Abstract
MicroRNAs (miRNA) are endogenously produced, short RNAs that repress and thus regulate the expression of almost half of known protein-coding genes. miRNA-mediated gene repression is an important regulatory mechanism to modulate fundamental cellular processes such as the cell cycle, growth, proliferation, phenotype, and death, which in turn have major influences on pathophysiological outcomes. In kidneys, miRNAs are indispensable for renal development and homeostasis. Emerging evidence has further pinpointed the pathogenic roles played by miRNAs in major renal diseases, including diabetic nephropathy, acute kidney injury, renal carcinoma, polycystic kidney disease, and others. Although the field of renal miRNA research is still in its infancy and important questions remain, future investigation on miRNA regulation in kidneys has the potential to revolutionize both the diagnosis and treatment of major renal diseases.
Keywords: Dicer, diabetic nephropathy, acute kidney injury, renal cell carcinoma, allograft rejection
microRNAs (miRNAs) are endogenously produced, short RNAs of 21–25 nucleotides that are important regulators of gene expression at the posttranscriptional level (4, 12, 52). By binding to the 3′-untranslated region (UTR) of the target gene mRNAs, miRNAs can induce mRNA degradation or, more frequently, result in repression of protein translation (18). miRNAs are produced by gene transcription followed by sequential processing or editing. A few hundred miRNAs have been identified in various organisms, and current estimates indicate that these miRNAs may regulate almost half of protein-coding genes (10). By regulating gene expression, miRNAs play critical roles in a variety of cellular and physiological activities (32). In human diseases, miRNA expression is frequently altered, contributing to pathogenesis (14, 15, 20, 22, 30, 37, 65, 75, 76, 90).
The role of miRNAs in the regulation of renal development, physiology, and pathology has emerged as an important and potentially fruitful area of research (47, 48, 72). The identification and characterization of the miRNAs in various renal diseases may lead to breakthroughs in the development of novel diagnostic tools and therapeutic interventions. The research field of miRNA regulation in kidneys and renal diseases is still in its infancy, and despite its enormous potential, significant roadblocks remain. In this review, we aim to discuss the fundamental aspects of miRNA biogenesis and regulation, analyze the roles of miRNAs in renal pathophysiology, and identify future directions of research in this emerging field.
Biogenesis, Function, and Regulation of miRNAs
Bioinformatic analysis coupled with experimental validation has lead to the estimation that most animals have a few hundred miRNA-coding genes (12, 52). Humans are predicted to have ∼800 miRNA genes, although many of them have yet to be validated experimentally (18, 34). The miRNA coding regions are present in the genome as independent genes or are present in the introns of protein-coding genes (12, 52, 66). The miRNA genes are transcribed, leading to the production of large transcripts (pri-miRNAs), which are then processed and edited, resulting in the formation of mature, functional miRNAs of 21–25 nucleotides. miRNAs are finally loaded onto a multiprotein complex that mediates mRNA degradation or translational repression (12, 26, 34, 95).
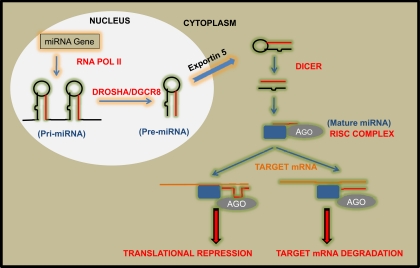
Transcription of miRNA genes is the first step in the biogenesis of miRNAs (52). RNA polymerase II is usually responsible for the transcription, although a minor group of miRNA genes can also be transcribed via RNA polymerase III (18). The transcription generally results in primary transcripts (called pri-miRNAs) that are several kilobases in length. Notably, these large transcripts have well-defined hairpin structures, which direct the pri-miRNAs to a multiprotein complex called the Microprocessor complex in the nucleus (6, 12, 26, 52). The Microprocessor complex contains two major proteins, Drosha (an RNase III-like protein) and its cofactor DiGeorge syndrome critical region gene 8 (DGCR8). DGCR8 recognizes and interacts with the hairpin structure in the pri-miRNAs and recruits Drosha. Drosha then cleaves pri-miRNAs precisely at the stem-loop structure, leading to the production of secondary precursor RNAs of ∼70-nucleotides, called pre-miRNAs (52). The pre-miRNAs are then exported to the cytoplasm by exportin 5, a nuclear transport receptor family member that recognizes the stem-loop structure of pre-miRNAs (12, 27, 52).
In the cytoplasm, the pre-miRNAs are further cleaved to release the mature miRNA of 21–25 nucleotides (12, 27). This cleavage involves a multiprotein complex, whose major component is Dicer, an RNase III type enzyme. Dicer is a large (∼200 kDa) protein that is remarkably conserved in almost all eukaryotic organisms. Although Dicer is directly involved in the cleavage of pre-miRNA to mature miRNA, its activity is thought to be modulated by other associated proteins such as TAR RNA-binding protein (TRBP) and PACT (52). Of note, in addition to its involvement in mature miRNA generation, the Dicer-containing protein complex also regulates the assembly of mature miRNA into the effector complex (12, 27, 52).
The miRNA effector complex is also called the RNA-induced silencing complex or RISC, which includes argonaute (AGO) proteins, the catalytic enzymes with endonuclease activity that are responsible for target mRNA degradation (52). Most organisms have multiple AGO proteins with sometimes distinct biological functions. However, in humans the four AGO proteins, AGO1–4, have overlapping roles in RISC formation and mRNA silencing (12, 27, 52). As depicted in Fig. 1, mature miRNA is loaded onto RISC. Then, one of the miRNA strands (the passenger strand) is degraded, while the other strand (the guide strand) complexes with AGO to form an active RISC, which is directed to the target mRNA based on the complementary sequences in the miRNA and mRNA. It is noteworthy that the AGO proteins possess robust endonuclease activity to degrade the target mRNA when a perfect complementary miRNA is present; however, the endonuclease activity of AGO is not always essential for RISC formation and translational repression of target mRNAs (10–12, 18, 27, 52).
Fig. 1.
Biogenesis and function of microRNAs (miRNA). Most of miRNA genes are transcribed via RNA polymerase II, leading to the generation of pri-miRNAs that are long transcripts with multiple hairpin loop structures. Pri-miRNAs are processed by the Microprocessor protein complex containing Drosha, resulting in the generation of smaller precursor molecules called pre-miRNAs. pre-miRNAs are then exported from the nucleus via exportin-5. In the cytoplasm, the pre-miRNAs are further processed via Dicer to generate short, double-stranded miRNAs, which are then converted to mature, single-stranded miRNAs via RISC, a protein complex containing argonaute (AGO). Finally, mature miRNAs direct RISC to target gene mRNA, resulting in mRNA degradation (miRNA finds perfect complementary sequences in mRNA) or translational repression (imperfect complementary sequences in mRNA).
miRNAs can repress target gene expression by either inducing mRNA degradation or by blocking protein translation; nevertheless, the latter mechanism is much more common in mammals (10, 11, 18, 52). It remains unclear as to how the binding of RISC (AGO/miRNA complex) to the 3′-UTR of target mRNA prevents protein translation. Several possibilities have been postulated: 1) deadenylation of the polyadenylated 3′-end may result in mRNA degradation; 2) the AGO-miRNA complex may compete with translation initiation factors, resulting in reduced translation initiation; 3) binding of the AGO-miRNA complex may induce premature termination and impaired elongation; and 4) the AGO-miRNA complex may recruit peptidases to degrade the growing polypeptide during translation (10, 11, 18).
The amount of a specific miRNA is determined by its biogenesis and decay. miRNA biogenesis is critically regulated at the level of gene transcription (52). Many of the miRNA genes are flanked by promoter regions that are very similar to those of protein-coding genes (12, 18, 52). The promoter activity of miRNA genes is governed by specific transcription factors like MYC, p53, and hypoxia-inducible factor 1 (HIF1α) (21, 25, 80). As a result, under conditions of genotoxic stress where p53 is activated and during hypoxia where HIF1α is activated, miRNAs such as miR-34 and miR-210 are upregulated, respectively, in a p53- or HIF1α-dependent manner (39, 60). These observations suggest that the expression of miRNAs is regulated at the gene transcription level in response to pathophysiological challenges. In addition, global miRNA expression can also be modulated by regulating the proteins or enzymes involved in miRNA processing, e.g., Drosha, Dicer, and AGO (52). miRNAs are also regulated via decay or degradation. It has been recognized that miRNAs can rapidly rise and then decrease under various cellular conditions, although the molecular machinery responsible for miRNA degradation is largely unknown (52).
miRNAs in Renal Pathophysiology
Renal diseases, including progressive kidney disease and acute kidney injury (AKI), are associated with high mortality and morbidity rates with very few effective treatment options. To identify novel treatment modalities, research in recent years has been focused on two major aspects: 1) new and more sensitive biomarkers for early diagnosis, and 2) pathogenic molecular targets or pathways that can be specifically targeted for therapy. The discovery of miRNAs as critical regulators of the fundamental activities of a cell, from proliferation and differentiation to apoptosis, suggests the involvement of miRNAs in the pathogenesis of various diseases including those of the kidneys. Indeed, research during the last couple of years has unveiled an emerging role of miRNAs in both chronic and acute kidney diseases. These studies fall into three general categories: 1) global depletion of miRNAs from specific cell types in kidneys by using conditional Dicer-knockout mouse models; 2) analysis of differential miRNA expression in renal diseases to identify potential pathogenic miRNA species; and 3) study of miRNA regulation of specific genes that play pathogenic roles in renal disease.
Dicer-knockout studies implicating miRNAs in renal pathophysiology.
Dicer is the enzyme responsible for the processing of pre-miRNAs into mature, functional miRNAs. As a result, genetic ablation of Dicer leads to global depletion of miRNAs (52). Germline knockout of Dicer results in embryonic lethality in mice, underscoring the critical role played by miRNAs in normal development (12, 52). Recently, tissue-specific deletion of Dicer has been employed as a strategy to study the effect of global miRNA repression in different organ systems (42). In kidneys, conditional knockout of Dicer has thus far been reported in podocytes (38, 41, 77), proximal tubules (92), and juxtaglomerular cells (74). Studies using these conditional knockout models have demonstrated striking phenotypes, providing compelling evidence for the involvement of miRNAs not only in kidney development and maintenance of normal renal function but also in the pathogenesis of renal diseases.
The podocyte-specific Dicer knockout model was reported in 2008 separately by three research groups (38, 41, 77). Notably, their findings are fairly consistent (38, 41, 77). Dicer ablation from podocytes results in proteinuria, tubulointerstitial fibrosis, glomerulosclerosis, and foot process effacement at 2–4 wk after birth. Remarkably, the defects rapidly progress into end-stage renal diseases in about 2 mo, resulting in increased mortality (42). The pathological abnormalities shown in the mouse model clearly suggest that Dicer is critical for the maintenance of podocyte homeostasis and associated renal functions. Mechanistically, it is suggested that the loss of miR-30 family miRNAs from podocytes may be responsible for the observed phenotype in the conditional Dicer-knockout model (38, 41, 42, 77). In line with this possibility, a recent study has demonstrated that the miR-30 family is a critical regulator of pronephric renal development in Xenopus laevis (1). Thus the miR-30 family miRNAs may contribute to the maintenance of the homoeostasis and function of podocytes in kidneys.
Sequeira-Lopez and colleagues (74) have recently established a mouse model with Dicer deletion in the renin-secreting juxtaglomerular cells. Mice in this model show an acute loss of juxtaglomerular cells as evidenced by histological, biochemical, and physiological analyses. As a result, renin expression in kidneys and plasma renin concentration are markedly reduced. Functionally, these changes manifest in the form of a significant decrease in blood pressure. Renal histology of the mice shows prominent striped fibrosis and vascular abnormalities. The latest work by Nagalakshmi et al. (62) further showed that when Dicer is specifically ablated from the progenitors of nephron epithelium, nephrogenesis is abrogated, underlying the critical role played by Dicer and miRNAs in kidney development. These results, along with the podocyte Dicer-knockout studies, suggest that Dicer and associated miRNA production are indispensable for normal structure and proper function of renal cells in kidneys.
We have established a conditional knockout mouse model in which Dicer is specifically ablated from the proximal tubular cells in kidneys (92). In this model, there is a global depletion of miRNAs from renal cortical tissues. Somewhat surprisingly, mice from this model show normal kidney size, histology, and function. The absence of renal phenotypes is most likely due to the late turn-on feature of the PEPCK promoter that drives Cre expression in proximal tubules in this conditional knockout model. In kidneys, the PEPCK promoter is not activated in proximal tubular cells until 3 wk after birth, a time point when kidney development has been mostly completed in mice. While the results do not rule in or out a role of miRNAs in kidney development, it is suggested that global depletion of miRNAs does not markedly affect the normal function and physiology of differentiated proximal tubules in adult mice. Strikingly, although these mice do not show phenotypes under normal physiological conditions, they are remarkably resistant to ischemia-reperfusion-induced kidney injury (see below for further discussion) (92).
These conditional Dicer-knockout studies indicate that miRNAs play critical roles in normal renal function or physiology and, when altered, may lead to renal diseases. Nonetheless, it is important to point out that, while global depletion of miRNAs in kidneys may result in renal diseases, it is unlikely for this to occur in humans. It is more likely that specific miRNAs are differentially regulated in renal diseases and contribute to their pathogenesis. If identified, these miRNAs could be used as either diagnostic tools or molecular targets for the prevention and therapy of the disease.
miRNA regulation in diabetic nephropathy.
Diabetic nephropathy leads to chronic renal failure and accounts for almost half the cases of end-stage renal disease (48). The disease is characterized by profound changes in renal vasculature accompanied by increased arterial blood pressure, reduced glomerular filtration rate (GFR), and most notably, proteinuria. Most of the pathological changes observed during diabetic nephropathy are associated with increased extracellular depositions in the glomerulus. Mesangial expansion as a result of accumulation of extracellular matrix and thickening of the glomerular basement membrane (GBM) is a key pathological feature of diabetic nephropathy (48). Although the exact molecular mechanism responsible for these pathological changes remains unclear, numerous studies have suggested the involvement of transforming growth factor-β (TGF-β), which may induce pathogenic collagen synthesis and cellular hypertrophy (48). Consistently, high levels of TGF-β are detected in the glomeruli of diabetic patients. Interestingly, recent studies have demonstrated compelling evidence for TGF-β regulation of miRNAs and its pathogenic role in diabetic nephropathy. In one of the first studies implicating miRNA regulation in renal diseases, Natarajan et al. (51) have identified miR-192 as a critical regulator of collagen production in diabetic nephropathy. In cultured mesangial cells and diabetic kidneys, miR-192 is induced via TGF-β and following induction, miR-192 targets the E-box repressor protein smad-interacting protein 1 (SIP1). Downregulation of SIP1 by miR-192 leads to the relief of the repression of col1a2 gene expression, resulting in increased collagen production and deposition in the mesangium (51), a hallmark of diabetic nephropathy. A more recent study by Chung et al. (19) has further suggested a role of miR-192 in renal fibrosis. Interestingly, in renal fibrosis models, miR-192 is also induced in a TGF-β-dependent manner, resulting in collagen production (19). In addition to miR-192, TGF-β also regulates the expression of miR-216a and miR-217 in mesangial cells during diabetic nephropathy (49, 50). Notably, these two miRNAs target phosphatase and tensin homolog deleted on chromosome 10 (PTEN) and due to PTEN repression, Akt, a key mediator of diabetic nephropathy, is activated. The latest work by Natarajan and colleagues (50) has further identified the RNA binding protein Ybx1 as a target of miR-216a during TGF-β-induced collagen expression in kidney cells (50). Together, these studies have established a regulatory role of specific miRNAs in TGF-β-dependent renal pathologies observed during diabetic nephropathy.
Are these miRNAs involved during diabetic nephropathy in humans? To answer this question, a recent study examined human renal biopsy samples from patients with established diabetic nephropathy (55). However, the findings are quite surprising and apparently contradictory to the results from diabetic mouse models. In contrast to the observations in diabetic mice, renal biopsy samples from diabetic patients show significantly lower miR-192 (55). Moreover, the decreased expression of miR-192 seems to directly correlate with tubulointerstitial fibrosis and a low GFR in individual patients. In addition, TGF-β suppresses miR-192 expression in cultured proximal tubular cells in this study. As suggested, TGF-β may regulate miR-192 differently in mesangial vs. proximal tubular cells (55). However, this explanation is challenged by the observations of both up- and downregulation of miR-192 by TGF-β in the same NRK52E renal tubular cell line (19, 85). Downregulation of miR-192 by TGF-β has also been shown in mesangial cells and apoE-knockout diabetic mouse kidneys. The cause of the discrepancies in the results from these studies is currently unclear. A recent study by Kriegel and colleagues (54) has further revealed miR-382 induction by TGF-β in human renal epithelial cells. A direct target of miR-382 is identified to be superoxide dismutase 2 (SOD2). Interestingly, blocking miR-382 or overexpressing SOD2 leads to E-cadherin expression, suggesting that miR-382 is involved in the phenotypic change in the cells during renal fibrosis in diabetic kidneys (54).
In addition to TGF-β induction and diabetic mouse models, miRNA expression has been examined during high-glucose or hyperglycemic treatment of cultured renal cells. While no significant changes in miRNA expression are detected during high-glucose incubation of HK2 human proximal tubular cells (55), high glucose induces miR-377, -129, and -337 in cultured human and mouse mesangial cells (91). The molecular targets of miR-377 are further identified as p21-activated kinase (PAK1) and manganese superoxide dismutase (mnSOD), which when inhibited resulted in fibronection expression, a characteristic of mesangial cells during diabetic nephropathy. A very recent study by Long and colleagues (59) has systematically analyzed miRNA expression in high glucose-treated podocytes and kidney microvascular endothelial cells and glomeruli of diabetic db/db mice. While a number of miRNAs show expression changes under these conditions, miR-93 is decreased in all samples. Importantly, follow-up studies demonstrate that miR-93 targets vascular endothelial growth factor (VEGF) (59), a crucial regulator of microvascular complications in diabetes. These results raise the possibility that increased renal VEGF expression in animal models of diabetes could be at least partially attributed to decreased miR-93 expression. Together, these findings have suggested the involvement of miRNAs in diabetic nephropathy, uncovering novel therapeutic targets for this devastating disease.
miRNA regulation in AKI.
AKI is a major kidney disease associated with high mortality and morbidity. Notably, it has been recently recognized that AKI is associated with increased risk of chronic kidney disease (CKD) and may be a key contributing factor in CKD (83). Despite decades of investigation, effective therapeutic approaches for AKI are still lacking. Recent studies have unveiled the role and regulation of miRNAs in AKI, raising hopes for novel and effective diagnostic and therapeutic strategies.
The first evidence for a pathogenic role of miRNAs in AKI was demonstrated by using a conditional Dicer-knockout model, in which Dicer was ablated specifically from renal proximal tubular cells in kidneys (92). Mice in this model show normal kidney development, histology, and function. However, when challenged by bilateral renal ischemia-reperfusion, the conditional Dicer-null mice are remarkably resistant to the ensuing AKI compared with their wild-type littermates (92). Better renal function and histology as well as significantly improved animal survival of Dicer-deficient mice provides compelling evidence for a pathogenic role of Dicer and associated miRNAs in ischemic AKI.
Then, which miRNAs may contribute critically to ischemic AKI? To address this question, renal cortical tissues collected from C57BL/6 mice at various time points following bilateral renal ischemia were analyzed by miRNA microarray (92). The microarray analysis has revealed significant expression changes in multiple miRNAs following renal ischemia-reperfusion. Interestingly, while some miRNAs are induced, others are downregulated in injured tissues. In addition, while some miRNAs change only at one time point, other miRNA species (e.g., miRNA-132, -362, -379, -668, and -687) show a continuous change during 12–48 h of reperfusion (92). By miRNA microarray, a recent study by Godwin and colleagues (35) also demonstrates miRNA expression changes during renal ischemia-reperfusion in C57BL/6 mice. However, the miRNA species that show significant changes in these two studies (35, 92) do not overlap. Although the exact cause of the discrepancy in the results from these two studies is not entirely clear, several major differences between the experimental models and analysis are noted. First, our study used a bilateral renal ischemia-reperfusion model, whereas Godwin et al. (35) used a unilateral model. There are notable differences between these two models. The bilateral model induces more severe AKI following the same ischemic duration and marked renal functional loss, which is undetectable in the unilateral model. In addition, the unilateral model is complicated by the compensatory response in the contralateral “control” kidney that is not subjected to ischemic injury. Second, we profiled miRNA expression at the acute (12 and 48 h of reperfusion) phase of AKI, while Godwin et al. covered late time points, up to 30 days postischemia. Finally, we analyzed renal cortical tissues, while Godwin et al. used RNA samples extracted from whole kidneys, including papillary, medullary, and cortical tissues.
In another recent study, we have specifically examined miR-34a expression and regulation in the AKI model of cisplatin nephrotoxicity (7). Cisplatin is a widely used cancer therapy drug, which has notable side effects in normal tissues, especially the kidneys. One of the major pathways leading to tubular cell injury and death in cisplatin nephrotoxicity involves a rapid DNA damage response and activation of the tumor suppressor protein p53 (67, 68, 71). We show that miR-34a is induced during cisplatin treatment of renal tubular cells in vitro and mouse kidneys in vivo (7). Interestingly, the inductive response is abrogated by pifithrin-α (a p53 inhibitor) and in p53-deficient mice. Surprisingly, inhibition of miR-34a results in increased apoptosis and reduced cell survival. Together, the results demonstrate a p53-dependent induction of miR-34a, which may play a protective role during cisplatin nephrotoxicity (7). By profiling of miRNA expression and analysis of specific miRNAs, future studies are expected to reveal miRNA changes in different models of AKI, resulting in the identification of potential targets for therapeutic intervention.
miRNA regulation in polycystic kidney disease.
Polycystic kidney diseases (PKD) are by far the most common genetically inherited renal diseases (33). The diseases are frequently associated with the mutation of specific genes, including PKD1, PKD2, and PKHD1. It is generally believed that dysregulated expression of these genes leads to abnormal cell division and proliferation, resulting in cyst formation in the kidneys (33). The role of miRNAs in PKD has only been recently implicated, mainly by two important findings. First, the PKD genes are targets of specific miRNAs, indicating a direct regulation of PKD gene expression and cystogenesis by miRNAs. Second, the overall miRNA expression pattern is changed in PKD models, suggesting that miRNAs may also act downstream of PKDs to affect disease progression. Translational repression of PKD2 by miR-17 has been reported by two groups (79, 81). Functionally, ectopic overexpression of miR-17 can promote cell proliferation in HEK cells by targeting PKD2 (79). In line with these findings, transgenic mice expressing artificial miRNAs targeting PKD1 develop PKD (86). In a rat model of PKD, 30 miRNAs are differentially expressed (mostly downregulated) in diseased tissues compared with normal animal tissues (69). Interestingly, bioinformatic analysis suggests that these miRNAs may target genes directly involved in the regulation of cell proliferation and cyst formation (69), a possibility that needs to be verified experimentally. A miRNA that may indeed link cell cycle regulation and PKD is miR-15a. In a rat PKD model and PKD patients, miR-15a is downregulated in cystic liver tissues. Moreover, ectopic expression of miR-15a can reduce cyst formation in an in vitro model. One target of miR-15a may be Cdc25A, a key cell cycle regulator. The results suggest that reduced miR-15a expression in PKD may contribute to hepatic cystogenesis by increasing Cdc25A expression and consequent cell proliferation (56). These studies support a profound role of miRNAs in PKD and related proliferative renal diseases.
miRNAs in renal allograft rejection.
Acute rejection and chronic allograft nephropathy are responsible for renal failure during kidney transplantation (9, 61). Using renal biopsies from patients with established acute rejection of transplanted kidneys, two studies have shed some light on the role of miRNAs in this pathological condition. In the first study, 8 miRNAs are upregulated and 12 are downregulated in the renal biopsies from acute rejection patients, compared with those of normal patients (78). Although the functional significance of the changes has not been studied, the differentially expressed miRNAs seem to have potential targets that may affect various processes during renal transplantation. In the second study, 17 miRNAs were found to be differentially expressed in acute rejection biopsies (3). Notably, there is a strong correlation between the expression of a subset of miRNAs (e.g.. miR-142–5p and miR-155) and acute rejection, suggesting that these miRNAs can be used as predictive markers for renal allograft function and rejection. In addition, this study raises the interesting possibility that some of the differentially regulated miRNAs could be from an extrarenal source, most probably immunological cells (3). Despite these reports, whether and how the differentially expressed miRNAs contribute to allograft rejection remains to be investigated.
miRNAs in kidney cancer.
Renal cell carcinoma (RCC) is the most common type of kidney cancer in adults. Changes in miRNA expression in RCC have been documented extensively, although the reported patterns are not very consistent (16, 17, 36, 40, 43–45, 63, 70, 93, 97). RCC is frequently associated with inactivation of the von Hippel-Lindau (VHL) tumor suppressor, resulting in elevated levels of hypoxia-inducible transcription factors (HIF) (46). Thus some of the miRNA changes observed in RCC may well be HIF dependent. In line with this idea, the hypoxia-dependent miRNA, miR-210, is upregulated in several types of tumors, including RCC (64), and may be a prognostic factor for cancer patients (60). Of note, Neal and colleagues (64) have shown recently that VHL may regulate miRNAs in both HIF-dependent and -independent manners. Compared with adjacent normal tissues, there is a significantly higher expression of miR-210, miR-155, and miR-21 in clear cell RCC tissues. While miR-210 expression correlates with VHL inactivation and HIF induction, several other miRNAs (e.g.. miR-21) are not expressed in response to HIF activation, suggesting HIF-independent regulation of these miRNAs. On the other hand, it is also important to recognize that some miRNAs are oncogenic and can act upstream of VHL, HIF, and other key factors in RCC to promote tumorigenesis (94).
In addition to RCC, recent research has also suggested the involvement of miRNAs in Wilm's tumor, a childhood kidney cancer. Notably, Wilm's tumor seems to involve the regulation of oncomir-1 (an oncogenic cluster of miRNAs located on chromosome 13) by the transcription factor E2F3 (53). By profiling of both mRNA and miRNA expression, Kort and colleagues (53) detected the highest oncomir-1 family expression in Wilm's tumors among the kidney tumor types analyzed. Interestingly, in tumor tissues oncomir-1 expression is paralleled by the expression of E2F3 (53), a transcription factor that has been implicated in governing oncomir-1 expression (96). More recent studies have further detected decreased expression miR-562 and -185, and increased expression of miR-483–3p in Wilms' tumors, and suggested their potential involvements in tumorigenesis (29, 84). The roles played by these and other miRNAs in renal cancer development, progression and treatment are being intensively investigated.
miRNAs in other renal diseases.
Preliminary evidence has also demonstrated differential miRNA expression in other renal diseases, including lupus nephritis, IgA nephropathy, and hypertensive renal injury. In renal biopsy samples from class II lupus nephritis patients, 66 miRNAs show significant changes in miRNA microarray analysis compared with normal kidney samples (23). A more recent study using peripheral blood mononuclear cells and Epstein-Barr virus-transformed cell lines derived from lupus nephritis patients has further identified five miRNAs (miR-371–5P, -423–5P, -638, -1224–3P, -663) that are differentially expressed in lupus nephritis across different racial groups. In IgA nephropathy, 35 miRNAs (of 132 miRNAs detected) are shown to be differentially expressed in renal biopsy samples. Interestingly, Wang and colleagues (87) have recently detected the expression of miR-200c, -141, -205, and -192 in renal biopsy of IgA nephropathy patients, correlating with the severity and progression of the disease. The same investigators also demonstrated increased expression of several miRNAs (miR-200a, -200b, -141, -429, miR-205, and miR-192) in hypertensive sclerosis of kidneys (88). It is important to note that despite these reports, the functional significance of the miRNAs in these renal diseases remains to be delineated. In this regard, the latest study by Liang and his laboratory (58) has suggested a protective role of miR-29b in renal injury during high salt-induced hypertension. miR-29b is upregulated in renal medullary tissues in Dahl salt-sensitive rats fed a high-salt diet. Importantly, knockdown of miR-29b results in expression of several collagen genes in the kidneys. In cultured renal medullary epithelial cells, miR-29b can suppress collagen expression. These findings demonstrate a negative regulatory role of miR-29b in collagen and extracellular matrix accumulation in hypertensive renal injury. Obviously, one of the major areas of emphasis and technical challenge in future investigations is identifying the functional significance of individual miRNAs in specific renal diseases.
miRNAs as diagnostic tools in renal diseases.
In the last few years, there has been a major thrust to identify novel and reliable biomarkers for renal diseases (8, 28, 82). As discussed above, miRNA studies in various renal diseases have shown not only that miRNA expression is differentially regulated but also that the expression pattern itself could be a useful tool for disease diagnosis. Multiple studies, especially in the cancer diagnostic field, have provided proof of principle that microRNA expression can be used as a useful diagnostic tool (5, 13, 31, 57, 61, 73). The relatively consistent changes of miRNAs in diseases, reliable methods of miRNA analysis, tissue-specific expression patterns, and their presence in blood and urine make miRNAs ideal candidates as renal disease biomarkers.
In 2007, Gottardo and colleagues (36) reported the upregulation of four miRNAs (miR-28, -185, -27, and let-7f-2) in RCC tissues. However, more recent studies by Huang and colleagues (44) compared the miRNA expression profiles in clear cell RCC and normal kidney tissues and detected changes in a much broader range of miRNAs. Apparently, these miRNA microarray analyses did not show consistent results, and more systematic profiling is required to identify a miRNA expression pattern that is applicable as a useful diagnostic tool. Nevertheless, these studies have suggested the potential use of miRNAs for RCC diagnosis and stage classification. miRNA expression has also been proposed to be a diagnostic marker for IgA nephropathy and lupus nephritis (23, 24). For example, by miRNA microarray analysis Dai and colleagues (24) identified 35 miRNAs that were upregulated in renal biopsy of IgA nephropathy patients. A more recent study further showed specific changes in several miRNAs in the urinary sediment of IgA nephropathy patients (89). Diagnostic use of miRNAs has also been suggested in acute renal allograft rejection in human patients. The Suthanthiran laboratory (3) demonstrated a distinguished intragraft miRNA profile of human allografts from patients with acute rejection. Moreover, the miRNA profile appears predictive of renal allograft function. The strong association between intragraft miRNA expression and renal allograft function or acute rejection indicates that miRNAs may serve as biomarkers of human renal allograft status.
Strategies and Challenges in Renal miRNA Research
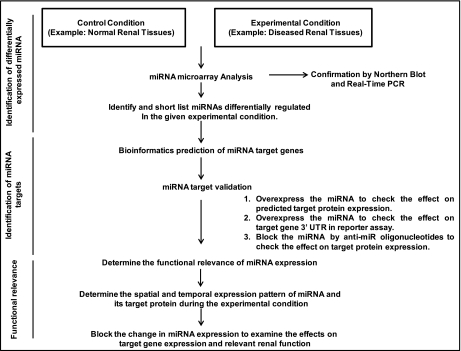
A broad strategy to identify and study miRNA regulation in renal physiology and pathology is described in Fig. 2. The general experimental strategy can be roughly divided into three parts: identification and characterization of miRNAs, delineation of miRNA target genes, and determination of the functional significance of miRNA regulation. These experimental strategies have been widely used to identify miRNA regulation in renal and other pathophysiological conditions.
Fig. 2.
General scheme to study miRNA regulation of renal pathophysiology. The research can be divided into 3 phases to identify the miRNAs, determine their targets, and delineate their pathophysiological roles. A flow chart is presented to depict the relevant studies.
As fascinating as it is, the research of miRNA in renal pathophysiology is highly challenging. In addition to the general research strategy discussed above, technical issues are frequently encountered. In the initial phase of research, miRNA expression changes have to be carefully verified. As discussed earlier, microarray analysis needs to be repeated with samples from separate experiments for better identification of differentially regulated miRNAs. Moreover, the miRNAs identified from the microarray analysis as pertinent to the study need to be subjected to further verification individually by real-time PCR analysis and Northern blotting. It is also noteworthy that the real-time PCR technique used for miRNA detection is not well standardized in general, and as a result inconsistent and sometimes erroneous results (noise) may be generated. It is therefore useful to confirm the real-time PCR results using other techniques like Northern blot analysis. However, probably, the most critical and challenging step is to predict and then verify the miRNA target genes. Multiple bioinformatic programs or databases are available that generally predict several potential target genes for a particular miRNA (2). Usually, the target prediction softwares offer broad sensitivity and specificity, generating numerous putative target genes of a specific miRNA (10). Thus biologically significant target gene identification that includes shortlisting and experimental verification of these targets can be a very tedious, subjective, and challenging process.
Kidneys are composed of different types of cells which may be differentially affected in various renal diseases. To understand the pathophysiological role played by a miRNA, it would be necessary to identify the cell type(s) that expresses the miRNA in a particular disease condition. The major tools used to study miRNAs are miRNA mimics and antisense oligonucleotides to overexpress or inhibit the function of a particular miRNA. These oligonucleotides are sometimes difficult to introduce in certain cell lines with low transfection efficiencies and may have some off-target or nonspecific effects when used in the in vivo experimental models. Transgenic animal models with conditional overexpression or knockdown of a particular miRNA might provide the best model to study the function and regulation of miRNA in renal diseases. Once the target protein/s has been experimentally validated, it is very important to identify its functional significance and its contribution to the pathophysiological condition. Identifying the functional significance of a change in miRNA regulation and its target genes under a certain condition can be very difficult because a single miRNA can target several proteins and a single protein can be targeted by several miRNAs.
As a final note, although miRNA regulation of renal diseases is an exciting emerging field of research, we should not be inundated by the information from numerous studies identifying miRNAs differentially regulated in various disease conditions. It is important to generate a coherent picture of the miRNAs, their targets, alterations in renal diseases, and pathophysiological roles, which provides a comprehensive view of the pathogenesis and hopefully leads to the development of novel therapeutics.
GRANTS
The study was supported in part by grants from the National Institutes of Health and Department of Veterans Affairs to Z. Dong and a predoctoral fellowship from the American Heart Association to K. Bhatt. Z. Dong is a Research Career Scientist of the Department of Veterans Affairs.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
REFERENCES
- 1. Agrawal R, Tran U, Wessely O. The miR-30 miRNA family regulates Xenopus pronephros development and targets the transcription factor Xlim1/Lhx1. Development 136: 3927–3936, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Analysis Databases. http://www.mirbase.org/http://www.microrna.org/microrna/home.dohttp://www.targetscan.org/
- 3. Anglicheau D, Sharma VK, Ding R, Hummel A, Snopkowski C, Dadhania D, Seshan SV, Suthanthiran M. MicroRNA expression profiles predictive of human renal allograft status. Proc Natl Acad Sci USA 106: 5330–5335, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bartel DP. MicroRNAs: target recognition and regulatory functions. Cell 136: 215–233, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bartels CL, Tsongalis GJ. MicroRNAs: novel biomarkers for human cancer. Clin Chem 55: 623–631, 2009 [DOI] [PubMed] [Google Scholar]
- 6. Beezhold KJ, Castranova V, Chen F. Microprocessor of microRNAs: regulation and potential for therapeutic intervention. Mol Cancer 9: 134, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bhatt K, Zhou L, Mi QS, Huang S, She JX, Dong Z. MicroRNA-34a is induced via p53 during cisplatin nephrotoxicity and contributes to cell survival. Mol Med 16: 409–416, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bonventre JV, Vaidya VS, Schmouder R, Feig P, Dieterle F. Next-generation biomarkers for detecting kidney toxicity. Nat Biotechnol 28: 436–440, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bosmans JL, Ysebaert DK, Verpooten GA. Chronic allograft nephropathy: what have we learned from protocol biopsies? Transplantation 85: S38–S41, 2008 [DOI] [PubMed] [Google Scholar]
- 10. Brodersen P, Voinnet O. Revisiting the principles of microRNA target recognition and mode of action. Nat Rev Mol Cell Biol 10: 141–148, 2009 [DOI] [PubMed] [Google Scholar]
- 11. Cai Y, Yu X, Hu S, Yu J. A brief review on the mechanisms of miRNA regulation. Genomics Proteomics Bioinformatics 7: 147–154, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Carthew RW, Sontheimer EJ. Origins and mechanisms of miRNAs and siRNAs. Cell 136: 642–655, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Casalini P, Iorio MV. MicroRNAs and future therapeutic applications in cancer. J Buon 14, Suppl 1: S17–S22, 2009 [PubMed] [Google Scholar]
- 14. Chang S, Wen S, Chen D, Jin P. Small regulatory RNAs in neurodevelopmental disorders. Hum Mol Genet 18: R18–R26, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Chen JF, Callis TE, Wang DZ. microRNAs and muscle disorders. J Cell Sci 122: 13–20, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Chow TF, Mankaruos M, Scorilas A, Youssef Y, Girgis A, Mossad S, Metias S, Rofael Y, Honey RJ, Stewart R, Pace KT, Yousef GM. The miR-17–92 cluster is over expressed in and has an oncogenic effect on renal cell carcinoma. J Urol 183: 743–751, 2010 [DOI] [PubMed] [Google Scholar]
- 17. Chow TF, Youssef YM, Lianidou E, Romaschin AD, Honey RJ, Stewart R, Pace KT, Yousef GM. Differential expression profiling of microRNAs and their potential involvement in renal cell carcinoma pathogenesis. Clin Biochem 43: 150–158, 2010 [DOI] [PubMed] [Google Scholar]
- 18. Chua JH, Armugam A, Jeyaseelan K. MicroRNAs: biogenesis, function and applications. Curr Opin Mol Ther 11: 189–199, 2009 [PubMed] [Google Scholar]
- 19. Chung AC, Huang XR, Meng X, Lan HY. miR-192 mediates TGF-beta/Smad3-driven renal fibrosis. J Am Soc Nephrol 21: 1317–1325, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Cordes KR, Srivastava D. MicroRNA regulation of cardiovascular development. Circ Res 104: 724–732, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Crosby ME, Devlin CM, Glazer PM, Calin GA, Ivan M. Emerging roles of microRNAs in the molecular responses to hypoxia. Curr Pharm Des 15: 3861–3866, 2009 [DOI] [PubMed] [Google Scholar]
- 22. da Costa Martins PA, Leptidis S, Salic K, De Windt LJ. MicroRNA regulation in cardiovascular disease. Curr Drug Targets 11: 900–906, 2010 [DOI] [PubMed] [Google Scholar]
- 23. Dai Y, Sui W, Lan H, Yan Q, Huang H, Huang Y. Comprehensive analysis of microRNA expression patterns in renal biopsies of lupus nephritis patients. Rheumatol Int 29: 749–754, 2009 [DOI] [PubMed] [Google Scholar]
- 24. Dai Y, Sui W, Lan H, Yan Q, Huang H, Huang Y. Microarray analysis of micro-ribonucleic acid expression in primary immunoglobulin A nephropathy. Saudi Med J 29: 1388–1393, 2008 [PubMed] [Google Scholar]
- 25. Dang CV, Le A, Gao P. MYC-induced cancer cell energy metabolism and therapeutic opportunities. Clin Cancer Res 15: 6479–6483, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Davis-Dusenbery BN, Hata A. Mechanisms of control of microRNA biogenesis. J Biochem 148: 381–392, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Davis BN, Hata A. Regulation of microRNA biogenesis: a miRiad of mechanisms. Cell Commun Signal 7: 18, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Devarajan P, Mishra J, Supavekin S, Patterson LT, Steven Potter S. Gene expression in early ischemic renal injury: clues towards pathogenesis, biomarker discovery, and novel therapeutics. Mol Genet Metab 80: 365–376, 2003 [DOI] [PubMed] [Google Scholar]
- 29. Drake KM, Ruteshouser EC, Natrajan R, Harbor P, Wegert J, Gessler M, Pritchard-Jones K, Grundy P, Dome J, Huff V, Jones C, Aldred MA. Loss of heterozygosity at 2q37 in sporadic Wilms' tumor: putative role for miR-562. Clin Cancer Res 15: 5985–5992, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Erson AE, Petty EM. MicroRNAs in development and disease. Clin Genet 74: 296–306, 2008 [DOI] [PubMed] [Google Scholar]
- 31. Fabbri M. miRNAs as molecular biomarkers of cancer. Expert Rev Mol Diagn 10: 435–444, 2010 [DOI] [PubMed] [Google Scholar]
- 32. Flynt AS, Lai EC. Biological principles of microRNA-mediated regulation: shared themes amid diversity. Nat Rev Genet 9: 831–842, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Gallagher AR, Germino GG, Somlo S. Molecular advances in autosomal dominant polycystic kidney disease. Adv Chronic Kidney Dis 17: 118–130, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Ghildiyal M, Zamore PD. Small silencing RNAs: an expanding universe. Nat Rev Genet 10: 94–108, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Godwin JG, Ge X, Stephan K, Jurisch A, Tullius SG, Iacomini J. Identification of a microRNA signature of renal ischemia reperfusion injury. Proc Natl Acad Sci USA 107: 14339–14344, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Gottardo F, Liu CG, Ferracin M, Calin GA, Fassan M, Bassi P, Sevignani C, Byrne D, Negrini M, Pagano F, Gomella LG, Croce CM, Baffa R. Micro-RNA profiling in kidney and bladder cancers. Urol Oncol 25: 387–392, 2007 [DOI] [PubMed] [Google Scholar]
- 37. Guller I, Russell AP. MicroRNAs in skeletal muscle: their role and regulation in development, disease and function. J Physiol 588: 4075–4087, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Harvey SJ, Jarad G, Cunningham J, Goldberg S, Schermer B, Harfe BD, McManus MT, Benzing T, Miner JH. Podocyte-specific deletion of dicer alters cytoskeletal dynamics and causes glomerular disease. J Am Soc Nephrol 19: 2150–2158, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Hermeking H. The miR-34 family in cancer and apoptosis. Cell Death Differ 17: 193–199, 2010 [DOI] [PubMed] [Google Scholar]
- 40. Hildebrandt MA, Gu J, Lin J, Ye Y, Tan W, Tamboli P, Wood CG, Wu X. Hsa-miR-9 methylation status is associated with cancer development and metastatic recurrence in patients with clear cell renal cell carcinoma. Oncogene 29: 5724–5728, 2010 [DOI] [PubMed] [Google Scholar]
- 41. Ho J, Ng KH, Rosen S, Dostal A, Gregory RI, Kreidberg JA. Podocyte-specific loss of functional microRNAs leads to rapid glomerular and tubular injury. J Am Soc Nephrol 19: 2069–2075, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Ho JJ, Marsden PA. Dicer cuts the kidney. J Am Soc Nephrol 19: 2043–2046, 2008 [DOI] [PubMed] [Google Scholar]
- 43. Horikawa Y, Wood CG, Yang H, Zhao H, Ye Y, Gu J, Lin J, Habuchi T, Wu X. Single nucleotide polymorphisms of microRNA machinery genes modify the risk of renal cell carcinoma. Clin Cancer Res 14: 7956–7962, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Huang Y, Dai Y, Yang J, Chen T, Yin Y, Tang M, Hu C, Zhang L. Microarray analysis of microRNA expression in renal clear cell carcinoma. Eur J Surg Oncol 35: 1119–1123, 2009 [DOI] [PubMed] [Google Scholar]
- 45. Juan D, Alexe G, Antes T, Liu H, Madabhushi A, Delisi C, Ganesan S, Bhanot G, Liou LS. Identification of a microRNA panel for clear-cell kidney cancer. Urology 75: 835–841, 2010 [DOI] [PubMed] [Google Scholar]
- 46. Kaelin WG., Jr The von Hippel-Lindau tumor suppressor gene and kidney cancer. Clin Cancer Res 10: 6290S–6295S, 2004 [DOI] [PubMed] [Google Scholar]
- 47. Karolina DS, Wintour EM, Bertram J, Jeyaseelan K. Riboregulators in kidney development and function. Biochimie 92: 217–225, 2010 [DOI] [PubMed] [Google Scholar]
- 48. Kato M, Arce L, Natarajan R. MicroRNAs and their role in progressive kidney diseases. Clin J Am Soc Nephrol 4: 1255–1266, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Kato M, Putta S, Wang M, Yuan H, Lanting L, Nair I, Gunn A, Nakagawa Y, Shimano H, Todorov I, Rossi JJ, Natarajan R. TGF-beta activates Akt kinase through a microRNA-dependent amplifying circuit targeting PTEN. Nat Cell Biol 11: 881–889, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Kato M, Wang L, Putta S, Wang M, Yuan H, Sun G, Lanting L, Todorov I, Rossi JJ, Natarajan R. Posttranscriptional upregulation of Tsc-22 by Ybx1, a target of miR-216a, mediates TGF-β-induced collagen expression in kidney cells. J Biol Chem 285: 34004–34015, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Kato M, Zhang J, Wang M, Lanting L, Yuan H, Rossi JJ, Natarajan R. MicroRNA-192 in diabetic kidney glomeruli and its function in TGF-beta-induced collagen expression via inhibition of E-box repressors. Proc Natl Acad Sci USA 104: 3432–3437, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Kim VN, Han J, Siomi MC. Biogenesis of small RNAs in animals. Nat Rev Mol Cell Biol 10: 126–139, 2009 [DOI] [PubMed] [Google Scholar]
- 53. Kort EJ, Farber L, Tretiakova M, Petillo D, Furge KA, Yang XJ, Cornelius A, Teh BT. The E2F3-Oncomir-1 axis is activated in Wilms' tumor. Cancer Res 68: 4034–4038, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Kriegel AJ, Fang Y, Liu Y, Tian Z, Mladinov D, Matus IR, Ding X, Greene AS, Liang M. MicroRNA-target pairs in human renal epithelial cells treated with transforming growth factor β1: a novel role of miR-382. Nucleic Acids Res 38: 8338–8347, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Krupa A, Jenkins R, Luo DD, Lewis A, Phillips A, Fraser D. Loss of MicroRNA-192 promotes fibrogenesis in diabetic nephropathy. J Am Soc Nephrol 21: 438–447, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Lee SO, Masyuk T, Splinter P, Banales JM, Masyuk A, Stroope A, Larusso N. MicroRNA15a modulates expression of the cell-cycle regulator Cdc25A and affects hepatic cystogenesis in a rat model of polycystic kidney disease. J Clin Invest 118: 3714–3724, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Liang M, Liu Y, Mladinov D, Cowley AW, Jr, Trivedi H, Fang Y, Xu X, Ding X, Tian Z. MicroRNA: a new frontier in kidney and blood pressure research. Am J Physiol Renal Physiol 297: F553–F558, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Liu Y, Taylor NE, Lu L, Usa K, Cowley AW, Jr, Ferreri NR, Yeo NC, Liang M. Renal medullary microRNAs in Dahl salt-sensitive rats: miR-29b regulates several collagens and related genes. Hypertension 55: 974–982, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Long J, Wang Y, Wang W, Chang BH, Danesh FR. Identification of microRNA-93 as a novel regulator of vascular endothelial growth factor in hyperglycemic conditions. J Biol Chem 285: 23457–23465, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. McCormick R, Buffa FM, Ragoussis J, Harris AL. The role of hypoxia regulated microRNAs in cancer. Curr Top Microbiol Immunol 345: 47–70, 2010 [DOI] [PubMed] [Google Scholar]
- 61. Naesens M, Sarwal MM. Molecular diagnostics in transplantation. Nat Rev Nephrol 6: 614–628, 2010 [DOI] [PubMed] [Google Scholar]
- 62. Nagalakshmi VK, Ren Q, Pugh MM, Valerius MT, McMahon AP, Yu J. Dicer regulates the development of nephrogenic and ureteric compartments in the mammalian kidney. Kidney Int 79: 317–330, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Nakada C, Matsuura K, Tsukamoto Y, Tanigawa M, Yoshimoto T, Narimatsu T, Nguyen LT, Hijiya N, Uchida T, Sato F, Mimata H, Seto M, Moriyama M. Genome-wide microRNA expression profiling in renal cell carcinoma: significant down-regulation of miR-141 and miR-200c. J Pathol 216: 418–427, 2008 [DOI] [PubMed] [Google Scholar]
- 64. Neal CS, Michael MZ, Rawlings LH, Van der Hoek MB, Gleadle JM. The VHL-dependent regulation of microRNAs in renal cancer. BMC Med 8: 64, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. O'Connell RM, Rao DS, Chaudhuri AA, Baltimore D. Physiological and pathological roles for microRNAs in the immune system. Nat Rev Immunol 10: 111–122, 2010 [DOI] [PubMed] [Google Scholar]
- 66. Olena AF, Patton JG. Genomic organization of microRNAs. J Cell Physiol 222: 540–545, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Pabla N, Dong Z. Cisplatin nephrotoxicity: mechanisms and renoprotective strategies. Kidney Int 73: 994–1007, 2008 [DOI] [PubMed] [Google Scholar]
- 68. Pabla N, Huang S, Mi QS, Daniel R, Dong Z. ATR-Chk2 signaling in p53 activation and DNA damage response during cisplatin-induced apoptosis. J Biol Chem 283: 6572–6583, 2008 [DOI] [PubMed] [Google Scholar]
- 69. Pandey P, Brors B, Srivastava PK, Bott A, Boehn SN, Groene HJ, Gretz N. Microarray-based approach identifies microRNAs and their target functional patterns in polycystic kidney disease. BMC Genomics 9: 624, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Petillo D, Kort EJ, Anema J, Furge KA, Yang XJ, Teh BT. MicroRNA profiling of human kidney cancer subtypes. Int J Oncol 35: 109–114, 2009 [DOI] [PubMed] [Google Scholar]
- 71. Price PM, Safirstein RL, Megyesi J. The cell cycle and acute kidney injury. Kidney Int 76: 604–613, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Saal S, Harvey SJ. MicroRNAs and the kidney: coming of age. Curr Opin Nephrol Hypertens 18: 317–323, 2009 [DOI] [PubMed] [Google Scholar]
- 73. Schaefer A, Stephan C, Busch J, Yousef GM, Jung K. Diagnostic, prognostic and therapeutic implications of microRNAs in urologic tumors. Nat Rev Urol 7: 286–297, 2010 [DOI] [PubMed] [Google Scholar]
- 74. Sequeira-Lopez ML, Weatherford ET, Borges GR, Monteagudo MC, Pentz ES, Harfe BD, Carretero O, Sigmund CD, Gomez RA. The microRNA-processing enzyme dicer maintains juxtaglomerular cells. J Am Soc Nephrol 21: 460–467, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Shenouda SK, Alahari SK. MicroRNA function in cancer: oncogene or a tumor suppressor? Cancer Metastasis Rev 28: 369–378, 2009 [DOI] [PubMed] [Google Scholar]
- 76. Shi M, Guo N. MicroRNA expression and its implications for the diagnosis and therapeutic strategies of breast cancer. Cancer Treat Rev 35: 328–334, 2009 [DOI] [PubMed] [Google Scholar]
- 77. Shi S, Yu L, Chiu C, Sun Y, Chen J, Khitrov G, Merkenschlager M, Holzman LB, Zhang W, Mundel P, Bottinger EP. Podocyte-selective deletion of dicer induces proteinuria and glomerulosclerosis. J Am Soc Nephrol 19: 2159–2169, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Sui W, Dai Y, Huang Y, Lan H, Yan Q, Huang H. Microarray analysis of MicroRNA expression in acute rejection after renal transplantation. Transpl Immunol 19: 81–85, 2008 [DOI] [PubMed] [Google Scholar]
- 79. Sun H, Li QW, Lv XY, Ai JZ, Yang QT, Duan JJ, Bian GH, Xiao Y, Wang YD, Zhang Z, Liu YH, Tan RZ, Yang Y, Wei YQ, Zhou Q. MicroRNA-17 post-transcriptionally regulates polycystic kidney disease-2 gene and promotes cell proliferation. Mol Biol Rep 37: 2951–2958, 2010 [DOI] [PubMed] [Google Scholar]
- 80. Takwi A, Li Y. The p53 pathway encounters the MicroRNA world. Curr Genomics 10: 194–197, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Tran U, Zakin L, Schweickert A, Agrawal R, Doger R, Blum M, De Robertis EM, Wessely O. The RNA-binding protein bicaudal C regulates polycystin 2 in the kidney by antagonizing miR-17 activity. Development 137: 1107–1116, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Vaidya VS, Waikar SS, Ferguson MA, Collings FB, Sunderland K, Gioules C, Bradwin G, Matsouaka R, Betensky RA, Curhan GC, Bonventre JV. Urinary biomarkers for sensitive and specific detection of acute kidney injury in humans. Clin Transl Sci 1: 200–208, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Venkatachalam MA, Griffin KA, Lan R, Geng H, Saikumar P, Bidani AK. Acute kidney injury: a springboard for progression in chronic kidney disease. Am J Physiol Renal Physiol 298: F1078–F1094, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Veronese A, Lupini L, Consiglio J, Visone R, Ferracin M, Fornari F, Zanesi N, Alder H, D'Elia G, Gramantieri L, Bolondi L, Lanza G, Querzoli P, Angioni A, Croce CM, Negrini M. Oncogenic role of miR-483–3p at the IGF2/483 locus. Cancer Res 70: 3140–3149, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Wang B, Herman-Edelstein M, Koh P, Burns W, Jandeleit-Dahm K, Watson A, Saleem M, Goodall GJ, Twigg SM, Cooper ME, Kantharidis P. E-cadherin expression is regulated by miR-192/215 by a mechanism that is independent of the profibrotic effects of transforming growth factor-beta. Diabetes 59: 1794–1802, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Wang E, Hsieh-Li HM, Chiou YY, Chien YL, Ho HH, Chin HJ, Leo Wang CK, Liang SC, Jiang ST. Progressive renal distortion by multiple cysts in transgenic mice expressing artificial microRNAs against Pkd1. J Pathol 222: 238–248, 2010 [DOI] [PubMed] [Google Scholar]
- 87. Wang G, Kwan BC, Lai FM, Choi PC, Chow KM, Li PK, Szeto CC. Intrarenal expression of microRNAs in patients with IgA nephropathy. Lab Invest 90: 98–103, 2010 [DOI] [PubMed] [Google Scholar]
- 88. Wang G, Kwan BC, Lai FM, Choi PC, Chow KM, Li PK, Szeto CC. Intrarenal expression of miRNAs in patients with hypertensive nephrosclerosis. Am J Hypertens 23: 78–84, 2010 [DOI] [PubMed] [Google Scholar]
- 89. Wang G, Kwan BC, Lai FM, Chow KM, Kam-Tao Li P, Szeto CC. Expression of microRNAs in the urinary sediment of patients with IgA nephropathy. Dis Markers 28: 79–86, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Wang N, Zhou Z, Liao X, Zhang T. Role of microRNAs in cardiac hypertrophy and heart failure. IUBMB Life 61: 566–571, 2009, 2010 [DOI] [PubMed] [Google Scholar]
- 91. Wang Q, Wang Y, Minto AW, Wang J, Shi Q, Li X, Quigg RJ. MicroRNA-377 is up-regulated and can lead to increased fibronectin production in diabetic nephropathy. FASEB J 22: 4126–4135, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Wei Q, Bhatt K, He HZ, Mi QS, Haase VH, Dong Z. Targeted deletion of Dicer from proximal tubules protects against renal ischemia-reperfusion injury. J Am Soc Nephrol 21: 756–761, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Weng L, Wu X, Gao H, Mu B, Li X, Wang JH, Guo C, Jin JM, Chen Z, Covarrubias M, Yuan YC, Weiss LM, Wu H. MicroRNA profiling of clear cell renal cell carcinoma by whole-genome small RNA deep sequencing of paired frozen and formalin-fixed, paraffin-embedded tissue specimens. J Pathol 222: 41–51, 2010 [DOI] [PubMed] [Google Scholar]
- 94. White NM, Yousef GM. MicroRNAs: exploring a new dimension in the pathogenesis of kidney cancer. BMC Med 8: 65, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Winter J, Jung S, Keller S, Gregory RI, Diederichs S. Many roads to maturity: microRNA biogenesis pathways and their regulation. Nat Cell Biol 11: 228–234, 2009 [DOI] [PubMed] [Google Scholar]
- 96. Woods K, Thomson JM, Hammond SM. Direct regulation of an oncogenic micro-RNA cluster by E2F transcription factors. J Biol Chem 282: 2130–2134, 2007 [DOI] [PubMed] [Google Scholar]
- 97. Yi Z, Fu Y, Zhao S, Zhang X, Ma C. Differential expression of miRNA patterns in renal cell carcinoma and nontumorous tissues. J Cancer Res Clin Oncol 136: 855–862, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]