Abstract
Endothelin-1 (ET-1), a potent vasoconstrictor, has been implicated in the pathogenesis of collagen accumulation, extracellular matrix remodeling, and renal and cardiac fibrosis in diabetes. However, the mechanism by which ET-1 promotes collagen accumulation remains unclear. Here, we analyzed the gene expression profile of ET-1-stimulated mesangial cells to identify determinants of collagen accumulation. In human mesangial cells (a microvascular pericyte that secretes excess collagen in diabetic glomerulosclerosis), ET-1 increased mRNA and protein for MCP-1 (macrophage chemoattractant protein-1) and IL-6. ET-1-induced MCP-1 and IL-6 mRNAs and proteins were blocked by an ETA (but not ETB) receptor antagonist. ET-1/ETA receptor signaling evoked a 7.4-fold increase in collagen accumulation. Exogenous addition of either recombinant MCP-1 or IL-6 increased collagen accumulation by 3.5-fold. Co-stimulation with both MCP-1 and IL-6 did not elevate collagen accumulation further. Neither an MCP-1-neutralizing antibody nor an MCP-1 receptor antagonist inhibited ET-1-induced collagen accumulation. Similarly, neutralizing antibodies against IL-6 or the gp130 subunit of the IL-6 receptor did not attenuate ET-1-induced collagen accumulation. However, co-incubation with MCP-1- and IL-6-neutralizing antibodies inhibited ET-1-induced collagen accumulation by 52%, suggesting a robust autocrine loop wherein MCP-1 and IL-6 are redundant. Taken together, these results demonstrate that an autocrine signaling loop involving MCP-1 and IL-6 contributes to ET-1-induced collagen accumulation.
Keywords: Diabetes, Extracellular Matrix, G Protein-coupled Receptors (GPCRs), Kidney, Signal Transduction, Endothelin-1, Fibrosis
Introduction
Endothelin-1 (ET-1)2 is an endothelial cell-derived vasoconstrictor and growth factor (1). Elevated secretion of ET-1 is a hallmark of endothelial dysfunction associated with insulin resistance, obesity, and diabetes (2). Chronic overexpression of ET-1 in transgenic mice leads to remodeling of the extracellular matrix with collagen deposition, fibrosis, and loss of organ function in the kidney, heart, and lung (3–6). In the context of diabetes, elevated secretion of ET-1 has been implicated in the development of renal and cardiac fibrosis (7), and ET-1 receptor antagonists represent a potential antifibrotic therapy. Interestingly, ET-1 promotes fibrosis in most experimental models without increasing vasoconstriction or blood pressure. However, the blood pressure-independent mechanism by which ET-1 stimulates fibrosis remains unclear.
To investigate mechanisms of ET-1-induced collagen accumulation and fibrosis, we (8, 9) and others (10) have studied ET-1 signal transduction in human mesangial cells, a microvascular pericyte that secretes excess collagen in diabetic kidney disease (11, 12). Deposition of excess mesangial matrix occurs early in transgenic mice that overexpress ET-1 and contributes to the development of glomerulosclerosis and a 35% decline in glomerular filtration rate (3, 4). In rat and mouse models of diabetic kidney disease, ET-1 receptor antagonists attenuate matrix deposition in the mesangium and slow or prevent diabetic glomerulosclerosis (13–18). In most models, ET-1 promotes mesangial matrix accumulation and glomerulosclerosis without increasing mean arterial pressure or altering glucose homeostasis (3, 4, 17).
How ET-1 promotes collagen accumulation independent of changes in blood pressure or blood glucose remains largely unclear. Previously, we have shown that exogenous ET-1 induces the genes encoding type I collagen (COL1A1 and COL1A2) in human mesangial cells (19). Consistent with accumulation of type I collagen, ET-1 induces a myofibroblast-like phenotype in mesangial cells with secretion and deposition of excess extracellular matrix proteins (20). ET-1 has been shown to induce the myofibroblast phenotype in primary fibroblasts isolated from the lung (21) and heart (22), suggesting that ET-1 stimulates this profibrotic phenotype in mesenchymal cells from multiple tissues. Stimulation of the MAPKs has been linked to ET-1-induced collagen accumulation (9), but these signals occur within minutes of ET-1 receptor activation, whereas the increase in collagen accumulation is detected 24–48 h after the addition of exogenous ET-1.
To identify determinants of ET-1-induced collagen accumulation, we analyzed the genome-wide gene expression profile of ET-1-stimulated mesangial cells. We report that ET-1 induces mRNA and protein secretion of MCP-1 (macrophage chemoattractant protein-1) and IL-6, forming an autocrine signaling pathway that stimulates collagen accumulation in the extracellular matrix.
EXPERIMENTAL PROCEDURES
Bioinformatics Analysis of the ET-1-induced Global Gene Expression Profile
To identify potential determinants of collagen accumulation, we employed gene set enrichment analysis with the DAVID bioinformatics platform (23, 24), using data from the Gene Ontology Consortium (25), to identify biological processes associated with our previously published set of 117 genes induced or repressed by ET-1 in human mesangial cells (available at the Gene Expression Omnibus under GEO accession numbers GSM9920–GSM9931) (19). The statistical significance of enrichment for biological processes was calculated using a modified Fisher's exact p value, a cutoff of p < 0.05, and a Benjamini correction for multiple testing (26).
Cultured Mesangial Cells
Human mesangial cells (Cambrex Corp., Walkersville, MD) were cultured and maintained as described previously (27, 28). Cells were positive for desmin, vimentin, and myosin IIA but did not stain for factor VIII, keratin, or common leukocyte antigen. In a typical experiment, cells in passages 4–9 were incubated in 0.5% fetal bovine serum for 24 h before the addition of 100 nm ET-1 (Peptides International). The media and cell monolayer were harvested for analysis of MCP-1 and IL-6 mRNAs, protein secretion, and collagen accumulation as described below. In some experiments, cells in 0.5% serum were preincubated for 3 h with the following receptor antagonists or neutralizing mouse monoclonal antibodies before the addition of ET-1: BQ-123 (250 nm) and BQ-788 (1.0 μm) (both from Peptides International), ETA- and ETB-selective receptor antagonists, respectively; anti-MCP-1 (5 μg/ml; clone 24822), anti-IL-6 (0.1 μg/ml; clone 6708), and anti-gp130 (2.0 μg/ml; clone 28126) (R&D Biosystems); and RS504393 (10 μm; Tocris Bioscience), an MCP-1 receptor antagonist. Actinomycin D (Sigma) was added at 5 μg/ml to block transcription. In other experiments, human recombinant MCP-1 and IL-6 (R&D Biosystems) were added to cells made quiescent for 24 h in 0.5% serum.
Measurements of ET-1-induced Gene Expression by Quantitative PCR (qPCR)
Total RNA was extracted for measurement of MCP-1 and IL-6 mRNA levels by qPCR (29). Gene-specific primer pairs were designed using Primer 3 (available upon request), and mRNA levels were normalized by GAPDH mRNA in the same sample. A template-negative control was included in each primer/probe set reaction. A standard dilution curve was constructed to ensure that the amount of input cDNA was within the linear dynamic range of detection (30).
Measurements of MCP-1 and IL-6 Secretion
Cells in 24-well plates were held in 0.5% FBS for 24 h before the addition of ET-1 or ET-1 receptor antagonists. MCP-1 and IL-6 secretion into the supernatant was measured by ELISA (R&D Systems) and corrected for cell number. Absorbance was recorded in 96-well plates using a SpectraMax 190 microplate reader (Molecular Devices). Wells with medium alone served as the blank.
Quantitative Assessment of Collagen Accumulation in the Extracellular Matrix
Collagen accumulation in the extracellular matrix was measured as a fraction of total protein using differential binding of Sirius red F3B and fast green FCF to collagen and non-collagen proteins, respectively, in methanol-fixed cells in the presence of picric acid (31, 32). Sirius red dye binds specifically to the (Gly-X-Y)n helical structure found in all collagens and thus does not discriminate between collagen subtypes. The amount of collagen produced was expressed as micrograms of collagen divided by milligrams of total protein (i.e. collagen + non-collagenous protein) exactly as described (31, 32).
Measurement of p44 Phospho-MAPK or Phospho-ERK1 (Thr-202/Tyr-204) as a Readout of MCP-1 and IL-6 Signaling
After treating mesangial cells as described above, the monolayers were scraped into lysis buffer (20 mm Tris (pH 7.5), 150 mm NaCl, 1 mm EDTA, 1 mm EGTA, 1% Triton X-100, 2.5 mm sodium pyrophosphate, 1 mm β-glycerophosphate, 1 mm Na3VO4, 1 μg/ml leupeptin, and 1 mm phenylmethylsulfonyl fluoride) at 4 °C, followed by sonification and centrifugation at 10,00 × g for 10 min. The amount of p44 phospho-MAPK normalized for total MAPK was measured by ELISA (Cell Signaling Technology) exactly as described by the manufacturer.
Statistical Analysis
Data are means ± S.D. for at least three independent experiments performed in duplicate. Statistical significance was calculated by unpaired Student's t test for single comparisons or by analysis of variance followed by a Bonferroni post hoc test for multiple comparisons as appropriate using IBM SPSS Version 17.
RESULTS
ET-1/ETA Receptor Signaling Induces Secretion of MCP-1 and IL-6
To identify genes that might function in ET-1-induced collagen accumulation, we analyzed a set of 117 genes up-regulated or down-regulated in response to ET-1 in mesangial cells (19) using gene set enrichment and annotation for biological processes (Table 1). The most statistically significant annotation related to fibrosis was the Gene Ontology (GO) term “response to wounding” (Table 1). Eighteen of the 117 ET-1-regulated genes were present in the response to wounding gene set, including the first messengers MCP-1 (also known as CCL2) and IL-6 (supplemental Table 1). Because mesangial cells express cognate receptors for MCP-1 and IL-6 (11, 33), we postulated that ET-1 promotes collagen accumulation by activating an autocrine signaling loop involving these mediators.
TABLE 1.
Enriched GO annotations for biological processes in the ET-1-regulated gene set ranked by p value
The ET-1-regulated gene set was uploaded into the DAVID bioinformatics platform, and the highest ranked GO annotations for biological processes were determined using data from the Gene Ontology Consortium (25). The number of ET-1-regulated genes within the specific annotation category was used to calculate p values by Fisher's exact method with Benjamini correction for multiple testing.
| Biological process | GO ID | p value | No. of ET-1-regulated genes | p value (Benjamini) |
|---|---|---|---|---|
| Response to organic substance | GO:0010033 | 2.1 × 10−8 | 23 | 3.2 × 10−5 |
| Response to nutrient | GO:0007584 | 1.2 × 10−7 | 11 | 8.7 × 10−5 |
| Response to wounding | GO:0009611 | 5.4 × 10−7 | 18 | 2.7 × 10−4 |
| Negative regulation of apoptosis | GO:0043066 | 1.7 × 10−5 | 13 | 2.3 × 10−3 |
| TGF-β receptor signaling pathway | GO:0007179 | 9.5 × 10−5 | 6 | 9.5 × 10−3 |
| Collagen fibril organization | GO:0030199 | 1.4 × 10−3 | 4 | 5.5 × 10−2 |
| Extracellular matrix organization | GO:0030198 | 8.4 × 10−3 | 5 | 1.8 × 10−1 |
| Cytokine-mediated signaling pathways | GO:0019221 | 1.7 × 10−2 | 4 | 2.5 × 10−1 |
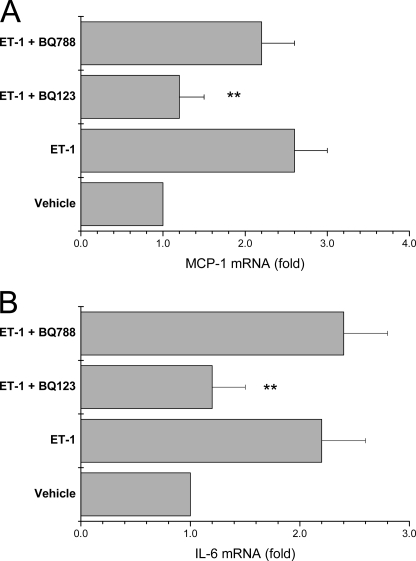
We validated the microarray measurements of ET-1-stimulated MCP-1 and IL-6 mRNAs by qPCR. ET-1 increased MCP-1 and IL-6 mRNAs by 2.9- and 2.6-fold, respectively, at 24 h (Fig. 1, A and B). Pretreatment of mesangial cells with actinomycin D to inhibit RNA polymerase II blocked induction of MCP-1 and IL-6 mRNAs by ET-1 (Fig. 1, A and B). Human mesangial cells express both ETA and ETB receptors (9). However, increments in MCP-1 and IL-6 mRNAs were blocked by the ETA-selective receptor antagonist BQ-123, but not by the ETB-selective receptor antagonist BQ-788 (Fig. 2, A and B).
FIGURE 1.
ET-1-induced MCP-1 and IL-6 mRNAs require de novo RNA synthesis. ET-1 (100 nm) was added to serum-starved mesangial cells for the times indicated, and mRNA levels for MCP-1 (A) and IL-6 (B) were quantified by qPCR normalized for GAPDH. Vehicle is medium alone without ET-1. Cells were preincubated for 30 min with 5 μg/ml actinomycin D (ActD) to block RNA polymerase II activity before adding ET-1 at time 0. Data are means ± S.D. for four independent experiments performed in duplicate. **, p < 0.01 versus vehicle or ET-1 + actinomycin D.
FIGURE 2.
ETA receptor signaling induces MCP-1 and IL-6 mRNAs. Serum-starved cells were preincubated for 3 h with an ETA-selective (BQ-123, 250 nm) or ETB-selective (BQ-788, 1.0 μm) receptor antagonist before stimulation with 100 nm ET-1 for 24 h and measurement of MCP-1 (A) and IL-6 (B) mRNAs by qPCR. Vehicle is medium alone without ET-1. Data are means ± S.D. for three independent experiments performed in duplicate. **, p < 0.01 versus cells stimulated with ET-1 or with ET-1 and BQ-788.
Whether the observed increments in MCP-1 and IL-6 mRNAs result in increased protein secretion was assessed in mesangial cells exposed to exogenous ET-1 (Fig. 3, A and B). MCP-1 and IL-6 secretion was elevated in mesangial cells stimulated with ET-1, with maximal -fold stimulation at 24 h. Consistent with the mRNA data, the ETA (but not ETB)-selective receptor antagonist blocked secretion of MCP-1 and IL-6 proteins in cells treated with ET-1 (Fig. 3, A and B).
FIGURE 3.
ETA receptor signaling stimulates secretion of MCP-1 and IL-6 proteins by human mesangial cells. Serum-starved cells were preincubated for 3 h with BQ-123 (250 nm) or BQ-788 (1.0 μm) before the addition of 100 nm ET-1. Secretion of MCP-1 (A) and IL-6 (B) proteins into the supernatant was measured by ELISA normalized for cell number. Data are means ± S.D. for three independent experiments performed in duplicate. **, p < 0.01 versus cells stimulated with ET-1 or with ET-1 and BQ-788.
To determine whether ET-1-induced MCP-1 secretion is dependent on IL-6 secretion, we pretreated cells with anti-IL-6 antibody before stimulation with ET-1. The neutralizing antibody against IL-6 did not alter ET-1-induced MCP-1 secretion (Fig. 4A). Similarly, IL-6 secretion was not blocked by a neutralizing antibody against MCP-1 (Fig. 4B). Collectively, these results demonstrate that ET-1 increases MCP-1 and IL-6 mRNAs and peptide secretion in mesangial cells. Moreover, the ET-1-induced effect was mediated by the ETA receptor.
FIGURE 4.
Effect of neutralizing antibodies on ET-1-induced MCP-1 and IL-6 protein secretion. Serum-starved mesangial cells were preincubated for 1 h with neutralizing monoclonal antibodies against IL-6 (0.1 μg/ml; clone 6708) (A) and MCP-1 (5 μg/ml; clone 24822) (B) before the addition of 100 nm ET-1 at time 0. MCP-1 and IL-6 peptide secretion was measured by ELISA. To control for nonspecific effects, an irrelevant monoclonal antibody (No IgG; 5 μg/ml) was added. Data are means ± S.D. for three experiments performed in duplicate, and there was not a statistically significant difference in MCP-1 or IL-6 secretion at any time point.
Characterization of Agonist-stimulated Accumulation of Total Collagen in Mesangial Cells
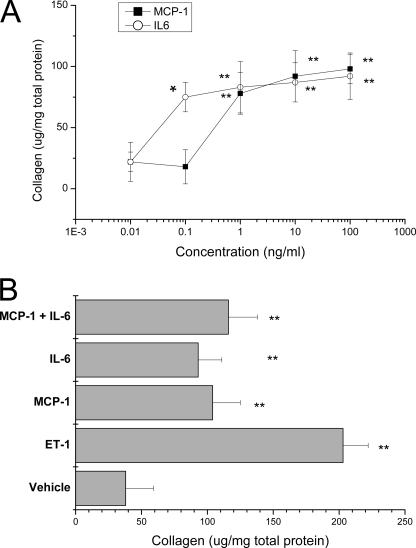
The effect of agonists on accumulation of total collagen in the mesangial cell matrix has not been determined. The addition of exogenous ET-1 increased total collagen accumulation by 7.4-fold compared with cells treated with vehicle (Fig. 5). Collagen accumulation in ET-1-treated cells was blocked by the ETA (but not ETB) receptor antagonist (Fig. 5). To determine whether MCP-1 or IL-6 elevates accumulation of total collagen, exogenous human recombinant MCP-1 or IL-6 alone and in combination were added to serum-starved mesangial cells. MCP-1 stimulated a dose-dependent increase in collagen accumulation, with a maximal 3.6-fold elevation at ∼1.0 ng/ml (Fig. 6A). Exogenous IL-6 also increased collagen accumulation dose-dependently, with a 3.8-fold elevation at 0.1 ng/ml (Fig. 6A). Co-stimulation with 1.0 ng/ml MCP-1 and 0.1 ng/ml IL-6 did not increase collagen accumulation more than either agonist alone (Fig. 6B), demonstrating that the increase in collagen accumulation by MCP-1 and IL-6 is not additive. Moreover, co-stimulation with exogenous MCP-1 and IL-6 increased collagen accumulation less than ET-1 alone (Fig. 6B).
FIGURE 5.
ET-1/ETA receptor signaling elevates collagen secretion in mesangial cells. Quiescent mesangial cells were pretreated for 3 h with BQ-123 (250 nm) and BQ-788 (1.0 μm) before the addition of 100 nm ET-1 at time 0 and measurement of total collagen in the cell monolayer at the times indicated. Data are means ± S.D. for three independent experiments performed in duplicate. **, p < 0.01 versus cells treated with vehicle or with ET-1 and BQ-123 at the same time point.
FIGURE 6.
Exogenous recombinant MCP-1 and IL-6 increase collagen accumulation in mesangial cells. A, serum-starved cells were treated with increasing concentrations of human recombinant MCP-1 or IL-6 for 48 h, and collagen in the extracellular matrix was assessed. *, p < 0.05; **, p < 0.01 versus cells treated with 0.01 ng/ml MCP-1 or IL-6. B, cells were treated for 48 h with 1.0 ng/ml MCP-1 or 0.1 ng/ml IL-6 alone and in combination, and collagen accumulation was measured. In concurrent wells, ET-1 at 100 nm was added for 48 h. Data are means ± S.D. for three independent experiments. **, p < 0.01 versus cells treated with vehicle.
ET-1/ETA Receptor Signaling Stimulates Collagen Accumulation through an Autocrine Signaling Loop Involving MCP-1 and IL-6
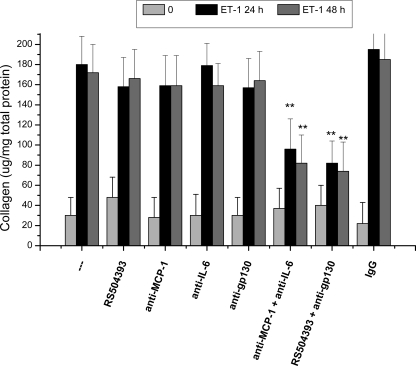
We next asked whether secretion of MCP-1 or IL-6 contributes to collagen accumulation in mesangial cells stimulated with ET-1. To determine whether ET-1-induced collagen accumulation requires autocrine signaling by MCP-1, we pretreated mesangial cells with an MCP-1-selective receptor antagonist (RS504393) or an MCP-1-neutralizing antibody before the addition of exogenous ET-1. In cells exposed to ET-1, collagen accumulation was not significantly reduced by RS504393 (Fig. 7). That the concentration of RS504393 employed was sufficient to inhibit signaling from the MCP-1 G protein-coupled receptor was demonstrated in mesangial cells exposed to exogenous MCP-1 using activation of p44 MAPK as a readout of MCP-1 receptor signaling (supplemental Fig. 1). Similarly, a neutralizing antibody that binds human MCP-1 and prevents receptor activation did not reduce ET-1-induced collagen accumulation (Fig. 7 and supplemental Fig. 1). As a control for possible effects of the MCP-1 receptor antagonist or MCP-1-neutralizing antibody on ET-1 receptor signaling, we confirmed that RS504393 and the MCP-1-neutralizing antibody did not block ET-1-induced p44 MAPK activation (data not shown). In addition, nonspecific murine IgG did not inhibit ET-1-induced collagen accumulation in mesangial cells (Fig. 7). Collectively, these results suggest that inhibition of MCP-1 secretion or MCP-1 receptor activation does not attenuate ET-1-induced collagen accumulation.
FIGURE 7.
Autocrine signaling by MCP-1 and IL-6 contributes to ET-1-induced collagen accumulation. Serum-starved human mesangial cells were preincubated for 1 h with an MCP-1 receptor antagonist (RS504393, 10 μm), an MCP-1-neutralizing antibody (5 μg/ml), a neutralizing antibody against the gp130 subunit of the IL-6 receptor (2.0 μg/ml), or a neutralizing antibody against IL-6 (0.1 μg/ml) alone and in combination before the addition of 100 nm ET-1 at time 0. As a control, nonspecific IgG (last bars) was added at a concentration of 5 μg/ml. Collagen accumulation was assessed in the cell monolayer in three experiments performed in duplicate. **, p < 0.01 versus cells treated with ET-1 alone at the same time point.
To determine the role of autocrine signaling by IL-6, we pretreated mesangial cells with neutralizing antibodies directed against either the IL-6 protein or the gp130 component of the IL-6 receptor complex. Neither antibody was sufficient to inhibit ET-1-induced collagen accumulation (Fig. 7). We confirmed that the neutralizing antibodies were added at concentrations that block IL-6 signaling using p44 MAPK activation as a readout (supplemental Fig. 1).
We next asked whether simultaneous antagonism of MCP-1 and IL-6 signaling inhibits ET-1-induced collagen accumulation. Co-incubation with MCP-1- and IL-6-neutralizing antibodies inhibited 48% of ET-1-induced collagen accumulation at 24 h and 53% at 48 h (Fig. 7). Similarly, co-incubation with RS504393 and anti-gp130 antibody inhibited ET-1-induced collagen accumulation by 53% at 24 h and by 56% at 48 h (Fig. 7).
DISCUSSION
ET-1 promotes collagen accumulation and fibrosis in vivo by incompletely characterized mechanisms that do not involve alterations of blood pressure or glucose homeostasis (3, 4, 17). We present evidence that ET-1 increases collagen deposition in the extracellular matrix by inducing an autocrine signaling loop involving secretion of MCP-1 and IL-6. In addition, this autocrine signaling loop is biologically robust in that MCP-1 and IL-6 appear to be redundant.
To identify putative downstream determinants of ET-1-induced collagen accumulation, we analyzed the ET-1-regulated gene set in human mesangial cells using enrichment analysis to identify genes that participate in biological pathways that control fibrosis. The ET-1-regulated gene set was significantly enriched in GO annotations for response to wounding (GO:0009611), defined as genes that regulate wounding responses to biological stress and cell injury. The ET-1-regulated gene set was also associated with additional annotations related to extracellular matrix accumulation, including “collagen fibril organization” (GO:0030199) and “extracellular matrix organization” (GO:0030198). In this study, we elucidated the role of ET-1-induced MCP-1 and IL-6 in autocrine regulation of collagen accumulation.
We demonstrated that ET-1 elevates MCP-1 and IL-6 mRNA levels and MCP-1 and IL-6 secretion via activation of ETA receptors, consistent with the requirement for ETA receptors in ET-1-stimulated collagen accumulation. Previous studies have shown that ET-1/ETA receptor signaling elevates MCP-1 and IL-6 secretion in vascular smooth muscle cells (34) (35) and IL-6 secretion in human fibroblasts (36). MCP-1 mRNA is elevated in the myocardia of transgenic mice that conditionally overexpress ET-1 (5), suggesting that ET-1-induced chemokine or cytokine secretion might be relevant in vivo. However, whether chemokine or cytokine secretion contributes to ET-1-induced phenotypes has not been established.
Our results suggest that ET-1-induced MCP-1 and IL-6 function in an autocrine signaling loop that stimulates collagen accumulation in the extracellular matrix. An important finding was that inhibition of either MCP-1 or IL-6 signaling alone does not block ET-1-induced collagen accumulation. Inhibition of both MCP-1 and IL-6 signaling was necessary to inhibit collagen accumulation in mesangial cells. The redundancy of mediators in autocrine signaling loops, as we have documented for MCP-1 and IL-6 in mesangial cells, is a feature of biologically robust signaling pathways that are resistant to perturbation (37). The robustness of the ET-1-regulated autocrine signaling loop might have important consequences for the use of therapeutic strategies aimed at antagonizing MCP-1 or IL-6 signaling in diabetic kidney injury.
Another important finding is that inhibition of autocrine signaling by both MCP-1 and IL-6 reduced collagen by ∼52% in mesangial cells exposed to ET-1. The additional pathway(s) involved in ET-1-induced collagen accumulation is unclear, but putative candidates might be found in the ET-1-regulated set of genes with annotations related to wound healing and fibrosis (supplemental Table 1). In particular, ET-1 induces two genes that encode the extracellular regulatory molecules TGF-β and SPP1 (secreted phosphoprotein-1; also called osteopontin), which elevate extracellular matrix accumulation in cultured mesangial cells (38, 39). Additional studies are necessary to determine whether TGF-β and SPP1 function independently of the MCP-1 and IL-6 autocrine loop to mediate ET-1-induced collagen accumulation in mesangial cells.
Supplementary Material
Acknowledgment
We thank Ragnath Mishra for excellent technical assistance.
This work was supported by the Rosenberg Foundation of the Centers for Dialysis Care and the Divisions of Nephrology and Hypertension and Endocrinology at Case Western Reserve University.

The on-line version of this article (available at http://www.jbc.org) contains supplemental Fig. 1 and Table 1.
- ET-1
- endothelin-1
- qPCR
- quantitative PCR
- GO
- Gene Ontology.
REFERENCES
- 1. Yanagisawa M., Kurihara H., Kimura S., Tomobe Y., Kobayashi M., Mitsui Y., Yazaki Y., Goto K., Masaki T. (1988) Nature 332, 411–415 [DOI] [PubMed] [Google Scholar]
- 2. Rask-Madsen C., King G. L. (2007) Nat. Clin. Pract. Endocrinol. Metab. 3, 46–56 [DOI] [PubMed] [Google Scholar]
- 3. Hocher B., Thöne-Reineke C., Rohmeiss P., Schmager F., Slowinski T., Burst V., Siegmund F., Quertermous T., Bauer C., Neumayer H. H., Schleuning W. D., Theuring F. (1997) J. Clin. Invest. 99, 1380–1389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Shindo T., Kurihara H., Maemura K., Kurihara Y., Ueda O., Suzuki H., Kuwaki T., Ju K. H., Wang Y., Ebihara A., Nishimatsu H., Moriyama N., Fukuda M., Akimoto Y., Hirano H., Morita H., Kumada M., Yazaki Y., Nagai R., Kimura K. (2002) J. Mol. Med. 80, 105–116 [DOI] [PubMed] [Google Scholar]
- 5. Yang L. L., Gros R., Kabir M. G., Sadi A., Gotlieb A. I., Husain M., Stewart D. J. (2004) Circulation 109, 255–261 [DOI] [PubMed] [Google Scholar]
- 6. Amiri F., Virdis A., Neves M. F., Iglarz M., Seidah N. G., Touyz R. M., Reudelhuber T. L., Schiffrin E. L. (2004) Circulation 110, 2233–2240 [DOI] [PubMed] [Google Scholar]
- 7. Clozel M., Salloukh H. (2005) Ann. Med. 37, 2–12 [DOI] [PubMed] [Google Scholar]
- 8. Simonson M. S., Wann S., Mené P., Dubyak G. R., Kester M., Nakazato Y., Sedor J. R., Dunn M. J. (1989) J. Clin. Invest. 83, 708–712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Simonson M. (2001) in Endothelin and Its Inhibitors (Warner T. ed) pp. 115–140, Springer, Berlin [Google Scholar]
- 10. Sorokin A., Kohan D. E. (2003) Am. J. Physiol. Renal Physiol. 285, F579–F589 [DOI] [PubMed] [Google Scholar]
- 11. Mené P., Simonson M. S., Dunn M. J. (1989) Physiol. Rev. 69, 1347–1424 [DOI] [PubMed] [Google Scholar]
- 12. Fioretto P., Caramori M. L., Mauer M. (2008) Diabetologia 51, 1347–1355 [DOI] [PubMed] [Google Scholar]
- 13. Benigni A., Colosio V., Brena C., Bruzzi I., Bertani T., Remuzzi G. (1998) Diabetes 47, 450–456 [DOI] [PubMed] [Google Scholar]
- 14. Hocher B., Schwarz A., Reinbacher D., Jacobi J., Lun A., Priem F., Bauer C., Neumayer H. H., Raschack M. (2001) Nephron 87, 161–169 [DOI] [PubMed] [Google Scholar]
- 15. Sasser J. M., Sullivan J. C., Hobbs J. L., Yamamoto T., Pollock D. M., Carmines P. K., Pollock J. S. (2007) J. Am. Soc. Nephrol. 18, 143–154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Dhein S., Hochreuther S., Aus Dem Spring C., Bollig K., Hufnagel C., Raschack M. (2000) J. Pharmacol. Exp. Ther. 293, 351–359 [PubMed] [Google Scholar]
- 17. Watson A. M., Li J., Schumacher C., de Gasparo M., Feng B., Thomas M. C., Allen T. J., Cooper M. E., Jandeleit-Dahm K. A. (2010) Diabetologia 53, 192–203 [DOI] [PubMed] [Google Scholar]
- 18. Gagliardini E., Corna D., Zoja C., Sangalli F., Carrara F., Rossi M., Conti S., Rottoli D., Longaretti L., Remuzzi A., Remuzzi G., Benigni A. (2009) Am. J. Physiol. Renal Physiol. 297, F1448–F1456 [DOI] [PubMed] [Google Scholar]
- 19. Mishra R., Leahy P., Simonson M. S. (2003) Am. J. Physiol. Cell Physiol. 285, C1109–C1115 [DOI] [PubMed] [Google Scholar]
- 20. Simonson M. S. (2007) Kidney Int. 71, 846–854 [DOI] [PubMed] [Google Scholar]
- 21. Shi-Wen X., Chen Y., Denton C. P., Eastwood M., Renzoni E. A., Bou-Gharios G., Pearson J. D., Dashwood M., du Bois R. M., Black C. M., Leask A., Abraham D. J. (2004) Mol. Biol. Cell 15, 2707–2719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Chintalgattu V., Katwa L. C. (2004) J. Pharmacol. Exp. Ther. 311, 691–699 [DOI] [PubMed] [Google Scholar]
- 23. Huang D. W., Sherman B. T., Lempicki R. A. (2009) Nat. Protoc. 4, 44–57 [DOI] [PubMed] [Google Scholar]
- 24. Dennis G., Jr., Sherman B. T., Hosack D. A., Yang J., Gao W., Lane H. C., Lempicki R. A. (2003) Genome Biol. 4, P3. [PubMed] [Google Scholar]
- 25. Ashburner M., Ball C. A., Blake J. A., Botstein D., Butler H., Cherry J. M., Davis A. P., Dolinski K., Dwight S. S., Eppig J. T., Harris M. A., Hill D. P., Issel-Tarver L., Kasarskis A., Lewis S., Matese J. C., Richardson J. E., Ringwald M., Rubin G. M., Sherlock G. (2000) Nat. Genet. 25, 25–29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Benjamini Y., Hochberg Y. (1995) J. R. Stat. Soc. Ser. B Stat. Methodol. 57, 289–300 [Google Scholar]
- 27. Shultz P. J., DiCorleto P. E., Silver B. J., Abboud H. E. (1988) Am. J. Physiol. 255, F674–F684 [DOI] [PubMed] [Google Scholar]
- 28. Mishra R., Leahy P., Simonson M. S. (2002) Am. J. Physiol. Renal Physiol. 283, F1151–F1159 [DOI] [PubMed] [Google Scholar]
- 29. Mishra R., Simonson M. S. (2008) Arterioscler. Thromb. Vasc. Biol. 28, 541–547 [DOI] [PubMed] [Google Scholar]
- 30. Bustin S. A., Benes V., Garson J. A., Hellemans J., Huggett J., Kubista M., Mueller R., Nolan T., Pfaffl M. W., Shipley G. L., Vandesompele J., Wittwer C. T. (2009) Clin. Chem. 55, 611–622 [DOI] [PubMed] [Google Scholar]
- 31. López-De León A., Rojkind M. (1985) J. Histochem. Cytochem. 33, 737–743 [DOI] [PubMed] [Google Scholar]
- 32. Jimenez W., Parés A., Caballería J., Heredia D., Bruguera M., Torres M., Rojkind M., Rodés J. (1985) Hepatology 5, 815–818 [DOI] [PubMed] [Google Scholar]
- 33. Schlondorff D., Banas B. (2009) J Am Soc Nephrol 20, 1179–1187 [DOI] [PubMed] [Google Scholar]
- 34. Sutcliffe A. M., Clarke D. L., Bradbury D. A., Corbett L. M., Patel J. A., Knox A. J. (2009) Br. J. Pharmacol. 157, 436–450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Browatzki M., Schmidt J., Kübler W., Kranzhöfer R. (2000) Basic Res. Cardiol. 95, 98–105 [DOI] [PubMed] [Google Scholar]
- 36. Solini A., Santini E., Madec S., Cuccato S., Ferrannini E. (2007) Growth Factors 25, 392–399 [DOI] [PubMed] [Google Scholar]
- 37. Stelling J., Sauer U., Szallasi Z., Doyle F. J., 3rd, Doyle J. (2004) Cell 118, 675–685 [DOI] [PubMed] [Google Scholar]
- 38. Mason R. M., Wahab N. A. (2003) J. Am. Soc. Nephrol. 14, 1358–1373 [DOI] [PubMed] [Google Scholar]
- 39. Sodhi C. P., Phadke S. A., Batlle D., Sahai A. (2001) Am. J. Physiol. Renal Physiol. 280, F667–F674 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.