Abstract
Hepatocytes show endoplasmic reticulum (ER) stress when exposed to lipotoxic stimuli such as hyperlipidemia. Recent work has revealed that AMP- activated protein kinase (AMPK) can mitigate ER stress. In this study we investigated the impact of AMPK on lipid-induced ER stress in hepatocytes and its underlying molecular mechanism. Treatment with 5-aminoimidazole-4-carboxamide ribonucleotide (AICAR), an AMPK agonist, or overexpression of a constitutively active AMPK significantly suppressed lipid-mediated ER stress, leading to marked protection against lipotoxic death. Incubation with AICAR and constitutively active AMPK overexpression induced the expression of an ER-associated chaperone, 150-kDa oxygen-regulated protein (ORP150), at both the mRNA and protein levels in hepatocytes. Forkhead box O1 (FOXO1) was identified as the critical transcription factor regulating ORP150 expression because silencing FOXO1 expression prevented the induction of ORP150 expression by AMPK. In contrast, overexpression of FOXO1-ADA promoted ORP150 expression in hepatocytes. FOXO1 bound directly to the ORP150 promoter, which was enhanced in the presence of AICAR. AMPK acts to activate FOXO1 by increasing its deacetylation and transcriptional activity via silent mating type information regulation 2 homolog 1 (SIRT1). Furthermore, AICAR infusion enhanced ORP150 expression, resulting in the marked amelioration of hepatic ER stress and apoptosis in C57BL/6J mice fed a high fat diet. Our results reveal a novel mechanism by which AMPK regulates ER homeostasis in hepatocytes and suggest that AMPK has a protective role against hypercholesterolemia-related liver damage.
Keywords: AMP-activated Kinase (AMPK), Apoptosis, Endoplasmic Reticulum (ER), ER Stress, Hepatocyte
Introduction
Endoplasmic reticulum (ER)3 stress has been recently identified as a new pathophysiological paradigm that determines whether cells survive or die and may, therefore, be involved in many chronic metabolic diseases (1–4). In conjunction with its central role in lipid synthesis, protein folding, and transportation, the ER serves as a major signal transduction organelle that integrates cellular responses to stress (3, 4). The accumulation of misfolded proteins and other stresses leads to the activation of an adaptive program by the ER, known as the unfolded protein response (UPR), to reestablish equilibrium (5). Initiation of the canonical UPR engages three distinct signaling branches mediated by pancreatic ER kinase (PERK), inositol-requiring transmembrane kinase/endonuclease-1 (IRE-1) and activating transcription factor-6 (ATF6) (6, 7). The combined action of PERK/IRE-1/ATF6 pathways leads to inhibition of mRNA translation, stimulation of protein degradation, and production of chaperone proteins, resulting in either recovery of ER function or cell death (8). An increasing amount of evidence has indicated that hypercholesterolemia is a strong trigger of ER stress, and it has been identified as a mechanism driving lipid-induced cell death in a model of cholesterol loading (9–11). Hence, it is possible that the ER serves as an organelle that senses stresses related to exposure of cells to lipid (12).
Adenosine monophosphate-activated protein kinase (AMPK), a key sensor of cellular energy status, plays a critical role in controlling the cellular energy balance and metabolism (13, 14). AMPK activation exhibits multiple protective effects, including inhibiting inflammation, oxidative stress, cell proliferation, and insulin resistance and reduces the risk for developing obesity and type 2 diabetes (15). Interestingly, several recent studies have shown that AMPK activation protects against hypoxic injury (16) and atherosclerosis (12, 17) by suppressing ER stress. However, the impact of AMPK on lipid-induced ER stress had not been investigated. The 150-kDa oxygen-regulated protein (ORP150) is an ER-associated chaperone (18) induced by hypoxia and oxidative stress that exerts critical cytoprotective properties (19, 20). ORP150 had been reported to prevent oxidized low density lipoprotein-induced apoptosis, probably as a consequence of its capacity to limit ER stress (21, 22). Because of the potential role of ORP150 in the protection of ER homeostasis disruption, our present study was designed to investigate whether AMPK-mediated induction of regulates ORP150 expression and its impact on hepatic ER stress and apoptosis. Notably, we uncovered novel evidence suggesting that AMPK is a potent activator in up-regulation of ORP150 expression by inducing forkhead box O1 (FOXO1) transcriptional activity and, thus, suppresses hepatic ER stress and apoptosis in high fat diet (HFD)-fed C57BL/6J mice.
EXPERIMENTAL PROCEDURES
Reagents
5-Aminoimidazole-4-carboxyamide ribonucleoside (AICAR) and polyclonal antibodies against phosphor- AMPK at Thr-172, acetyl-lysine, and phospho-Thr24 of FOXO1, FOXO1, phospho-eIF2α, eIF2α, phospho-PERK, PERK, and β-actin were purchased from Cell Signaling Technology (Beverly, MA). AMPK antagonist compound C was obtained from Calbiochem. Metformin was purchased from Sigma. A-769662 was provided by Symansis Pty Ltd. Anti-ORP150 antibody (ab85233, the band size is 111 kDa) was obtained from Abcam (Cambridge, CA). The non-radioactive FOXO1 transcription factor assay kit was provided by Upstate Biotechnology (Billerica, MA).
Cell Culture
HepG2 cells (human hepatoma cell line, ATCC #HB 8065, American Type Culture Collection) cells were cultured in Dulbecco's modified Eagle's medium supplemented with 10% fetal bovine serum and antibiotics at 37°C in a humidified 5% CO2, 95% air atmosphere.
Adenoviral Vectors and Infection
The adenoviral vector expressing CA-AMPK and a dominant-negative AMPKα mutant (DN-AMPK) were kindly provided by Dr J. Ha (Dept. of Molecular Biology, Kyung Hee University, College of Medicine, Seoul, Korea). The cDNA encoding CA-AMPK or DN-AMPK was inserted into the EcoRI/XhoI sites of the pAdTrack-CMV shuttle vector. The resulting vector was then electroporated into BJ5138 cells containing the AdEasy adenoviral vector to produce the recombinant adenoviral plasmid. The recombinant viruses were amplified in HEK293 cells and isolated by cesium chloride density-gradient centrifugation. The viruses were collected and desalted, and the titers were measured using Adeno-XTM Rapid titer (BD Bioscience) according to the manufacturer's instructions (23).
Adenoviruses encoding hemagglutinin-tagged constitutively nuclear (ADA) and dominant-negative (Δ256) FOXO1 were constructed as previously described and obtained from Addgene (Cambridge, MA) (24). FOXO1-ADA has mutated Akt phosphorylation sites (T24A, S256D, and S316A). FOXO1-Δ256 lacks the transactivation domain and competes with endogenous FOXO1.
Gene Silencing by Small Interfering RNA (siRNA)
For experiments with RNAi, SMARTpool for human ORP150 (ONTARGETplus SMARTpool targeting human ORP150, L-003678-00-0005), FOXO1 (L-003006-00-0005), and SIRT1 (L-094699-01-0010) were obtained from Dharmacon (Chicago). Scrambled oligonucleotides (ONTARGETplus siCONTROL Non-Targeting Pool, D-001810–10−20) were used as control. For transient expression experiments, 80% confluent cells were transfected by using Lipofectamine 2000.
Measurement of Caspase-3 Activity
Caspase-3 activity was measured by using the Caspase-3 Colorimetric Activity Assay kit with Ac-DEVD-p-nitroanilide as the substrate (Catalogue no. APT165, Chemicon). After treatment, cellular lysates were incubated with reaction buffer containing Ac-DEVD-p-nitroanilide with or without caspase-3-specific inhibitor (0.1 μm Ac-DEVD-CHO) for 2 h at 37°C. Caspase-3 activity was determined by absorbance at 405 nm in a microplate reader.
TUNEL Assay
TUNEL staining was performed according to the manufacturer's protocol (In Situ Cell Death Detection kit, Roche Applied Science). Fluorescein labels incorporated in nucleotide polymers were detected under a Zeiss Axioskop at an excitation wavelength of 488 nm. Apoptotic cell percentage was quantified by calculating the ratio of TUNEL-positive cells to DAPI-stained total nuclei in at least 15 random high power fields (×400 at final magnification) (25).
Quantitative Real-Time Reverse-transcription Polymerase Chain Reaction
Cells were harvested in TRIzol (Invitrogen), and total RNA was isolated according to the manufacturer's instructions. Real-time PCR was performed with the QuantiTect SYBR Green PCR kit (Qiagen, Valencia, CA) on the ABI 7500 DNA Sequence Detection System with standard fluorescent chemistries. The primers were ORP150 forward (5′-TGCAGTGATCACCGTGCCAG-3′) and reverse (5′-TCTTTCCGGCGGAAGACACC-3′), sXBP-1 forward (5′-GGGAATGAAGTGAGGCCAG-3′) and reverse (5′-CAATACCGCCAGAATCCATG-3′), Ddit3 forward (5′-CCAGCAGAGGTCACAAGCAC-3′) and reverse (5′-CGCACTGACCACTCTGTTTC-3′), and GAPDH forward (5′-GAGTCAACGGATTTGGTCGT-3′) and reverse (5′-GACAAGCTTCCCGTTCTCAG-3′).
Acetylation Assay
Cells were washed with ice-cold PBS, and then cold lysis buffer (25 mm Tris-HCl, pH 7.9, 5 mm MgCl2, 10% glycerol, 100 mm KCl, 1% Nonidet P-40, 0.3 mm dithiothreitol, 5 mm sodium pyrophosphate, 1 mm sodium orthovanadate, and 50 mm sodium fluoride) containing protease inhibitor mixture (Calbiochem) was added. For acetylation studies, 5 mm nicotinamide and 1 mm sodium butyrate were added to the buffer. Then cells were transferred into an Eppendorf tube, left on ice for 15 min, and centrifuged at 10,000 × g for 10 min. The supernatant was collected and stored at −80 °C. FOXO1 acetylation was evaluated by immunoprecipitating 1000 μg of total proteins from cells with anti-FOXO1 antibody followed by immunoblotting for acetyl-lysine. The blots were detected by using ultrasensitive horseradish peroxidase chemiluminescence (Pierce).
Immunoprecipitation and Western Blot
Routinely, 500 mg of protein from cultured cells was used for immunoprecipitation. Forty microliters of protein A-Sepharose resuspended in lysis buffer was used for preclearing the sample and immunoprecipitation after conjugating the beads with 3–5 mg of antibody. The resulting immunoprecipitate was boiled with Laemmli sample buffer and used for Western blot. Bands were visualized with an enhanced chemiluminescence detection system (Pierce).
Chromatin Immunoprecipitation (ChIP)
We used the ChIP assay kit (Upstate). Briefly, HepG2 cells (10-cm dishes) were cross-linked with formaldehyde, washed twice with cold phosphate-buffered saline, and centrifuged. Pellets were resuspended, and cellular lysates were then sonicated to shear cross-linked chromatin into 500–1000-bp pieces. Each immunoprecipitation reaction consisted of 0.06 mg of chromatin and 5 μg of antibody (anti-AMPK antibody or control immunoglobulin G) in conjugation with protein A/G-Sepharose beads. The eluted immunoprecipitates were digested with proteinase K, and DNA was extracted with phenol/chloroform/isoamyl alcohol, precipitated with EtOH, and amplified by PCR. The following primers were used to amplify the human ORP150 gene: forward (5′-CCGCACTAAACCGCTGTGTC-3′) and reverse (5′-CTCCGGAATTTACCGTGACC-3′). The PCR program used 30 cycles (94 °C, 30 s; 60 °C, 1 min; 72 °C, 1 min). The relative levels of DNA were normalized to the input DNA and expressed as the percentage of the untreated control (26).
SIRT1 Deacetylase Activity
SIRT1 deacetylase activity was measured using the fluorometric SIRT1 Assay kit (Sigma, CS1040) according to the manufacturer's instructions. Fluorescence intensity at 444 nm (excitation 355 nm) was recorded and normalized to micrograms of protein. Values are represented as -fold of control.
Animals and Diets
Animal experiments were conducted in accordance with institutional guidelines of Sun Yat-sen University. Male C57BL/6J mice at 6 weeks of age (The Jackson Laboratory, Bar Harbor, ME) were used for this study. The mice were fed a standard purified mouse diet for 2 weeks before the start of the experiment. Then the animals were switched to a HFD (60% of calories from fat) and received either AICAR (n = 8, 200 mg/kg/day) or vehicle (n = 8, 0.9% NaCl) via subcutaneous injection once daily. In the compound C group, mice were intraperitoneally injected with AICAR (200 mg/kg/day) simultaneously with compound C (n = 8, 20 mg/kg/day). The animals were maintained in a 22 °C room with a 12-h light/dark cycle and received drinking water ad libitum.
Statistics
Continuous data are expressed as the mean ± S.E. Comparison between groups was analyzed via one-way analysis of variance followed by the Student-Newman-Keuls test. p < 0.05 was considered significant. Nonquantitative results are representative of at least three independent experiments.
RESULTS
AMPK Prevents Lipid-induced ER Stress in Hepatocytes
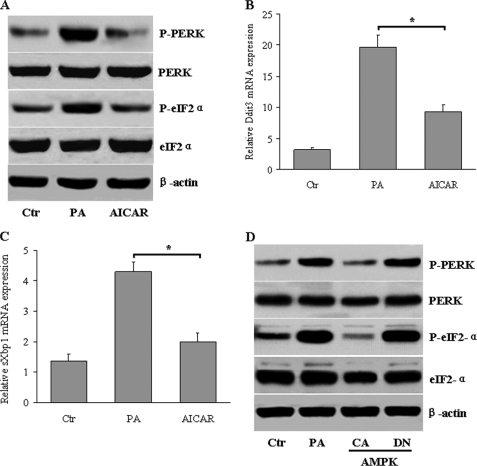
We first tested whether AICAR, a pharmacological activator of AMPK, can alter ER stress induced in hepatocytes when exposed to saturated fatty acids (27, 28). Treatment of HepG2 cells with palmitate induced obvious ER stress markers, as determined by phosphorylation of PERK (P-PERK) (Fig. 1A) and eukaryotic translation initiation factor 2α (P-eIF2-α) (Fig. 1A). However, concurrent incubation with AICAR almost completely protected against palmitate-induced ER stress in HepG2 cells. Moreover, AICAR treatment largely suppressed palmitate-induced splicing of XBP-1 (sXBP-1) and C/EBP homologous protein expression (CHOP, which is encoded by Ddit3), which are two elements of the UPR-induced transcriptional program (Fig. 1, B and C). To exclude potential off-target effects of AICAR, we transfected the HepG2 cells with CA-AMPK or DN-AMPK. CA-AMPK also reduced the palmitate-activated ER stress, but DN-AMPK had no influence (Fig. 1D).
FIGURE 1.
Activation of AMPK inhibits lipid-induced ER stress in hepatocytes. HepG2 cells were treated with AICAR (1 mm) in the absence or presence of palmitic acid (PA, 500 μm) for 24 h. A, shown are P-PERK and P-eIF2-α, as determined by Western blot. B and C, shown are mRNA levels of Ddit3 and sXbp1, as determined by quantitative RT-PCR. Data represent the means ± S.E. *, p < 0.05 versus PA. D, HepG2 cells were infected with adenoviruses encoding constitutively active (CA) or dominant-negative (DN) forms of AMPK in the presence of palmitic acid (500 μm). P-PERK and P-eIF2-α levels were examined by Western blot.
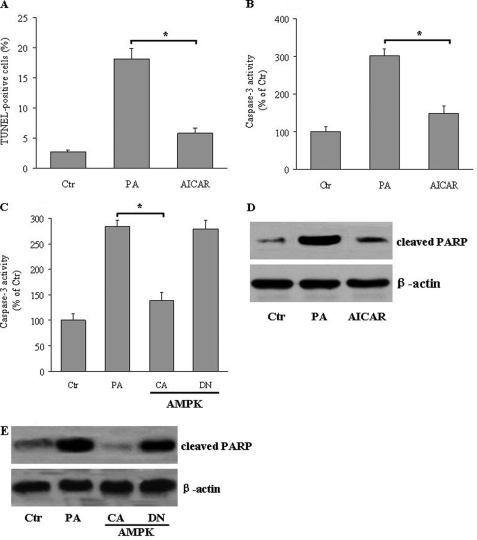
Because saturated fatty acids or modified lipoproteins can induce apoptotic pathways (29), we next asked whether altering ER stress under this condition could modulate lipotoxic cell death in hepatocytes. Compared with vehicle-treated hepatocytes, palmitate treatment resulted in a significant increase of cellular apoptosis, as determined by TUNEL assays, which was markedly attenuated in the presence of AICAR (Fig. 2A). Caspase-3 is partially or totally responsible for the proteolytic cleavage of many key proteins, such as the nuclear enzyme PARP, a key executioner of apoptosis (30, 31). The effects of AMPK on apoptosis were further investigated by monitoring caspase-3 activity and the cleavage of PARP. As expected, palmitate loading caused significant elevation of caspase-3 activity, which was attenuated in the presence of AICAR (Fig. 2B). In accordance with these data, overexpression of CA-AMPK suppressed caspase-3 activity, whereas overexpression of DN-AMPK aggravated caspase-3 activation in hepatocytes (Fig. 2C). Furthermore, palmitate increased the cleavage of PARP. Activation of AMPK by AICAR (Fig. 2D) or CA-AMPK suppressed (Fig. 2E), whereas overexpression of DN-AMPK (Fig. 2E) increased the effects of palmitate. Together, these results show that AMPK can protect cultured hepatocytes against lipid-induced ER stress and apoptosis.
FIGURE 2.
AMPK activation protects against apoptosis in hepatocytes. HepG2 cells were treated with AICAR (1 mm) in the absence or presence of palmitic acid (PA, 500 μm) for 24 h. A, apoptosis rate was determined by TUNEL assay, and results are shown as the means ± S.E. *, <0.05. B, caspase-3 activity was determined by a colorimetric method with Ac-DEVD-p-nitroanilide as the substrate. *, p < 0.05. C, HepG2 cells were infected with adenoviruses encoding constitutively active (CA) or dominant-negative (DN) forms of AMPK in the presence of PA (500 μm). Caspase-3 activity was determined as indicated under “Experimental Procedures.” *, p < 0.05. D and E, the expression of cleaved PARP from AICAR-treated (D) and CA-AMPK-transfected (E) hepatocytes was measured by Western blot. Representative blots from three separate assays are shown.
AMPK Up-regulates ORP150 Expression in Hepatocytes
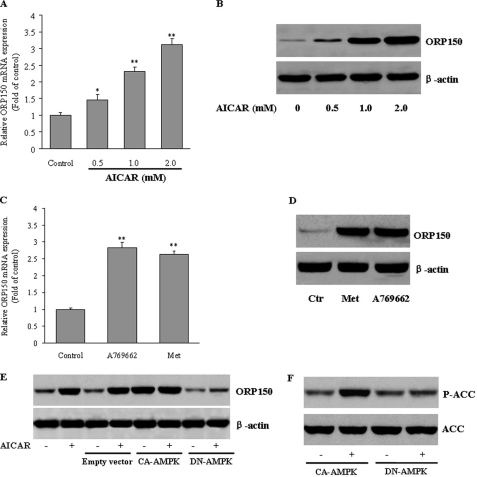
ORP150 is an anti-apoptotic ER resident chaperone that exerts a protective effect against ER stress-dependent apoptosis (21, 22). We next examined the effects of AMPK on ORP150 expression in hepatocytes. Exposure of HepG2 cells to AICAR led to a concentration-dependent increase of ORP150 mRNA (Fig. 3A) and protein (Fig. 3B) expression, as determined by quantitative RT-PCR and Western blot, respectively. The unrelated AMPK activators metformin (32) and A-769662 (33) also increased ORP150 mRNA and protein expression in HepG2 cells (Fig. 3, C and D). To further substantiate these results, we transfected the HepG2 cells with CA-AMPK or DN-AMPK. Overexpression of CA-AMPK caused a significant induction of ORP150 protein expression that could not be further enhanced by AICAR. In contrast, when DN-AMPK was transfected, AICAR was unable to increase ORP150 expression (Fig. 3E). Thus, AMPK activation strongly triggers ORP150 expression in hepatocytes.
FIGURE 3.
Activation of AMPK increases ORP150 gene and protein expression in hepatocytes. A and B, cultured HepG2 cells were treated with AICAR (0.5, 1.0, or 2.0 mm) for 24 h. ORP150 expression was evaluated by real-time RT-PCR and Western blot. Results are representative of at least three independent experiments. The abundance of ORP150 mRNA in untreated control cells was defined as 1, and the amounts of ORP150 mRNA from AICAR-treated cells were then expressed as -fold changes over this control value. *, p < 0.05; **, p < 0.01 compared with control. C and D, HepG2 cells were treated with metformin (1 mm, Met) or A769662 (100 μm) for 24 h. ORP150 mRNA and protein expression were measured by quantitative RT-PCR and Western blot, respectively. **p < 0.01 compared with control. E, HepG2 cells were infected with adenoviruses encoding constitutively active (CA) or dominant-negative (DN) forms of AMPKα in the presence of AICAR (1 mm) or vehicle. Forty micrograms of total protein was used for Western blot to evaluate the level of ORP150 protein. *, p < 0.05; **, p < 0.01 versus control. F, AMPK activation was verified by measuring acetyl-CoA carboxylase (ACC) phosphorylation levels by Western blot.
ORP150 Blockade Abrogates the Reduction of Lipid-induced ER Stress and Apoptosis by AMPK
To further examine whether the suppressive effect of AMPK on ER stress depends on ORP150 expression, HepG2 cells were transfected with ORP150 siRNA or scrambled siRNA as a control. As shown in Fig. 4A, ORP150 siRNA significantly decreased the endogenous ORP150 protein level in HepG2 cells. As a result, in ORP150 siRNA-transfected hepatocytes, the suppressive effects of AICAR on ER stress markers (Fig. 4, B–D) and apoptosis, as evaluated by both TUNEL staining (Fig. 4E) and caspase-3 activity (Fig. 4F), were largely reversed. However, control siRNA did not affect ER stress or apoptosis. These results indicate that AMPK inhibits cholesterol-induced ER stress via induction of ORP150 in hepatocytes.
FIGURE 4.
Suppression of ORP150 expression abolishes the effects of AMPK on ER stress and apoptosis. A, HepG2 cells were transfected with ORP150-specific or scrambled siRNA (100 nm) for 48 h. Proteins were extracted and immunoblotted for ORP150 and β-actin. B–F, forty-eight hours after the siRNA transfection, HepG2 cells were treated with palmitic acid (PA) alone or in the presence of AICAR (1 mm) for 24 h. ER stress markers (B), Ddit3 (C), and sXbp1 mRNA levels (D), TUNEL staining (E), and caspase-3 activity (F). *, p < 0.05.
ORP150 Interacts with ER Stress Sensors in Hepatocytes
Because ORP150 can prevent structural alteration of proteins in ER, we investigated whether ORP150 could bind to ER stress sensors, thereby inhibiting UPR induction. This binding was explored by coimmunoprecipitating ORP150 with the ER stress sensors PERK and eIF2-α in empty vector- and ORP150-transfected HepG2 cells. As shown in Fig. 5, A and B, in ORP150-transfected hepatocytes, ORP150 was detected in the immunoprecipitates of each ER stress sensor (PERK and eIF2α) under basal and cholesterol-stimulated conditions. In contrast, ORP150 was present only in small amounts in ER stress sensor immunoprecipitates from vector-transfected cells. These data suggest that in cells constitutively expressing ORP150, this ER chaperone may directly bind to ER stress sensors. This binding may further limit ER stress activation and maintain the stress sensors in an inactive state (Fig. 5, C and D), thereby contributing to the inhibition of UPR activation.
FIGURE 5.
ORP150 interacts with ER stress sensors. PERK (A) and eIF2α (B) were immunoprecipitated (IP) from cell extracts of empty vector-transfected and ORP150-transfected HepG2 cells under basal quiescent conditions and after 14 h of incubation with PA (500 μm) and were immunoblotted with their corresponding antibodies and with the anti-ORP150 antibody. These data are representative of three separate experiments. The time course of phosphorylation of PERK (C) and eIF2α (D) induced by PA in vector- and ORP150-transfected hepatocytes. The data are representative of three independent experiments. E, ORP150, PERK, and eIF2α in crude extracts were measured by Western blot.
AMPK Increases ORP150 Transcription through FOXO1
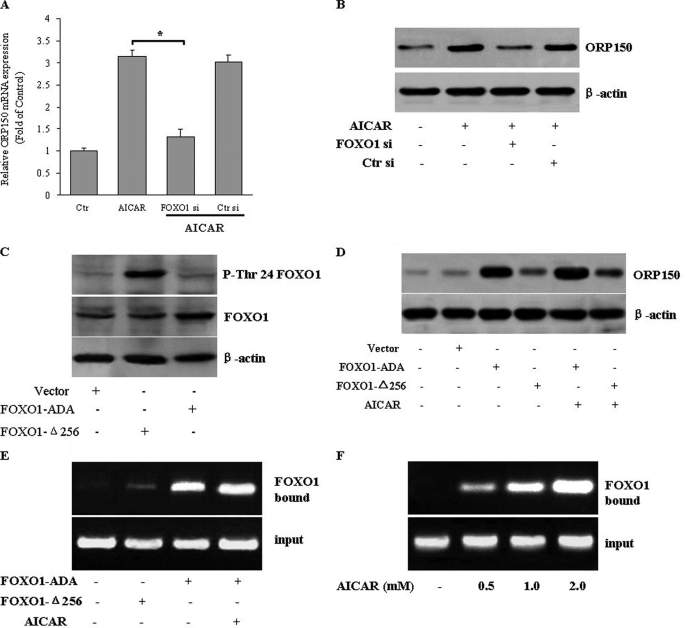
Because AMPK-mediated down-regulation of ER stress and apoptosis requires ORP150, we further investigated how AMPK regulates ORP150 expression. We identified FOXO1 as one of these transcriptional factors that may mediate the increase in ORP150 transcription by AMPK. Silencing FOXO1 with siRNA significantly prevented the AICAR-induced expression of ORP150 expression at both the mRNA (Fig. 6A) and protein levels (Fig. 6B), indicating that FOXO1 is involved in the AMPK-induced up-regulation of ORP150.
FIGURE 6.
FOXO1 mediates the AMPK-induced up-regulation of ORP150. A and B, HepG2 cells were transfected with FOXO1 siRNA (FOXO1 si) or control siRNA (Ctr si) and then treated with AICAR (1 mm) for 24 h. A, ORP150 mRNA was measured by quantitative RT-PCR, and results are expressed as -fold of control. *p < 0.05. The effectiveness of FOXO1 knockdown was examined by anti-FOXO1 antibody (upper panel). B, ORP150 protein expression was analyzed by Western blot. Representative data and quantitative analysis from three independent experiments are shown. *, p < 0.05. C, a representative Western blot shows the relative levels of endogenous and ectopic FOXO1. FOXO1 Thr-24 phosphorylation, total FOXO1 in hepatocytes infected with FOXO1-ADA, and FOXO1-Δ256 expression vectors are shown. The Δ256 form of FOXO1 lacks the epitope recognized by the FOXO1 antibody. D, overexpression of FOXO1 increased ORP150 protein levels. HepG2 cells were transfected with FOXO1-ADA or FOXO1-Δ256 in the absence or presence of AICAR (1 mm). The protein expression of ORP150 was examined. Representative blots from three independent experiments are shown. HepG2 cells transfected with FOXO1-ADA or FOXO1-Δ256 were treated with AICAR (1 mm) for 24 h (E) or HepG2 cells were incubated with AICAR (0–2 mm) for 24 h (F). After treatment, the protein-DNA complex was immunoprecipitated with an anti-FOXO1 antibody. The FOXO binding site in the DNA-protein complex was amplified by PCR. Representative blots from three independent experiments are shown.
To further determine the essential role of FOXO1, we analyzed the ability of gain- and loss-of-function FOXO1 mutants to affect AMPK-mediated ORP150 expression. Transduction of HepG2 cells with adenoviral vectors encoding FOXO1-Δ256 increased the phosphorylation of Thr-24, which is responsible for FOXO nuclear exclusion (34). In contrast, phosphorylation of FOXO1-ADA on T24 was not detected (Fig. 6C).
Furthermore, FOXO1-ADA overexpression markedly increased ORP150 expression in the absence or presence of AICAR, but FOXO1-Δ256 dramatically decreased ORP150 expression (Fig. 6D). These data support the hypothesis that FOXO1 is necessary and sufficient for the induction of ORP150 expression by AMPK. The ability of FOXO1 to bind to the ORP150 promoter was tested by ChIP assays using nuclei isolated from HepG2 cells. FOXO1-ADA associated with the ORP150 promoter in the absence or presence of AICAR (Fig. 6E). In addition, AICAR dose-dependently increased the binding of endogenous FOXO1 to ORP150 promoter (Fig. 6F), further confirming that FOXO1 may mediate ORP150 expression induced by AMPK.
AMPK Enhances FOXO1 Transcriptional Activity via Deacetylation of FOXO1
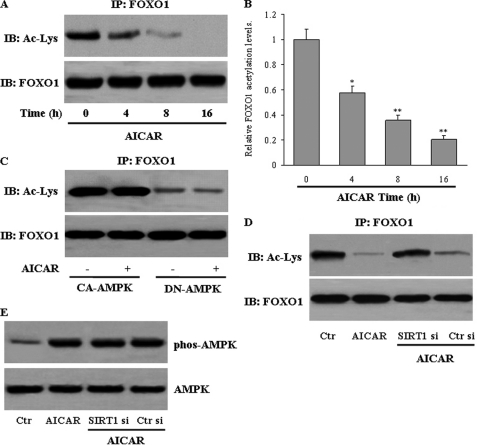
We investigated the potential mechanisms by which AMPK regulates FOXO1. We speculated that AMPK increases ORP150 expression by changing the FOXO1 acetylation status and activity. Immunoprecipitation using anti-acetyl-lysine antibody followed by blotting with anti-FOXO1 antibody revealed that AICAR treatment decreased FOXO1 acetylation as early as 4 h, which became more evident throughout the 16-h treatment period. (Fig. 7, A and B). Furthermore, overexpression of CA-AMPK led to a robust deacetylation of FOXO1 that could not be further enhanced by AICAR. In contrast, DN-AMPK expression abrogated the ability of AICAR to deacetylate FOXO1 (Fig. 7C). There was no change in FOXO1 expression at the mRNA or protein level upon AICAR treatment (data not shown). The activation of AMPK, therefore, initiates FOXO1 deacetylation in hepatocytes.
FIGURE 7.
AMPK promotes FOXO1 deacetylation. A, HepG2 cells were treated with AICAR for the times indicated. Then total protein lysates were used for immunoprecipitation (IP) of FOXO1, and FOXO1 acetylation levels were measured by Western blot (IB). B, relative band intensities were quantified. Data represent the means ± S.E. *, p < 0.05; or **, p < 0.01 versus 0 h. HepG2 cells were infected with adenoviruses encoding constitutively active (CA) or dominant-negative (DN) forms of AMPK (C) or transfected with either control (Ctr) or SIRT1 siRNA (Si) (D). Next, cells were treated with vehicle or AICAR (1 mm) for 8 h. Then, the acetyl-lysine level was determined in FOXO1 immunoprecipitates. E, the phosphorylation of Thr-172 of AMPK in the cellular extracts was measured by Western blot. The data represent results of three independent experiments.
Because SIRT1 interacts with and deacetylates FOXO1 (35), we next evaluated whether SIRT1 mediates the AICAR-induced deacetylation of FOXO1. AICAR failed to decrease FOXO1 acetylation when SIRT1 expression was knocked down with specific siRNA (Fig. 7D). The lack of SIRT1 did not affect AICAR-induced AMPK phosphorylation in this cell model (Fig. 7E).
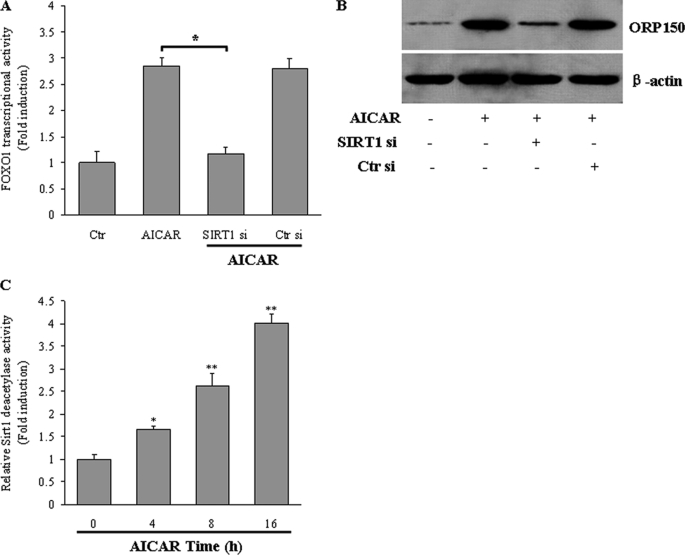
We further monitored whether AMPK-mediated FOXO1 deacetylation influences FOXO1 transcriptional activity. AICAR treatment robustly increased FOXO1 action in HepG2 cells, and this increase was reduced ∼60% when SIRT1 was silenced by siRNA (Fig. 8A). The lack of SIRT1 also compromised the induction of ORP150 expression by AICAR (Fig. 8B). We further carried out a SIRT1 deacetylase activity assay using a fluorometric kit. In parallel with reduced FOXO1 deacetylation, activation of AMPK by AICAR increased SIRT1 deacetylase activity in a time-dependent manner in HepG2 cells (Fig. 8C). This result indicates that SIRT1 is necessary for AMPK to increase FOXO1-mediated ORP150 expression.
FIGURE 8.
AMPK enhances FOXO1 transcriptional activity. HepG2 cells were transfected with either control (Ctr) or SIRT1 siRNA (Si). The cells were then treated with AICAR (1 mm) for 8 h. FOXO1 transcriptional activity (A) and ORP150 protein (B) were measured. Data are expressed as the mean ± S.E. of representative blots from three independent experiments. *, p < 0.05. C, HepG2 cells were treated with AICAR (1 mm) for the times indicated. Then, SIRT1 deacetylase activity was determined by using a fluorometric kit. Data represent the mean ± S.E. *, p < 0.05; **, p < 0.01 compared with 0 h.
Involvement of ORP150 in the Suppression of Hepatic ER Stress by AMPK in Vivo
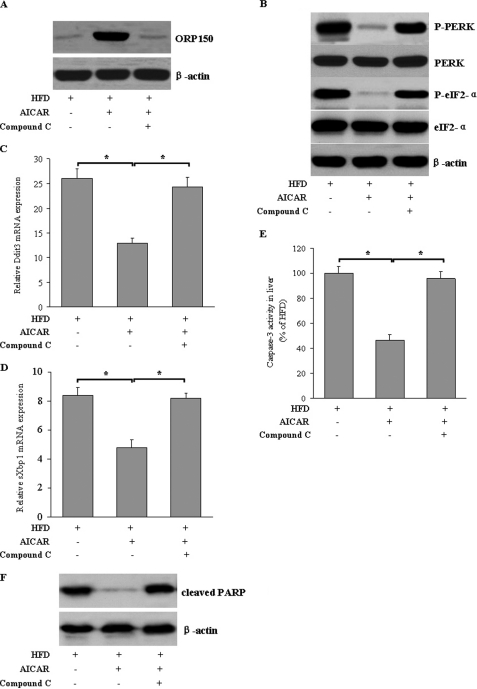
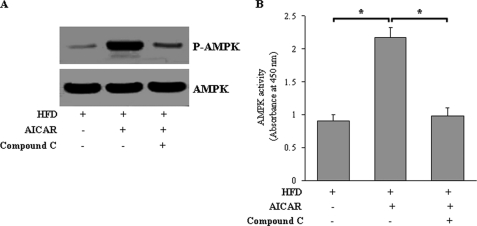
To further determine whether our in vitro findings were applicable in vivo, C57BL/6J mice fed a HFD were intravenously injected with AICAR or vehicle for 4 weeks. AICAR treatment markedly increased ORP150 protein expression (Fig. 9A), which was accompanied by attenuated ER stress markers (Fig. 9, B–D), reduced caspase-3 activity (Fig. 9E), and PARP cleavage (Fig. 9F) in liver of C57BL/6J mice. Furthermore, in AICAR-treated mice administered compound C, both ORP150 protein expression and the protective effects of AICAR on hepatic ER stress were largely abolished, confirming that the specific effects are mainly attributable to the activation of AMPK (Fig. 9, A–E). Hepatic AMPK activity was confirmed by the level of phosphorylated AMPK at Thr-172 (Fig. 10A) and direct measurement using the SAMS peptide as a substrate (Fig. 10B). Taken together, these results indicate that ORP150 plays a critical role in the amelioration of hepatic ER stress and apoptosis by AMPK in high fat diet-fed mice.
FIGURE 9.
AICAR increases ORP150 expression and decreases ER stress in vivo. A, C57BL/6J mice were treated with AICAR (n = 8, 200 mg/kg/d) or vehicle (n = 8, 0.9% NaCl) in the absence or presence of compound C (n = 8, 20 mg/kg/d) for 4 weeks. B, ORP150 protein expression and ER stress indicators were measured by Western blot in liver tissue extracts. Ddit3 (C), sXbp1 (D), and caspase-3 activity (E) were measured by quantitative RT-PCR and a colorimetric method. Results are expressed as the mean ± S.E. of representative blots. *, p < 0.05. n = 6 per group. F, cleaved PARP was measured by Western blot. Representative blots from three independent assays are shown.
FIGURE 10.
Infusion with AICAR increases the activation of AMPK in vivo. C57BL/6J mice fed a HFD were treated with AICAR (200 mg/kg/day, n = 8) or vehicle (0.9% NaCl, n = 8) in the absence or presence of compound C (n = 8, 20 mg/kg/day) for 4 weeks. A, AMPK Thr-172 phosphorylation in liver tissue extracts was analyzed by Western blot. The data represent results of three independent experiments. B, AMPK activity assays with the use of SAMS peptide as a substrate were also performed on aorta extracts. Data represent the means ± S.E. n = 6 per group. *, p < 0.05.
DISCUSSION
Abnormal ER stress contributes substantially to hepatocyte cell death during altered lipid metabolism (36, 37). Thus, modulation of hepatic ER stress becomes crucial in understanding the extent of its contribution to the pathogenesis of various hepatic disorders. The present study provides the first evidence that AMPK suppresses the lipid-induced hepatic ER stress and apoptosis in vitro and in vivo. First, we show that AMPK activation by AICAR or CA-AMPK overexpression provides marked protection against lipid-mediated ER stress and cell death in HepG2 cells. Second, AMPK strongly induces the mRNA and protein expression of ORP150, which then attenuates PA-triggered ER stress in hepatocytes via interacting directly with ER stress sensors. Third, AMPK regulates ORP150 expression by deacetylation of FOXO1 through increasing SIRT1 deacetylase activity. Finally, activation of AMPK in vivo by AICAR administration confers protective effects against HFD-mediated ER stress response and apoptosis in liver. Based on our findings, we propose that ORP150 is a novel target of AMPK in the protection against lipid-induced hepatic ER stress.
ORP150 is an ER-resident stress protein that functions as a chaperone and is induced by stressful conditions such as oxygen or glucose deprivation (38, 39). In the current study we report the novel finding that AMPK up-regulates ORP150 expression in hepatocytes in vitro and in vivo. We observed that pharmacological activation of AMPK by AICAR increases ORP150 mRNA and protein expression in hepatocytes. This result was confirmed by complementary AMPK agonist metformin and A-769662. Furthermore, CA-AMPK overexpression strongly increases ORP150 expression levels, which points to an AMPK-specific effect.
Induction of ORP150 exhibits protective roles in the prevention of ER stress (21) and deregulation of calcium homeostasis and apoptosis (22), which supports the speculation that ORP150 expression could be beneficial under the local challenging conditions of ER stress. We wondered whether ORP150 participates in the anti-ER stress action of AMPK. ORP150 down-regulation by specific siRNA almost completely overcame the effect of AMPK on lipid-induced hepatic ER stress indicators and apoptosis in HepG2 cells. In addition to the better-known properties of ORP150, mainly, calcium buffering and protection against ER calcium store depletion, ORP150 binds misfolded proteins, thereby releasing the activated ER stress sensors that become activated and trigger adaptive responses to ER dysfunction (21). We report here that ORP150 can directly bind to the ER stress sensors PERK and eIF2α, which probably maintains them in an inactive state, thereby inhibiting the UPR and down-regulating ER stress. Therefore, ORP150 may be primarily responsible for the anti-ER stress and anti-apoptosis effects of AMPK. Regarding the mechanism by which the AMPK pathway induces ORP150 expression, our study suggests that FOXO1 may be the critical transcription factor that mediates the effects of AMPK on ORP150 expression. FOXO transcription factors are important regulators of metabolism, cell-cycle progression, apoptosis, and oxidative stress resistance (40–42). ChIP revealed direct binding of FOXO1 to a motif at the transcription initiation site of human ORP150, which may lead to activation of ORP150 transcription.
FOXO transcription factors are generally regulated at the post-transcriptional level by phosphorylation, dephosphorylation, acetylation, and deacetylation (43–45). We demonstrated that AMPK activation by AICAR strongly decreased the FOXO1 acetylation level. In accordance with these results, AICAR incubation strongly enhanced FOXO1 transcriptional activity. SIRT1, the mammalian ortholog of yeast silent information regulator (Sir) 2, is a class III histone deacetylase (46). SIRT1 deacetylates FOXO1, FOXO3, and FOXO4 and regulates FOXO-dependent gene transcription either positively or negatively (47, 48). A physiological interaction of FOXO with SIRT1, an NAD+-dependent deacetylase, has been recently demonstrated (49). We observed that knockdown of SIRT1 impairs FOXO1 deacetylation, FOXO1 transactivation activity, and ORP150 expression. Exposure to AICAR caused a marked increase in SIRT1 deacetylase activity. Thus, the deacetylation of FOXO by SIRT1 also appears to reverse the inhibitory effect of acetylation on its transactivation activity. Conversely, knockdown of SIRT1 reverses the deacetylation of FOXO1 by AICAR and consequently diminishes FOXO1 transcriptional activity and its mediated up-regulation of ORP150 expression. Our data provide a direct link between SIRT1 and ER stress, the detailed mechanisms of which need to be defined in further studies.
In summary, our data allow us to integrate AMPK into the transcriptional network that regulates ORP150 expression and preserves normal ER stress under pathophysiologic conditions. To our knowledge, this is the first time AMPK has been identified as a positive regulator of ORP150 expression through the induction of FOXO1 transcriptional activity via SIRT1-mediated deacetylation of FOXO1. The induction of ORP150 mediates the amelioration of hepatic ER stress and apoptosis by AMPK in vitro and in vivo. Thus, AMPK could be an effective therapeutic target in the prevention and treatment of hyperlipidemia-related hepatic disorders.
Acknowledgment
We greatly appreciate the gift of CA-AMPK and DN-AMPK donated by Dr. J. Ha (Kyung Hee University College of Medicine, Seoul, Korea).
This research was supported by grants from the National Natural Science Foundation (81072301), grants from the Guangdong Natural Science Foundation (9151012003000002), Foundation for the Author of National Excellent Doctoral Dissertation of PR China (200978), and the Fundamental Research Funds for the Central Universities of Sun Yat-Sen University (10ykzd04).
- ER
- endoplasmic reticulum
- AMPK
- adenosine monophosphate-activated protein kinase
- CA-AMPK
- constitutively active AMPK
- DN-AMPK
- dominant-negative AMPK
- AICAR
- 5-aminoimidazole-4-carboxamide ribonucleotide
- ORP150
- 150-kDa oxygen-regulated protein
- FOXO1
- Forkhead transcription factor 1
- UPR
- unfolded protein response
- PERK
- pancreatic ER kinase
- IRE-1
- inositol requiring transmembrane kinase/endonuclease-1
- ATF6
- activating transcription factor-6
- HFD
- high fat diet
- PARP
- poly(ADP-ribose) polymerase
- SAMS
- HMRSAMSGLHLVKRR
- ADA
- T24A/S253D/S316A.
REFERENCES
- 1. Lin J. H., Walter P., Yen T. S. (2008) Annu. Rev. Pathol. 3, 399–425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Hotamisligil G. S. (2010) Cell 140, 900–917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hotamisligil G. S. (2010) Nat. Med. 16, 396–399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Xu C., Bailly-Maitre B., Reed J. C. (2005) J. Clin. Invest. 115, 2656–2664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ron D., Walter P. (2007) Nat. Rev. Mol. Cell Biol. 8, 519–529 [DOI] [PubMed] [Google Scholar]
- 6. Marciniak S. J., Ron D. (2006) Physiol. Rev. 86, 1133–1149 [DOI] [PubMed] [Google Scholar]
- 7. Wu J., Kaufman R. J. (2006) Cell Death Differ. 13, 374–384 [DOI] [PubMed] [Google Scholar]
- 8. Rutkowski D. T., Hegde R. S. (2010) J. Cell Biol. 189, 783–794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Yao P. M., Tabas I. (2000) J. Biol. Chem. 275, 23807–23813 [DOI] [PubMed] [Google Scholar]
- 10. Yao P. M., Tabas I. (2001) J. Biol. Chem. 276, 42468–42476 [DOI] [PubMed] [Google Scholar]
- 11. Feng B., Yao P. M., Li Y., Devlin C. M., Zhang D., Harding H. P., Sweeney M., Rong J. X., Kuriakose G., Fisher E. A., Marks A. R., Ron D., Tabas I. (2003) Nat. Cell Biol. 5, 781–792 [DOI] [PubMed] [Google Scholar]
- 12. Erbay E., Babaev V. R., Mayers J. R., Makowski L., Charles K. N., Snitow M. E., Fazio S., Wiest M. M., Watkins S. M., Linton M. F., Hotamisligil G. S. (2009) Nat. Med. 15, 1383–1391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kahn B. B., Alquier T., Carling D., Hardie D. G. (2005) Cell Metab. 1, 15–25 [DOI] [PubMed] [Google Scholar]
- 14. Hardie D. G. (2007) Nat. Rev. Mol. Cell Biol. 8, 774–785 [DOI] [PubMed] [Google Scholar]
- 15. Shaw R. J., Lamia K. A., Vasquez D., Koo S. H., Bardeesy N., Depinho R. A., Montminy M., Cantley L. C. (2005) Science 310, 1642–1646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Terai K., Hiramoto Y., Masaki M., Sugiyama S., Kuroda T., Hori M., Kawase I., Hirota H. (2005) Mol. Cell. Biol. 25, 9554–9575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Dong Y., Zhang M., Liang B., Xie Z., Zhao Z., Asfa S., Choi H. C., Zou M. H. (2010) Circulation 121, 792–803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kuwabara K., Matsumoto M., Ikeda J., Hori O., Ogawa S., Maeda Y., Kitagawa K., Imuta N., Kinoshita T., Stern D. M., Yanagi H., Kamada T. (1996) J. Biol. Chem. 271, 5025–5032 [DOI] [PubMed] [Google Scholar]
- 19. Tamatani M., Matsuyama T., Yamaguchi A., Mitsuda N., Tsukamoto Y., Taniguchi M., Che Y. H., Ozawa K., Hori O., Nishimura H., Yamashita A., Okabe M., Yanagi H., Stern D. M., Ogawa S., Tohyama M. (2001) Nat. Med. 7, 317–323 [DOI] [PubMed] [Google Scholar]
- 20. Ozawa K., Kuwabara K., Tamatani M., Takatsuji K., Tsukamoto Y., Kaneda S., Yanagi H., Stern D. M., Eguchi Y., Tsujimoto Y., Ogawa S., Tohyama M. (1999) J. Biol. Chem. 274, 6397–6404 [DOI] [PubMed] [Google Scholar]
- 21. Sanson M., Augé N., Vindis C., Muller C., Bando Y., Thiers J. C., Marachet M. A., Zarkovic K., Sawa Y., Salvayre R., Nègre-Salvayre A. (2009) Circ. Res. 104, 328–336 [DOI] [PubMed] [Google Scholar]
- 22. Sanson M., Ingueneau C., Vindis C., Thiers J. C., Glock Y., Rousseau H., Sawa Y., Bando Y., Mallat Z., Salvayre R., Nègre-Salvayre A. (2008) Cell Death Differ. 15, 1255–1265 [DOI] [PubMed] [Google Scholar]
- 23. Park K. G., Min A. K., Koh E. H., Kim H. S., Kim M. O., Park H. S., Kim Y. D., Yoon T. S., Jang B. K., Hwang J. S., Kim J. B., Choi H. S., Park J. Y., Lee I. K., Lee K. U. (2008) Hepatology 48, 1477–1486 [DOI] [PubMed] [Google Scholar]
- 24. Nakae J., Kitamura T., Silver D. L., Accili D. (2001) J. Clin. Invest. 108, 1359–1367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Akao M., Ohler A., O'Rourke B., Marbán E. (2001) Circ. Res. 88, 1267–1275 [DOI] [PubMed] [Google Scholar]
- 26. Qin X., Xie X., Fan Y., Tian J., Guan Y., Wang X., Zhu Y., Wang N. (2008) Hepatology 48, 432–441 [DOI] [PubMed] [Google Scholar]
- 27. Borradaile N. M., Han X., Harp J. D., Gale S. E., Ory D. S., Schaffer J. E. (2006) J. Lipid Res. 47, 2726–2737 [DOI] [PubMed] [Google Scholar]
- 28. Lai E., Bikopoulos G., Wheeler M. B., Rozakis-Adcock M., Volchuk A. (2008) Am. J. Physiol. Endocrinol Metab. 294, E540–E550 [DOI] [PubMed] [Google Scholar]
- 29. Schaffer J. E. (2003) Curr. Opin. Lipidol. 14, 281–287 [DOI] [PubMed] [Google Scholar]
- 30. Fernandes-Alnemri T., Litwack G., Alnemri E. S. (1994) J. Biol. Chem. 269, 30761–30764 [PubMed] [Google Scholar]
- 31. Nicholson D. W., Ali A., Thornberry N. A., Vaillancourt J. P., Ding C. K., Gallant M., Gareau Y., Griffin P. R., Labelle M., Lazebnik Y. A., et al. (1995) Nature 376, 37–43 [DOI] [PubMed] [Google Scholar]
- 32. Zhou G., Myers R., Li Y., Chen Y., Shen X., Fenyk-Melody J., Wu M., Ventre J., Doebber T., Fujii N., Musi N., Hirshman M. F., Goodyear L. J., Moller D. E. (2001) J. Clin. Invest. 108, 1167–1174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Cool B., Zinker B., Chiou W., Kifle L., Cao N., Perham M., Dickinson R., Adler A., Gagne G., Iyengar R., Zhao G., Marsh K., Kym P., Jung P., Camp H. S., Frevert E. (2006) Cell Metab. 3, 403–416 [DOI] [PubMed] [Google Scholar]
- 34. Nakae J., Barr V., Accili D. (2000) EMBO J. 19, 989–996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Yang Y., Hou H., Haller E. M., Nicosia S. V., Bai W. (2005) EMBO J. 24, 1021–1032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Malhi H., Guicciardi M. E., Gores G. J. (2010) Physiol. Rev. 90, 1165–1194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Flamment M., Kammoun H. L., Hainault I., Ferré P., Foufelle F. (2010) Curr. Opin. Lipidol. 21, 239–246 [DOI] [PubMed] [Google Scholar]
- 38. Bando Y., Ogawa S., Yamauchi A., Kuwabara K., Ozawa K., Hori O., Yanagi H., Tamatani M., Tohyama M. (2000) Am. J. Physiol. Cell Physiol. 278, C1172–C1182 [DOI] [PubMed] [Google Scholar]
- 39. Tsukamoto Y., Kuwabara K., Hirota S., Ikeda J., Stern D., Yanagi H., Matsumoto M., Ogawa S., Kitamura Y. (1996) J. Clin. Invest. 98, 1930–1941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Tothova Z., Kollipara R., Huntly B. J., Lee B. H., Castrillon D. H., Cullen D. E., McDowell E. P., Lazo-Kallanian S., Williams I. R., Sears C., Armstrong S. A., Passegué E., DePinho R. A., Gilliland D. G. (2007) Cell 128, 325–339 [DOI] [PubMed] [Google Scholar]
- 41. Lehtinen M. K., Yuan Z., Boag P. R., Yang Y., Villén J., Becker E. B., DiBacco S., de la Iglesia N., Gygi S., Blackwell T. K., Bonni A. (2006) Cell 125, 987–1001 [DOI] [PubMed] [Google Scholar]
- 42. Nakamura T., Sakamoto K. (2008) Mol. Cell Endocrinol. 281, 47–55 [DOI] [PubMed] [Google Scholar]
- 43. Accili D., Arden K. C. (2004) Cell 117, 421–426 [DOI] [PubMed] [Google Scholar]
- 44. Matsuzaki H., Daitoku H., Hatta M., Aoyama H., Yoshimochi K., Fukamizu A. (2005) Proc. Natl. Acad. Sci. U.S.A. 102, 11278–11283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Frescas D., Valenti L., Accili D. (2005) J. Biol. Chem. 280, 20589–20595 [DOI] [PubMed] [Google Scholar]
- 46. Lavu S., Boss O., Elliott P. J., Lambert P. D. (2008) Nat. Rev. Drug Discov. 7, 841–853 [DOI] [PubMed] [Google Scholar]
- 47. Brunet A., Sweeney L. B., Sturgill J. F., Chua K. F., Greer P. L., Lin Y., Tran H., Ross S. E., Mostoslavsky R., Cohen H. Y., Hu L. S., Cheng H. L., Jedrychowski M. P., Gygi S. P., Sinclair D. A., Alt F. W., Greenberg M. E. (2004) Science 303, 2011–2015 [DOI] [PubMed] [Google Scholar]
- 48. Motta M. C., Divecha N., Lemieux M., Kamel C., Chen D., Gu W., Bultsma Y., McBurney M., Guarente L. (2004) Cell 116, 551–563 [DOI] [PubMed] [Google Scholar]
- 49. Cantó C., Gerhart-Hines Z., Feige J. N., Lagouge M., Noriega L., Milne J. C., Elliott P. J., Puigserver P., Auwerx J. (2009) Nature 458, 1056–1060 [DOI] [PMC free article] [PubMed] [Google Scholar]