects in normal rats, however, administration of tacrolimus to the Dahl SS rat decreased infiltration of T cells in the kidney, decreased renal oxidative stress, and attenuated sodium-dependent hypertension and renal disease in these rats. A ready explanation is not apparent for this observation; it may be, however, that the reduction in infiltrating cells has a far greater impact on the progression of disease in this model than the results of any change in NO production, particularly in the Dahl SS rat which is known to have a predisposition to endothelial dysfunction.
In conclusion, the data presented in this manuscript indicate that high-salt intake is associated with increased infiltration of T lymphocytes, oxidative stress, and expression of NADPH oxidase in the renal tissue of Dahl SS rats. Chronic immunosuppression with tacrolimus ameliorates hypertension and renal damage by decreasing renal infiltration of T cells and their production of free radicals. All these data together indicate that renal infiltrating T lymphocytes are capable of participating in the development or progression of hypertension and renal damage by increasing the intrarenal oxidative stress.
GRANTS
This work was partially supported by National Institutes of Health Grants HL-29587 and DK-62803.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
REFERENCES
- 1. Berry C, Hamilton CA, Brosnan MJ, Magill FG, Berg GA, McMurray JJV, Dominiczak AF. Investigation into the sources of superoxide in human vessels: angiotensin II increases superoxide production in human internal mammary arteries. Circulation 101: 2206–2212, 2000 [DOI] [PubMed] [Google Scholar]
- 2. Beswick R, Zhang H, Marable D, Catravas JD, Hill WD, Webb RC. Long-term antioxidant administration attenuates mineralocorticoid hypertension and renal inflammatory response. Hypertension 37: 781–786, 2001 [DOI] [PubMed] [Google Scholar]
- 3. Bravo Y, Quiroz Y, Ferrebuz A, Vaziri ND, Rodriguez-Iturbe B. Mycophenolate mofetil administration reduces renal inflammation, oxidative stress, and arterial pressure in rats with lead-induced hypertension. Am J Physiol Renal Physiol 293: F616–F623, 2007 [DOI] [PubMed] [Google Scholar]
- 4. Cabassi A, Bouchard JF, Dumont EC, Girouard H, Le Jossec M, Lamontagne D, Besner JG, de Chaplain J. Effect of antioxidant treatments on nitrate tolerance delopment in normotensive and hypertensive rats. J Hypertens 18: 187–196, 2000 [DOI] [PubMed] [Google Scholar]
- 5. Cathcart M. Regulation of superoxide anion production by NADPH oxidase in monocytes/macrophages: contributions to atherosclerosis. Arterioscler Thromb Vasc Biol 24: 23–28, 2004 [DOI] [PubMed] [Google Scholar]
- 6. Chabrashvili T, Kitiyakara C, Blau J, Karber A, Aslam S, Welch WJ, Wilcox CS. Effects of Ang II type 1 and 2 receptors on oxidative stress, renal NADPH oxidase and SOD expression. Am J Physiol Regul Integr Comp Physiol 285: R117–R124, 2003 [DOI] [PubMed] [Google Scholar]
- 7. Chen X, Touyz RM, Park JB, Schiffrin EL. Antioxidant effects of vitamin C and E are associated with altered activation of vascular NADPH oxidase and superoxide dismutase in stroke-prone SHR. Hypertension 38: 606–611, 2000 [DOI] [PubMed] [Google Scholar]
- 8. Cifuentes M, Rey FE, Carretero OA, Pagano JP. Upregulation of p67phox and gp91phox in aortas from angiotensin II-infused mice. Am J Physiol Heart Circ Physiol 279: H2234–H2240, 2000 [DOI] [PubMed] [Google Scholar]
- 9. Cook LG, Chiasson VL, Long C, Wu GY, Mitchell BM. Tacrolimus reduces nitric oxide synthase function by binding to FKBP rather than its calcineurin effect. Kidney Int 75: 719–726, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. De Miguel C, Das S, Lund H, Mattson DL. T lymphocytes mediate hypertension and kidney damage in Dahl salt-sensitive rats. Am J Physiol Regul Integr Comp Physiol 298: R1136–R1142, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Dröge W. Free radicals in the physiologic control of cell function. Physiol Rev 82: 47–95, 2002 [DOI] [PubMed] [Google Scholar]
- 12. Endemann D, Schiffrin EL. Nitric oxide, oxidative excess, and vascular complications of diabetes mellitus. Curr Hypertens Rep 6: 85–89, 2004 [DOI] [PubMed] [Google Scholar]
- 13. Franco M, Martinez F, Quiroz Y, Galicia O, Bautista R, Johnson RJ, Rodriguez-Iturbe B. Renal angiotensin II concentration and interstitial infiltration of immune cells are correlated with blood pressure levels in salt-sensitive hypertension. Am J Physiol Regul Integr Comp Physiol 293: R251–R256, 2007 [DOI] [PubMed] [Google Scholar]
- 14. Griendling K, Sorescu D, Ushio-Fukai M. NAD(P)H oxidase: role in cardiovascular biology and disease. Circ Res 86: 494–501, 2000 [DOI] [PubMed] [Google Scholar]
- 15. Guzik T, Hoch N, Brown K, McCann L, Rahman A, Dikalov S, Goronzy J, Weyand C, Harrison D. Role of T cells in the genesis of angiotensin II induced hypertension and vascular dysfunction. J Exp Med 204: 2449–2460, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Heitzer T, Wenzel U, Hink U, Krollner D, Skatchkov M, Stahl RA, MacHarzina R, Brasen JH, Meinertz T, Munzel T. Increased NAD(P)H oxidase-mediated superoxide production in renovascular hypertension: evidence for an involvement of protein kinase C. Kidney Int 55: 252–260, 1999 [DOI] [PubMed] [Google Scholar]
- 17. Herrera J, Ferrebuz A, García MacGregor E, Rodriguez-Iturbe B. Mycophenolate mofetil treatment improves hypertension in patients with psoriasis and rheumatoid arthritis. J Am Soc Nephrol 17: 218–225, 2006 [DOI] [PubMed] [Google Scholar]
- 18. Hisaki R, Fujita H, Saito F, Kushiro T. Tempol attenuates the development of hypertensive renal injury in Dahl salt-sensitive rats. Am J Hypertens 18: 707–713, 2005 [DOI] [PubMed] [Google Scholar]
- 19. Jackson S, Devadas S, Kwon J, Pinto LA, Williams MS. T cells express a phagocyte-type NADPH oxidase that is activated after T cell receptor stimulation. Nat Immunol 5: 818–827, 2004 [DOI] [PubMed] [Google Scholar]
- 20. Jung O, Schreiber JG, Geiger H, Pedrazzini T, Busse R, Brandes RP. gp91-phox-containing NADPH oxidase mediates endothelial dysfunction in renovascular hypertension. Circulation 109: 1795–1801, 2004 [DOI] [PubMed] [Google Scholar]
- 21. Kobayashi M, Sugiyama H, Wang DH, Toda N, Maeshima Y, Yamasaki Y, Masuoka N, Yamada M, Kira S, Makino H. Catalase deficiency renders remnant kidneys more susceptible to oxidant tissue injury and renal fibrosis in mice. Kidney Int 68: 1018–1031, 2005 [DOI] [PubMed] [Google Scholar]
- 22. Lacy F, Kailasam MT, O'Connor DT, Schmid-Schonbein GW, Parmer RJ. Plasma hydrogen peroxide production in human essential hypertension: role of heredity, gender and ethnicity. Hypertension 36: 878–884, 2000 [DOI] [PubMed] [Google Scholar]
- 23. Li Q, Subbulakshmi V, Fields AP, Murray NR, Cathcart MK. Protein kinase C alpha regulates human monocyte O-2 production and low density lipoprotein lipid oxidation. J Biol Chem 274: 3764–3771, 1999 [DOI] [PubMed] [Google Scholar]
- 24. Makino A, Skelton MM, Zou AP, Cowley AW., Jr Increased renal medullary H2O2 leads to hypertension. Hypertension 42: 25–30, 2003 [DOI] [PubMed] [Google Scholar]
- 25. Mattson D, James L, Berdan EA, Meister CJ. Immune suppression attenuates hypertension and renal disease in the Dahl salt-sensitive rat. Hypertension 48: 149–156, 2006 [DOI] [PubMed] [Google Scholar]
- 26. Meng S, Roberts 2nd LJ, Cason GW, Curry TS, Manning RD., Jr Superoxide dismutase and oxidative stress in Dahl salt-sensitive and -resistant rats. Am J Physiol Regul Integr Comp Physiol 283: R732–R738, 2002 [DOI] [PubMed] [Google Scholar]
- 27. Muller D, Shagdarsuren E, Park JK, Dechend R, Mervaala E, Hampich F, Fiebeler A, Ju X, Finckenberg P, Theuer J, Viedt C, Kreuzer J, Heidecke H, Haller H, Zenke M, Luft F. Immunosuppressive treatment protects against angiotensin II-induced renal damage. Am J Pathol 161: 1679–1693, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Nishikawa T, Edelstein D, Du XL, Yamagishi S, Matsumura T, Kaneda Y, Yorek MA, Beebe D, Oates PJ, Hammes HP, Giardino I, Brownlee M. Normalizing mitochondrial superoxide production blocks three pathways of hyperglycaemic damage. Nature 404: 787–790, 2000 [DOI] [PubMed] [Google Scholar]
- 29. Ozawa Y, Kobori H, Suzaki Y, Navar LG. Sustained renal interstitial macrophage infiltration following chronic angiotensin II infusions. Am J Physiol Renal Physiol 292: F330–F339, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Park J, Touyz RM, Chen X, Schriffin EL. Chronic treatment with a superoxide dismutase mimetic prevents remodeling, and progression of hypertension in salt-loaded stroke-prone spontaneously hypertensive rats. Am J Hypertens 15: 78–84, 2002 [DOI] [PubMed] [Google Scholar]
- 31. Pechman K, Basile DP, Lund H, Mattson DL. Immune suppression blocks sodium-sensitive hypertension following recovery from ischemic acute renal failure. Am J Physiol Regul Integr Comp Physiol 294: R1234–R1239, 2008 [DOI] [PubMed] [Google Scholar]
- 32. Polla B, Bachelet M, Elia G, Santoro MG. Stress proteins in inflammation. Ann NY Acad Sci 851: 75–85, 1998 [DOI] [PubMed] [Google Scholar]
- 33. Quiroz Y, Pons H, Gordon K, Rincon J, Chavez M, Parra G, Herrera-Acosta J, Gomez-Garre D, Larfo R, Egido J, Johnson RJ, Rodriguez-Iturbe B. Mycophenolate mofetil prevents salt-sensitive hypertension resulting from nitric oxide synthase inhibition. Am J Physiol Renal Physiol 281: F38–F47, 2001 [DOI] [PubMed] [Google Scholar]
- 34. Rajagopalan S, Kurz S, Münzel T, Tarpey M, Freeman BA, Griendling KK, Harrison DG. Angiotensin II-mediated hypertension in the rat increases vascular superoxide production via membrane NADH/NADPH oxidase activation: contribution to alterations of vasomotor tone. J Clin Invest 97: 1916–1923, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Redón J, Oliva MR, Tormos C, Giner V, Chaves J, Iradi A, Sáez GT. Antioxidant activities and oxidative stress byproducts in human hypertension. Hypertension 41: 1096–1101, 2003 [DOI] [PubMed] [Google Scholar]
- 36. Rodriguez-Iturbe B, Johnson RJ. The role of inflammatory cells in the kidney in the induction and maintenance of hypertension. Nephrol Dial Transplant 21: 260–263, 2006 [DOI] [PubMed] [Google Scholar]
- 37. Rodriguez-Iturbe B, Sepassi L, Quiroz Y, Ni Z, Wallace DC, Vaziri ND. Association of mitochondrial SOD deficiency with salt-sensitive hypertension and accelerated renal senescence. J Appl Physiol 102: 255–260, 2007 [DOI] [PubMed] [Google Scholar]
- 38. Rodriguez-Iturbe B, Vaziri ND, Herrera-Acosta J, Johnson RJ. Oxidative stress, renal infiltration of immune cells and salt-sensitive hypertension: all for one and one for all. Am J Physiol Renal Physiol 286: F606–F616, 2004 [DOI] [PubMed] [Google Scholar]
- 39. Schieffer B, Luchtefeld M, Braun S, Hilfiker A, Hilfiker-Kleiner D, Drexler H. Role of NADPH oxidase in angiotensin II-induced JAK/STAT signaling and cytokine induction. Circ Res 87: 1195–1201, 2000 [DOI] [PubMed] [Google Scholar]
- 40. Schnackenberg C, Wilcox CS. Two-week administration of tempol attenuates both hypertension and renal excretion of 8-iso prostaglandin F2alpha. Hypertension 33: 424–428, 1999 [DOI] [PubMed] [Google Scholar]
- 41. Seaberg EC, Muñoz A, Lu M, Detels R, Margolick JB, Riddler SA, Williams CM, Phair JP, Multicenter AIDS Cohort Study Association between highly active antiretroviral therapy and hypertension in a large cohort of men followed from 1984 to 2003. AIDS 19: 953–960, 2005 [DOI] [PubMed] [Google Scholar]
- 42. Somers M, Mavromatis K, Galis ZS, Harrison DG. Vascular superoxide production and vasomotor function in hypertension induced by deoxycorticosterone acetate-salt. Circulation 101: 1722–1728, 2000 [DOI] [PubMed] [Google Scholar]
- 43. Takeda Y, Miyamori I, Furukawa K, Inaba S, Mabuchi H. Mechanisms of FK 506-induced hypertension in the rat. Hypertension 33: 130–136, 1999 [DOI] [PubMed] [Google Scholar]
- 44. Taylor N, Cowley AW. Effects of renal medullary H2O2 on salt-induced hypertension and renal injury. Am J Physiol Regul Integr Comp Physiol 289: R1573–R1579, 2005 [DOI] [PubMed] [Google Scholar]
- 45. Touyz R, Schiffrin EL. Increased generation of superoxide by angiotensin II in smooth muscle cells from resistance arteries of hypertensive patients: role of phospholipase d-dependent NADPH oxidase-sensitive pathways. J Hypertens 19: 1245–1254, 2001 [DOI] [PubMed] [Google Scholar]
- 46. Vaz S, Augusto O. Inhibition of myeloperoxidase-mediated protein nitration by tempol: kinetics, mechanism and implications. Proc Natl Acad Sci USA 105: 8191–8196, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Vaziri N, Dicus M, Ho N, Sindhu RK. Oxidative stress, and dysregulation of superoxide dismutase, NAD(P)H oxidase and xanthine oxidase in chronic renal insufficiency. Kidney Int 63: 179–185, 2003 [DOI] [PubMed] [Google Scholar]
- 48. Vaziri ND, Ni Z, Oveisi F, Trnavsky-Hobbs DL. Effect of antioxidant therapy on blood pressure and NO synthase expression in hypertensive rats. Hypertension 36: 957–964, 2000 [DOI] [PubMed] [Google Scholar]
- 49. Wu R, Millete E, Wu L, de Champlain J. Enhanced superoxide anion formation in vascular tissues from spontaneously hypertensive and deoxycorticosterone acetate-salt hypertensive rats. J Hypertens 19: 741–748, 2001 [DOI] [PubMed] [Google Scholar]
- 50. Yagi K. Simple assay for the level of total lipid peroxides in serum or plasma. Methods Mol Biol 108: 101–106, 1998 [DOI] [PubMed] [Google Scholar]