Abstract
Chondrosarcoma is a type of highly malignant tumor with a potent capacity to invade locally and cause distant metastasis. Chondrosarcoma shows a predilection for metastasis to the lungs. IL-6 is a multifunctional cytokine that is associated with the disease status and outcomes of cancers. However, the effect of IL-6 on the migration activity of human chondrosarcoma cells is mostly unknown. Here, we found that IL-6 increased the migration and expression of MMP-13 in human chondrosarcoma cells. We also found that human chondrosarcoma tissues had significant expression of IL-6, which was higher than that in normal cartilage. IL-6-mediated migration and MMP-13 up-regulation were attenuated by anti-IL-6 receptor antibody, Ras, Raf-1, and a MEK inhibitor. Activation of the Ras, Raf-1, MEK, ERK, and NF-κB signaling pathways after IL-6 treatment was demonstrated, and IL-6-induced MMP-13 expression and migration activity were inhibited by the specific inhibitor and mutant Ras, Raf-1, MEK, ERK, and NF-κB cascades. In addition, migration-prone sublines demonstrated that cells with increasing migration ability had greater expression of IL-6 and MMP-13. Taken together, these results indicate that IL-6 and IL-6 receptor interaction enhances migration of chondrosarcoma through an increase in MMP-13 production.
Keywords: Cell Migration, Cell Motility, Interleukin, Matrix Metalloproteinase, Ras
Introduction
Chondrosarcoma is the second most common malignancy of bone, and it has a poor response to the chemotherapy or radiation treatment currently used, making the management of chondrosarcomas a complicated challenge (1). Clinically, surgical resection remains the primary mode of therapy for chondrosarcoma. In the absence of an effective adjuvant therapy, this mesenchymal malignancy has a poor prognosis, and therefore, it is important to explore novel and adequate remedies (2). Because chondrosarcoma is a type of highly malignant tumor with a potent capacity to invade locally and metastasize distantly (2), an approach that decreases its ability to invade and metastasize may facilitate the development of effective adjuvant therapy.
The tumor metastatic cascade consists of multiple successive steps, including adhesion of tumor cells at the primary site, invasion into the intravascular space, dissemination to distant sites, adhesion of tumor cells to the vascular endothelium of distant tissues, extravasation and invasion into surrounding tissues, and finally formation of secondary tumor colonies (3). To facilitate the cell motility, invading cells need to change the cell-cell adhesion properties, rearrange the extracellular matrix environment, suppress anoikis, and reorganize their cytoskeletons (4). Matrix metalloproteinases (MMPs)3 have important roles in these processes because their proteolytic activities assist in degradation of the extracellular matrix and basement membrane (5, 6). MMPs, cytokines, growth factors, and chemokines have been shown to regulate tumor cell invasion through autocrine or paracrine pathways (4). Previous studies demonstrated the expression of MMP-1, -2, -3, -9, and MMP-13 in human chondrosarcoma cells (7, 8). MMP-13 also mediates the chemokine-induced migration of chondrosarcoma (8, 9).
IL-6, originally identified as a T-cell-derived cytokine that induces final maturation of B-cells into antibody-producing cells (10), exhibits multiple biological activities that differ widely among various types of tissues and cells. Many investigators have reported that IL-6 can enhance or inhibit the proliferation of carcinoma cells (11–14) and that a variety of malignant tumors, including oral squamous cell carcinomas and adenocarcinomas, contain or synthesize IL-6, and autocrine growth stimulation has been suggested as the possible mechanism for the action of IL-6 (15–17). IL-6 is implicated in the development and progression of tumors of various organs, including myeloma and renal, prostate, and ovarian cancers (18, 19).
Ras has been found to couple with multiple effector systems to activate distinct physiological and pathological responses such as cell proliferation and proinflammatory mediator release (20, 21). An important class of Ras effectors is the MAPK family. The classic Ras-mediated pathway involves the binding of Raf-1 and subsequent phosphorylation of Raf-1 at Ser338 by many kinases (22, 23), which in turn activates ERKs (23) and consequently phosphorylates many target proteins, including transcription factors and protein kinases (24). A role for Ras in cancer migration has been implied in many cell types (25, 26). However, the role of the Ras/Raf-1/MEK/ERK pathway in IL-6-mediated cancer migration has not been investigated in chondrosarcoma. Here, we show that IL-6 increases migration and up-regulates MMP-13 expression in human chondrosarcoma cells. In addition, the IL-6 receptor, Ras, Raf-1, MEK, ERK, and NF-κB signaling pathways are involved.
EXPERIMENTAL PROCEDURES
Materials
Protein A/G beads; horseradish peroxidase-conjugated anti-mouse and anti-rabbit IgG; rabbit polyclonal antibodies specific for phospho-MEK, MEK, phospho-ERK, ERK, Raf-1, p65, and β-actin; MMP-13 and control siRNAs; and control shRNA and IL-6 shRNA plasmids were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). U0126, PD98059, l-1-tosylamido-2-phenylethyl chloromethyl ketone (TLCK), and pyrrolidine dithiocarbamate (PDTC) were purchased from Calbiochem. Rabbit polyclonal antibodies specific for phospho-Raf-1 (Ser338) and phospho-p65 (Ser536) were purchased from Cell Signaling Technology (Danvers, MA). A Ras activity assay kit was purchased from Upstate Biotechnology (Lake Placid, NY). Recombinant human IL-6 was purchased from PeproTech (Rocky Hill, NJ). The MEK1 dominant-negative mutant was a gift from Dr. W. M. Fu (National Taiwan University, Taipei, Taiwan). The ERK2 dominant-negative mutant was a gift from Dr. M. Cobb (University of Texas Southwestern Medical Center, Dallas, TX). The NF-κB-luciferase plasmid was purchased from Stratagene (La Jolla, CA). The pSV-β-galactosidase vector and luciferase assay kit were purchased from Promega (Madison, MA). All other chemicals were purchased from Sigma.
Cell Culture
The human chondrosarcoma cell line JJ012 was kindly provided by the laboratory of Dr. Sean P. Scully (University of Miami School of Medicine, Miami, FL). The human chondrosarcoma cell line SW1353 was obtained from American Type Culture Collection. The cells were cultured in Dulbecco's modified Eagle's medium/α-minimum essential medium supplemented with 10% fetal bovine serum and maintained at 37 °C in a humidified atmosphere of 5% CO2.
Patients and Specimen Preparation
Upon approval by the local ethics committee, specimens of tumor tissue or normal cartilage tissue were obtained from patients who had been pathologically diagnosed with chondrosarcoma or knee osteoarthritis (the articular cartilage was collected) and had undergone surgical resection at the China Medical University Hospital. Tissue specimens were ground and then sonicated in lysis buffer. The protein level was analyzed by Western blotting.
Migration and Invasion Assay
The migration assay was performed using Transwell 24-well plates (pore size of 8 μm; Costar). For invasion assay, filters were precoated with 25 μl of Matrigel basement membrane matrix (BD Biosciences) for 30 min. The following procedures were the same for both migration and invasion assays. Cells were pretreated for 30 min with different concentrations of inhibitors, including U0126, PD98059, or the vehicle control (0.1% Me2SO). Approximately 1 × 104 cells in 200 μl of serum-free medium were placed in the upper chamber, and 300 μl of the same medium containing IL-6 was placed in the lower chamber. The plates were incubated for 24 h at 37 °C in 5% CO2, and then cells were fixed in methanol for 15 min and stained with 0.05% crystal violet in PBS for 15 min. Cells on the upper side of the filters were removed with cotton-tipped swabs, and the filters were washed with PBS. Cells on the underside of the filters were examined and counted under a microscope. Each clone was plated in triplicate in each experiment, and each experiment was repeated at least three times. The number of invading cells in each experiment was adjusted by the cell viability assay to correct for proliferation effects of IL-6 treatment (corrected invading cell number = counted invading cell number/percentage of viable cells) (27).
Wound-healing Migration Assay
For the wound-healing migration assay, cells were seeded on 12-well plates at a density of 1 × 105 cells/well in culture medium. Twenty-four h after seeding, the confluent monolayer of the culture was scratched with a fine pipette tip, and migration was visualized under a microscope with magnification. The rate of wound closure was observed at the indicated times.
Western Blot Analysis
The cell lysates were prepared as described previously (28). Proteins were resolved by SDS-PAGE and transferred to Immobilon PVDF membranes. The blots were blocked with 4% BSA for 1 h at room temperature and then probed with rabbit anti-human antibody against MEK, phospho-MEK, ERK, or phospho-ERK (1:1000) for 1 h at room temperature. After three washes, the blots were subsequently incubated with peroxidase-conjugated donkey anti-rabbit secondary antibody (1:1000) for 1 h at room temperature. The blots were visualized by enhanced chemiluminescence using Kodak X-Omat LS film. The activities of ERK were determined using a kit from Cell Signaling Technology according to the manufacturer's instructions.
Ras Activity Assay
Ras activity was measuring using a Ras activity assay kit. The assay was performed according to the manufacturer's instructions. Briefly, cells were washed twice with ice-cold PBS, lysed in magnesium lysis buffer (25 mm HEPES (pH 7.5), 150 mm NaCl, 5% IGEPAL CA-630 (Upstate Biotechnology), 10 mm MgCl2, 5 mm EDTA, 10% glycerol, 10 μg/ml aprotinin, and 10 μg/ml leupeptin), and centrifuged. An equal volume of lysate was incubated with 5 μg of the Raf-1 Ras-binding domain at 4 °C overnight, and beads were washed three times with magnesium lysis buffer. Bound Ras proteins were then solubilized in 2× Laemmli sample buffer and quantitatively detected by Western blotting (10% SDS-PAGE) using mouse anti-Ras monoclonal antibody with the ECL system and by densitometry of the corresponding bands using scientific imaging systems.
Zymography Analysis
The supernatants of JJ012 cells were mixed with sample buffer without reducing agent or heating. The sample was loaded onto a gelatin (1 mg/ml)-containing SDS-polyacrylamide gel and subjected to electrophoresis with constant voltage. Afterward, the gel was washed with 2.5% Triton X-100 to remove SDS, rinsed with 50 mm Tris-HCl (pH 7.5), and then incubated overnight at room temperature with developing buffer (50 mm Tris-HCl (pH 7.5), 5 mm CaCl2, 1 μm ZnCl2, 0.02% thimerosal, and 1% Triton X-100) (8).
Quantitative Real-time PCR
Total RNA was extracted from chondrosarcomas using a TRIzol kit (MDBio, Inc., Taipei, Taiwan). Two μg of total RNA was reverse-transcribed into cDNA using an oligo(dT) primer. Quantitative real-time PCR (qPCR) analysis was carried out using TaqMan® One-Step PCR Master Mix (Applied Biosystems). Two μl of total cDNA was added per 25-μl reaction with sequence-specific primers and TaqMan® probes. Sequences for all target gene primers and probes were purchased commercially (with GAPDH used as an internal control) (Applied Biosystems). qPCR assays were carried out in triplicate on an StepOnePlus sequence detection system. The cycling conditions were 10 min of polymerase activation at 95 °C, followed by 40 cycles at 95 °C for 15 s and at 60 °C for 60 s. The threshold was set above the non-template control background and within the linear phase of target gene amplification to calculate the cycle number at which the transcript was detected (denoted CT).
Transfection and Reporter Gene Assay
Human chondrosarcoma cells were cotransfected with 0.8 μg of NF-κB-luciferase plasmid and 0.4 μg of β-galactosidase expression vector. JJ012 cells were grown to 80% confluence in 12-well plates and transfected on the following day with Lipofectamine 2000 (Invitrogen). DNA and Lipofectamine 2000 were premixed for 20 min and then applied to cells. After 24 h of transfection, the cells were incubated with the indicated agents. After a further 24 h of incubation, the media were removed, and cells were washed once with cold PBS. To prepare lysates, 100 μl of reporter lysis buffer (Promega) was added to each well, and cells were scraped from the dishes. The supernatant was collected after centrifugation at 13,000 rpm for 2 min. Aliquots of cell lysates (20 μl) containing equal amounts of protein (20–30 μg) were placed into the wells of an opaque black 96-well microplate. An equal volume of luciferase substrate was added to all samples, and luminescence was measured in a microplate luminometer. The value of luciferase activity was normalized to transfection efficiency monitored by the cotransfected β-galactosidase expression vector.
Chromatin Immunoprecipitation Assay
ChIP analysis was performed as described previously (27). DNA immunoprecipitated by anti-p65 antibody was purified. The DNA was then extracted with phenol/chloroform. The purified DNA pellet was subjected to PCR. PCR products were then resolved by 1.5% agarose gel electrophoresis and visualized by UV light. Primers 5′-AACAAGAGATGCTCTCA-3′ and 5′-TGAATGGTGATGCCTGG-3′ were utilized to amplify the human MMP-13 promoter region (−182 to +27).
Establishment of Migration-prone Sublines
Subpopulations of JJ012 cells were selected according to their differential migration ability using the cell culture insert system as described above. After 24 h of migration, cells that penetrated through pores and migrated to the underside of the filters were trypsinized and harvested for a second round of selection. The original cells that did not pass through membrane pores were designated as JJ012(S0). After 10 rounds of selection, the migration-prone subline was designated as JJ012(S10) (29).
Establishment of Stably Transfected Cells
IL-6 or control shRNA plasmids were transfected into cancer cells with Lipofectamine 2000 transfection reagent. Twenty-four h after transfection, stable transfectants were selected in puromycin (Invitrogen) at a concentration of 10 μg/ml. Thereafter, the selection medium was replaced every 3 days. After 2 weeks of selection in puromycin, clones of resistant cells were isolated.
Statistics
The values given are means ± S.E. The significant difference between the experimental groups and controls was assessed by Student's t test. The difference was significant if the p value was <0.05.
RESULTS
IL-6-directed Chondrosarcoma Cell Migration via the IL-6 Receptor
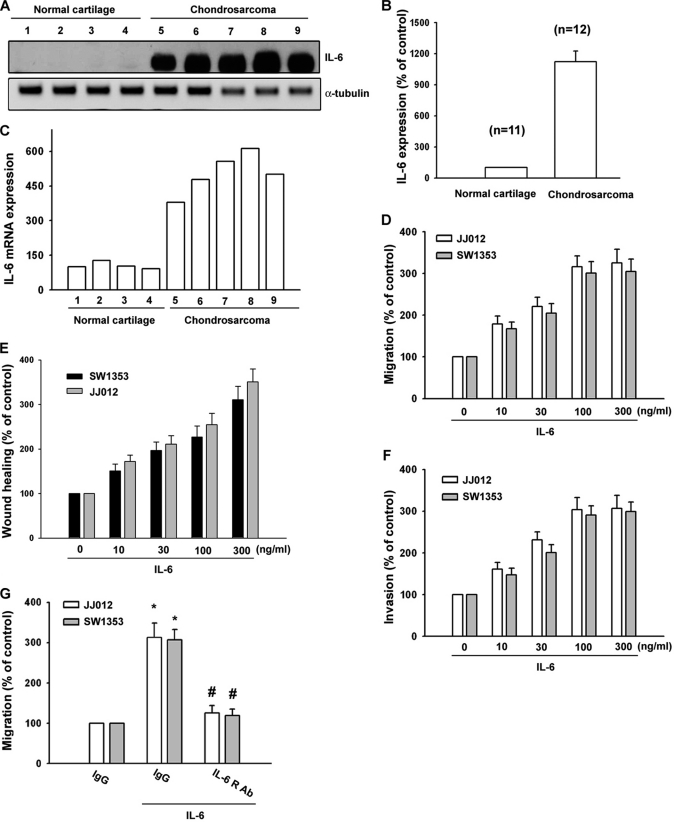
IL-6 has been reported to stimulate directional migration and invasion of human cancer cells. However, little is known about the expression of IL-6 in human chondrosarcoma cells. We examined human chondrosarcoma patients for expression of IL-6 by Western blotting and qPCR. The protein and mRNA levels of IL-6 in chondrosarcoma patients (Fig. 1, A and C, lanes 1–4) were significantly higher than in normal cartilage (Fig. 1, A and C, lanes 5–9). The qualification data of IL-6 protein expression are shown in Fig. 1B. We next examined the migration activity of human chondrosarcoma cells using the Transwell assay. IL-6 directed human chondrosarcoma cell (JJ012 and SW1353) migration (Fig. 1D). On the other hand, IL-6 also increased wound-healing activity in human chondrosarcoma cells (Fig. 1E). The invasion ability of chondrosarcoma cells through the Matrigel basement membrane matrix was increased by IL-6 stimulation (Fig. 1F). Thus, expression of IL-6 was associated with an invasive and/or metastatic phenotype of chondrosarcoma cells. In addition, pretreatment of cells for 30 min with anti-IL-6 receptor (IL-6R) Ab (5 μg/ml) markedly inhibited the IL-6-induced cell migration (Fig. 1G). These data suggest that IL-6-induced cancer migration may occur via the IL-6 receptor.
FIGURE 1.
IL-6 induces the migration activity of human chondrosarcoma cells. A, total proteins were extracted from chondrosarcoma patients and normal cartilage and subjected to Western blot analysis for IL-6. B, quantitative data are shown. C, total RNAs were extracted from chondrosarcoma patients and normal cartilage and subjected to qPCR analysis for IL-6. D and F, cells were incubated with IL-6 for 24 h, and in vitro migration and invasion were measured with the Transwell assay after 24 h. E, cells were treated with IL-6 (10–300 ng/ml) for 48 h, and the wound-scratching assay was performed. G, cells were pretreated with anti-IL-6R Ab (5 μg/ml) for 30 min followed by stimulation with IL-6 for 24 h, and in vitro migration was measured with the Transwell assay after 24 h. Results are expressed as means ± S.E. *, p < 0.05 compared with the control; #, p < 0.05 compared with the IL-6-treated group.
Involvement of MMP-13 in the IL-6-directed Cell Migration of Chondrosarcoma
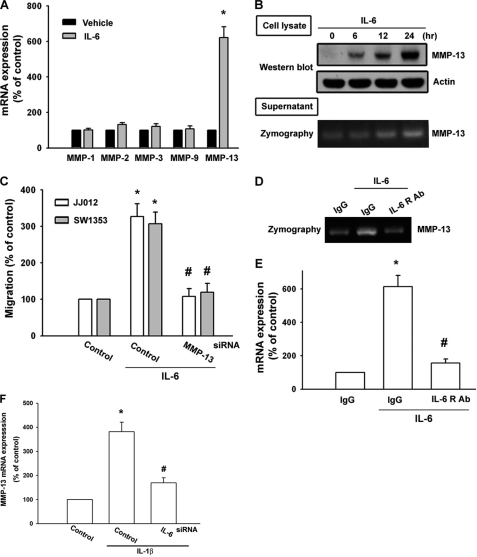
Previous studies have shown a significant expression of MMP-1, -2, -3, -9, and -13 in human chondrosarcoma cells (7, 8). We therefore hypothesized that any of these MMPs may be involved in IL-6-directed chondrosarcoma migration. Treatment of cells with IL-6 induced the expression of MMP-13 but not other MMPs as determined by qPCR (Fig. 2A). Furthermore, IL-6 increased protein expression of MMP-13 in JJ012 cells (Fig. 2B). MMP-13 expression was also increased in the supernatant, and its enzyme activity was up-regulated (Fig. 2B). Transfection of cells with MMP-13 siRNA reduced the IL-6-increased cell migration (Fig. 2C). On the other hand, MMP-13 mRNA expression and enzyme activity were abolished by anti-IL-6R Ab (Fig. 2, D and E), confirming the involvement of IL-6 in MMP-13 regulation. It has been reported that IL-1β induces IL-6 and MMP-13 expression in chondrosarcoma cells (39). Inhibition of IL-6 by IL-6 siRNA following IL-1β treatment diminished MMP-13 expression (Fig. 2F). Whether IL-6 and MMP-13 are downstream molecules in IL-1β-mediated inflammatory responses needs further examination.
FIGURE 2.
IL-6-directed migration activity of human chondrosarcoma cells involves up-regulation of MMP-13. A, JJ012 cells were incubated with IL-6 for 24 h, and the mRNA levels of MMP-1, -2, -3, -9, and -13 were determined by qPCR. B, JJ012 cells were incubated with IL-6 for the indicated time periods. The cultured medium and cell lysates were then collected. The protein level of MMP-13 in cell lysates and the enzyme activity of MMP-13 in the supernatant were examined by Western blotting and zymography. C, cells were transfected with MMP-13 siRNA for 24 h followed by stimulation with IL-6, and in vitro migration was measured after 24 h. D and E, JJ012 cells were pretreated with anti-IL-6R Ab for 30 min followed by stimulation with IL-6 for 24 h. The mRNA level of MMP-13 in cell lysates and the enzyme activity of MMP-13 in the supernatant were examined by qPCR and zymography. F, JJ012 cells were transfected with IL-6 or control siRNA for 24 h followed by stimulation with IL-1β, and mRNA expression of MMP-13 was examined by qPCR after 24 h. Results are expressed as means ± S.E. *, p < 0.05 compared with the control; #, p < 0.05 compared with the IL-6-treated group.
Ras and Raf-1 Signaling Pathways Are Involved in the IL-6-mediated Migration of Chondrosarcoma Cells
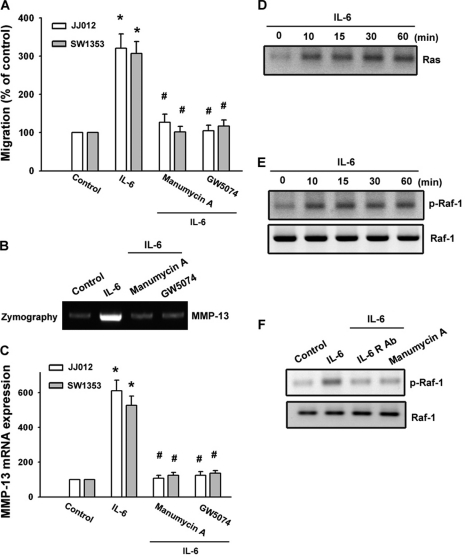
It has been demonstrated that Ras mediates cancer migration (25, 26). To explore whether Ras might mediate IL-6-induced cancer migration, the Ras inhibitor manumycin A was used. As shown in Fig. 3 (A–C), pretreatment of cells with manumycin A inhibited IL-6-induced cell migration and MMP-13 expression. Next, we directly measured the Ras activity in response to IL-6. Fig. 3D shows that exposure of JJ012 cells to IL-6 induced an increase in Ras activity in a time-dependent manner, as assessed by immunoblotting samples for Ras immunoprecipitated from lysates using the Raf-1 Ras-binding domain. To examine whether Raf-1 (a target protein for Ras) might play a crucial role in IL-6-induced cancer migration, the Raf-1 inhibitor GW5074 was used. As shown in Fig. 3 (A–C), pretreatment of cells with GW5074 inhibited IL-6-induced migration and MMP-13 activity. Raf-1 is associated with Ras-GTP and, then by additional modifications such as phosphorylation at Ser338, becomes the active form (30). The activated Raf-1 then triggers sequential activation of downstream molecules. Thus, phosphorylation of Raf-1 at Ser338 is a critical step in Raf-1 activation. Next, we further examined Raf-1 Ser338 phosphorylation by IL-6 stimulation. Stimulation of cells with IL-6 increased Raf-1 Ser338 phosphorylation (Fig. 3E). In addition, IL-6-induced Raf-1 Ser338 phosphorylation was inhibited by treatment with anti-IL-6R Ab and manumycin A (Fig. 3F). The results indicate that Raf-1 is a downstream molecule of Ras and is involved in IL-6-mediated migration and MMP-13 expression.
FIGURE 3.
Involvement of Ras and Raf-1 signaling pathways in response to IL-6 in chondrosarcoma cells. A, cells were pretreated with manumycin A (3 μm) and GW5074 (3 μm) for 30 min followed by stimulation with IL-6 for 24 h, and in vitro migration was measured with the Transwell assay after 24 h. B and C, cells were pretreated with manumycin A and GW5074 for 30 min followed by stimulation with IL-6 for 24 h. The mRNA level of MMP-13 in cell lysates and the enzyme activity of MMP-13 in the supernatant were examined by qPCR and zymography. D, JJ012 cells were incubated with IL-6 for the indicated time periods, and cell lysates were immunoprecipitated with an antibody specific for the Raf-1 Ras-binding domain. E and F, JJ012 cells were incubated with IL-6 for the indicated time periods or pretreated for 30 min with anti-IL-6R Ab or manumycin A followed by stimulation with IL-6 for 30 min, and phospho-Raf-1 (p-Raf-1) was examined by Western blotting. Results are expressed as means ± S.E. *, p < 0.05 compared with the control; #, p < 0.05 compared with the IL-6-treated group.
The Signaling Pathways of MEK and ERK Are Involved in the Potentiating Action of IL-6 Stimulation
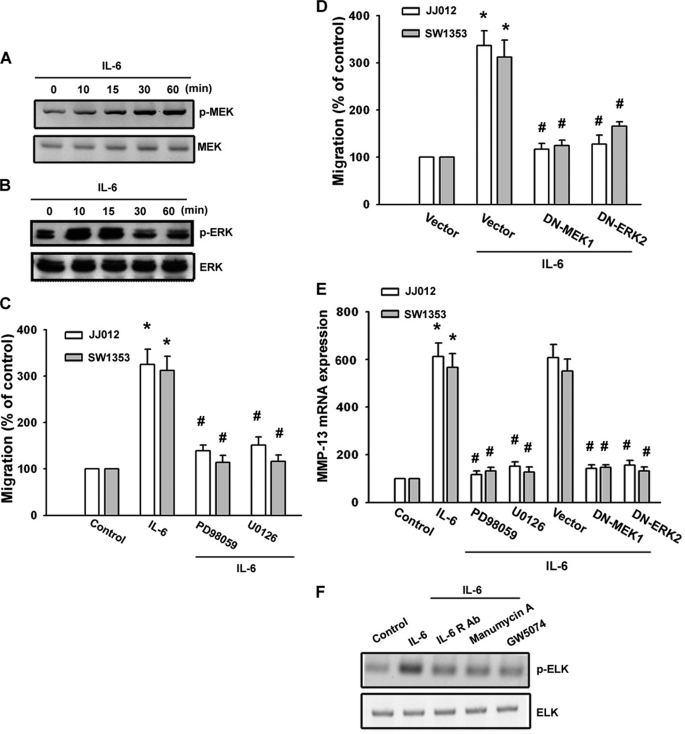
We next wished to determine whether IL-6 is able to activate MEK/ERK, a critical downstream target of Raf-1 (31) that has been shown to induce gene expression (32). Stimulation of cells with IL-6 induced MEK and ERK phosphorylation (Fig. 4, A and B). IL-6-induced migration of cells was greatly reduced by treatment with the MEK inhibitors PD98059 and U0126 (Fig. 4C). PD98059 and U0126 also inhibited IL-6-increased MMP-13 expression (Fig. 4E). Transfection of cells with mutant MEK1 or ERK2 reduced IL-6-mediated cell migration and MMP-13 expression (Fig. 4, D and E). Furthermore, IL-6 induced ERK kinase activity because phosphorylation of one of its substrates, ELK, was markedly inhibited if cells were pretreated for 30 min with anti-IL-6R Ab, manumycin A, and GW5074 (Fig. 4F). Taken together, these results indicate that the IL-6 receptor/Ras/Raf-1/MEK and ERK pathways are involved in IL-6-induced migration activity and MMP-13 up-regulation in human chondrosarcoma cells.
FIGURE 4.
MEK/ERK pathway is involved in IL-6-mediated migration of human chondrosarcoma cells. A and B, JJ012 cells were incubated with IL-6 for the indicated time periods, and phospho-MEK (p-MEK) and phospho-ERK (p-ERK) expression was determined by Western blot analysis. C and D, cells were pretreated with PD98059 (30 μm) and U0126 (30 μm) for 30 min or transfected with a dominant-negative (DN) mutant of MEK1 or ERK2 for 24 h followed by stimulation with IL-6 for 24 h, and in vitro migration was measured with the Transwell assay after 24 h. E, cells were pretreated with PD98059 and U0126 for 30 min or transfected with a dominant-negative mutant of MEK1 or ERK2 for 24 h followed by stimulation with IL-6 for 24 h, and IL-6 mRNA was measured by qPCR. F, JJ012 cells were pretreated for 30 min with anti-IL-6R Ab, manumycin A, or GW5074 followed by stimulation with IL-6 for 30 min, and the ERK kinase activity was examined using an ERK kinase assay kit. Results are expressed as means ± S.E. *, p < 0.05 compared with the control; #, p < 0.05 compared with the IL-6-treated group.
Involvement of NF-κB in IL-6-induced Cell Migration and MMP-13 Expression
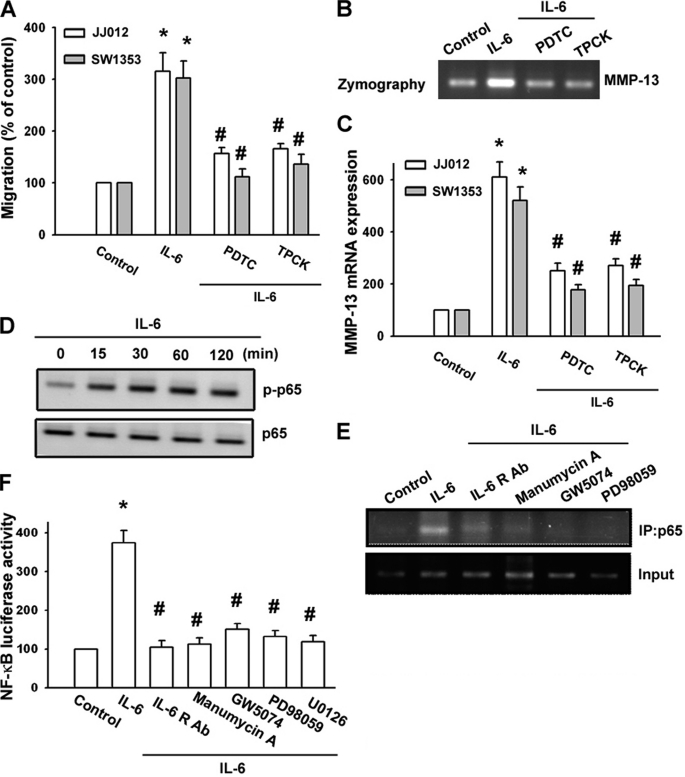
As mentioned above, NF-κB activation is necessary for the migration and invasion of human chondrosarcoma cells (33). To examine whether NF-κB activation is involved in the signal transduction pathway leading to migration and MMP-13 expression caused by IL-6, the NF-κB inhibitor PDTC was used. Fig. 5 (A--C) shows that PDTC inhibited the enhancement of migration and MMP-13 production induced by IL-6. Furthermore, pretreatment of cells with an IκB protease inhibitor (TLCK) also antagonized the potentiating action of IL-6 (Fig. 5, A--C). Previous studies showed that p65 Ser536 phosphorylation increases NF-κB transactivation (34), and the antibody against p65 phosphorylated at Ser536 was used to examine p65 phosphorylation. Treatment of chondrosarcoma cells with IL-6 for various time periods resulted in p65 Ser536 phosphorylation (Fig. 5D). We next investigated whether p65 binds to the NF-κB element on the MMP-13 promoter after IL-6 stimulation. The in vivo recruitment of p65 to the MMP-13 promoter (−182 to +27) was assessed by the ChIP assay. In vivo binding of p65 to the NF-κB element of the MMP-13 promoter occurred after IL-6 stimulation (Fig. 5E). Binding of p65 to the NF-κB element by IL-6 was attenuated by anti-IL-6R Ab, manumycin A, GW5074, and PD98059 (Fig. 5E). To directly determine NF-κB activation after IL-6 treatment, chondrosarcoma cells were transiently transfected with NF-κB-luciferase as an indicator of NF-κB activation. As shown in Fig. 5F, IL-6 treatment of chondrosarcoma cells for 24 h caused an increase in NF-κB-luciferase activity. In addition, the IL-6-induced increase in NF-κB-luciferase activity was also inhibited by treatment with anti-IL-6R Ab, manumycin A, GW5074, PD98059, and U0126 (Fig. 5F). Taken together, these data suggest that activation of the IL-6 receptor, Ras, Raf-1, MEK, and ERK is required for IL-6-induced NF-κB activation in human chondrosarcoma cells.
FIGURE 5.
IL-6 induces cell migration and MMP-13 up-regulation through NF-κB. A, cells were pretreated for 30 min with PDTC (10 μm) or TLCK (3 μm) followed by stimulation with IL-6, and in vitro migration was measured with the Transwell assay after 24 h. B and C, cells were pretreated with PDTC or TLCK for 30 min followed by stimulation with IL-6 for 24 h. The mRNA level of MMP-13 in cell lysates and the enzyme activity of MMP-13 in the supernatant were examined by qPCR and zymography. D, JJ012 cells were incubated with IL-6 for the indicated time periods, and phospho-p65 (p-p65) expression was determined by Western blot analysis. E, JJ012 cells were pretreated with anti-IL-6R Ab, manumycin A, GW5074, or PD98059 and then stimulated with IL-6 for 120 min, and the ChIP assay was performed. Chromatin was immunoprecipitated (IP) with anti-phospho-65 antibody. One percent of the precipitated chromatin was assayed to verify equal loading (Input). F, JJ012 cells were pretreated with anti-IL-6R Ab, manumycin A, GW5074, or PD98059 for 30 min and then stimulated with IL-6 for 24 h. NF-κB-luciferase activity was measured, and the results were normalized to β-galactosidase activity. Results are expressed as means ± S.E. *, p < 0.05 compared with the control; #, p < 0.05 compared with the IL-6-treated group.
Increase in IL-6 and MMP-13 Expression in Migration-prone Cells
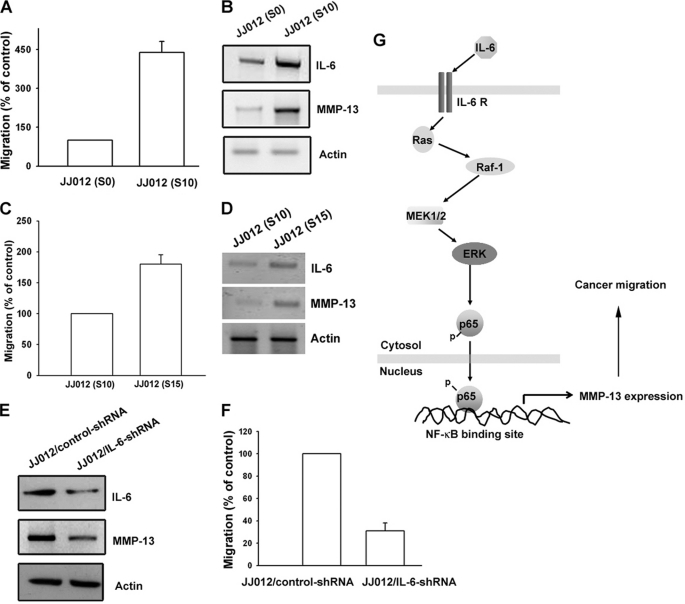
To confirm the IL-6-mediated cell migration and MMP-13 expression in human chondrosarcoma cells, we selected JJ012 sublines with higher cell mobility according to the methods described under “Experimental Procedures.” The migration-prone JJ012(S10) subline had higher cell motility compared with the original JJ012(S0) line (Fig. 6A). Moreover, JJ012(S10) markedly increased the protein expression of IL-6 and MMP-13 (Fig. 6B). We also established a migration-prone subline from JJ012(S10). We found that JJ012(S15) had higher cell motility compared with JJ012(S10) (Fig. 6C). In addition, JJ012(S15) markedly increased the protein expression of IL-6 and MMP-13 (Fig. 6D). To further confirm the IL-6-mediated cell migration and MMP-13 expression in human chondrosarcoma cells, IL-6 shRNA-expressing cells were established. The IL-6 expression level in stable transfectants was compared by Western blotting. The expression of IL-6 was dramatically inhibited by IL-6 shRNA orientation in JJ012/IL-6 shRNA cells (Fig. 6E). Because IL-6 has already been reported to act as a mitogen in osteoblasts (35), we sought to characterize the cell growth rate of control cells and transfectants by performing the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide assay 1–6 days after cell seeding. No appreciable difference in cell growth ability was evident among these cells (data not shown), suggesting that IL-6 does not have any mitogenic effect in human chondrosarcoma cells. Furthermore, the migration ability of these transfectants was analyzed using a Transwell migration assay. Knockdown of IL-6 expression inhibited the migration ability by ∼70% in JJ012 cells (Fig. 6F). In addition, knockdown IL-6 also reduced MMP-13 expression in JJ012 cells (Fig. 6E). Therefore, human chondrosarcoma cells with a greater ability to migrate expressed more IL-6 and MMP-13.
FIGURE 6.
Up-regulation of IL-6 and MMP-13 expression in migration-prone cells. A, after 10 rounds of selection of JJ012 cells by the cell culture insert system, the migration-prone subline JJ012(S10) exhibited greater migration than the original JJ012(S0) line. B, JJ012(S10) cells expressed higher IL-6 and MMP-13 protein levels compared with the original JJ012(S0) cells. C, after five rounds of selection of JJ012(S10) cells by the cell culture insert system, the migration-prone subline JJ012(S15) exhibited greater migration than JJ012(S10) cells. D, JJ012(S15) cells expressed higher IL-6 and MMP-13 protein levels compared with JJ012(S10) cells. E, the protein levels of IL-6 and MMP-13 in JJ012/control shRNA and JJ012/IL-6 shRNA cells were examined by Western blot analysis. F, the in vitro migration activity of JJ012/control shRNA and JJ012/IL-6 shRNA cells was measured with the Transwell assay. Results are expressed as means ± S.E. G, schematic presentation of the signaling pathways involved in IL-6-induced migration and MMP-13 expression in chondrosarcoma. IL-6 and IL-6 receptor interaction activates the Ras, Raf-1, MEK, and ERK pathways, which in turn induces NF-κB activation, which leads to MMP-13 expression and increases the migration of human chondrosarcoma cells.
DISCUSSION
Unlike other mesenchymal malignancies such as osteosarcoma and Ewing sarcoma, for which dramatic increases in long-term survival have been made possible with the advent of systemic chemotherapy, chondrosarcoma continues to have a poor prognosis due to the absence of an effective adjuvant therapy (36). The metastatic potential for conventional chondrosarcomas correlates well with the histologic grade of the tumor, but because of the relatively indolent growth rates of many low- and moderate-grade chondrosarcomas, ∼15% of patients dying from metastatic disease do so >5 years after the initial diagnosis (36). Therefore, it is important to develop effective adjuvant therapy for preventing chondrosarcoma metastasis. We hypothesized that IL-6 would help to direct the metastasis of chondrosarcoma cells. We found that IL-6 increased the migration of chondrosarcoma cells. One of the mechanisms underlying IL-6-directed migration was transcriptional up-regulation of MMP-13 and activation of the IL-6 receptor, Ras, Raf-1, MEK, ERK, and NF-κB pathways. In this study, we used two high-grade (grade II) chondrosarcoma cell lines to examine the migration activity. Therefore, the same signaling pathways are involved in high-grade chondrosarcoma. However, whether the same signaling pathways are involved in all chondrosarcoma cells needs further examination. Using Western blot analysis, we found that the expression of IL-6 in human chondrosarcoma tissues (n = 12) was significantly higher than that in normal tissues (n = 11). Therefore, the high expression of IL-6 in chondrosarcomas is a common feature of all chondrosarcomas.
IL-6 activates target genes involved in differentiation, survival, apoptosis, and proliferation. It also manipulates the process of tumorigenesis and tumor progression (37, 38). However, the effect of IL-6 on the migration activity of chondrosarcoma is mostly unknown. Using Western blotting and qPCR analysis, we found that expression of the protein and mRNA levels of IL-6 in chondrosarcoma patients was significantly higher than that in normal cartilage. In addition, exogenous IL-6 increased migration of chondrosarcoma. Moreover, overexpression of IL-6 shRNA inhibited the migration ability by ∼70% in JJ012 cells. In addition, the migration-prone sublines JJ012(S10) and JJ012(S15) demonstrated that cells with increasing migration ability had greater expression of IL-6 and MMP-13. Our data provide evidence that the expression of IL-6 is associated with a metastatic phenotype of chondrosarcoma cells. IL-6 exerts it effects through interaction with specific IL-6 receptors (35). However, the effect of the IL-6 receptor in chondrosarcoma cells is largely unknown. We found that anti-IL-6R Ab reduced IL-6-mediated migration and MMP-13 expression. Therefore, our data suggest a critical role for the IL-6 receptor in IL-6-mediated cell migration and MMP-13 expression in human chondrosarcoma cells. IL-6 is a prominent cytokine released during the inflammatory response.
Enzymatic degradation of the extracellular matrix is one of the crucial steps in cancer invasion and metastasis. In human chondrosarcoma cells, MMPs have been found to correlate with malignancy grade and metastasis (40). MMP-13 expression has been detected in several pathological conditions that are characterized by the destruction of normal collagen tissue architecture (41). In cancer cells, the constitutive expression of MMP-13 has been detected in 27.3% of breast carcinomas, 85.7% of squamous cell carcinomas in the head and neck, 75% of cell lines established from invasive squamous carcinomas of the vulva, and 52.2% of malignant melanomas (42). In this study, we found that IL-6 induced MMP-13 expression and secretion in human chondrosarcoma cells without significantly changing the expression of MMP-1, -2, -8, and -9 mRNAs. In addition, the inhibition of IL-6-enhanced MMP-13 protein expression by siRNA significantly suppressed IL-6-induced migration. Therefore, MMP-13 may be the IL-6-responsive mediator, and it causes the degradation of the extracellular matrix, leading to subsequent cancer migration.
Ras proteins are members of the superfamily of small GTPases claimed to play a key role in signaling pathways leading to cell proliferation, differentiation, and transformation (43). Several reports have indicated that Ras might play a critical role in the induction of cancer migration (20, 22). In this study, we have shown that manumycin A (a Ras farnesyltransferase inhibitor) inhibited the IL-6-induced increase in migration and MMP-13 expression. In addition, IL-6 stimulation also increased the kinase activity of Ras. These results suggest that Ras activation might be involved in IL-6-mediated migration and MMP-13 expression.
The prognosis of patients with chondrosarcoma distant metastasis is generally considered very poor; hence, preventing human chondrosarcoma metastasis is an important issue nowadays. Our study has shown that IL-6 increases the activity of MMP-13 via the IL-6 receptor, Ras, Raf-1, MEK, ERK, and NF-κB pathways and enhances migration of human chondrosarcoma cells (Fig. 6G). Furthermore, the discovery of the IL-6-mediated signaling pathway helps us understand the mechanism of human chondrosarcoma metastasis and may lead us to develop effective therapy in the future.
Acknowledgments
We thank Dr. W. M. Fu for providing the MEK1 mutant and Dr. M. Cobb for providing the ERK2 mutant.
This work was supported by National Science Council of Taiwan Grant NSC99-2320-B-039-003-MY3 and China Medical University Hospital Grant DMR-100-086.
- MMP
- matrix metalloproteinase
- TLCK
- l-1-tosylamido-2-phenylethyl chloromethyl ketone
- PDTC
- pyrrolidine dithiocarbamate
- qPCR
- quantitative real-time PCR
- IL-6R
- IL-6 receptor.
REFERENCES
- 1. Terek R. M., Schwartz G. K., Devaney K., Glantz L., Mak S., Healey J. H., Albino A. P. (1998) J. Orthop. Res. 16, 585–590 [DOI] [PubMed] [Google Scholar]
- 2. Yuan J., Dutton C. M., Scully S. P. (2005) J. Orthop. Res. 23, 1467–1474 [DOI] [PubMed] [Google Scholar]
- 3. Joyce J. A., Pollard J. W. (2009) Nat. Rev. Cancer 9, 239–252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Woodhouse E. C., Chuaqui R. F., Liotta L. A. (1997) Cancer 80, 1529–1537 [DOI] [PubMed] [Google Scholar]
- 5. Egeblad M., Werb Z. (2002) Nat. Rev. Cancer 2, 161–174 [DOI] [PubMed] [Google Scholar]
- 6. Kerkelä E., Saarialho-Kere U. (2003) Exp. Dermatol. 12, 109–125 [DOI] [PubMed] [Google Scholar]
- 7. Tan T. W., Yang W. H., Lin Y. T., Hsu S. F., Li T. M., Kao S. T., Chen W. C., Fong Y. C., Tang C. H. (2009) Carcinogenesis 30, 258–268 [DOI] [PubMed] [Google Scholar]
- 8. Hou C. H., Hsiao Y. C., Fong Y. C., Tang C. H. (2009) Bone 44, 233–242 [DOI] [PubMed] [Google Scholar]
- 9. Tan T. W., Lai C. H., Huang C. Y., Yang W. H., Chen H. T., Hsu H. C., Fong Y. C., Tang C. H. (2009) J. Cell. Biochem. 107, 345–356 [DOI] [PubMed] [Google Scholar]
- 10. Rose-John S., Scheller J., Elson G., Jones S. A. (2006) J. Leukocyte Biol. 80, 227–236 [DOI] [PubMed] [Google Scholar]
- 11. Okamoto M., Lee C., Oyasu R. (1997) Cancer Res. 57, 141–146 [PubMed] [Google Scholar]
- 12. Okamoto M., Hattori K., Oyasu R. (1997) Int. J. Cancer 72, 149–154 [DOI] [PubMed] [Google Scholar]
- 13. Miki S., Iwano M., Miki Y., Yamamoto M., Tang B., Yokokawa K., Sonoda T., Hirano T., Kishimoto T. (1989) FEBS Lett. 250, 607–610 [DOI] [PubMed] [Google Scholar]
- 14. Chen L., Mory Y., Zilberstein A., Revel M. (1988) Proc. Natl. Acad. Sci. U.S.A. 85, 8037–8041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Okamoto M., Lee C., Oyasu R. (1997) Endocrinology 138, 5071–5074 [DOI] [PubMed] [Google Scholar]
- 16. Eustace D., Han X., Gooding R., Rowbottom A., Riches P., Heyderman E. (1993) Gynecol. Oncol. 50, 15–19 [DOI] [PubMed] [Google Scholar]
- 17. Lu C., Kerbel R. S. (1993) J. Cell Biol. 120, 1281–1288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Culig Z., Steiner H., Bartsch G., Hobisch A. (2005) J. Cell. Biochem. 95, 497–505 [DOI] [PubMed] [Google Scholar]
- 19. Berek J. S., Chung C., Kaldi K., Watson J. M., Knox R. M., Martínez-Maza O. (1991) Am. J. Obstet. Gynecol. 164, 1038–1042; Discussion 1042–1043 [DOI] [PubMed] [Google Scholar]
- 20. Campbell S. L., Khosravi-Far R., Rossman K. L., Clark G. J., Der C. J. (1998) Oncogene 17, 1395–1413 [DOI] [PubMed] [Google Scholar]
- 21. Blaine S. A., Wick M., Dessev C., Nemenoff R. A. (2001) J. Biol. Chem. 276, 42737–42743 [DOI] [PubMed] [Google Scholar]
- 22. Marshall C. J. (1996) Curr. Opin. Cell Biol. 8, 197–204 [DOI] [PubMed] [Google Scholar]
- 23. Zhang X. F., Settleman J., Kyriakis J. M., Takeuchi-Suzuki E., Elledge S. J., Marshall M. S., Bruder J. T., Rapp U. R., Avruch J. (1993) Nature 364, 308–313 [DOI] [PubMed] [Google Scholar]
- 24. Whitmarsh A. J., Davis R. J. (1998) Trends Biochem. Sci. 23, 481–485 [DOI] [PubMed] [Google Scholar]
- 25. Lahsnig C., Mikula M., Petz M., Zulehner G., Schneller D., van Zijl F., Huber H., Csiszar A., Beug H., Mikulits W. (2009) Oncogene 28, 638–650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Lu K. W., Tsai M. L., Chen J. C., Hsu S. C., Hsia T. C., Lin M. W., Huang A. C., Chang Y. H., Ip S. W., Lu H. F., Chung J. G. (2008) Anticancer Res. 28, 1093–1099 [PubMed] [Google Scholar]
- 27. Fong Y. C., Li T. M., Wu C. M., Hsu S. F., Kao S. T., Chen R. J., Lin C. C., Liu S. C., Wu C. L., Tang C. H. (2008) J. Cell. Physiol. 217, 846–855 [DOI] [PubMed] [Google Scholar]
- 28. Tang C. H., Chuang J. Y., Fong Y. C., Maa M. C., Way T. D., Hung C. H. (2008) Carcinogenesis 29, 1483–1492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Chuang J. Y., Yang W. H., Chen H. T., Huang C. Y., Tan T. W., Lin Y. T., Hsu C. J., Fong Y. C., Tang C. H. (2009) J. Cell. Physiol. 220, 418–426 [DOI] [PubMed] [Google Scholar]
- 30. Zang M., Hayne C., Luo Z. (2002) J. Biol. Chem. 277, 4395–4405 [DOI] [PubMed] [Google Scholar]
- 31. Friday B. B., Adjei A. A. (2008) Clin. Cancer Res. 14, 342–346 [DOI] [PubMed] [Google Scholar]
- 32. Lee H. W., Ahn D. H., Crawley S. C., Li J. D., Gum J. R., Jr., Basbaum C. B., Fan N. Q., Szymkowski D. E., Han S. Y., Lee B. H., Sleisenger M. H., Kim Y. S. (2002) J. Biol. Chem. 277, 32624–32631 [DOI] [PubMed] [Google Scholar]
- 33. Chiu Y. C., Shieh D. C., Tong K. M., Chen C. P., Huang K. C., Chen P. C., Fong Y. C., Hsu H. C., Tang C. H. (2009) Carcinogenesis 30, 1651–1659 [DOI] [PubMed] [Google Scholar]
- 34. Madrid L. V., Mayo M. W., Reuther J. Y., Baldwin A. S., Jr. (2001) J. Biol. Chem. 276, 18934–18940 [DOI] [PubMed] [Google Scholar]
- 35. Li Y., Bäckesjö C. M., Haldosén L. A., Lindgren U. (2008) Cytokine 43, 165–173 [DOI] [PubMed] [Google Scholar]
- 36. Fong Y. C., Yang W. H., Hsu S. F., Hsu H. C., Tseng K. F., Hsu C. J., Lee C. Y., Scully S. P. (2007) J. Orthop. Res. 25, 1106–1114 [DOI] [PubMed] [Google Scholar]
- 37. Walter M., Liang S., Ghosh S., Hornsby P. J., Li R. (2009) Oncogene 28, 2745–2755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Hov H., Tian E., Holien T., Holt R. U., Våtsveen T. K., Fagerli U. M., Waage A., Børset M., Sundan A. (2009) Eur. J. Haematol. 82, 277–287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Shi J., Schmitt-Talbot E., DiMattia D. A., Dullea R. G. (2004) Inflamm. Res. 53, 377–389 [DOI] [PubMed] [Google Scholar]
- 40. Scully S. P., Berend K. R., Qi W. N., Harrelson J. M. (1999) Braz. J. Med. Biol. Res. 32, 885–889 [DOI] [PubMed] [Google Scholar]
- 41. Ala-aho R., Kähäri V. M. (2005) Biochimie 87, 273–286 [DOI] [PubMed] [Google Scholar]
- 42. Leeman M. F., Curran S., Murray G. I. (2002) Crit. Rev. Biochem. Mol. Biol. 37, 149–166 [DOI] [PubMed] [Google Scholar]
- 43. Satoh T., Nakafuku M., Kaziro Y. (1992) J. Biol. Chem. 267, 24149–24152 [PubMed] [Google Scholar]