Abstract
Minocycline is a tetracycline family antibiotic that has anti-inflammatory and immunomodulatory properties. These properties have shown promise in the treatment of conditions such as rheumatoid arthritis, Huntington disease, and multiple sclerosis. As lymphocyte activation is involved in the pathogenesis of many of these diseases, T cells are postulated to be a primary target in minocycline therapy. Previous studies have demonstrated attenuation of CD4+ T cell activation by minocycline, but a specific mechanism has not been elucidated. In this study, we investigated the effect of minocycline on the activity of three key transcription factors regulating CD4+ T cell activation: NF-κB, AP-1 (activator protein 1), and NFAT (nuclear factor of activated T) cells. Our data demonstrate that minocycline selectively impairs NFAT-mediated transcriptional activation, a result of increased phosphorylation and reduced nuclear translocation of the isoform NFAT1. Minocycline increased the activity of the NFAT kinase GSK3 and decreased intracellular Ca2+ flux, both of which facilitate NFAT1 phosphorylation. These findings provide a novel mechanism for minocycline induced suppression of CD4+ T cell activation and may better inform the application of minocycline as an immunomodulatory agent.
Keywords: Cellular Immune Response, Immunology, Immunosupressor, Lymphocyte, NFAT Transcription Factor, Signal Transduction, Transcription Factors, Transcription Regulation
Introduction
The tetracycline family of antibiotics is known to have multiple biological activities distinct from their antimicrobial function. Over the past two decades, the tetracycline derivative minocycline has demonstrated neuroprotective and immunomodulatory effects in animal models of diseases, including multiple sclerosis and Parkinson and Huntington diseases (1–3). Interest in the mechanism of its neuroprotective abilities has driven extensive research on minocycline-induced effects on inflammatory signaling in monocyte lineage cells, particularly the brain-resident microglia (4–6). Modulation of T cell activation and function has also been proposed as one of the major mechanisms of action of minocycline.
T cell activation contributes to disease pathogenesis by creating an inflammatory environment that leads to cellular damage and death as well as decreased immune function. Minocycline has been found to be therapeutic in multiple diseases whose pathogenesis is significantly T cell driven by modulating cellular activation and function. In rheumatoid arthritis patients, minocycline significantly improved clinical symptoms and increased the frequency of remissions leading to its usage as a disease modifying anti-rheumatic drug (7–9). Studies using CD4+ T cell clones derived from the synovium of an rheumatoid arthritis patient showed that minocycline decreased activation-induced proliferation and inflammatory cytokine production (10). Minocycline has also shown therapeutic effects on T cells in experimental autoimmune encephalomyelitis, an animal model of multiple sclerosis. Minocycline treatment of rodents with experimental autoimmune encephalomyelitis attenuated the severity of encephalitis, decreased T cell transmigration in vitro, and reduced CD4+ and CD8+ T cell infiltration into the spinal cord in vivo (1, 11).
In the context of HIV infection, T cell activation is closely correlated with disease progression and remains elevated even after long term administration of highly active antiretroviral therapy (12–14). No treatments currently exist for this ongoing, damaging process. We have previously found that minocycline can alleviate the incidence and severity of encephalitis in our simian immunodeficiency virus macaque model of HIV-associated neurological disease (15). In this model, minocycline treatment decreased cytotoxic lymphocyte infiltration into the brain, and reduced simian immunodeficiency virus and HIV replication along with p38 activation in primary lymphocytes in vitro (15). We recently reported that minocycline treatment suppressed CD4+ T cell activation, decreasing activation marker expression (CD25, HLADR), cytokine secretion, and proliferation (16). These effects resulted in attenuation of both HIV infection and reactivation of latent virus in CD4+ T cells in vitro and that attenuation occurs at the level of transcription (16).
Despite numerous studies demonstrating suppressive effects of minocycline on CD4+ T cell activation, a specific mechanism of action remains to be elucidated. Classical T lymphocyte activation is characterized by changes in gene expression in response to two signals: 1) an antigen-specific signal through the T cell receptor (TCR)2 complex when it binds antigen:MHC molecules on the surface of antigen presenting cells, and 2) a co-stimulatory signal delivered through a molecule such as CD28 binding a B7-family member on the antigen presenting cell surface (17). These signals activate diverse signaling pathways, including those mediated by the second messengers calcium (Ca2+) and diacylglycerol. These pathways converge on the activation of three major transcription factor families to modulate gene expression: NF-κB, AP-1 (activator protein 1), and NFAT (nuclear factor of activated T cells). To better understand the effects of minocycline in CD4+ T cells, we investigated its impact on the activity of these three transcription factors. Here, we show that minocycline mediates selective suppression of NFAT-mediated transcriptional activation in primary human CD4+ T cells using a luciferase reporter assay. Minocycline increases rephosphorylation of NFAT1 which reduces its nuclear translocation after several hours of activation. Treatment with minocycline enhances the activity of GSK3 and attenuates intracellular [Ca2+] by reducing extracellular Ca2+ influx. These findings demonstrate a novel and specific mechanism of action for minocycline in CD4+ T cells that is critical to understanding its immunomodulatory properties.
EXPERIMENTAL PROCEDURES
Cell Culture
Whole blood from healthy human donors was isolated in syringes with heparin. Informed consent was obtained under a Johns Hopkins Medicine Institutional Review Board-approved protocol. Peripheral blood mononuclear cells were separated by centrifugation on Ficoll (GE Healthcare). Purified CD4+ T cells were isolated using a CD4+ T cell isolation kit II (Miltenyi Biotec); purity was routinely >95% CD3+CD4+ by flow cytometry. Cells were cultured in R10 (Roswell Park Memorial Institute 1640 medium, 10% fetal bovine serum, l-glutamine, HEPES, gentamicin) and maintained at 37 °C and 5% CO2. CD4+ T cells (1 × 106) were cultured in 96-well plates and pretreated with minocycline (0–80 μg/ml) for 3 h before activation using either 10 ng/ml phorbol 12-myristate 13-acetate (PMA; Sigma), PMA and 1 μm ionomycin (Sigma), or ionomycin.
Luciferase Reporter Assays
NFAT and AP-1 luciferase reporters were obtained from Dr. Joel Pomerantz. pNF-κB-luciferase was obtained from Stratagene. CD4+ T cells (5 × 106) were transfected by electroporation with 8 μg reporter plasmid and 200 ng pRL TK Renilla (Promega) using a human T cell Nucleofector kit (Amaxa). After transfection, cells were resuspended in warm R10 and incubated overnight. After pretreatment and activation as above, cells were spun down and washed, and luciferase activity was measured by Dual-Luciferase assay (Promega).
Western Blots
Whole cell lysates were prepared in radioimmune precipitation assay buffer supplemented with protease and phosphatase inhibitor mixtures (Sigma). Marligen Nuclear Extraction kits were used for isolation of nuclei, followed by lysis in radioimmune precipitation assay buffer. Lysates were run on Bio-Rad Criterion 10% Tris-HCl. Antibodies used: NFAT1 (Abcam), GAPDH, transcription factor II B (Santa Cruz Biotechnology), phospho-p38 Thr-180/Tyr-182, p38, GSK3α, GSK3β, phospho-GSK3α/β S21/9 (Cell Signaling).
Phosphatase Assay
Calcineurin activity was measured following the manufacturer's protocol using Calcineurin Phosphatase Assay (Enzo Life Sciences). Minocycline (20 or 40 μg/ml) was added to some reactions prior to addition of calcineurin and maintained throughout the duration of the assay.
Intracellular Calcium Flux
CD4+T cells were loaded using Fluo-4 AM direct assay (Invitrogen) following manufacturer's protocol. After 30 min at 37 °C, aliquots of 1 × 106 cells were diluted to 500 μl in 1x Ca2+ assay buffer (Invitrogen) with or without 40 μg/ml minocycline. Cells were left at room temperature in the dark for 30 min. In some samples, 5 μl of 1 m EGTA (10 mm final) was added immediately before samples were run on a BD LSRFortessa. A 30-s baseline was recorded followed by addition of 5 μl of stimuli and vortexing. Stimuli used were ionomycin (10 nm and 100 nm final) and thapsigargin (100 nm final) in DMSO. Fluorescence was measured in linear mode in FL1 channel, and data were analyzed in Flowjo (Treestar).
RESULTS
Minocycline Reduces Transcriptional Activity of NFAT
Upon TCR recognition of antigen or cross-linking the TCR with antibodies, phospholipase C γ is activated leading to phophatidylinositol 4,5-bisphosphate cleavage into inositol 1,4,5-triphosphate and diacylglycerol (17). Inositol 1,4,5-triphosphate binds to the inositol 1,4,5-triphosphate receptor and releases Ca2+ from the endoplasmic reticulum (ER) store, subsequently activating the Ca2+ release-activated Ca2+ (CRAC) channels in the plasma membrane and raising intracellular Ca2+ levels. This pathway can be triggered with the release of inositol 1,4,5-triphosphate-sensitive stores by the Ca2+ ionophore ionomycin (18). Diacylglycerol activates the Ras MAPK and protein kinase C θ cascades, and these pathways can be specifically activated by the diacylglycerol analog PMA (19). These signals result in changes in gene expression through the activation of three major transcription factor families: NF-κB, NFAT, and AP-1.
To determine which pathways contributed to the suppressive effect of minocycline on activation, primary human CD4+ T cells from healthy donors were co-transfected with a reporter plasmid expressing firefly luciferase responsive to NF-κB, NFAT, or AP-1, along with a plasmid expressing Renilla luciferase under herpes simplex virus thymidine kinase promoter to control for transfection efficiency. Cells were pretreated with minocycline and then stimulated with PMA alone or in combination with ionomycin. After 4 h, the effect of minocycline pretreatment on transcriptional activation was assessed by measuring luciferase activity. Minocycline pretreatment did not significantly impact transcriptional activation mediated by NF-κB or AP-1 when cells were activated by PMA alone or PMA with ionomycin (Fig. 1, A and B). Transcriptional activity of the Ca2+-dependent NFAT was induced only by PMA in combination with ionomycin, and not PMA alone (Fig. 1C). Minocycline significantly decreased NFAT activity stimulated by PMA and ionomycin by ∼5-fold relative to stimulated untreated control (Fig. 1C, untreated mean fluorescence intensity, 19.47 versus treated mean fluorescence intensity, 3.793; p = .0313).
FIGURE 1.
Minocycline inhibits the transcriptional activity of NFAT. Primary human CD4+ T cells isolated from healthy donors were transfected with an herpes simplex virus thymidine kinase Renilla reporter plasmid and a firefly luciferase reporter plasmid responsive to NF-κB (A), AP-1 (B), or NFAT (C). After resting overnight in R10, cells were pretreated or left untreated for 2–4 h with 20 μg/ml minocycline. Cells were then activated by 10 ng/ml PMA alone or PMA and 1 μm ionomycin. Luciferase activity was measured 4 h post-activation, and fold induction was calculated by normalizing firefly:Renilla ratios to unactivated controls. Each point represents one independent experiment; bars represent means. p values calculated by Wilcoxon matched pairs test.
Nuclear Translocation of NFAT1 Is Decreased by Minocycline Treatment
The transcriptional activity of NFAT is strictly regulated by phosphorylation (20). In resting cells, NFAT is maintained in a constitutively phosphorylated state in the cytoplasm. Upon T cell stimulation that initiates a rise in intracellular Ca2+, NFAT is dephosphorylated by the Ca2+-dependent phosphatase calcineurin and translocates to the nucleus where it forms homo- or heterodimers to activate gene transcription (21). As intracellular Ca2+ returns to resting levels, calcineurin is deactivated, and NFAT kinases rephosphorylate NFAT to facilitate its nuclear export.
NFAT1 is the NFAT family member predominantly expressed in primary human CD4+ T cells; this isoform has also been shown to represent the majority of NFAT DNA-binding activity in the first 4 h after activation by PMA and ionomycin (22). Therefore, we hypothesized that minocycline impairs NFAT activity by reducing nuclear translocation of NFAT1. Primary human CD4+ T cells were pretreated for 3 h with minocycline (20 or 40 μg/ml) and activated by PMA and ionomycin. Nuclear lysates were isolated for analysis by Western blot. Minocycline pretreatment mediated a dose-dependent decrease in nuclear translocation of NFAT1 relative to untreated, activated controls at both 2 and 4 h post-activation (Fig. 2A).
FIGURE 2.
Nuclear translocation and dephosphorylation of NFAT1 is reduced by minocycline. Primary human CD4+ T cells were pretreated with indicated amounts of minocycline for ∼3 h. Cells were then activated for indicated amounts of time by 10 ng/ml PMA and 1 μm ionomycin or ionomycin alone. Unactivated controls are the far left lane in each blot. A, nuclear lysates showing nuclear translocation of NFAT1 and nuclear control transcription factor II B (TFIIB). B, whole cell lysates showing NFAT1 phosphorylation at 4h post-activation. Whole cell lysates showing NFAT1 phosphorylation over time after activation by PMA and ionomycin (C) or ionomycin alone (D). Each blot is representative of at least three independent experiments.
In minocycline-treated samples, the NFAT1 band had a higher apparent molecular mass compared with untreated samples (Fig. 2A). Previous studies have demonstrated that NFAT1 can migrate as a phosphorylated band with apparent molecular mass ∼140 kDa and a dephosphorylated band with mass ∼120 kDa (23). Because NFAT1 is exported from the nucleus upon phosphorylation, we hypothesized that minocycline-mediated nuclear export of NFAT results from increased phosphorylation of NFAT1. To investigate this possibility, primary human CD4+ T cells were pretreated with minocycline and then activated with PMA and ionomycin for 4 h. Whole cell lysates were isolated and analyzed by Western blot. Minocycline mediated a dose-dependent shift favoring the higher molecular mass, phosphorylated form of NFAT1 (Fig. 2B). At the highest dose (80 μg/ml), all detectable NFAT1 shifted to its higher molecular mass, a phosphorylated, deactivated form that results in nuclear export.
Effects of Minocycline Are Synergistic with PMA-mediated Deactivation of NFAT1
Loh et al. (24) demonstrated that after TCR cross-linking with antibodies, NFAT1 activation progresses through two phases: a rapid, calcineurin-dependent dephosphorylation in minutes followed by a gradual, slow deactivation and rephosphorylation taking place over hours. This contrasts with ionomycin-stimulated NFAT1 activation, which persists for several hours with relatively little rephosphorylation. When cells are stimulated with both PMA and ionomycin, early activation of NFAT1 remains unaffected, but late deactivation is facilitated.
Because we observed that minocycline mediated a slow decline in nuclear NFAT1 levels over the course of hours, we hypothesized that early activation of NFAT1 was not affected, but rather that minocycline acts by facilitating gradual rephosphorylation and deactivation. Consistent with our hypothesis, we observed that minocycline did not alter NFAT1 dephosphorylation in whole cell lysates of CD4+ T cells after 1 h of activation by PMA and ionomycin (Fig. 2C). Instead, minocycline accelerated the shift to phosphorylated NFAT1. In treated cells, the phosphorylated band appears at 2 and 4 h post-activation, whereas in untreated cells, the phosphorylated form does not appear until 4 h post-activation (Fig. 2C). Cells stimulated with ionomycin alone (Fig. 2D) had much less phosphorylated NFAT1 after 4 h of activation compared with cells stimulated with PMA and ionomycin (Fig. 2C). Minocycline treatment took relatively longer to elicit NFAT1 deactivation when stimulated by ionomycin alone: only the highest dose (40 μg/ml) resulted in detectable rephosphorylation of NFAT1 after 2 h of activation (Fig. 2D). At 4 h post-activation with ionomycin, a low dose minocycline (20 μg/ml) resulted in incomplete rephosphorylation, whereas a high dose (40 μg/ml) resulted in complete rephosphorylation and deactivation. In contrast, both doses resulted in complete rephosphorylation at 4 h post-activation with PMA and ionomycin. These data support a mechanism for minocycline acting directly on Ca2+ signaling and demonstrates that its effect is synergistic with negative NFAT1 regulation by PMA.
Calcineurin Is Not Inhibited by Minocycline in a Cell-free Assay
The extent of NFAT1 dephosphorylation, and the corresponding length of NFAT1 nuclear residency, is dependent on two opposing forces: dephosphorylation by the phosphatase calcineurin and rephosphorylation by kinases (both constitutive and inducible) that restore and maintain phosphorylation to facilitate NFAT1 export (20). Multiple immunosuppressive agents target NFAT activation by acting on the phosphatase calcineurin, preventing binding and activation by the Ca2+-dependent protein calmodulin (25). It has been suggested that the ability of minocycline to suppress T cell proliferation may be related to chelation of Ca2+, but others were unable to demonstrate that minocycline altered intracellular Ca2+ concentrations (10). We utilized an in vitro phosphatase assay to determine whether the effects of minocycline on NFAT dephosphorylation could be explained by direct chelation of available Ca2+ reducing calcineurin activity or a direct interaction between minocycline and calcineurin, thereby inhibiting its activity (Fig. 3A). We found that minocycline (up to 40 μg/ml) did not significantly affect the activity of calcineurin on a phosphopeptide substrate in a cell-free assay. EGTA treatment, a calcium-chelating control, decreased phosphatase activity to below the limit of detection (data not shown). These results demonstrate that the effect of minocycline on NFAT1 cannot be explained by direct chelation of available Ca2+ or direct molecular interaction of calcineurin and minocycline.
FIGURE 3.
Minocycline decreases activity of the NFAT kinase p38 and increases activity of GSK3. A, an in vitro cell-free phosphatase assay was performed as described under “Experimental Procedures” incubating purified calcineurin with a peptide substrate in the presence or absence of minocycline. Activity is expressed as percent phosphate released normalized to untreated control. Bars represent mean ± S.E. (n = 3). B, primary human CD4+ T cells were pretreated with minocycline and activated for indicated times with 10 ng/ml PMA and 1 μm ionomycin. Levels of activated p38 (p-p38) and inhibited GSK3 (p-GSK3 α/β) were analyzed by Western blot. Results are representative of at least three independent experiments.
Minocycline Decreases Inhibition of NFAT Kinase GSK3
We hypothesized that minocycline may mediate increased phosphorylation and nuclear export of NFAT1 by increasing the activity of NFAT kinases. NFAT1 can be phosphorylated by p38, an activation-inducible kinase, and GSK3, a constitutive kinase that is inhibited by activation (26, 27). After activation with PMA and ionomycin, p38 is activated by phosphorylation; it has been shown that NFAT1 is a p38 substrate, and activation-induced p38 has been hypothesized to be a negative regulator of NFAT1 (27). At 1 h post-activation, minocycline pretreatment did not alter p38 activation; by 2 and 4 h post-activation, minocycline decreased p38 activation (Fig. 3B). This indicates that minocycline does not mediate increased NFAT1 phosphorylation through p38 activation.
GSK3 is a constitutively active kinase that assists in keeping NFAT1 deactivated in resting cells (26, 28). Upon activation of CD4+ T cells, GSK3 isoforms are inhibited by Akt-mediated phosphorylation of serine residues (29). Minocycline treatment did not affect inhibitory phosphorylation of GSK3 at 1 or 2 h post activation (Fig. 3B). However, by 4 h, post-activation minocycline treatment reduced the level of phosphorylated GSK3α and -β, suggesting that minocycline treatment may enhance NFAT1 rephosphorylation through increased GSK3 activity. Although this observation suggests one potential mechanism for minocycline-mediated increases in NFAT1 phosphorylation at 4 h post-activation, this does not explain increased NFAT1 phosphorylation at 2 h post-activation.
Minocycline Attenuates Intracellular Ca2+ Flux by Reducing store-operated Ca2+ Entry
NFAT is known to be a sensitive integrator of Ca2+ signaling in T lymphocytes. Intracellular Ca2+ responds to strong stimulation or artificial store depletion in a biphasic manner; the first, peak phase consists of a transient rise in concentration representing the rapid release of intracellular Ca2+ stores (specifically the ER) followed by a second, plateau phase of extracellular Ca2+ influx through STIM1-mediated activation of store-operated CRAC/Orai1 channels at the plasma membrane, sometimes termed capacitative or store-operated Ca2+ entry (SOCE) (30). Previous studies have shown that NFAT-driven gene expression requires 1) intracellular Ca2+ concentrations at or above 3-fold resting levels and 2) sustaining elevated Ca2+ for a minimum of 25 min, optimally ∼2 h (31, 32). By contrast, activation of NF-kB is dependent on the initial rapid Ca2+ transient from intracellular store release, not sustained Ca2+ (33, 34).
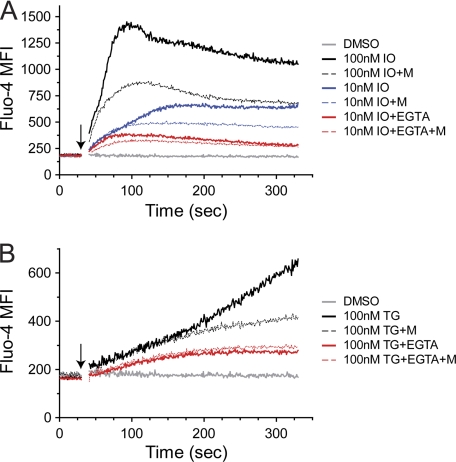
In light of our data showing that 1) minocycline does not inhibit early activation of NFAT1, 2) minocycline does not reduce transcriptional activity of NF-κB, and 3) the direct chelation of minocycline of Ca2+ does not affect calcineurin activity, we hypothesized that minocycline acts by suppressing SOCE in CD4+ T cells. We utilized the intracellular Ca2+ indicator dye Fluo-4 to measure the effect of minocycline on intracellular Ca2+ flux induced by ionomycin. It has been previously reported that concentrations of ionomycin <100 nm are sufficient to release the majority of intracellular Ca2+ stores without significantly permeabilizing the plasma membrane to Ca2+ entry (35). Ca2+ flux initiated by 100 nm ionomycin generates the expected biphasic response consisting of a fast peak followed by a slowly decaying plateau phase, and minocycline treatment reduces peak and plateau levels of Ca2+ in response to ionomycin (Fig. 4A, solid versus dashed black traces). Similar effects are seen on Ca2+ flux initiated by 10 nm ionomycin (Fig. 4A, solid versus dashed blue traces). In the presence of EGTA, extracellular Ca2+ is chelated, enabling analysis of Ca2+ flux released from intracellular stores. Stimulating with 10 nm ionomycin in the presence of EGTA, intracellular Ca2+ peak and plateau are both suppressed, and minocycline treatment has no effect on them (Fig. 4A, solid versus dashed red traces). In the presence of EGTA, peak Ca2+ is reached earlier (t ∼ 75s) followed by the slowly decaying plateau phase. This indicates that the portion of the ionomycin-induced Ca2+ response after this time requires extracellular Ca2+. These data strongly suggest that minocycline acts at the level of SOCE through the plasma membrane and not by reducing the release of Ca2+ stores or their Ca2+ content.
FIGURE 4.
Minocycline attenuates extracellular Ca2+ influx. Primary human CD4+ T cells were loaded with Fluo-4 as described under “Experimental Procedures.” Cells were then pretreated at room temperature with 40 μg/ml minocycline (M; A, B) for 30 min. Fluo-4 mean fluorescence intensity was measured by flow cytometry with addition (arrows) of indicated concentrations of ionomycin (IO; A) or thapsigargin (TG; B) in the presence or absence of 10 mm EGTA. Results are representative of three independent experiments.
Intracellular Ca2+ levels during SOCE are determined by rates of influx through plasma membrane CRAC channels, efflux across the plasma membrane via plasma membrane Ca2+-ATPase and Na+/Ca2+ exchanger, and reuptake into the ER and mitochondria by the sarco/endoplasmic reticulum Ca2+-ATPase and mitochondrial Ca2+ uniporter, respectively. We utilized the sarco/endoplasmic reticulum Ca2+-ATPase pump inhibitor thapsigargin to irreversibly empty ER stores and determine whether minocycline decreases intracellular Ca2+ levels by enhancing Ca2+ reuptake (36). After treatment with 100 nm thapsigargin, intracellular Ca2+ levels steadily increase, and minocycline treatment attenuates this increase at t > 175 s (Fig. 4B, solid versus dashed black traces). In the presence of EGTA, thapsigargin increases intracellular Ca2+ levels until a plateau is reached at t ∼ 175 s, indicating that Ca2+ increases after this time point require extracellular Ca2+ (Fig. 4B, solid red trace). Minocycline does not decrease intracellular Ca2+ flux in the presence of EGTA, indicating that minocycline does not affect the capacity of the ER to retain or efflux Ca2+ (Fig. 4B, dashed red trace). Taken together, these data suggest that minocycline acts on SOCE, suppressing activation-induced intracellular Ca2+ flux over time, which is consistent with our findings of reduced NFAT1 dephosphorylation and increased nuclear export several hours after activation.
DISCUSSION
In this study, we demonstrate that minocycline reduces activation of NFAT1 in CD4+ T cells by increasing its phosphorylation and nuclear export several hours after activation by PMA and ionomycin. Furthermore, our data suggest two potential mechanisms for the effects of minocycline: 1) increased activity of GSK3 and 2) attenuated intracellular Ca2+ flux. Our data favor the latter as the major mechanism of action because GSK3 activity is not enhanced until 4 h post-activation, and minocycline increases NFAT1 phosphorylation as early as 2 h post-activation. By contrast, minocycline mediates early attenuation of Ca2+ flux in the first 5 min after stimulation, providing a more plausible mechanism for enhanced NFAT1 phosphorylation.
Minocycline has long been hypothesized to act by altering Ca2+ signaling. Multiple studies demonstrated that tetracycline family antibiotics including minocycline can act as a weak chelator of Ca2+ (37, 38). Our data on calcineurin activity and Ca2+ flux are consistent with this and strongly suggest that direct chelation does not significantly reduce available Ca2+ concentrations. Our results also indicate that minocycline does not affect the content or release of Ca2+ from intracellular stores, such as the ER. Instead, minocycline decreases the amount of extracellular Ca2+ influx, suggesting that minocycline is acting at the level of SOCE. The steady-state plateau concentration of Ca2+ during SOCE is determined by rates of influx (CRAC/Orai1) and efflux (plasma membrane Ca2+-ATPase, Na+/Ca2+ exchanger) at the plasma membrane and reuptake into the major intracellular stores, mitochondria (mitochondrial Ca2+ uniporter) and the ER (sarco/endoplasmic reticulum Ca2+-ATPase). The ability of minocycline to reduce thapsigargin-mediated Ca2+ flux rules out a mechanism affecting sarco/endoplasmic reticulum Ca2+-ATPase pump activity. Our findings that minocycline mediates no change in Ca2+ flux in the presence of EGTA suggest that the drug does not alter the rate of plasma membrane efflux. Taken together, the literature and our data suggest that minocycline acts through a mitochondria-based mechanism to reduce NFAT1 activation in CD4+ T cells.
Mitochondrial Ca2+ buffering is known to be important in controlling Ca2+ signaling in many cell types. Recent studies have discovered that mitochondria play an active role in shaping intracellular Ca2+ signaling during T cell activation (39–41). Mitochondria relocate to immunological synapses and are critical to sustained Ca2+ signaling (39). In addition, mitochondria have been shown to translocate toward the plasma membrane after thapsigargin-induced Ca2+ flux. In this study, CRAC channel activity was found to be dependent upon mitochondrial translocation and Ca2+ uptake (40). Mitochondria are proposed to sustain CRAC channel activity by reducing closure due to negative feedback of high Ca2+ concentrations, thereby enhancing local Ca2+ entry (40, 41). Further evidence supporting this model showed that mitochondrial uncouplers prevent T cells from sustaining SOCE and ultimately reduce local Ca2+ entry (41, 42).
We hypothesize that minocycline acts by reducing the capacity for mitochondria to buffer Ca2+, resulting in decreased SOCE in CD4+ T cells. In isolated mitochondria from rat liver and brain, minocycline has been shown to bind the inner mitochondrial membrane in a Ca2+-dependent manner (37). Minocycline forms Ca2+-dependent small ion channels that result in partial uncoupling of mitochondria and reduced Ca2+ retention as determined by Ca2+-induced mitochondrial swelling (37, 43). Our data demonstrate that minocycline does not affect early Ca2+ mobilization or early NFAT1 activation. Instead, minocycline accelerated NFAT1 rephosphorylation several hours after activation and reduced levels of sustained extracellular Ca2+ entry. Previous studies have shown that PMA and TCR stimulation mediate a late-phase deactivation of NFAT1 and that inhibitors of SOCE produce similar effects (24). Our results indicate that minocycline synergizes with PMA-mediated effects to accelerate NFAT1 deactivation via phosphorylation and that this is consistent with an accelerated return of intracellular Ca2+ to basal levels.
The transcription factor NFAT is a critical, Ca2+-dependent regulator of T cell activation. We demonstrate for the first time that minocycline can significantly decrease transcriptional activation by NFAT1 in human CD4+ T cells. We showed previously that minocycline decreased HIV transcription during infection and reactivation from latency (16). Our data indicated that minocycline impairs HIV at the transcriptional level and that this effect was linked to attenuation of T cell activation, suggesting minocycline might have therapeutic effects on both HIV infection and immunopathogenesis. Our findings in this study provide a mechanism for these effects because NFAT1 is known to activate viral gene transcription from the HIV long terminal repeat (44–46). NFAT1 also plays a role in up-regulation of immune response genes such as the IL-2 and TNFα, as well as controlling expression of cell cycle regulators such as CDK4 (47, 48). This is consistent with our previous data in activated CD4+ T cells showing reduced cytokine secretion and proliferation (16).
Chronic immune activation is hypothesized to be a major pathogenic mechanism in HIV, underlying CD4+ T cell depletion and immune dysfunction, as well as playing an important role in virus replication by generating activated host cells permissive to HIV infection. In HIV infection, minocycline may have use as a novel antiretroviral, as well as an immunomodulatory therapy to alleviate the effects of chronic CD4+ T cell activation. Current evidence supports a model of HIV pathogenesis where disease progression and cellular destruction is driven by immune activation. In HIV-infected patients, activated CD4+ T cells (HLADR+CD45RO+) have been shown to be more prone to both spontaneous and activation-induced apoptosis ex vivo and that this correlated closely with disease progression (49). In a portion of discordant highly active antiretroviral therapy-treated patients, virological suppression does not lead to satisfactory rebound of absolute CD4+ T cell counts (immunological nonresponders). Immunological nonresponders display significantly higher levels of proliferating (Ki67+) and activated (HLADR+CD95+) CD4+ T cells, higher propensity for ex vivo CD4+ T cell apoptosis, and lower levels of circulating naïve (CD45RA+CD62L+) CD4+ T cells relative to virologically suppressed immunologic responders (50, 51).
The use of immune modulation to avert HIV pathogenesis is not unprecedented (52, 53). An effective immunomodulatory therapy for HIV should dampen immune activation to within optimal levels while avoiding complete immunosuppression, achieving a balance between the destructive effects of exuberant immune activation and the deficiency of immunosuppression. We speculate that minocycline would be a potential candidate for this approach. We have previously shown that minocycline treatment of CD4+ T cells in vitro results in decreased levels of Ki67+ and HLADR+ cells, and increased levels of CD45RA+ cells after activation (16). Minocycline-mediated reduction of cellular activation in HIV-infected patients could decrease the sensitivity of CD4+ T cells to death, decrease proliferation to reduce cellular turnover, and attenuate damaging inflammatory responses.
We previously demonstrated that the suppressive effect of minocycline on CD4+ T cell activation is dose-dependent, with significant changes in activation marker profile at concentrations as low as 5 μg/ml (16). The results of our work indicate that 20 μg/ml is the optimal concentration for suppression of T cell activation. Concentrations below this threshold, such as the 1–2 μg/ml achieved in sera of patients during routine oral dosing, would be expected to provide partial blunting of T cell responses. There are two major caveats to consider; 1) it is difficult to relate in vitro concentrations to serum levels of minocycline during routine oral dosing, and 2) it is difficult to extrapolate serum levels to concentrations in critical immune tissues such as lung, brain, or the secondary lymphoid organs. However, even if minocycline only partially blunts T cell activation in standard oral regimens, a recent study demonstrated that IV administration of minocycline can achieve concentrations exceeding 20 μg/ml in sera with no short term toxicity (54). This indicates that more aggressive dosing regimens are a viable option to achieve higher in vivo concentrations and increase suppression of T cell activation if necessary.
An advantage of minocycline is that our in vitro measurements in this study indicate complete inhibition of the NFAT pathway would require concentrations in excess of 40 μg/ml. This dose lies far outside the range of the highest reported in vivo concentrations, making the danger of total immunosuppression remote (54). The dose-dependent nature of the effects of minocycline suggests that the immune response could be equilibrated to a desired set point by therapeutic dose monitoring.
For example, minocycline is a potential trigger of drug-induced lupus, and recent studies demonstrate a clear association between increased NFAT1 activity in T cells and systemic lupus erythematosus (55–58). Paradoxically, in cases of minocycline-induced lupus, higher doses might prove therapeutic through increased suppression of NFAT1.
Given its function as a key regulatory factor in T cell activation, NFAT is one of the major targets of traditional immunosuppressive drugs in the immunophilin ligand class (cyclosporin A, FK506). Suppression of T cell activation with these drugs is therapeutic in many conditions including transplant rejection, graft versus host disease, autoimmune, and inflammatory diseases, but carries with them a host of associated risks. Cyclosporin has a narrow therapeutic range due to the balance between adequate immunosuppression and toxic side effects such as nephrotoxicity; slow and variable absorption, as well as high intra- and interpatient variability in dose responses require intensive therapeutic dose monitoring and make HIV immunomodulation a challenge (59). Minocycline is well poised to reduce risks in these areas; the drug has been used safely for long term treatment of acne and shows excellent tissue penetration with near 100% bioavailability, and our in vitro data show that its immunomodulatory effects are dose-dependent and NFAT-specific. This last property makes minocycline an interesting candidate drug that can be used in an immunomodulatory manner. Therapeutic opportunities are open to minocycline in diseases such as HIV where the goal of therapy is not immunosuppression but a new equilibrium for the immune response. Altogether, the results of this study suggest that minocycline has potential as a novel immunomodulatory agent and warrants further clinical investigation.
Acknowledgments
Medical editor Michael E. Linde contributed to the editing of the manuscript. We thank Lucio Gama, Julia Drewes, Jeanne Sisk, and Kelly Meulendyke for productive discussion and critical reading of the manuscript.
This work was supported, in whole or in part, by National Institutes of Health Grants NS055648 and MH087233.
- TCR
- T cell receptor
- ER
- endoplasmic reticulum
- CRAC
- Ca2+ release-activated Ca2+
- PMA
- phorbol 12-myristate 13-acetate
- SOCE
- store-operated calcium entry
- GSK3
- glycogen synthase kinase 3.
REFERENCES
- 1. Brundula V., Rewcastle N. B., Metz L. M., Bernard C. C., Yong V. W. (2002) Brain 125, 1297–1308 [DOI] [PubMed] [Google Scholar]
- 2. Du Y., Ma Z., Lin S., Dodel R. C., Gao F., Bales K. R., Triarhou L. C., Chernet E., Perry K. W., Nelson D. L., Luecke S., Phebus L. A., Bymaster F. P., Paul S. M. (2001) Proc. Natl. Acad. Sci. U.S.A. 98, 14669–14674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Chen M., Ona V. O., Li M., Ferrante R. J., Fink K. B., Zhu S., Bian J., Guo L., Farrell L. A., Hersch S. M., Hobbs W., Vonsattel J. P., Cha J. H., Friedlander R. M. (2000) Nat. Med. 6, 797–801 [DOI] [PubMed] [Google Scholar]
- 4. Giuliani F., Hader W., Yong V. W. (2005) J. Leukoc. Biol. 78, 135–143 [DOI] [PubMed] [Google Scholar]
- 5. Nikodemova M., Watters J. J., Jackson S. J., Yang S. K., Duncan I. D. (2007) J. Biol. Chem. 282, 15208–15216 [DOI] [PubMed] [Google Scholar]
- 6. Yrjänheikki J., Keinänen R., Pellikka M., Hökfelt T., Koistinaho J. (1998) Proc. Natl. Acad. Sci. U.S.A. 95, 15769–15774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Tilley B. C., Alarcón G. S., Heyse S. P., Trentham D. E., Neuner R., Kaplan D. A., Clegg D. O., Leisen J. C., Buckley L., Cooper S. M., Duncan H., Pillemer S. R., Tuttleman M., Fowler S. E. (1995) Ann. Intern. Med. 122, 81–89 [DOI] [PubMed] [Google Scholar]
- 8. O'Dell J. R., Haire C. E., Palmer W., Drymalski W., Wees S., Blakely K., Churchill M., Eckhoff P. J., Weaver A., Doud D., Erikson N., Dietz F., Olson R., Maloley P., Klassen L. W., Moore G. F. (1997) Arthritis Rheum. 40, 842–848 [DOI] [PubMed] [Google Scholar]
- 9. O'Dell J. R., Paulsen G., Haire C. E., Blakely K., Palmer W., Wees S., Eckhoff P. J., Klassen L. W., Churchill M., Doud D., Weaver A., Moore G. F. (1999) Arthritis Rheum. 42, 1691–1695 [DOI] [PubMed] [Google Scholar]
- 10. Kloppenburg M., Verweij C. L., Miltenburg A. M., Verhoeven A. J., Daha M. R., Dijkmans B. A., Breedveld F. C. (1995) Clin. Exp. Immunol. 102, 635–641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Nikodemova M., Lee J., Fabry Z., Duncan I. D. (2010) J. Neuroimmunol. 219, 33–37 [DOI] [PubMed] [Google Scholar]
- 12. Giorgi J. V., Hultin L. E., McKeating J. A., Johnson T. D., Owens B., Jacobson L. P., Shih R., Lewis J., Wiley D. J., Phair J. P., Wolinsky S. M., Detels R. (1999) J. Infect. Dis. 179, 859–870 [DOI] [PubMed] [Google Scholar]
- 13. Simmonds P., Beatson D., Cuthbert R. J., Watson H., Reynolds B., Peutherer J. F., Parry J. V., Ludlam C. A., Steel C. M. (1991) Lancet 338, 1159–1163 [DOI] [PubMed] [Google Scholar]
- 14. Benito J. M., López M., Lozano S., Ballesteros C., Martinez P., González-Lahoz J., Soriano V. (2005) J. Acquir. Immune Defic. Syndr. 38, 373–381 [DOI] [PubMed] [Google Scholar]
- 15. Zink M. C., Uhrlaub J., DeWitt J., Voelker T., Bullock B., Mankowski J., Tarwater P., Clements J., Barber S. (2005) JAMA 293, 2003–2011 [DOI] [PubMed] [Google Scholar]
- 16. Szeto G. L., Brice A. K., Yang H. C., Barber S. A., Siliciano R. F., Clements J. E. (2010) J. Infect. Dis. 201, 1132–1140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Smith-Garvin J. E., Koretzky G. A., Jordan M. S. (2009) Annu. Rev. Immunol. 27, 591–619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Yoshida S., Plant S. (1992) J. Physiol. 458, 307–318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Niedel J. E., Kuhn L. J., Vandenbark G. R. (1983) Proc. Natl. Acad. Sci. U.S.A. 80, 36–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Rao A., Luo C., Hogan P. G. (1997) Annu. Rev. Immunol. 15, 707–747 [DOI] [PubMed] [Google Scholar]
- 21. Hogan P. G., Chen L., Nardone J., Rao A. (2003) Genes Dev. 17, 2205–2232 [DOI] [PubMed] [Google Scholar]
- 22. Lyakh L., Ghosh P., Rice N. R. (1997) Mol. Cell Biol. 17, 2475–2484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Shaw K. T., Ho A. M., Raghavan A., Kim J., Jain J., Park J., Sharma S., Rao A., Hogan P. G. (1995) Proc. Natl. Acad. Sci. U.S.A. 92, 11205–11209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Loh C., Carew J. A., Kim J., Hogan P. G., Rao A. (1996) Mol. Cell Biol. 16, 3945–3954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ho S., Clipstone N., Timmermann L., Northrop J., Graef I., Fiorentino D., Nourse J., Crabtree G. R. (1996) Clin. Immunol. Immunopathol. 80, S40–45 [DOI] [PubMed] [Google Scholar]
- 26. Okamura H., Garcia-Rodriguez C., Martinson H., Qin J., Virshup D. M., Rao A. (2004) Mol. Cell Biol. 24, 4184–4195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Gómez del Arco P., Martínez-Martínez S., Maldonado J. L., Ortega-Pérez I., Redondo J. M. (2000) J. Biol. Chem. 275, 13872–13878 [DOI] [PubMed] [Google Scholar]
- 28. Gwack Y., Sharma S., Nardone J., Tanasa B., Iuga A., Srikanth S., Okamura H., Bolton D., Feske S., Hogan P. G., Rao A. (2006) Nature 441, 646–650 [DOI] [PubMed] [Google Scholar]
- 29. Cross D. A., Alessi D. R., Cohen P., Andjelkovich M., Hemmings B. A. (1995) Nature 378, 785–789 [DOI] [PubMed] [Google Scholar]
- 30. Hogan P. G., Lewis R. S., Rao A. (2010) Annu. Rev. Immunol. 28, 491–533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Negulescu P. A., Shastri N., Cahalan M. D. (1994) Proc. Natl. Acad. Sci. U.S.A. 91, 2873–2877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Timmerman L. A., Clipstone N. A., Ho S. N., Northrop J. P., Crabtree G. R. (1996) Nature 383, 837–840 [DOI] [PubMed] [Google Scholar]
- 33. Dolmetsch R. E., Lewis R. S., Goodnow C. C., Healy J. I. (1997) Nature 386, 855–858 [DOI] [PubMed] [Google Scholar]
- 34. Dolmetsch R. E., Xu K., Lewis R. S. (1998) Nature 392, 933–936 [DOI] [PubMed] [Google Scholar]
- 35. Albert P. R., Tashjian A. H., Jr. (1984) J. Biol. Chem. 259, 15350–15363 [PubMed] [Google Scholar]
- 36. Lytton J., Westlin M., Hanley M. R. (1991) J. Biol. Chem. 266, 17067–17071 [PubMed] [Google Scholar]
- 37. Antonenko Y. N., Rokitskaya T. I., Cooper A. J., Krasnikov B. F. (2010) J. Bioenerg. Biomembr. 42, 151–163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Brion M., Berthon G., Fourtillan J. B. (1981) Inorganica Chimica Acta 55, 47–56 [Google Scholar]
- 39. Quintana A., Schwindling C., Wenning A. S., Becherer U., Rettig J., Schwarz E. C., Hoth M. (2007) Proc. Natl. Acad. Sci. U.S.A. 104, 14418–14423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Quintana A., Schwarz E. C., Schwindling C., Lipp P., Kaestner L., Hoth M. (2006) J. Biol. Chem. 281, 40302–40309 [DOI] [PubMed] [Google Scholar]
- 41. Schwindling C., Quintana A., Krause E., Hoth M. (2010) J. Immunol. 184, 184–190 [DOI] [PubMed] [Google Scholar]
- 42. Hoth M., Fanger C. M., Lewis R. S. (1997) J. Cell Biol. 137, 633–648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Fernandez-Gomez F. J., Galindo M. F., Gomez-Lazaro M., González-García C., Ceña V., Aguirre N., Jordán J. (2005) Neuroscience 133, 959–967 [DOI] [PubMed] [Google Scholar]
- 44. Cron R. Q., Bartz S. R., Clausell A., Bort S. J., Klebanoff S. J., Lewis D. B. (2000) Clin. Immunol. 94, 179–191 [DOI] [PubMed] [Google Scholar]
- 45. Robichaud G. A., Barbeau B., Fortin J. F., Rothstein D. M., Tremblay M. J. (2002) J. Biol. Chem. 277, 23733–23741 [DOI] [PubMed] [Google Scholar]
- 46. Pessler F., Cron R. Q. (2004) Genes Immun. 5, 158–167 [DOI] [PubMed] [Google Scholar]
- 47. Luo C., Burgeon E., Carew J. A., McCaffrey P. G., Badalian T. M., Lane W. S., Hogan P. G., Rao A. (1996) Mol. Cell Biol. 16, 3955–3966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Baksh S., Widlund H. R., Frazer-Abel A. A., Du J., Fosmire S., Fisher D. E., DeCaprio J. A., Modiano J. F., Burakoff S. J. (2002) Mol. Cell 10, 1071–1081 [DOI] [PubMed] [Google Scholar]
- 49. Gougeon M. L., Lecoeur H., Dulioust A., Enouf M. G., Crouvoiser M., Goujard C., Debord T., Montagnier L. (1996) J. Immunol. 156, 3509–3520 [PubMed] [Google Scholar]
- 50. Marchetti G., Gori A., Casabianca A., Magnani M., Franzetti F., Clerici M., Perno C. F., Monforte A., Galli M., Meroni L. (2006) AIDS 20, 1727–1736 [DOI] [PubMed] [Google Scholar]
- 51. Massanella M., Negredo E., Pérez-Alvarez N., Puig J., Ruiz-Hernández R., Bofill M., Clotet B., Blanco J. (2010) AIDS 24, 959–968 [DOI] [PubMed] [Google Scholar]
- 52. Lori F. (2008) Curr. Opin. HIV AIDS 3, 99–103 [DOI] [PubMed] [Google Scholar]
- 53. Cron R. Q. (2001) DNA Cell Biol. 20, 761–767 [DOI] [PubMed] [Google Scholar]
- 54. Fagan S. C., Waller J. L., Nichols F. T., Edwards D. J., Pettigrew L. C., Clark W. M., Hall C. E., Switzer J. A., Ergul A., Hess D. C. (2010) Stroke 41, 2283–2287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Schlienger R. G., Bircher A. J., Meier C. R. (2000) Dermatology 200, 223–231 [DOI] [PubMed] [Google Scholar]
- 56. Fujii Y., Fujii K., Iwata S., Suzuki K., Azuma T., Saito K., Tanaka Y. (2006) Clin. Immunol. 119, 297–306 [DOI] [PubMed] [Google Scholar]
- 57. Kyttaris V. C., Wang Y., Juang Y. T., Weinstein A., Tsokos G. C. (2007) J. Immunol. 178, 1960–1966 [DOI] [PubMed] [Google Scholar]
- 58. Mehta J., Genin A., Brunner M., Scalzi L. V., Mishra N., Beukelman T., Cron R. Q. (2010) Arthritis Rheum. 62, 2499–2509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Midtvedt K. (2004) Transplant Proc. 36, 430S–433S [DOI] [PubMed] [Google Scholar]