Abstract
Lantibiotics are ribosomally synthesized and post-translationally modified peptide antibiotics that contain unusual amino acids such as dehydro and lanthionine residues. Nukacin ISK-1 is a class II lantibiotic, whose precursor peptide (NukA) is modified by NukM to form modified NukA. ATP-binding cassette (ABC) transporter NukT is predicted to cleave off the N-terminal leader peptide of modified NukA and secrete the mature peptide. Multiple sequence alignments revealed that NukT has an N-terminal peptidase domain (PEP) and a C-terminal ATP binding domain (ABD). Previously, in vitro reconstitution of NukT has revealed that NukT peptidase activity depends on ATP hydrolysis. Here, we constructed a series of NukT mutants and investigated their transport activity in vivo and peptidase activity in vitro. Most of the mutations of the conserved residues of PEP or ABD resulted in failure of nukacin ISK-1 production and accumulation of modified NukA inside the cells. NukT(N106D) was found to be the only mutant capable of producing nukacin ISK-1. Asn106 is conserved as Asp in other related ABC transporters. Additionally, an in vitro peptidase assay of NukT mutants demonstrated that PEP is on the cytosolic side and all of the ABD mutants as well as PEP (with the exception of NukT(N106D)) did not have peptidase activity in vitro. Taken together, these observations suggest that the leader peptide is cleaved off inside the cells before peptide secretion; both PEP and ABD are important for NukT peptidase activity, and cooperation between these two domains inside the cells is indispensable for proper functioning of NukT.
Keywords: ABC Transporter, Bacteria, Membrane Proteins, Peptidases, Peptides, Lantibiotics, Leader Peptide
Introduction
Lantibiotics are lanthionine-containing antibiotic peptides, which display a number of favorable characteristics. For example, they have no taste or odor, they are tolerant to high heat and acid, and they have stable activity at nanomolar concentrations against antibiotic-resistant pathogens. These attractive qualities are expected to lead to the broad application potentials of lantibiotics (1–4). One of the best known lantibiotics, nisin, which is produced by Lactococcus lactis, has been used as a food preservative for decades. Lantibiotics are divided into two different classes based on the biosynthetic pathway and the enzymes from which they are produced (5, 6). Ribosomally synthesized prepeptide (LanA; “Lan” is an abbreviation for enzymes and/or proteins related to lantibiotic biosynthesis) is inactive and consists of an N-terminal leader peptide and a C-terminal propeptide portion. In class I lantibiotics such as nisin, LanB dehydrates serine and threonine residues of the propeptide, LanC cyclizes dehydrated residues with cysteine, LanT secretes the peptide, and the extracellular protein LanP cleaves off the N-terminal leader peptide. In class II lantibiotics such as nukacin ISK-1, a single enzyme, LanM, catalyzes both dehydration and cyclization, and ABC2 transporter LanT removes the leader peptide concomitantly with secretion.
LanT of the class II lantibiotics is a member of the ABC transporter maturation and secretion (AMS) protein family. Members of this ABC transporter subfamily are involved in the secretion of proteinaceous compounds and contain an additional N-terminal domain involved in the processing of their substrates at the so-called double glycine site at the C terminus of the leader peptide (7–9). There are only a few studies on the N-terminal peptidase domain (PEP) of AMS proteins. The PEPs of LagD, CvaB, ComA, and LctT have been purified and been proven to cleave off the leader peptide of their cognate precursor peptide (7, 9–11). The PEPs of these proteins have Cys, His, or Asp residues, which are conserved among the papain-like cysteine proteases. Substitution of these residues results in loss of their peptidase activity, demonstrating that PEP also belongs to the cysteine protease family (9–11). The N-terminal leader peptide sequence of the precursor peptide has been reported to be important for peptidase activity of PEP. Furgerson Ihnken et al. found that the double glycine motif and the α-helical structure of the leader peptide are important for proteolysis by PEP of LctT (9). Kotake et al. also demonstrated that PEP of ComA cleaves off the leader peptide after the double glycine site and proposed the presence of hydrophobic patch on the surface of the PEP, which interacts with the hydrophobic face of the helix of the precursor peptide (12). A three-dimensional crystal structure of ComA has been recently clarified, and PEP was shown to have an overall structure similar to the structure of the papain-like cysteine proteases (13). The Cys-His active site of PEP at the bottom of a narrow cleft is suitable for binding of the double glycine site, and a shallow hydrophobic concave surface was proposed to accommodate the helical structure of the leader peptide (13).
There is an ATP binding domain (ABD) at the C terminus of the AMS protein. Walker A, Walker B, and H-loop are distinctive motifs for ABD of the ABC transporters (Fig. 1). The membrane topology of LcnC, an AMS protein involved in the production of lactococcin, includes four membrane helices that span the cytoplasmic membrane. Both the N- and C-terminal parts are in the cytoplasm (8). The peptidase activity of full-length AMS, which has both PEP and ABD, has been reported to require nucleotide hydrolysis as its driving force (14, 15).
FIGURE 1.
Sequence alignment of ABC transporter containing the N-terminal peptidase domain. The amino acid sequence alignments of NukT, LctT, CvaB, LcnC, and ComA were performed using the ClustalW program. The conserved active sites in the peptidase domain are marked with asterisks. The conserved motifs in the ABD (Walker A, Walker B, H-loop) are indicated under the sequence. The mutation points in NukT are shown above the sequence.
Nukacin ISK-1 is a class II lantibiotic produced by Staphylococcus warneri ISK-1 (16, 17). NukA is a precursor peptide that consists of an N-terminal leader peptide and a C-terminal propeptide. NukM introduces unusual amino acids into NukA to form modified NukA. NukT is an AMS protein that is responsible for leader peptide cleavage of modified NukA and secretion of mature nukacin ISK-1 (18). A previous in vitro study of NukT has clarified that NukT cannot cleave off the leader peptide if the unusual amino acids are not included in the propeptide and that peptidase activity requires energy from ATP hydrolysis (15). However, the functions of PEP and ABD and the details regarding the cooperation between these two domains in full-length NukT have not been characterized. For further understanding of the molecular characteristics of lantibiotic biosynthetic enzymes, we have focused on the conserved residues in PEP and ABD of the AMS protein and constructed a series of NukT mutants. Both in vivo and in vitro experiments indicate that C-terminal ABD is important not only for transport activity but also for peptidase activity of NukT.
EXPERIMENTAL PROCEDURES
Bacterial Strains and Growth Conditions
The bacterial strains and plasmids used in this study are listed in Table 1. L. lactis was grown in M17 medium (OXOID; Hampshire, UK) supplemented with 0.5% glucose (GM17) or chemically defined medium (19) at 30 °C. Staphylococcus carnosus and Staphylococcus aureus were grown in Luria-Bertani (LB) medium or B2 medium (1% casein hydrolysate, 2.5% yeast extract, 0.5% glucose, 2.5% NaCl, 0.1% K2HPO4, pH adjusted to 7.5) at 37 °C. Lactobacillus sakei was grown in MRS medium (OXOID) at 30 °C. Media were supplemented, when appropriate, with chloramphenicol (5 μg/ml for L. lactis), erythromycin (5 μg/ml for L. lactis), or tetracycline (25 μg/ml for S. aureus and S. carnosus).
TABLE 1.
Bacterial strains and plasmids used in this study
Cmr, chloramphenicol resistance; Emr, erythromycin resistance; Tcr, tetracycline resistance. PnisA is a nisA promoter that is used for the nisin-controlled expression system (42). Pxyl is a xylose-inducible xylA promoter (43). JCM, Japan Collection of Microorganisms, Wako, Japan.
| Strain or plasmid | Description | Source or reference |
|---|---|---|
| Strains | ||
| Lactococcus lactis NZ9000 | MG1363 derivative; nisRK::pep | 38 |
| Staphylococcus carnosus TM300 | Plasmid-free | 39 |
| Staphylococcus aureus RN4220 | Cloning host | 40 |
| Lactobacillus sakei subsp. sakei JCM 1157T | Indicator strain of nukacin ISK-1 | JCM |
| Plasmids | ||
| pNZ8048 | Cmr, L. lactis expression vector with PnisA | 38 |
| pInuk | Emr, nukAMTFEGH | 18 |
| pInukdT | Emr, nukAMFEGH, disruption of nukT in pInuk | 18, 41 |
| pNZT | pNZ8048 derivative containing nukT downstream of PnisA | 18 |
| pNZT mutant | pNZT derivative; mutation introduced at indicated residue of NukT | This study |
| pTX15 | Tcr, staphylococcal expression vector with Pxyl | 39 |
| pTXnukT | pTX15 derivative containing nukT downstream of Pxyl | 15 |
| pTXnukT mutant | pTXnukT derivative; mutation introduced at indicated residue of NukT | This study |
Molecular Biology Protocols
Established molecular biology protocols were followed (20). PCR was performed with KOD Plus DNA polymerase (Toyobo, Osaka, Japan). The PCR products were purified with PCR SV (Geneall, Seoul, Korea). Plasmid DNA was prepared using Plasmid SV Mini (Geneall), according to the manufacturer's instructions, except that the staphylococcal cells were incubated in 250 μl of cell suspension buffer containing 10 μg/ml lysostaphin (Wako, Osaka, Japan) for 30 min at 37 °C. For the construction of staphylococcal plasmid DNA, restriction-deficient S. aureus RN4220 was used as the host.
Site-directed Mutagenesis of nukT
Site-directed mutagenesis of nukT was performed by inverse PCR, using pNZT or pTXnukT as the template. The primers used in this study are listed in supplemental Table S1. Self-ligated fragments of pNZT mutants were transformed in L. lactis NZ9000 (pInukdT) according to the method developed by Holo and Nes (21). Self-ligated fragments of pTXnukT mutants were transformed into restriction-deficient S. aureus RN4220 as a host and then into S. carnosus TM300 (22).
Detection of Nukacin ISK-1 in Culture Supernatant
Detection of nukacin ISK-1 in chemically defined medium culture supernatant of recombinant L. lactis strain was performed according to a previously described method (23, 24). A 10-ml volume of the culture supernatant was loaded onto a Sep-Pak C18 cartridge (Waters) for partial purification, and the eluate was applied to liquid chromatography/mass spectrometry (LC/MS) coupled to an AccuTOF T100LC mass spectrometer (JEOL, Tokyo, Japan). The relative productivity of nukacin ISK-1 was assayed by the spot-on-lawn method (25). Lactobacilli Agar AOAC (Becton Dickinson) was overlaid on MRS agar medium with L. sakei subsp. sakei JCM 1157T as an indicator strain, and 10 μl of serial 1.5-fold diluted supernatant was spotted onto the surface of the medium. After overnight incubation at 30 °C, the bacterial lawns were checked for inhibition zones and are defined as arbitrary units/ml.
Detection of Modified NukA Inside the Cell
Modified NukA accumulated inside the cells was detected by MALDI-TOF/MS of the cell-free extract (CFE). L. lactis recombinant strains were precultured in GM17 overnight at 30 °C. Expression of the peptides was induced in 1 ml of GM17 containing 10 ng/ml nisin A. After a 7-h incubation period, the cells were harvested, washed with 50 mm Tris-HCl (pH 7.4), and resuspended in 500 μl of the same buffer. The suspension was transferred to a 2-ml screw-cap polypropylene tube containing 300-mg glass beads (diameter, 0.1 mm; Yasui Kikai, Osaka, Japan). The tube was shaken at 2,700 rpm for 3 min at 4 °C in a bead-beater instrument (Multi-beads Shocker; Yasui Kikai). The treated sample was centrifuged at 13,000 × g at 4 °C for 2 min, and the supernatant was collected as a CFE sample. Peptides were purified from the CFE by using C18 ZipTips® (Millipore). The ZipTips® were wetted and equilibrated with 50% acetonitrile (0.1% TFA) followed by MilliQ water (0.1% TFA). The CFE was bound and washed with 5% methanol (0.1% TFA), eluted with 50% acetonitrile (0.1% TFA), and 5 μl was directly used for MALDI-TOF/MS analysis (AXIMA-CFR plus mass spectrometer; Shimadzu, Kyoto, Japan) with α-cyano-4-hydroxycinnamic acid as the matrix.
Membrane Preparation
Membrane vesicles were prepared from the NukT/NukT mutant expressing S. carnosus TM300. S. carnosus was cultured at 37 °C for 18 h in LB medium containing 0.5% xylose and 25 μg/ml tetracycline. Cells were lysed with 5 μg/ml lysostaphin in 50 mm K-HEPES (pH 7.4) containing 10 μg/ml DNase, 10 mm MgSO4, and Protease Inhibitor Mixture (EDTA-free, 100×; Nacalai Tesque, Kyoto, Japan). The cell lysate was passed through a French pressure cell (Thermo Fisher Scientific), which yields predominantly inside-out (ISO) membrane vesicles (15, 26–28). The method developed by Kaback was used to yield predominantly right-side-out (RSO) membrane vesicles (26, 27, 29). Cells were lysed in the same buffer described above, with the exception of addition of 10% sucrose. Spheroplasts were suspended in a minimal volume of 50 mm K-HEPES buffer containing 10% sucrose and rapidly diluted into 200 volumes of 50 mm K-HEPES buffer containing 10 μg/ml DNase, 10 mm MgSO4, and Protease Inhibitor Mixture to cause disruption of the spheroplasts by osmotic pressure. Membrane vesicles were harvested by ultracentrifugation (210,000 × g, 1 h), washed with K-HEPES buffer, resuspended in 20 mm K-HEPES buffer (pH 7.4) containing 10% glycerol, and stored at −80 °C.
In Vitro Peptidase Assay
Modified His-tagged NukA (modified His-NukA) was prepared (30) and incubated with ISO or RSO membrane vesicles as described previously (15) with the exception that the final protein concentration of membrane vesicles was 1 mg/ml. To detect the nukacin ISK-1, which was produced by leader peptide cleavage, the reaction mixture was subjected to 10% Tricine SDS-PAGE, overlaid with L. sakei as the indicator strain, and incubated overnight at 30 °C. The remaining substrates in the reaction mixture were visualized by Western blotting with an anti-His tag antibody. The signals of modified His-NukA were quantified by Multi Gauge software (Fujifilm, Tokyo, Japan).
RESULTS
Multiple Sequence Alignment of AMS Proteins and Construction of NukT Mutants
Fig. 1 shows the multiple sequence alignment of the primary structures of AMS proteins. NukT possesses N-terminal PEP and C-terminal ABD. Cys12, His90, and Asn106, which are conserved as Asp in other AMS proteins, appear to constitute an active site catalytic triad within the N-terminal cysteine protease-like domain. Gln6 is also conserved and has been predicted to function as an oxyanion hole (11). The C-terminal domain of NukT has motifs known as Walker A, Walker B, and H-loop. These motifs are conserved in the ABD of the ABC transporter. NukT mutants were constructed to investigate the role of these conserved residues. Gln6, Cys12, and His90 in PEP were replaced with Ala, and Asn106 was replaced with Asp. The conserved residues in ABD were substituted to residues that impair ATPase activity (K503R), ATP binding (D615N), or transport activity (H649R), according to the previous study of the MalK ABC transporter (31–33).
NukT Mutants Affect the Production of Nukacin ISK-1 in Vivo
L. lactis NZ9000 (pInukdT/pNZT) can heterologously produce nukacin ISK-1 (18). The effect of the mutation of NukT was investigated in vivo with L. lactis NZ9000 (pInukdT/pNZT mutant). SDS-PAGE followed by Western blotting using NukT antiserum showed that the NukT mutants were expressed at similar levels relative to wild-type NukT (Fig. 2). The culture supernatant was partially purified using a Sep-Pak C18 cartridge, and nukacin ISK-1 production was evaluated by LC/MS (Table 2 and supplemental Fig. S1). The vector control, without NukT expression (dNukT), did not produce nukacin ISK-1 in the culture supernatant. Alanine substitution at residues of the putative active site for cysteine protease in PEP (C12A, H90A) resulted in failure of nukacin ISK-1 production. A mutant at the site of the predicted oxyanion hole, NukT(Q6A), was found to be incapable of secreting nukacin ISK-1 in the culture supernatant as well. Asn106 is a residue conserved as Asp in other AMS proteins. NukT(N106D) was found to be capable of producing nukacin ISK-1, suggesting that Asn106 in NukT can be replaced by Asp to mimic the AMS proteins. Substitutions of conserved motifs in ABD (K503R, D615N, H649R) were predicted to have no transport activity. As expected, these mutations also resulted in depletion of nukacin ISK-1 production.
FIGURE 2.
Expression levels of NukT and its mutants in L. lactis. The cell-free extract of the recombinant L. lactis strain was prepared as described under “Experimental Procedures,” and 10 μl of the sample was subjected to SDS-PAGE, followed by Western blotting using NukT antiserum. The arrow indicates the position of NukT (80 kDa).
TABLE 2.
Peptides in culture supernatant and CFE
| NukT mutants | Nukacin ISK-1 in culture supernatanta | Modified NukA in CFEb |
|---|---|---|
| dNukT | − | + |
| NukT | + | − |
| NukT(Q6A) | − | + |
| NukT(C12A) | − | + |
| NukT(H90A) | − | + |
| NukT(N106D) | + | − |
| NukT(K503R) | − | + |
| NukT(D615N) | − | + |
| NukT(H649R) | − | + |
a Nukacin ISK-1 production in culture supernatant was evaluated by LC/MS.
b Modified NukA in CFE was evaluated by MALDI-TOF/MS after cell disruption. +, peptide detected; −, peptide not detected.
NukT Mutants Accumulate Precursor Peptide (Modified NukA) Inside the Cell
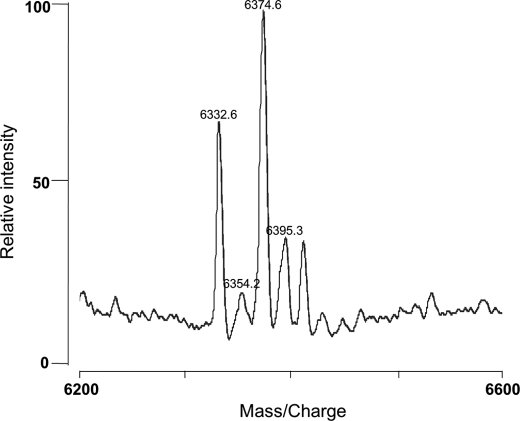
L. lactis recombinant strains were also used for detection of the peptide remaining inside the cell. CFE was prepared by the cell bead-beating method. The sample was partially purified with C18 ZipTips®, and the peptides accumulated inside the cell were detected by MALDI-TOF/MS (supplemental Fig. S2). The NukT-deficient strain, L. lactis NZ9000 (pInukdT), cannot produce nukacin ISK-1 (18). The CFE of this strain has a peak at approximately m/z = 6,330 (Fig. 3), which cannot be detected in the CFE of the nukacin ISK-1-producing strain L. lactis NZ9000 (pInuk) (data not shown). These peaks correspond to protonated, sodium adduct, potassium adduct, and sodium/potassium adduct of modified NukA (6,331 Da) at m/z = 6332.6, 6354.2, 6374.6, and 6395.3, respectively. NukT(Q6A), NukT(C12A), and NukT(H90A) were found to accumulate modified NukA inside the cells, suggesting that these NukT mutants could not cleave off the leader peptide (Table 2). Because NukT(N106D) could produce nukacin ISK-1 as NukT, this mutant strain did not accumulate modified NukA. Substitutions of conserved motifs in ABD (NukT(K503R), NukT(D615N), NukT(H649R)) also resulted in accumulation of modified NukA, suggesting that these mutants could not cleave off the leader peptide of modified NukA and thus could not transport the peptide (Table 2).
FIGURE 3.
MALDI-TOF/MS spectrum of the cell-free extract of L. lactis NZ9000 (pInukdT). Modified NukA that had accumulated inside the cells was monitored by MALDI-TOF/MS. Each peak corresponds to a protonated, sodium adduct, potassium adduct, and sodium/potassium adduct at m/z 6332.6, 6354.2, 6374.6, and 6395.3, respectively.
Peptidase Activity of NukT, using ISO or RSO Membrane Vesicles
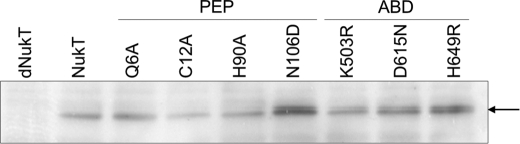
The in vitro peptidase assay using ISO membrane vesicles prepared from S. carnosus (pTXnukT) revealed that NukT cleaves off the leader peptide of modified His-NukA (modified NukA containing N-terminal His tag) (15). The same assay was performed using RSO membrane vesicles prepared from the same strain. ISO and RSO membrane vesicles were prepared by cell disruption by using the French pressure cell and osmotic shock, respectively. The former procedure yields predominantly ISO vesicles, whose membrane orientation is inverted, whereas the latter yields predominantly RSO vesicles having the same orientation as the parent cells (15, 26–29). The amount of NukT in the reaction mixture was confirmed to be at the same level by Western blotting (data not shown). After the reaction of modified His-NukA and membrane vesicles, the reaction mixture was applied to Tricine SDS-PAGE followed by an overlay assay, which enables the monitoring of nukacin ISK-1 whose leader peptide is cleaved off from modified His-NukA. The result showed that RSO membrane vesicles expressing NukT cannot cleave off the leader peptide of modified His-NukA (Fig. 4). This suggests that PEP of NukT is on the cytosolic side and not on the extracellular side.
FIGURE 4.
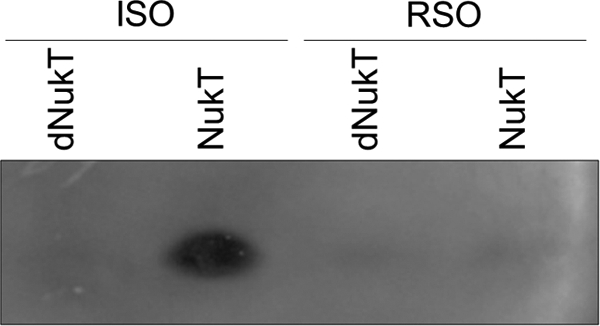
Peptidase activity of NukT using ISO or RSO membrane vesicles. Modified His-NukA (3 μm) was incubated with 1 mg/ml NukT membrane. Nukacin ISK-1 produced by cleavage of the leader peptide was detected from its antibacterial activity using the overlay assay method with L. sakei as the indicator strain.
Leader Peptide Cleavage by NukT Mutants
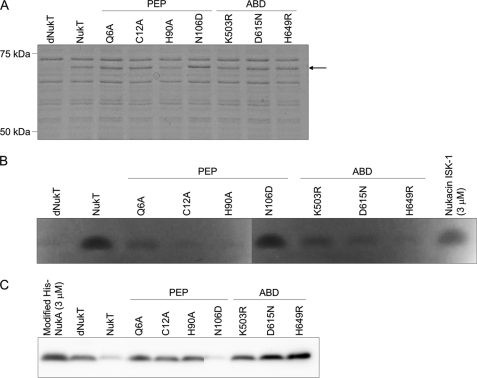
The peptidase activity of the NukT mutant was investigated in vitro with ISO membrane vesicles prepared from S. carnosus TM300 (pTXnukT mutant) (15). The same mutation was introduced in NukT as part of the in vivo study. SDS-PAGE followed by Coomassie Brilliant Blue staining showed that the NukT mutants were expressed at similar levels relative to wild-type NukT (Fig. 5A). Modified His-NukA was incubated with ISO membrane vesicles expressing the NukT mutant. Nukacin ISK-1 produced after the reaction was detected by an overlay assay (Fig. 5B), and the unreacted substrate remaining in the reaction mixture was visualized by Western blotting with an anti-His tag antibody (Fig. 5C). Alanine substitution of residues located at the putative active site for cysteine protease in PEP (NukT(C12A), NukT(H90A)) and at the predicted oxyanion hole (NukT(Q6A)) resulted in loss of nukacin ISK-1 production. Modified His-NukA remained in the reaction mixture of these mutants, indicating that they could not cleave off the leader peptide. NukT(N106D) could effectively cleave off the leader peptide of modified His-NukA. This observation supports the finding that NukT(N106D) in the nukacin ISK-1 heterologous expression system could produce nukacin ISK-1 (Table 2). Substitutions of residues in the conserved motifs in ABD (NukT(K503R), NukT(D615N), and NukT(H649R)) resulted in failure of leader peptide cleavage of modified His-NukA. This phenomenon was unexpected because these three derivatives do not have any mutations in the N-terminal PEP domain, which was predicted to contribute to the leader peptide cleavage.
FIGURE 5.
Expression of NukT mutants in S. carnosus and their peptidase activity in vitro. ISO vesicles were prepared from cells of recombinant S. carnosus, and 5 μg of membrane protein was subjected to SDS-PAGE, followed by Coomassie Brilliant Blue staining (A). The arrow indicates the position of NukT (80 kDa). Modified His-NukA (3 μm) was incubated with 1 mg/ml NukT membrane. Nukacin ISK-1 produced after the reaction was detected from its antibacterial activity by using the overlay assay method with L. sakei as the indicator strain (B), and the remaining substrates in the reaction mixture were visualized using Western blotting with anti-His tag antibody (C).
Correlation between Nukacin ISK-1 Production and Peptidase Activity
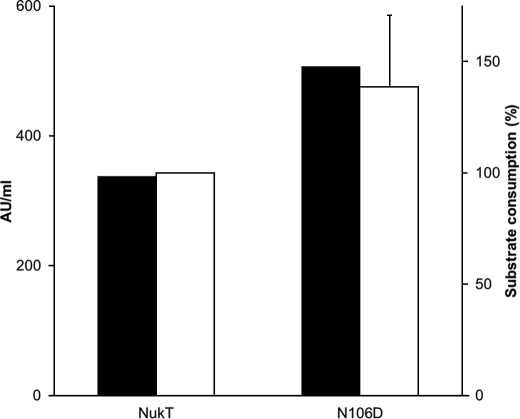
NukT(N106D) was the only mutant capable of producing nukacin ISK-1 in vivo (Table 2) and possesses peptidase activity in vitro (Fig. 5). Nukacin ISK-1 production and peptidase activity of wild-type NukT and NukT(N106D) were compared quantitatively (Fig. 6). The production level of nukacin ISK-1 was determined by antibacterial activity (arbitrary units/ml) against L. sakei as an indicator strain. Peptidase activity was determined by comparing the signal intensity of the modified NukA remaining after the in vitro peptidase assay by Western blotting. The signal intensity of the modified NukA remaining in the NukT or NukT(N106D) reaction mixture was subtracted from the signal intensity of the modified His-NukA (3 μm) and taken as substrate consumption. Compared with wild-type NukT, NukT(N106D) produced a 1.5-fold increase of nukacin ISK-1 in vivo. Peptidase activity in vitro showed that NukT(N106D) digested approximately a 1.4-fold greater amount of modified His-NukA than wild-type NukT.
FIGURE 6.
Quantitative analysis of nukacin ISK-1 production (black bars) and peptidase activity (white bars) with wild-type NukT and NukT(N106D). Nukacin ISK-1 production was assessed by measuring antibacterial activity (arbitrary units (AU)/ml) against L. sakei as an indicator strain. Peptidase activity was determined by the remaining substrates (modified His-NukA) in the reaction mixture of the in vitro assay, followed by Western blotting using anti-His antibody. The signals of the remaining substrates were quantified by Multi Gauge software. Substrate consumption by wild-type NukT was taken as 100%. This assay was performed in duplicate, and the average values are shown with error bars.
DISCUSSION
NukT is the biosynthetic enzyme for the production of nukacin ISK-1, a class II lantibiotic. NukT contributes to cleavage of the leader peptide of the precursor peptide (modified NukA), which leads to secretion of mature nukacin ISK-1 (18). AMS proteins are a family of proteins responsible for both processing (leader peptide cleavage of the precursor peptide) and secretion of the mature active peptide. AMS proteins such as NukT consist of an N-terminal PEP and a C-terminal ABD. Previous reports of purified PEPs (approximately 150 amino acids) of LagD, CvaB, ComA, and LctT have revealed that PEP is a papain-like cysteine protease that recognizes the helix structure and the double glycine motif in the leader peptide of the precursor and cleaves off the leader peptide at the double glycine site (7, 9–11). Despite the significant diversity of transport substrates, the sequences of the ABDs are remarkably well conserved among all of the ABC transporters. ABD powers the transporter by binding and hydrolyzing ATP. Presumably, ATP binding and/or hydrolysis is coupled to conformational changes in the membrane-spanning domain that mediate the unidirectional pumping of substrates across the membrane (34). Functional and structural information on PEP and ABD has been provided. However, the relationship between PEP and ABD in full-length AMS proteins is still poorly understood. Here, we focused on the conserved residues of PEP and ABD in the AMS protein. We selected mutation points in NukT at residues that are conserved in the PEP catalytic active site of cysteine proteases (Q6A, C12A, H90A, N106D) and conserved motifs that are important for ATPase activity (K503R), ATP binding (D615N), and transport activity (H649R) in ABD (Fig. 1). We constructed a series of NukT mutants and investigated their transport activity in vivo and peptidase activity in vitro.
The in vivo transport assay revealed that all of the NukT mutants except for NukT(N106D) cannot produce nukacin ISK-1 in the culture supernatant. Additionally, accumulation of the precursor peptide of modified NukA was detected in nukacin ISK-1 nonproducing recombinant strains (Table 2). NukT(Q6A), NukT(C12A), and NukT(H90A) have mutations in PEP but not in ABD. Accumulation of modified NukA in these mutants indicates that PEP mutants cannot transport the precursor peptide without cleavage of the leader peptide. On the other hand, ABD mutants have no PEP mutations but were observed to accumulate modified NukA. This indicates that cleavage of the leader peptide did not occur. From these results, it is suggested that NukT can function properly only when the peptidase and the transporter are both in the active form.
ISO membrane vesicles expressing NukT prepared from S. carnosus can effectively cleave off the leader peptide of modified His-NukA (15). However, the same experiment using RSO membrane instead of ISO membrane revealed that NukT in the correct orientation as in the parent cells cannot cleave off the leader peptide of modified His-NukA, which is added externally (Fig. 4). In the case of the class I lantibiotic nisin, NisT exports the fully modified prenisin, and then the extracellular serine protease NisP cleaves off the leader peptide (35). Although there have been no investigations on the membrane topology of LanT of class II lantibiotics, our results distinctively indicate that, unlike the class I lantibiotic, PEP of NukT is on the cytosolic side, and the leader peptide of nukacin ISK-1, a class II lantibiotic, is cleaved off inside the cells before or concomitantly with secretion.
The in vitro peptidase assay revealed that the PEP mutants NukT(Q6A), NukT(C12A), and NukT(H90A) cannot cleave off the leader peptide as expected. All of the ABD mutants (NukT(K503R), NukT(D615N), and NukT(H649R)) were also incapable of cleaving off the leader peptide (Fig. 5). These results strongly support the in vivo data described above and also the previous data which suggest that NukT peptidase activity requires ATP hydrolysis (15). According to the previous study of MalK, the H649R mutation has been predicted to not have an effect on ATP binding or hydrolysis but to repress transmembrane signaling or coupling of ATP hydrolysis to transport (32). In addition to ATP binding and ATP hydrolysis, there should be another important function of ABD for NukT peptidase activity. The three-dimensional crystal structure of PEP of ComA has revealed that PEP interacts with hydrophobic residues of the helix structure and the double glycine motif in the leader peptide (13). We propose that the conformational transition subsequent to ATP hydrolysis was blocked in the NukT mutants, and thus the interaction between PEP and modified NukA was prevented which resulted in failure to cleave off the leader peptide.
LctT is an AMS protein involved in the production of the class II lantibiotic lacticin 481. The purified PEP of LctT can cleave off the leader peptide of both the modified and unmodified precursor peptide (9). On the other hand, we previously reported that full-length NukT can only digest modified NukA (15). ABD of NukT might play an important role in recognition of lanthionine rings. In the future, further investigations of PEP or ABD alone should clearly answer this question.
NukT(N106D) was found to be the only mutant that could produce nukacin ISK-1 in vivo and cleave off the leader peptide in vitro. Quantitative analysis revealed that both the level of production of nukacin ISK-1 and the peptidase activity of NukT(N106D) are higher than those of wild-type NukT (Fig. 6). Asn106 is one of the residues in the catalytic triad of the cysteine protease and is conserved as Asp in most of the other AMS proteins (7). Among the papain-like cysteine proteases, a conserved Cys and His form a thiolate-imidazolium ion pair, and Asp or Asn is responsible for the correct orientation of the imidazolium ring of the active-site histidine (36, 37). Our results led us to propose that Asp is favored over Asn as a catalytic active residue for cleavage of the leader peptide by AMS proteins and thus Asp is highly conserved among this protein family.
In this study, we constructed a series of NukT mutants to investigate the importance of conserved residues in PEP and ABD as a full-length AMS protein. All of the NukT mutants except for NukT(N106D) could not produce nukacin ISK-1. The precursor peptide was accumulated inside the cells, and the leader peptide could not be cleaved off in vitro. We conclude from these data that both PEP and ABD are important for peptidase activity, and their cooperative functions are indispensable for proper functioning of NukT.
Supplementary Material
Acknowledgments
We are grateful to F. Götz (University of Tübingen) for providing S. carnosus TM300 and pTX15 and Hiroyuki Jikuya (Kyushu University) for assistance with the MALDI-TOF/MS analysis.
This study was supported in part by grants from the Japan Society for the Promotion of Science, the Japan Science Society, the Novartis Foundation (Japan) for the Promotion of Science, Novozymes Japan Research Fund, and the Nagase Science and Technology Foundation.

The on-line version of this article (available at http://www.jbc.org) contains Figs. S1 and S2 and Table S1.
- ABC
- ATP-binding cassette
- ABD
- ATP binding domain
- AMS
- ABC transporter maturation and secretion
- CFE
- cell-free extract
- ISO
- inside-out
- PEP
- peptidase
- RSO
- right-side-out.
REFERENCES
- 1. de Vos W. M., Kuipers O. P., van der Meer J. R., Siezen R. J. (1995) Mol. Microbiol. 17, 427–437 [DOI] [PubMed] [Google Scholar]
- 2. Chatterjee C., Paul M., Xie L., van der Donk W. A. (2005) Chem. Rev. 105, 633–684 [DOI] [PubMed] [Google Scholar]
- 3. Nagao J., Asaduzzaman S. M., Aso Y., Okuda K., Nakayama J., Sonomoto K. (2006) J. Biosci. Bioeng. 102, 139–149 [DOI] [PubMed] [Google Scholar]
- 4. Asaduzzaman S. M., Sonomoto K. (2009) J. Biosci. Bioeng. 107, 475–487 [DOI] [PubMed] [Google Scholar]
- 5. Pag U., Sahl H. G. (2002) Curr. Pharm. Des. 8, 815–833 [DOI] [PubMed] [Google Scholar]
- 6. Xie L., van der Donk W. A. (2004) Curr. Opin. Chem. Biol. 8, 498–507 [DOI] [PubMed] [Google Scholar]
- 7. Håvarstein L. S., Diep D. B., Nes I. F. (1995) Mol. Microbiol. 16, 229–240 [DOI] [PubMed] [Google Scholar]
- 8. Franke C. M., Tiemersma J., Venema G., Kok J. (1999) J. Biol. Chem. 274, 8484–8490 [DOI] [PubMed] [Google Scholar]
- 9. Furgerson Ihnken L. A., Chatterjee C., van der Donk W. A. (2008) Biochemistry 47, 7352–7363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Wu K. H., Tai P. C. (2004) J. Biol. Chem. 279, 901–909 [DOI] [PubMed] [Google Scholar]
- 11. Ishii S., Yano T., Hayashi H. (2006) J. Biol. Chem. 281, 4726–4731 [DOI] [PubMed] [Google Scholar]
- 12. Kotake Y., Ishii S., Yano T., Katsuoka Y., Hayashi H. (2008) Biochemistry 47, 2531–2538 [DOI] [PubMed] [Google Scholar]
- 13. Ishii S., Yano T., Ebihara A., Okamoto A., Manzoku M., Hayashi H. (2010) J. Biol. Chem. 285, 10777–10785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Zhong X., Kolter R., Tai P. C. (1996) J. Biol. Chem. 271, 28057–28063 [DOI] [PubMed] [Google Scholar]
- 15. Nishie M., Shioya K., Nagao J., Jikuya H., Sonomoto K. (2009) J. Biosci. Bioeng. 108, 460–464 [DOI] [PubMed] [Google Scholar]
- 16. Kimura H., Nagano R., Matsusaki H., Sonomoto K., Ishizaki A. (1997) Biosci. Biotechnol. Biochem. 61, 1049–1051 [DOI] [PubMed] [Google Scholar]
- 17. Kimura H., Sashihara T., Matsusaki H., Sonomoto K., Ishizaki A. (1998) Ann. N.Y. Acad. Sci. 864, 345–348 [DOI] [PubMed] [Google Scholar]
- 18. Aso Y., Nagao J., Koga H., Okuda K., Kanemasa Y., Sashihara T., Nakayama J., Sonomoto K. (2004) J. Biosci. Bioeng. 98, 429–436 [DOI] [PubMed] [Google Scholar]
- 19. Jensen P. R., Hammer K. (1993) Appl. Environ. Microbiol. 59, 4363–4366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Sambrook J., Fritsch E., Maniatis T. (1989) Molecular Cloning: A Laboratory Manual, 2nd Ed., Cold Spring Harbor Laboratory, Cold Spring Harbor, NY [Google Scholar]
- 21. Holo H., Nes I. F. (1995) Methods Mol. Biol. 47, 195–199 [DOI] [PubMed] [Google Scholar]
- 22. Augustin J., Götz F. (1990) FEMS Microbiol. Lett. 54, 203–207 [DOI] [PubMed] [Google Scholar]
- 23. Islam M. R., Shioya K., Nagao J., Nishie M., Jikuya H., Zendo T., Nakayama J., Sonomoto K. (2009) Mol. Microbiol. 72, 1438–1447 [DOI] [PubMed] [Google Scholar]
- 24. Nagao J., Morinaga Y., Islam M. R., Asaduzzaman S. M., Aso Y., Nakayama J., Sonomoto K. (2009) Peptides 30, 1412–1420 [DOI] [PubMed] [Google Scholar]
- 25. Asaduzzaman S. M., Nagao J., Aso Y., Nakayama J., Sonomoto K. (2006) Appl. Environ. Microbiol. 72, 6012–6017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Altendorf K. H., Staehelin L. A. (1974) J. Bacteriol. 117, 888–899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Futai M. (1974) J. Membr. Biol. 15, 15–28 [DOI] [PubMed] [Google Scholar]
- 28. Matsushita K., Inoue T., Adachi O., Toyama H. (2005) J. Bacteriol. 187, 4346–4352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kaback H. R. (1972) Biochim. Biophys. Acta 265, 367–416 [DOI] [PubMed] [Google Scholar]
- 30. Nagao J., Harada Y., Shioya K., Aso Y., Zendo T., Nakayama J., Sonomoto K. (2005) Biochem. Biophys. Res. Commun. 336, 507–513 [DOI] [PubMed] [Google Scholar]
- 31. Schneider E., Wilken S., Schmid R. (1994) J. Biol. Chem. 269, 20456–20461 [PubMed] [Google Scholar]
- 32. Davidson A. L., Sharma S. (1997) J. Bacteriol. 179, 5458–5464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Hunke S., Landmesser H., Schneider E. (2000) J. Bacteriol. 182, 1432–1436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Davidson A. L., Chen J. (2004) Annu. Rev. Biochem. 73, 241–268 [DOI] [PubMed] [Google Scholar]
- 35. Kuipers A., de Boef E., Rink R., Fekken S., Kluskens L. D., Driessen A. J., Leenhouts K., Kuipers O. P., Moll G. N. (2004) J. Biol. Chem. 279, 22176–22182 [DOI] [PubMed] [Google Scholar]
- 36. Drenth J., Kalk K. H., Swen H. M. (1976) Biochemistry 15, 3731–3738 [DOI] [PubMed] [Google Scholar]
- 37. Nicastro G., Menon R. P., Masino L., Knowles P. P., McDonald N. Q., Pastore A. (2005) Proc. Natl. Acad. Sci. U.S.A. 102, 10493–10498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. de Ruyter P. G., Kuipers O. P., de Vos W. M. (1996) Appl. Environ. Microbiol. 62, 3662–3667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Peschel A., Ottenwälder B., Götz F. (1996) FEMS Microbiol. Lett. 137, 279–284 [DOI] [PubMed] [Google Scholar]
- 40. Kreiswirth B. N., Löfdahl S., Betley M. J., O'Reilly M., Schlievert P. M., Bergdoll M. S., Novick R. P. (1983) Nature 305, 709–712 [DOI] [PubMed] [Google Scholar]
- 41. Nagao J., Aso Y., Shioya K., Nakayama J., Sonomoto K. (2007) J. Mol. Microbiol. Biotechnol. 13, 235–242 [DOI] [PubMed] [Google Scholar]
- 42. de Ruyter P. G., Kuipers O. P., Beerthuyzen M. M., van Alen-Boerrigter I., de Vos W. M. (1996) J. Bacteriol. 178, 3434–3439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Sizemore C., Buchner E., Rygus T., Witke C., Götz F., Hillen W. (1991) Mol. Gen. Genet. 227, 377–384 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.