Abstract
Diabetic cardiac dysfunction is associated with decreased rates of myocardial glucose oxidation (GO) and increased fatty acid oxidation (FAO), a fuel shift that has been shown to sensitize the heart to ischemic insult and ventricular dysfunction. We sought to evaluate the metabolic and functional consequences of chronic suppression of GO in heart as modeled by transgenic mice with cardiac-specific overexpression of pyruvate dehydrogenase kinase 4 (myosin heavy chain (MHC)-PDK4 mice), an inhibitor of pyruvate dehydrogenase. Hearts of MHC-PDK4 mice were shown to exhibit an insulin-resistant substrate utilization profile, characterized by low GO rates and high FAO flux. Surprisingly, MHC-PDK4 mice were not sensitized to cardiac ischemia-reperfusion injury despite a fuel utilization pattern that phenocopied the diabetic heart. In addition, MHC-PDK4 mice were protected against high fat diet-induced myocyte lipid accumulation, likely related to increased capacity for FAO. The high rates of mitochondrial FAO in the MHC-PDK4 heart were related to heightened activity of the AMP-activated protein kinase, reduced levels of malonyl-CoA, and increased capacity for mitochondrial uncoupled respiration. The expression of the known AMP-activated protein kinase target, peroxisome proliferator-activated receptor γ coactivator-1α (PGC-1α), a master regulator of mitochondrial function and biogenesis, was also activated in the MHC-PDK4 heart. These results demonstrate that chronic activation of PDK4 triggers transcriptional and post-transcriptional mechanisms that re-program the heart for chronic high rates of FAO without the expected deleterious functional or metabolic consequences.
Keywords: Cardiac Metabolism, Diabetes, Fatty Acid Oxidation, Gene Regulation, Glucose Metabolism
Introduction
The emerging pandemic of obesity is driving a dramatic increase in the incidence of type 2 diabetes mellitus and “metabolic syndrome” (1, 2). Diabetic individuals are more sensitive to co-morbidities known to cause heart failure, such as myocardial ischemic injury (3–5). The term “diabetic cardiomyopathy” has been coined to capture this unique condition. Although many factors certainly contribute, recent evidence suggests that diabetic cardiac dysfunction is driven at least in part by abnormalities in myocardial fuel metabolism (6–8).
The healthy adult mammalian heart exhibits energy substrate flexibility, utilizing a variety of energy substrates including fatty acids (FAs),3 glucose, ketones, and lactate to yield ATP to meet the high energy demands of a constant pump (9, 10). In most conditions, FAs are the preferred substrate for myocardial ATP production, although a significant proportion of ATP is derived from glycolysis and glucose oxidation (GO) (8). The insulin-resistant diabetic heart loses this substrate flexibility due to reduced insulin responsive glucose uptake and oxidation, together with increased delivery of FAs, resulting in near complete reliance on fatty acid oxidation (FAO) for ATP production (7, 11, 12). GO is inhibited in the insulin-resistant heart at the step catalyzed by the pyruvate dehydrogenase complex, due in part to increased expression of the pyruvate dehydrogenase kinase, PDK4 (13). PDK4-mediated phosphorylation of pyruvate dehydrogenase inhibits the activity of this enzyme complex and reduces capacity to oxidize glucose (14). Chronic dependence on FA as the cardiac fuel source in the diabetic heart is associated with accumulation of cardiac myocyte neutral lipid, toxic lipid intermediates, and reactive oxygen species, all of which have been implicated in the development of cardiomyopathy (7, 11, 12, 15, 16). Indeed, myocyte triacylglycerol (TG) accumulation is a metabolic signature of the diabetic heart and is considered a marker for “lipotoxic” cardiomyopathy. There is also evidence that the inability to oxidize glucose plays an etiologic role in the development of diabetic cardiomyopathy, especially in the context of ischemic insult (17, 18).
Studies conducted in genetically modified mice have begun to explore the role of metabolic derangements in the pathogenesis of diabetic cardiac dysfunction. For example, transgenic mice with chronic activation of the peroxisome proliferator-activated receptor α (PPARα), a lipid-activated nuclear receptor that regulates the expression of enzymes involved in cellular FA uptake and oxidation, have been studied. Cardiac-specific PPARα transgenics (MHC-PPARα mice) develop a metabolic phenotype remarkably similar to the diabetic heart including increased myocardial FA uptake and oxidation, TG accumulation, and development of cardiomyopathy (19). Similar phenotypes have been shown in transgenic mice with overexpression of PPARγ (20) and acyl-CoA synthetase, a key enzyme in the import and activation of FAs (21). However, the results of these studies have not clearly distinguished between the role of lipid accumulation (lipotoxic effects) versus substrate inflexibility in the cardiomyopathic phenotype. To further address the effects of chronic cardiac fuel inflexibility, we focused on mice that overexpress PDK4 in a cardiac-specific manner (MHC-PDK4 mice) (22). The MHC-PDK4 line is an ideal model to address this question because the hearts of these mice rely largely on FA as the chief cardiac energy substrate together with chronically suppressed GO (22). Assessment of substrate utilization in isolated MHC-PDK4 working hearts demonstrated that the fixed substrate utilization rates were unresponsive to insulin, similar to diabetic hearts. Surprisingly, MHC-PDK4 mice were resistant to high fat (HF) diet-induced myocyte lipid accumulation and, unlike diabetic or MHC-PPARα hearts, exhibited normal functional recovery after I/R injury. This adaptive metabolic re-programming is shown to involve activation of AMP-activated protein kinase (AMPK) and the transcriptional coactivator, PGC-1α.
EXPERIMENTAL PROCEDURES
Detailed methods can be found in the supplemental information.
Animal Studies
Mice with cardiac-specific overexpression of PDK4 driven by the α-myosin heavy chain promoter (MHC-PDK4 mice) have been previously described (22). Cardiac functional and metabolic endpoints were analyzed in sex- and age-matched MHC-PDK4 and non-transgenic (NTg) littermate controls. For diet studies, mice were allowed ad libitum access to either HF chow, which provided 43% of the calories from fat (TD 97268, Harlan Teklad, Madison, WI) or standard diet. All studies were performed on 10–12-week-old mice unless otherwise indicated.
To examine insulin signaling, mice were fasted for 4 h and injected intravenously with human insulin (10 milliunits/g of body weight). Mice were sacrificed 5 min later, and cardiac ventricles were harvested and snap-frozen in liquid nitrogen for protein extraction.
All experiments and protocols were conducted in strict accordance with the National Institutes of Health guidelines for humane treatment of animals and were reviewed and approved by the Washington University School of Medicine Animal Studies Committee.
Mouse Isolated Working Heart Preparation
Mouse isolated working heart preparations were performed as previously described (23). Glycolysis was assessed in separate studies using [5-3H]glucose (0.1 μCi/ml). The percent contribution of palmitate and glucose oxidation to the total acetyl-CoA production was also determined. Acetyl-CoA production was calculated in a similar manner as ATP production as previously described and assumes 100% coupled respiration (23).
For I/R studies, hearts underwent the following protocol: aerobic perfusion for 30 min and 18 min of ischemia followed by reperfusion for 40 min. Hearts were perfused with 5 mm glucose, 0.4 or 1.2 mm palmitate, and 100 microunits/ml insulin.
Echocardiographic Studies
Transthoracic M-mode and two-dimensional echocardiography was performed on conscious mice in the Washington University Mouse Cardiovascular Phenotyping Core using an Acuson Sequoia 256 Echocardiography system (Acuson Corp., Mountain View, CA) as previously described (24). Detailed methods can be found in the supplemental information. All tissue analyses protocols can be found in supplemental Information.
Statistical Analyses
Data were analyzed using a 2-tailed Student's t test with the level of significance set at p < 0.05 in all cases. Data are reported as the mean values ± S.E.
RESULTS
The Fuel Utilization Inflexibility of MHC-PDK4 Hearts Is Resistant to Insulin
As previously reported (22), cardiac PDK4 gene expression was increased ∼14-fold in the hearts of MHC-PDK4 transgenic mice compared with littermate NTg controls (supplemental Fig. 1, A and B), and cardiac function was normal (supplemental Fig. 1C). Additionally, there was no significant difference in body weight or circulating glucose and free fatty acid levels in the transgenic line (data not shown).
To explore cardiac fuel utilization rates and response to insulin, substrate oxidation rates were assessed in working hearts isolated from MHC-PDK4 mice and controls. NTg and MHC-PDK4 hearts were perfused with [9,10-3H]palmitate and [U-14C]glucose with either 10 or 100 microunits/ml insulin, and glucose and palmitate oxidation rates were determined. Hearts isolated from NTg and MHC-PDK4 mice performed similar levels of cardiac work during perfusion (supplemental Fig. 2). As expected, GO rates were strikingly lower in MHC-PDK4 hearts compared with littermate NTg mice (Fig. 1A). The higher concentration of insulin (100 microunits/ml) stimulated GO in NTg hearts but not in MHC-PDK4 hearts (Fig. 1A). Conversely, palmitate oxidation rates were increased in the MHC-PDK4 hearts compared with littermate controls, regardless of the insulin dose (Fig. 1A).
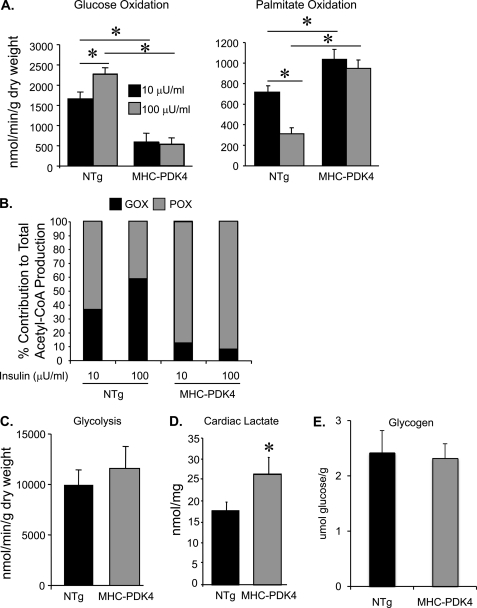
FIGURE 1.
Hearts of MHC-PDK4 mice exhibit increased rates of palmitate oxidation and concomitant decreased rates of glucose oxidation. A, substrate utilization rates in working hearts isolated from female NTg and MHC-PDK4 hearts (n = 5–6 per group) is shown. The bars denote mean (±S.E.) glucose oxidation (left) and palmitate oxidation (right) rates under low insulin (10 μm, black bars) or high insulin (100 μm, gray bars) perfusion conditions. B, the bars denote percent contribution to total acetyl-CoA production by glucose oxidation (GOX, black portion) or palmitate oxidation (POX, gray portion) (n = 5–6 per group). C, the bars denote mean rates of glycolysis in NTg and MHC-PDK4 hearts (n = 8–9 per group). D, bars denote mean levels of cardiac lactate in NTg and MHC-PDK4 hearts (n = 9–11 per group). E, bars denote mean levels of glycogen in NTg and MHC-PDK4 hearts (n = 8–10 per group). *, p < 0.05.
The percentage of acetyl-CoA produced from each of the oxidative pathways was calculated based on the comparison of substrate oxidation rates. When the insulin concentration of the perfusate was increased from 10 to 100 microunits/ml, the contribution of GO to total acetyl-CoA production increased from ∼38 to 60% in NTg hearts (Fig. 1B). In contrast, GO contributed only ∼8% to total acetyl-CoA production under both low and high insulin concentrations in MHC-PDK4 hearts, with the remainder accounted for by palmitate oxidation (Fig. 1B). Collectively, these results indicate that the energy substrate preference of MHC-PDK4 hearts is highly biased toward fatty acids and is resistant to the effects of insulin, similar to that of the diabetic heart.
The original characterization of myocardial glucose metabolism in MHC-PDK4 mice using NMR spectroscopy also demonstrated that glucose utilization was diminished (22) but did not delineate flux rates through glycolysis versus glucose oxidation. Therefore, isolated working heart perfusion using [3H]glucose was used to measure glycolytic rates in NTg and MHC-PDK4 mice. In contrast to GO, rates of glycolysis were not impaired and actually tended to be higher in MHC-PDK4 hearts compared with NTg littermate controls (Fig. 1C). Consistent with a mismatch between capacity for glycolysis and GO, myocardial tissue lactate levels were increased in MHC-PDK4 mice compared with their NTg counterparts (Fig. 1D). No significant difference in myocardial glycogen content was observed between NTg and MHC-PDK4 mice (Fig. 1E).
Insulin Signaling Remains Intact in MHC-PDK4 Mice
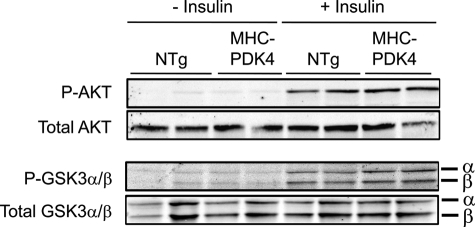
Given that the transgenic hearts are resistant to the effects of insulin, the phosphorylation status of key signaling proteins downstream of the insulin receptor was assessed. Insulin stimulated phosphorylation of serine 473 of AKT, serine 21 of GSK3α, and serine 9 of GSK3β in MHC-PDK4 and NTg hearts to a similar extent (Fig. 2). Western blot analyses also demonstrated that total levels of GLUT1 and GLUT4 proteins were not different between the groups (data not shown). These data suggest that the resistance of the PDK4 heart to insulin-stimulated glucose utilization involves mechanisms downstream of the insulin signaling pathway.
FIGURE 2.
Insulin receptor-mediated signaling is unchanged in MHC-PDK4 hearts. Male NTg and MHC-PDK4 mice were injected IV with (10 milliunits/g of body weight) insulin or saline (−insulin), and hearts were harvested 5 min later (n = 6 per group). Representative Western blots using heart lysates are shown.
The MHC-PDK4 Hearts Exhibit Normal Functional Recovery after I/R Injury despite Constrained Substrate Flexibility
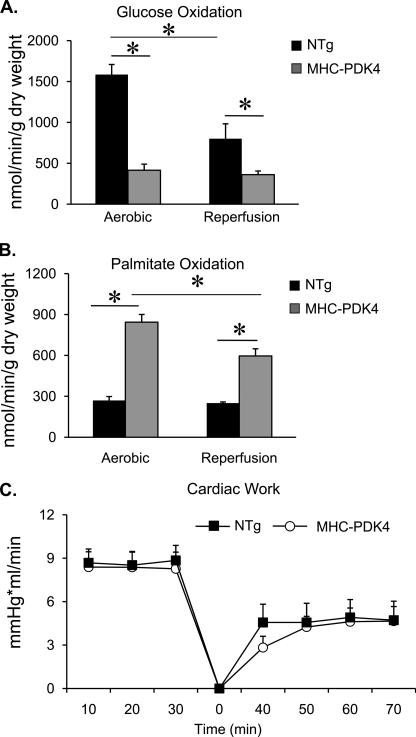
Diabetic humans and animals are susceptible to I/R injury and metabolic inflexibility. This response has been attributed in part to reduced capacity for GO and high FAO rates (23, 25–27). Accordingly, we sought to determine whether MHC-PDK4 hearts exhibit increased sensitivity to I/R injury. Hearts isolated from NTg and MHC-PDK4 mice were subjected to an I/R protocol (30 min of aerobic perfusion, 18 min of ischemia, 40 min of aerobic reperfusion, 5 mm glucose, 0.4 mm palmitate, and 100 microunits/ml insulin). GO rates were severely reduced, and FAO rates increased in MHC-PDK4 mice compared with NTg hearts under both aerobic and reperfusion conditions (Figs. 3, A and B). Surprisingly, however, the functional recovery (cardiac work) of the MHC-PDK4 hearts was not different than that of NTg controls (Fig. 3C). Similar results were observed when the I/R studies were conducted in conditions of high concentrations of palmitate in the perfusate (5 mm glucose, 1.2 mm palmitate, and 100 microunits/ml insulin; data not shown).
FIGURE 3.
MHC-PDK4 hearts have normal functional recovery after ischemia-reperfusion injury. Female NTg and MHC-PDK4 hearts were subjected to ischemia-reperfusion in the isolated working preparation as described under “Experimental Procedures” with 5 mm glucose, 0.4 mm palmitate, and 100 microunits/ml insulin. A, bars represent mean glucose oxidation rates (±S.E.) under aerobic (n = 11–15 per group) conditions and after reperfusion (n = 6–12 per group). B, bars denote palmitate oxidation rates (± S.E.) under aerobic (n = 11–15 per group) conditions and after reperfusion (n = 6–12 per group). C, the line graph depicts cardiac work (±S.E.) over time during the ischemia-reperfusion protocol in NTg (black squares) and MHC-PDK4 (open circles) hearts. Time 0 represents the beginning of the reperfusion period. *, p < 0.05.
MHC-PDK4 Transgenic Mice Are Protected against Diet-induced Myocardial TG Accumulation
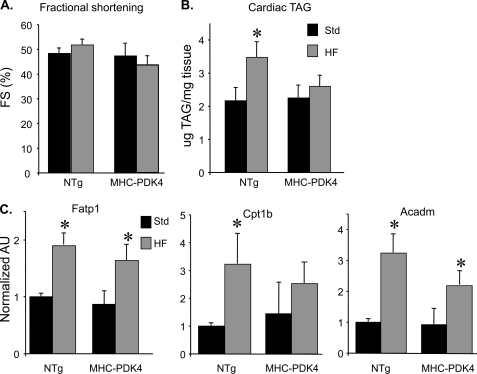
Myocardial lipid accumulation often occurs in the diabetic heart and in other metabolic cardiomyopathies (28). To determine whether the MHC-PDK4 mice develop this signature of cardiac lipotoxicity given their high rates of fatty acid uptake and oxidation rates, they were challenged with a HF diet (43% of calories from fat). After 4 weeks of HF chow, the MHC-PDK4 mice did not exhibit any evidence of systolic or diastolic ventricular dysfunction or remodeling in MHC-PDK4 hearts (Fig. 4A and data not shown). Moreover, although HF diet increased cardiac TG levels in NTg mice, TG levels were not increased in the hearts of MHC-PDK4 mice (Fig. 4B).
FIGURE 4.
MHC-PDK4 mice are protected from HF diet-induced TG accumulation in the myocardium. A, echocardiography was performed on male NTg and MHC-PDK4 mice (n = 4–6 per group after standard or high fat chow diet). Bars represent mean percent left ventricular fractional shortening (FS) % (±S.E.) after 4 weeks of HF diet (gray bars) versus standard chow (black bars). B, bars denote mean cardiac triglyceride (TG) levels (± S.E.) in male NTg and MHC-PDK4 hearts after 4 weeks of either standard (black bars) or HF chow (gray bars) (n = 6–9 per group). C, levels of mRNAs as determined by quantitative real-time-PCR were performed on total RNA isolated from heart ventricles of male mice from the genotypes indicated (n = 6–9 per group). Values are shown as arbitrary units (AU) normalized (=1.0) to the NTg on standard chow. *, p < 0.05.
The resistance of MHC-PDK4 hearts to TG accumulation together with the high FAO rates suggested that the MHC-PDK4 hearts have undergone a re-programming to increase capacity to oxidize FA. We assessed the expression of genes involved in FAO and FA uptake. The expression levels of Fatp1 (cellular FA import), Cpt1b (mitochondrial FA import), and Acadm (FAO) were not different in MHC-PDK4 hearts compared with NTg littermate controls on standard chow or after exposure to HF chow, the latter known to induce these genes (Fig. 4C). Thus, re-programming of the MHC-PDK4 hearts for increased FAO does not occur at the level of FAO enzyme gene expression.
Increased Activation of AMP-activated Protein Kinase and PGC-1 Signaling in MHC-PDK4 Hearts
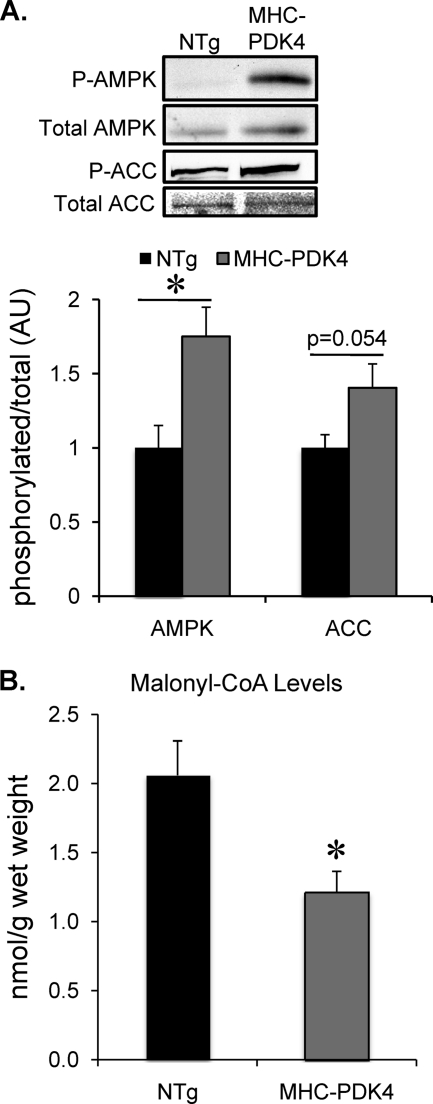
To assess the potential post-transcriptional mechanisms involved in the observed increased FAO rates in the MHC-PDK4 hearts, the activity of AMPK was assessed. AMPK is known to increase flux through the β-oxidation pathway by phosphorylating acetyl-CoA carboxylase, which inhibits its enzymatic activity and causes a decrease in malonyl-CoA levels, releasing suppression on carnitine palmitoyltransferase 1 (Cpt1b), the rate-limiting step for mitochondrial import of FA (29, 30). Phosphorylation of AMPK, a marker of its activity, was increased in MHC-PDK4 hearts compared with NTg controls (Fig. 5A). In addition, phosphorylation of acetyl-CoA carboxylase was also increased in the MHC-PDK4 hearts (p = .054, Fig. 5A). Consistent with these regulatory changes, malonyl-CoA levels were significantly lower in the hearts of the MHC-PDK4 transgenic mice when compared with their NTg littermate controls (Fig. 5B). Taken together, these data strongly suggest that the sustained increase in myocardial FAO in MHC-PDK4 myocardium involves reduced malonyl-CoA-mediated suppression of mitochondrial FAO via AMPK.
FIGURE 5.
Activation of AMPK and decreased malonyl-CoA levels in hearts of MHC-PDK4 mice. A, top, representative Western blot analyses were conducted on lysates of heart ventricles of female mice of indicated genotypes for phosphorylated (P) and total AMPK and acetyl-CoA carboxylase (ACC) as denoted. Bottom, bars represent the ratio in arbitrary units (AU) of phosphorylated/total levels of AMPK or acetyl-CoA carboxylase as indicated normalized (=1.0) to the NTg (n = 7 per group). *, p < 0.05 compared with NTg. B, bars represent the mean (±S.E.) cardiac malonyl-CoA levels as determined from heart ventricles of male mice from indicated genotypes (n = 6–9 per group). *, p < 0.05 compared with NTg.
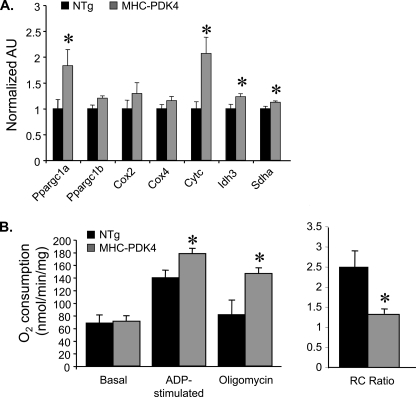
AMPK has also been shown to increase the expression and activity of the transcriptional coactivator PGC-1α (31), which in turn activates a network of genes involved in mitochondrial biogenesis and respiratory function in a variety of oxidative tissues including heart and skeletal muscle (32, 33). The expression of PGC-1α and a subset of its known target genes encoding enzymes involved in the citric acid cycle (Idh3, Sdha) and electron transport chain (Cytc) were increased in MHC-PDK4 hearts compared with NTg littermate controls at 10–12 weeks of age (Fig. 6A).
FIGURE 6.
Induction of PGC-1α gene expression and increased respiratory uncoupling in mitochondria isolated from MHC-PDK4 hearts. A, levels of specific mRNAs based on quantitative real-time-PCR performed on RNA isolated from heart ventricles of male mice from the genotypes indicated (n = 8–10 per group) are shown. Bars represent arbitrary uints (AU) normalized (=1.0) to the NTg. B, mitochondrial respiration rates determined by oxygen consumption (VO2) performed on mitochondria isolated from ventricle of male NTg and MHC-PDK4 hearts are shown. Mean values (±S.E.) are shown for the basal, ADP-stimulated, and oligomycin-inhibited states using succinate/rotenone as a substrate. The mean respiratory control (RC) ratio (±S.E.) is shown on the right. *, p < 0.05 compared with NTg.
Respiratory function was next assessed in isolated mitochondria from NTg and MHC-PDK4 mice in the presence of succinate/rotenone. Under basal conditions, oxygen consumption rates were not altered in MHC-PDK4 mitochondria (Fig. 6B). However, there was an increase in maximal ADP-stimulated respiration in the MHC-PDK4 mitochondria (Fig. 6B). The increased state 3 rate in the MHC-PDK4 mitochondria was more resistant to inhibition by oligomycin compared with controls indicative of increased respiratory uncoupling.
DISCUSSION
Abnormalities in cardiac fuel and energy metabolism are believed to contribute to the pathogenesis of heart failure due to many etiologies (7, 11, 12). For example, heart failure caused by hypertension is associated with reduced capacity for FA metabolism, causing the myocardium to rely heavily on glucose (34–36). Conversely, the diabetic heart uses FA almost exclusively, and GO is severely constrained, the latter due at least in part to increased PDK4 activity (19). Herein, we examined the effects of chronic suppression of myocardial GO in MHC-PDK4 transgenic mice. We were surprised to find that although the substrate utilization profile of the MHC-PDK4 heart is similar to that of the insulin-resistant diabetic myocardium, it was not sensitized to ischemia-reperfusion injury. Moreover, the MHC-PDK4 heart was resistant to myocyte lipid accumulation caused by HF diet. These data indicate that chronic inhibition of pyruvate dehydrogenase in heart results in an adaptive re-programming that allows for high capacity FAO and reduced GO. This inverse “Randle effect” is, to our knowledge unique and could be highly relevant to defining adaptive versus maladaptive metabolic responses relevant to new therapeutic approaches to prevent diabetic cardiac dysfunction.
This work confirms and extends the original characterization of the MHC-PDK4 transgenic mice (22). We found that the substrate utilization pattern in the isolated MHC-PDK4 working heart was consistent with the previous NMR-based measurements (22). In addition, our study unveils several new findings. First, the high FAO and low GO rates of the MHC-PDK4 hearts are resistant to the effects of insulin, an effect that is independent of alterations in the insulin signaling pathway. Second, anaerobic glycolytic rates were unaffected in MHC-PDK4 hearts despite marked impairments in GO, a mismatch that drives increased myocardial lactate production. Third, our data strongly suggest that the molecular mechanism whereby forced suppression of GO leads to chronically increased mitochondrial FAO rates involves activation of AMPK and PGC-1α signaling. Finally, despite insulin resistance and metabolic inflexibility, MHC-PDK4 hearts exhibit normal function, even after ischemia-reperfusion injury or administration of a high fat diet.
Perhaps the most important finding in this study is that MHC-PDK4 mice have undergone an adaptive cardiac metabolic re-programming that allows for high rates of FAO without development of lipotoxic cardiomyopathy or sensitivity to I/R. Previously, we generated and characterized MHC-PPARα transgenic mice toward understanding the role of lipid metabolic derangements that contribute to the pathogenesis of diabetic cardiomyopathy (19). Whereas the cardiac substrate utilization profile driven by chronic activation of PPARα is very similar to MHC-PDK4 hearts, only the former develop cardiomyopathy. These results suggest that substrate inflexibility per se is insufficient to cause cardiac dysfunction. Interestingly, MHC-PPARα mice exhibit cardiac myocyte lipid accumulation, but the MHC-PDK4 heart is protected against HF diet-induced myocyte lipid accumulation, presumably due to a high capacity for FAO. Increased myocyte triglyceride levels were also linked to cardiomyopathy in a variety of genetically engineered mouse lines (20, 21, 37, 38). Taken together, our results suggest that lipotoxicity rather than metabolic inflexibility underlies cardiomyopathic remodeling in diabetic heart. However, the molecular identity of the lipotoxic mediator(s) remains a matter of speculation.
Our data suggest that the adaptation in the MHC-PDK4 mice involves secondary activation of the AMPK pathway, which drives increased capacity for mitochondrial β-oxidation via effects on reducing malonyl-CoA levels. This likely explains the high rates of FAO in MHC-PDK4 hearts in the absence of corresponding changes in FAO enzyme gene expression. Increased import of FA into the mitochondrion must be coupled to a corresponding increase in the capacity of downstream mitochondrial oxidative pathways to allow complete oxidation of this fuel. Consistent with this, we found that the expression of PGC-1α, a master transcriptional regulator of mitochondrial functional capacity and biogenesis, was increased in MHC-PDK4 hearts. AMPK has been shown to increase PGC-1α levels and activity (31). We also found evidence for increased respiratory uncoupling in the MHC-PDK4 heart, which could also contribute to the increased FAO flux. The mechanism for this latter effect is unknown but does not involve increased expression of uncoupling proteins (data not shown). Recent studies by others suggest that chronic HF diet can enhance cardiac function and resistance to physiological stress in rats, further supporting the notion that multiple mechanisms for adaptive fuel metabolic reprogramming exist in the cardiac myocyte (39, 40). For example, our results do not exclude a PDK4-mediated kinase function that is independent of its known inhibitory effects on the pyruvate dehydrogenase complex.
Recovery after I/R injury is impaired in the insulin-resistant and diabetic heart as well as in the MHC-PPARα heart (23, 25–27). Surprisingly, impairments in GO and FAO did not sensitize the MHC-PDK4 heart to I/R injury. It has long been proposed that reduced GO and increased FAO is deleterious for the recovery of cardiac dysfunction after ischemic insult (23, 25–27). FAO requires more oxygen per mole of ATP produced, and in a hypoxic environment, this could promote increased cell death. Alternatively, or in addition, a mismatch between increased glycolysis and reduced GO capacity leads to increased lactate formation, altering the cellular pH and potentially perturbing calcium homeostasis (41). However, despite extremely low rates of GO in MHC-PDK4 hearts, recovery after ischemia-reperfusion was normal. This surprising observation provides additional support for the conclusion that a metabolic adaptation is triggered in response to the chronic inhibition of the pyruvate dehydrogenase complex. It is possible that the observed increase in AMPK activity or respiratory uncoupling, both of which have been proposed to improve the reperfusion response (42), counteract the effects of reduced GO after ischemic insult. Future studies aimed at the mechanisms involved in triggering the adaptive responses could yield novel therapeutic targets aimed at the prevention of diabetic cardiac dysfunction.
Supplementary Material
Acknowledgments
We are grateful to Dr. Steven Kliewer for providing the MHC-PDK4 mice and for helpful discussions and critique. We thank Genevieve DeMaria for help with manuscript preparation and Courtney Dey for expert technical assistance with mouse experiments.
This work was supported, in whole or in part, by National Institutes of Health Grants P30 DK56341 (to the Washington University Nutrition and Obesity Research Center Core), Diabetes Research and Training Core Grant P60 DK20579), and Grants HL077113 and HL101189 (to D. P. K.).

The on-line version of this article (available at http://www.jbc.org) contains supplemental Figs. 1 and 2.
- FA
- fatty acid
- GO
- glucose oxidation
- FAO
- fatty acid oxidation
- TG
- triacylglycerol
- PPARα
- peroxisome proliferator-activated receptor α
- HF
- high fat
- I/R
- ischemia reperfusion
- MHC
- myosin heavy chain
- NTg
- non-transgenic
- PDK4
- pyruvate dehydrogenase kinase 4
- AMPK
- AMP-activated protein kinase
- PGC-1α
- peroxisome proliferator-activated receptor γ coactivator-1α.
REFERENCES
- 1. Engelgau M. M., Geiss L. S., Saaddine J. B., Boyle J. P., Benjamin S. M., Gregg E. W., Tierney E. F., Rios-Burrows N., Mokdad A. H., Ford E. S., Imperatore G., Narayan K. M. (2004) Ann. Intern. Med. 140, 945–950 [DOI] [PubMed] [Google Scholar]
- 2. Zimmet P., Alberti K. G., Shaw J. (2001) Nature 414, 782–787 [DOI] [PubMed] [Google Scholar]
- 3. Fein F. S., Sonnenblick E. H. (1985) Prog. Cardiovasc. Dis. 27, 255–270 [DOI] [PubMed] [Google Scholar]
- 4. Regan T. J., Lyons M. M., Ahmed S. S., Levinson G. E., Oldewurtel H. A., Ahmad M. R., Haider B. (1977) J. Clin. Invest. 60, 884–899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Rubler S., Dlugash J., Yuceoglu Y. Z., Kumral T., Branwood A. W., Grishman A. (1972) Am. J. Cardiol. 30, 595–602 [DOI] [PubMed] [Google Scholar]
- 6. Stanley W. C., Lopaschuk G. D., McCormack J. G. (1997) Cardiovasc. Res. 34, 25–33 [DOI] [PubMed] [Google Scholar]
- 7. Taegtmeyer H., McNulty P., Young M. E. (2002) Circulation 105, 1727–1733 [DOI] [PubMed] [Google Scholar]
- 8. Rodrigues B., Cam M. C., McNeill J. H. (1995) J. Mol. Cell. Cardiol. 27, 169–179 [DOI] [PubMed] [Google Scholar]
- 9. Taegtmeyer H., Wilson C. R., Razeghi P., Sharma S. (2005) Ann. N.Y. Acad. Sci. 1047, 208–218 [DOI] [PubMed] [Google Scholar]
- 10. Bing R. J., Siegel A., Ungar I., Gilbert M. (1954) Am. J. Med. 16, 504–515 [DOI] [PubMed] [Google Scholar]
- 11. Paulson D. J., Crass M. F., 3rd. (1982) Am. J. Physiol. 242, H1084–H1094 [DOI] [PubMed] [Google Scholar]
- 12. Young M. E., McNulty P., Taegtmeyer H. (2002) Circulation 105, 1861–1870 [DOI] [PubMed] [Google Scholar]
- 13. Wu P., Sato J., Zhao Y., Jaskiewicz J., Popov K. M., Harris R. A. (1998) Biochem. J. 329, 197–201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Yeaman S. J., Hutcheson E. T., Roche T. E., Pettit F. H., Brown J. R., Reed L. J., Watson D. C., Dixon G. H. (1978) Biochemistry 17, 2364–2370 [DOI] [PubMed] [Google Scholar]
- 15. Frustaci A., Kajstura J., Chimenti C., Jakoniuk I., Leri A., Maseri A., Nadal-Ginard B., Anversa P. (2000) Circ. Res. 87, 1123–1132 [DOI] [PubMed] [Google Scholar]
- 16. Boudina S., Sena S., O'Neill B. T., Tathireddy P., Young M. E., Abel E. D. (2005) Circulation 112, 2686–2695 [DOI] [PubMed] [Google Scholar]
- 17. Burkart E. M., Sambandam N., Han X., Gross R. W., Courtois M., Gierasch C. M., Shoghi K., Welch M. J., Kelly D. P. (2007) J. Clin. Invest. 117, 3930–3939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Yang J., Sambandam N., Han X., Gross R. W., Courtois M., Kovacs A., Febbraio M., Finck B. N., Kelly D. P. (2007) Circ. Res. 100, 1208–1217 [DOI] [PubMed] [Google Scholar]
- 19. Finck B. N., Lehman J. J., Leone T. C., Welch M. J., Bennett M. J., Kovacs A., Han X., Gross R. W., Kozak R., Lopaschuk G. D., Kelly D. P. (2002) J. Clin. Invest. 109, 121–130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Son N. H., Park T. S., Yamashita H., Yokoyama M., Huggins L. A., Okajima K., Homma S., Szabolcs M. J., Huang L. S., Goldberg I. J. (2007) J. Clin. Invest. 117, 2791–2801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Chiu H. C., Kovacs A., Ford D. A., Hsu F. F., Garcia R., Herrero P., Saffitz J. E., Schaffer J. E. (2001) J. Clin. Invest. 107, 813–822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Zhao G., Jeoung N. H., Burgess S. C., Rosaaen-Stowe K. A., Inagaki T., Latif S., Shelton J. M., McAnally J., Bassel-Duby R., Harris R. A., Richardson J. A., Kliewer S. A. (2008) Am. J. Physiol. Heart Circ. Physiol. 294, H936–H943 [DOI] [PubMed] [Google Scholar]
- 23. Sambandam N., Morabito D., Wagg C., Finck B. N., Kelly D. P., Lopaschuk G. D. (2006) Am. J. Physiol. Heart Circ. Physiol. 290, H87–H95 [DOI] [PubMed] [Google Scholar]
- 24. Rogers J. H., Tamirisa P., Kovacs A., Weinheimer C., Courtois M., Blumer K. J., Kelly D. P., Muslin A. J. (1999) J. Clin. Invest. 104, 567–576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. McVeigh J. J., Lopaschuk G. D. (1990) Am. J. Physiol. 259, H1079–H1085 [DOI] [PubMed] [Google Scholar]
- 26. Lewandowski E. D., White L. T. (1995) Circulation 91, 2071–2079 [DOI] [PubMed] [Google Scholar]
- 27. Taegtmeyer H., Goodwin G. W., Doenst T., Frazier O. H. (1997) Am. J. Cardiol. 80, 3A–10A [DOI] [PubMed] [Google Scholar]
- 28. Kelly D. P., Strauss A. W. (1994) N. Engl. J. Med. 330, 913–919 [DOI] [PubMed] [Google Scholar]
- 29. Merrill G. F., Kurth E. J., Hardie D. G., Winder W. W. (1997) Am. J. Physiol. 273, E1107–E1112 [DOI] [PubMed] [Google Scholar]
- 30. Vavvas D., Apazidis A., Saha A. K., Gamble J., Patel A., Kemp B. E., Witters L. A., Ruderman N. B. (1997) J. Biol. Chem. 272, 13255–13261 [DOI] [PubMed] [Google Scholar]
- 31. Jäger S., Handschin C., St-Pierre J., Spiegelman B. M. (2007) Proc. Natl. Acad. Sci. U.S.A. 104, 12017–12022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Handschin C., Spiegelman B. M. (2006) Endocr. Rev. 27, 728–735 [DOI] [PubMed] [Google Scholar]
- 33. Kelly D. P., Scarpulla R. C. (2004) Genes Dev. 18, 357–368 [DOI] [PubMed] [Google Scholar]
- 34. de las Fuentes L., Herrero P., Peterson L. R., Kelly D. P., Gropler R. J., Dávila-Román V. G. (2003) Hypertension 41, 83–87 [DOI] [PubMed] [Google Scholar]
- 35. Huss J. M., Kelly D. P. (2005) J. Clin. Invest. 115, 547–555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Russell L. K., Finck B. N., Kelly D. P. (2005) J. Mol. Cell. Cardiol. 38, 81–91 [DOI] [PubMed] [Google Scholar]
- 37. Zhou Y. T., Grayburn P., Karim A., Shimabukuro M., Higa M., Baetens D., Orci L., Unger R. H. (2000) Proc. Natl. Acad. Sci. U.S.A. 97, 1784–1789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Haemmerle G., Lass A., Zimmermann R., Gorkiewicz G., Meyer C., Rozman J., Heldmaier G., Maier R., Theussl C., Eder S., Kratky D., Wagner E. F., Klingenspor M., Hoefler G., Zechner R. (2006) Science 312, 734–737 [DOI] [PubMed] [Google Scholar]
- 39. Berthiaume J. M., Bray M. S., McElfresh T. A., Chen X., Azam S., Young M. E., Hoit B. D., Chandler M. P. (2010) Am. J. Physiol. Heart Circ. Physiol. 299, H410–421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Okere I. C., Chess D. J., McElfresh T. A., Johnson J., Rennison J., Ernsberger P., Hoit B. D., Chandler M. P., Stanley W. C. (2005) Clin. Exp. Pharmacol. Physiol. 32, 825–831 [DOI] [PubMed] [Google Scholar]
- 41. Cairns S. P., Westerblad H., Allen D. G. (1993) J. Physiol. 464, 561–574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Lopaschuk G. D. (2008) Int. J. Obes. (Lond) 32, Suppl. 4, S29–S35 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.