Abstract
We previously identified and purified a human ATP-dependent chromatin remodeling complex with similarity to the Saccharomyces cerevisiae INO80 complex (Jin, J., Cai, Y., Yao, T., Gottschalk, A. J., Florens, L., Swanson, S. K., Gutierrez, J. L., Coleman, M. K., Workman, J. L., Mushegian, A., Washburn, M. P., Conaway, R. C., and Conaway, J. W. (2005) J. Biol. Chem. 280, 41207–41212) and demonstrated that it is composed of (i) a Snf2 family ATPase (hIno80) related in sequence to the S. cerevisiae Ino80 ATPase; (ii) seven additional evolutionarily conserved subunits orthologous to yeast INO80 complex subunits; and (iii) six apparently metazoan-specific subunits. In this report, we present evidence that the human INO80 complex is composed of three modules that assemble with three distinct domains of the hIno80 ATPase. These modules include (i) one that is composed of the N terminus of the hIno80 protein and all of the metazoan-specific subunits and is not required for ATP-dependent nucleosome remodeling; (ii) a second that is composed of the hIno80 Snf2-like ATPase/helicase and helicase-SANT-associated/post-HSA (HSA/PTH) domain, the actin-related proteins Arp4 and Arp8, and the GLI-Kruppel family transcription factor YY1; and (iii) a third that is composed of the hIno80 Snf2 ATPase domain, the Ies2 and Ies6 proteins, the AAA+ ATPases Tip49a and Tip49b, and the actin-related protein Arp5. Through purification and characterization of hINO80 complex subassemblies, we demonstrate that ATP-dependent nucleosome remodeling by the hINO80 complex is catalyzed by a core complex comprising the hIno80 protein HSA/PTH and Snf2 ATPase domains acting in concert with YY1 and the complete set of its evolutionarily conserved subunits. Taken together, our findings shed new light on the structure and function of the INO80 chromatin-remodeling complex.
Keywords: ATPases, Chromatin Remodeling, Gene Regulation, Histones, Nucleosome
Introduction
The Ino80 protein is a Snf2 family ATPase evolutionarily conserved from yeast to man (1, 2). The Ino80 protein was initially identified in the yeast Saccharomyces cerevisiae, where it was found to function as an integral component of a multisubunit ATP-dependent chromatin remodeling complex, called the INO80 complex, with roles in transcription, DNA replication, and DNA repair (1–4). We subsequently purified the human Ino80 ATPase (hIno80) and found that it is also a component of a multisubunit ATP-dependent chromatin remodeling complex possessing both similarities to and intriguing differences from the S. cerevisiae INO80 complex (5–7). Evidence suggests that, similar to its yeast counterpart, the human INO80 complex regulates transcription as well as DNA repair and replication processes (6, 8–11).
The hINO80 complex shares with the S. cerevisiae INO80 complex a set of eight evolutionarily conserved subunits, including the hIno80 Snf2-family ATPase, the AAA+ ATPases Tip49a and Tip49b (also called RuvBL1 and RuvBL2), actin-related proteins Arp4 (also called Baf53a), Arp5, and Arp8, and the Ies2 and Ies6 proteins; however, it lacks obvious orthologs of the remaining S. cerevisiae INO80 complex subunits Nhp10, Taf9, Ies1, Ies3, Ies4, and Ies5 (5). In their place, it contains several apparently metazoan-specific subunits, including the deubiquitinating enzyme Uch37 and the less well characterized Amida, INO80D (FLJ20309), INO80E (CCDC95 or FLJ90652), forkhead-associated domain containing MCRS1, and nuclear factor related to κB (NFRKB) proteins (5, 7). A Drosophila melanogaster INO80 complex with a collection of subunits similar to those of the hINO80 complex was recently described by Müller and co-workers (12). Both human and Drosophila INO80 complexes were found to include the GLI-Kruppel family zinc finger transcription factor YY1 (referred to as Pleiohomeotic (PHO) in flies) (6, 8, 12). Although YY1 and PHO were initially thought to be metazoan-specific subunits of the INO80 complex, the recently characterized Schizosaccharomyces pombe INO80 complex contains a GLI-Kruppel family zinc finger protein referred to as Iec1 (13), which may be orthologous to YY1 and PHO.
As part of our effort to understand the mechanism(s) by which the hINO80 complex regulates chromatin structure, we wish to define the architecture of the hINO80 complex and to learn how its individual subunits contribute to its ATP-dependent nucleosome remodeling activity. Ino80 proteins from yeast to humans share conserved Snf2-like ATPase/helicase and helicase-SANT-associated/Post-HSA (HSA/PTH)2 domains flanked by nonconserved N- and C-terminal regions (14). Previous studies have established that the catalytic activity of the Ino80 Snf2-like ATPase domain is required for ATP-dependent nucleosome remodeling by the S. cerevisiae INO80 complex (4). The Ino80 ATPase/helicase domain has also been proposed to provide a binding site for the AAA+ ATPases and Arp5, based on evidence (i) that the corresponding domain of a related Snf2-like ATPase, Swr1, is required for binding to the AAA+ ATPases and (ii) that binding of Arp5 to S. cerevisiae Ino80 depends on the AAA+ ATPases (15, 16). The HSA/PTH domain of S. cerevisiae Ino80 is also required for ATP-dependent nucleosome remodeling and serves as a docking site for actin and actin-related proteins Arp4 and Arp8 (17, 18). S. cerevisiae INO80 complexes lacking one or more of the actin-related proteins or the AAA+ ATPases exhibit greatly reduced nucleosome remodeling activities, suggesting that these proteins either participate directly in nucleosome remodeling or are required for proper assembly of active complexes (16, 18). We note that we have not yet determined whether actin is a bona fide subunit of the hINO80 complex. Our current evidence suggests that actin is present in our most highly purified preparations of the hINO80 complex in significantly smaller amounts than the actin-related proteins Arp4, Arp5, and Arp8.3
Although the information described above has provided useful preliminary insights into the organization of the INO80 complex and the functions of some of its subunits, major questions remain. In particular, the architecture of the conserved portion of INO80 complexes has not been fully defined, there is no information about which domain(s) of the hIno80 protein govern assembly of the metazoan-specific subunits into the hINO80 complex, and, importantly, there is no information about the potential contributions of the metazoan-specific subunits to the ATP-dependent nucleosome remodeling activity of the hINO80 complex.
To define further the organization of the hINO80 complex and to explore the contributions of various domains of the hIno80 protein and of the evolutionarily conserved and metazoan-specific subunits to its ATP-dependent nucleosome remodeling activity, we have carried out a systematic structure-function analysis of the hIno80 ATPase. Our findings reveal that ATP-dependent nucleosome remodeling in vitro can be carried out by a hINO80 complex subassembly composed of the hIno80 HSA/PTH and Snf2 ATPase domains acting in concert with YY1 and the seven evolutionarily conserved subunits of the complex. Furthermore, we observe that all six metazoan-specific subunits of the hINO80 complex assemble together with an N-terminal hIno80 region to form a module that is not essential for ATP-dependent nucleosome remodeling. Taken together, our findings shed new light on the roles of the hIno80 ATPase and its associated subunits in chromatin remodeling.
EXPERIMENTAL PROCEDURES
Antibodies
Rabbit polyclonal anti-FLAG antibody (7425) and anti-FLAG (M2) agarose were obtained from Sigma; rabbit polyclonal anti-MCRS1 antibody (11362-1-AP) and anti-NFRKB antibody (A301-459A) were obtained from ProteinTech Group, Inc. and Bethyl Laboratory, Inc., respectively. Mouse anti-Tip49a monoclonal antibodies (ab51500) and rabbit anti-Tip49b polyclonal antibodies (ab36569) were purchased from Abcam. Rabbit anti-Arp5 polyclonal antibodies were raised against recombinant Arp5 expressed in Escherichia coli (Cocalico Biologicals, Reamstown, PA) and were purified using Melon Gel IgG Purification Resin from Pierce, Thermo Scientific.
Purification of hINO80 Complexes Containing Full-length and Mutant hIno80 ATPase
Human INO80 complexes containing full-length hIno80 ATPase were purified from HEK293FRT cells stably expressing FLAG-INO80E (CCDC95/FLJ90652) (5). To generate cell lines expressing mutant hIno80 ATPase, a single point mutation was introduced into the hIno80 coding sequence in pCMV-3×FLAG-hIno80 (8) to change proline 107 to leucine to match the NCBI reference sequence NP_060023.1. A catalytically dead version of hIno80 was generated by mutating glutamate 653 to glutamine using the QuikChange II XL Site-directed Mutagenesis kit (Stratagene). Deletion mutants of hIno80 and catalytically dead hIno80 (hIno80 E653Q) were designed based on the secondary structure prediction provided by DomPred (19) and amplified by PCR amplification. Full-length and mutant hIno80 cDNAs were subcloned into pcDNA5-FRT in-frame with an N-terminal FLAG epitope tag, and stable HEK293-FRT cell lines expressing hIno80 and its derivatives were generated as described (20). To isolate cells stably expressing detectable levels of hIno80 derivatives, hygromycin-resistant clones were initially screened by PCR. Clones having the expected amplicons were expanded into a single roller bottle and screened for expression of FLAG-tagged hIno80 derivatives by Western blotting. Clones expressing the highest levels of each hIno80 protein were expanded to 20 roller bottles and used for preparation of nuclear extracts (21). FLAG-hIno80 derivatives and associated proteins were purified by anti-FLAG agarose immunoaffinity chromatography as described (20). The relative concentrations of purified complexes were estimated by semiquantitative Western blotting using anti-Arp5, anti-Tip49a, anti-Tip49b, and anti-FLAG antibodies, with Alexa Fluor 680-conjugated anti-rabbit IgG (Molecular Probes) and IRDye800-conjugated anti-mouse IgG (Rockland, Gilbertsville, PA) as secondary antibodies. All antibody dilutions were made in casein-blocking solution (LiCor, Lincoln, NE). Signal intensities were analyzed using the Odyssey infrared imaging system (LiCor). In addition, the molar concentration of Arp5 in purified complexes was determined by semiquantitative Western blotting with anti-Arp5 antibody, using purified recombinant Arp5 as a standard.
Mass Spectrometry
Identification of proteins was accomplished using a modification of the multidimensional protein identification technology (MudPIT) procedure (22, 23). Fully automated MudPIT runs were carried out on the electrosprayed peptides, as described (23). Tandem mass spectra were interpreted using SEQUEST (24) against a data base of 30,709 human proteins (downloaded from NCBI on July 2, 2009), 160 sequences from common contaminants (human keratins, IgGs, proteolytic enzymes), eight hINO80 mutant sequences, and, to estimate false discovery rates, 30,894 randomized amino acid sequences derived from each nonredundant protein entry. Peptide/spectrum matches were sorted and selected using DTASelect (25). To estimate relative protein levels, normalized spectral abundance factors (NSAFs) were calculated for each detected protein (26–29).
Chromatin Remodeling Assays
A 216-bp DNA fragment (601-Gal4) was generated by PCR from pGEM-3Z-601-G4, a derivative of pGEM-3Z-601 (30). PCR was performed in the presence of [α-32P]dCTP with the primers 5′-ACAGGATGTATATATCTGACACGTGCCTGG and 5′-AATACTCAAGCTTGGATGCCTGCAG. Mononucleosomes were reconstituted on the labeled DNA by dilution transfer from HeLa short oligonucleosomes as described (20, 31). hINO80 complex or hINO80 complex subassemblies were incubated at 37 °C with 0.5 μl of nucleosomes (∼2 fmol of labeled mononucleosomes and ∼50 fmol of HeLa short oligonucleosomes) in buffer containing 20 mm Hepes-NaOH (pH 7.9), 50 mm NaCl, 5 mm MgCl2, 1 mm dithiothreitol (DTT), 1 mm phenylmethanesulfonyl fluoride (PMSF), 0.1 mg/ml bovine serum albumin (BSA), 5% glycerol, 0.02% Nonidet P-40, 0.02% Triton X-100, and 1 mm ATP (USB/Affymetrix). After 60 min, reactions were terminated by the addition of a large excess of unlabeled nucleosomes and DNA, and reaction products were analyzed as described (20).
ATPase Assays
10-μl reaction mixtures contained 20 mm Tris-HCl (pH 7.5), 60 mm NaCl, 6.6 mm MgCl2, 0.8 mm EDTA, 0.015% Nonidet P-40, 2.5% glycerol, 0.1 mg/ml BSA, 1 mm DTT, 1 mm PMSF, 2 μm ATP, 2 μCi of [α-32P] ATP (3000 Ci/mmol, PerkinElmer), with or without 450 ng of closed circular plasmid DNA (pcDNA5), and the indicated hINO80 complex. After incubation at 30 °C for various times, a 0.5-μl aliquot of each reaction mixture was spotted onto a cellulose polyethyleneimine thin layer chromatography plate (J.T. Baker). Plates were developed with 0.375 m potassium phosphate (pH 3.5), and reaction products were detected and quantitated using a Typhoon PhosphorImager (GE Healthcare).
RESULTS
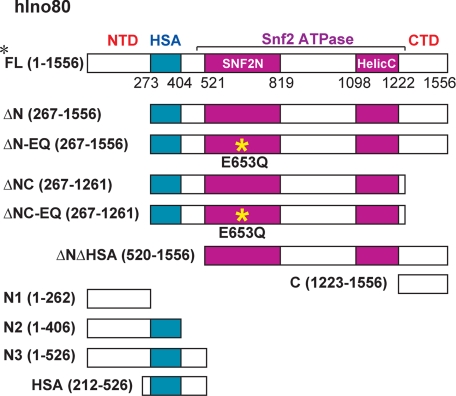
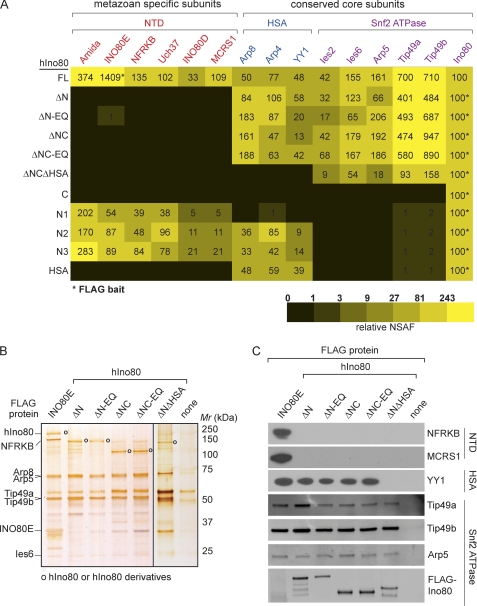
To begin to investigate the role of the hIno80 ATPase and individual subunits of the hINO80 complex in reconstitution of ATP-dependent nucleosome remodeling, we generated a series of human embryonic kidney (HEK) 293 cell lines stably expressing the N-terminally FLAG-tagged hIno80 mutants shown in Fig. 1. These mutants include deletion mutants lacking the N-terminal domain (NTD), the HSA/PTH domain, and/or the C-terminal domain (CTD), with or without a DEAD/H box point mutation (E653Q) predicted to interfere with hIno80 ATPase activity. In addition, we generated 293 cell lines expressing hIno80 fragments that contain the NTD alone, the NTD and HSA/PTH domain, and the CTD alone. Because we observed that neither full-length FLAG-hIno80 nor FLAG-hIno80 mutants containing both the N terminus and Snf2 ATPase domains could be stably expressed in HEK293 cells at levels sufficient for subsequent analyses, we purified intact hINO80 complexes for this study from an HEK293 cell line stably expressing FLAG-tagged INO80 subunit INO80E (5). Nuclear extracts prepared from HEK293 cells expressing FLAG-INO80E or the FLAG-hIno80 mutants were subjected to anti-FLAG agarose immunoaffinity chromatography, and proteins present in anti-FLAG agarose eluates were identified by MudPIT mass spectrometry (Fig. 2A) and analyzed by SDS-PAGE (Fig. 2B) and Western blotting (Fig. 2C).
FIGURE 1.
Schematic diagram showing the domain organization of the hIno80 ATPase and hIno80 mutants used in this study. HSA, HSA/PTH domain; Snf2N, Snf2 family N terminus; HelicC, helicase superfamily C terminus, conserved domains in the Snf2 ATPase domain of hIno80. Numbers refer to positions in the amino acid sequence of the hIno80 protein (accession number NP_060023.1). Yellow asterisk shows the position of the E653Q mutation used to inactivate the hIno80 ATPase.
FIGURE 2.
Modular organization of the hINO80 complex. A, MudPIT analysis of intact hINO80 complex and hINO80 complex subassemblies. The table shows hINO80 subunits detected by MudPIT mass spectrometry in complexes containing full-length hIno80 or the indicated hIno80 mutants. Red, subunits associating with the hIno80 NTD; blue, subunits associating with the hIno80 HSA/PTH domain; purple, subunits associating with the Snf2 ATPase domain. The subunit used as FLAG-bait for purification of each complex is indicated with an asterisk. NSAFs provide a rough estimate of the relative amounts of each protein detected in a MudPIT data set (26–29); relative NSAFs shown in the table were calculated by normalizing the NSAF for each subunit to the NSAF for hIno80 or hIno80 derivative in each complex. B, hINO80 complex and hINO80 subassemblies analyzed by SDS-PAGE and silver staining. Open circles indicate the positions of hIno80 and hIno80 derivatives. Lanes separated by black line are from separate gels. C, hINO80 complex and hINO80 subassemblies analyzed by Western blotting with anti-FLAG antibodies to detect FLAG-hIno80 fragments or with antibodies against the indicated subunits.
The results of these experiments argue that the hINO80 complex is composed of at least three modules and can be summarized as follows. The metazoan-specific subunits Amida, INO80E, INO80D, NFRKB, Uch37, and MCRS1 were lost from INO80 complexes containing hIno80ΔN, which lacks the first 266 amino acids of hIno80 but retains the HSA/PTH, Snf2 ATPase, and CTDs. Arguing that the hIno80 NTD is both necessary and sufficient to nucleate a hINO80 complex subassembly containing all of the metazoan-specific subunits, complexes containing just the hIno80 NTD (fragment N1) included each of the 6 metazoan-specific subunits (Fig. 2A). In contrast, the 295-amino acid nonconserved CTD fragment of hIno80 did not copurify with any of the INO80 subunits. In addition, deletion of the CTD from hIno80 did not result in the loss of any subunits from the complex. Complexes with either hIno80ΔN or a hIno80 fragment lacking both the NTD and the CTD (hIno80ΔNC) contained all of the conserved subunits, including actin-related proteins Arp4, Arp5, Arp8, the AAA+ ATPases Tip49a and Tip49b, Ies2, Ies6, and YY1, as did complexes containing the catalytically inactive hIno80ΔN EQ or hIno80ΔNC EQ mutants.
Arguing that the HSA/PTH domain nucleates assembly of a module containing YY1 and actin-related proteins Arp4 and Arp8, deletion of the HSA/PTH domain from hIno80 ΔNTD led to loss of YY1, Arp4, and Arp8, whereas all hINO80 fragments that include the HSA/PTH domain copurified with YY1, Arp4, and Arp8. These findings are consistent with previous results indicating that in the S. cerevisiae INO80 complex, the Ino80 HSA/PTH domain serves as a docking site for actin and actin-related proteins Arp4 and Arp8 (17, 18).
Finally, we observed that the remaining evolutionarily conserved subunits Ies2, Ies6, Arp5, and the AAA+ ATPases Tip49a and Tip49b are all capable of assembling into a module that includes just the hIno80 Snf2 ATPase domain. Although none of these subunits had previously been shown to assemble with a specific Ino80 domain, our observation that Arp5, Tip49a, and Tip49b are among the subunits associated with the hIno80 Snf2 ATPase domain is consistent with previous data suggesting that, in the yeast SWR1 remodeling complex, binding of the actin-related protein Arp6 and the yeast orthologs of Tip49a and Tip49b to Swr1 depends on the presence of an intact Swr1 Snf2-like ATPase domain (15).
In a previous study, we observed that, like the S. cerevisiae INO80 complex, the hINO80 complex is capable of catalyzing both ATP-dependent nucleosome sliding and DNA-dependent ATPase in vitro (5). To begin to investigate the potential roles of individual subunits of the hINO80 complex in these activities, we tested the purified hINO80 complex subassemblies generated above for their abilities to catalyze ATP-dependent nucleosome remodeling and DNA-stimulated ATP hydrolysis.
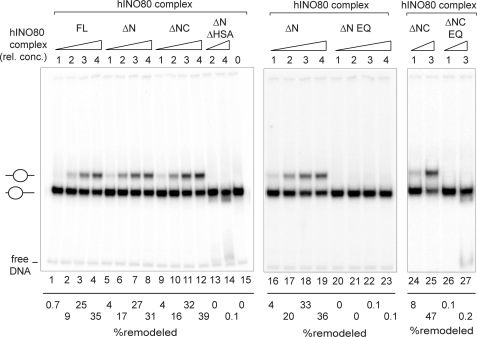
To assay ATP-dependent nucleosome remodeling, the FLAG-immunopurified hINO80 complex or hINO80 complex subassemblies shown in Fig. 2 were incubated in the presence of ATP with mononucleosomes assembled on a 216-bp, 32P-labeled DNA fragment with a 601-nucleosome positioning sequence near one end of the DNA. Following reactions, HeLa oligonucleosomes and free DNA were added to reaction mixtures as competitor to remove nucleosome- or DNA-binding proteins that might alter mononucleosome electrophoretic mobility, and reaction products were analyzed on native polyacrylamide gels.
The electrophoretic mobility in a native gel of a DNA fragment containing a nucleosome at one end is greater than that of the same DNA fragment containing a more centrally located nucleosome. The majority of nucleosomes used in our assays are initially located on the laterally positioned nucleosome positioning sequence; thus, nucleosome sliding toward the middle of the DNA can be readily detected by a decrease in electrophoretic mobility of the labeled nucleosome.
As shown in Fig. 3, lanes 1–4, the intact hINO80 complex is capable of sliding laterally positioned nucleosomes to a more central position in a dose-dependent manner. In addition, subassemblies containing hIno80ΔN (lanes 5–8) or hIno80ΔNC (lanes 9–12) and all of the conserved subunits exhibited nucleosome sliding activities very similar to that of the intact hINO80 complex, indicating that neither the NTD and CTD of hIno80 nor any of the metazoan-specific subunits is essential for nucleosome remodeling. Nucleosome sliding was strictly dependent on the presence of a catalytically active hIno80 Snf2 ATPase because complexes containing hIno80ΔN EQ or hIno80ΔNC EQ were inactive (compare lanes 16–19 with 20–23, and 24 and 25 with 26 and 27). Arguing that the hIno80 HSA/PTH domain and/or Arp4, Arp8, and YY1 are required for nucleosome sliding, subassemblies containing the hIno80 ΔNΔHSA and Ies2, Ies6, Arp5, Tip49a, and Tip49b, but lacking the HSA/PTH domain and Arp4, Arp8, and YY1 were not active (lanes 13 and 14).
FIGURE 3.
Nucleosome remodeling activities of hINO80 complex and hINO80 complex subassemblies. Nucleosome sliding assays were performed with the indicated complexes as described under “Experimental Procedures.” hINO80 complexes at a relative concentration (rel. conc.) of 4 contain ∼400 fmol of Arp5, equivalent to the amount of material loaded onto the gels shown in Fig. 2, B and C. Percent remodeled nucleosomes is equivalent to the amount of radioactivity in the upper band, corresponding to the centrally positioned remodeled nucleosome, divided by the total amount of radioactivity in the remodeled nucleosome and the prominent lower band, which corresponds to the laterally positioned, starting nucleosome (see lane 15). The faint band at the bottom of the gels is due to a small amount of free DNA in the nucleosome preparation. The data in lanes 1–15, 16–23, and 24–27 are from three separate experiments; quantitative comparisons should be made only within an individual experiment.
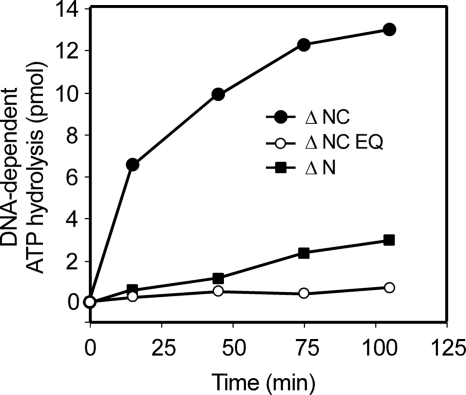
In parallel experiments, the intact hINO80 complex and hINO80 complex subassemblies were assayed for their abilities to catalyze DNA-dependent ATP hydrolysis in the presence of closed circular plasmid DNA (Table 1 and Fig. 4). The relative DNA-dependent ATPase activities of the intact hINO80 complex and complexes containing hIno80ΔN, hIno80ΔN EQ, hIno80ΔNC EQ, and hIno80ΔNΔHSA mirrored their relative activities in nucleosome sliding. Surprisingly, however, complexes containing hIno80ΔNC exhibited substantially higher DNA-dependent ATPase activity than intact complexes or hINO80ΔN complexes. Because complexes containing hIno80ΔNC EQ did not exhibit significant DNA-dependent ATPase, this activity depends on a catalytically active hIno80 Snf2-like ATPase. Although future studies will be required to define the underlying mechanisms, these observations suggest that the hIno80 CTD might function in some contexts as a negative regulator of ATP hydrolysis by the hINO80 complex. In addition, although our findings argue that the ATPase activity of hIno80 is required for nucleosome remodeling activity, they also suggest that ATPase activity may not be strictly coupled to nucleosome remodeling.
TABLE 1.
DNA-dependent ATPase activity associated with wild-type hINO80 complexes and hINO80 complex subassemblies
Reactions were performed with or without DNA as described under “Experimental Procedures” and contained 1×, 2×, 3×, or 4× wild-type hINO80, hINO80ΔN, hINO80ΔN EQ, or hINO80ΔNC complexes or 4× hINO80ΔNC EQ or hINO80ΔNΔHSA complexes, where 1× contains ∼100 fmol of Arp5. Aliquots of each reaction were removed at various time points between 15 and 120 min for measurement of ATP hydrolysis. Values shown for the hINO80ΔNC and hINO80ΔNΔHSA complexes are based on data from two independent reactions. Values for wild-type hINO80, hINO80ΔN, hINO80ΔN EQ, or hINO80ΔNC complexes are based on measurements from at least three independent reactions and include only data points in which less than ∼25% of the starting ATP was hydrolyzed.
| INO80 complex | DNA-dependent ATP hydrolysis (pmol/min per 1× complex) |
|---|---|
| Wild type | 0.007 ± 0.001 |
| Ino80ΔN | 0.007 ± 0.003 |
| Ino80ΔN EQ | <0.001 |
| Ino80ΔNC | 0.095 ± 0.021 |
| Ino80ΔNC EQ | <0.001 |
| Ino80 ΔNΔHSA | <0.001 |
FIGURE 4.
Modulation of DNA-dependent ATPase activity by the hIno80 CTD. Representative DNA-dependent ATPase assays were performed as described under “Experimental Procedures.” Each reaction included ∼400 fmol of Arp5, equivalent to the amount of material loaded onto the gels shown in Fig. 2, B and C.
DISCUSSION
In summary, our findings suggest that the hINO80 complex is composed of at least three modules that assemble on distinct regions of the hIno80 protein. Two of these modules assemble on the conserved HSA/PTH and ATPase domains of the hIno80 protein and, together, are sufficient to reconstitute the ATP-dependent nucleosome remodeling activity of the hINO80 complex. Associated with the hIno80 ATPase domain are a subset of the conserved subunits, including the AAA+ ATPases Tip49a and Tip49b, Ies2 and Ies6, and the actin-related protein Arp5, whereas the remaining conserved subunits Arp4, Arp8, and YY1 assemble on the HSA/PTH domain. HSA/PTH domains are found in multiple chromatin remodeling complexes from yeast to human and have been shown to function as docking sites for actin-related proteins (17, 18, 32). Evidence from this and prior studies argues that HSA/PTH domains are required for maximal ATPase and/or nucleosome remodeling activities catalyzed by HSA/PTH domain-containing Snf2 family ATPases (15, 17, 18); however, the exact function(s) of HSA/PTH domains and of their associated proteins are not known. Based on the observations (i) that yeast INO80 complexes lacking actin, Arp4, and Arp8 are defective in DNA binding, ATPase, and nucleosome remodeling activities (18), and (ii) that Arp4 and Arp8 can bind histones (18, 33), it is possible that the INO80 HSA/PTH-containing module may contribute to recognition of DNA and/or nucleosome substrates. In this regard, it is noteworthy that YY1 is a DNA-binding protein that in at least some contexts can target the hINO80 complex to YY1-responsive elements in cells (6, 13). Whether the YY1 DNA binding activity contributes to the nucleosome remodeling or ATPase activities of the INO80 complex remains to be determined.
The hIno80 NTD nucleates assembly of the third hINO80 module, which includes all of the metazoan-specific subunits. Notably, although this hIno80 region is not conserved from yeast to humans, comparison of the sequences of human and insect Ino80 proteins reveals several conserved sequence blocks (data not shown), and in the future it will be of interest to address the possibility that these sequences direct assembly of the metazoan-specific module. Our finding that the hIno80 NTD and the metazoan-specific subunits that assemble on it are dispensable for the ATPase and nucleosome sliding activities of the hINO80 complex suggests that these subunits are likely to have regulatory roles in vivo and paves the way for future studies on their contribution(s) to the function of the hINO80 complex in cells.
The hIno80 protein also includes an ∼330-amino acid CTD region that is apparently conserved only in mammals. The hIno80 CTD does not bind stably to any of the known subunits of the hINO80 complex, nor does its deletion affect ATP-dependent nucleosome remodeling in our assays. Intriguingly, however, deletion of this domain led to an increase in DNA-dependent ATP hydrolysis by the hINO80 complex, raising the possibility that this domain might contribute in some way to regulation of the activity of the hINO80 complex. In this regard, it is noteworthy that the hIno80 CTD has been shown to be a target for cell cycle-dependent phosphorylation (34), and, in the future, it will be of interest to determine whether phosphorylation of this domain regulates activity(s) of the hINO80 complex.
Acknowledgments
We thank Maria Katt, Valerie Neubauer, and Tari J. Parmely for invaluable help with tissue culture.
This work was supported, in whole or in part, by National Institutes of Health Grant GM41628 through the NIGMS (to R. C. C. and J. W. C.).
L. Chen, Y. Cai, J. Jin, L. Florens, S. K. Swanson, M. P. Washburn, J. W. Conaway, and R. C. Conaway, unpublished data.
- HSA/PTH
- Snf2-like ATPase/helicase and helicase-SANT-associated/post-HSA
- CTD
- C-terminal domain
- MudPIT
- multidimensional protein identification technology
- NSAF
- normalized spectral abundance factors
- NTD
- N-terminal domain.
REFERENCES
- 1. Bao Y., Shen X. (2007) Mutat. Res. 618, 18–29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Conaway R. C., Conaway J. W. (2009) Trends Biochem. Sci. 34, 71–77 [DOI] [PubMed] [Google Scholar]
- 3. Ebbert R., Birkmann A., Schüller H. J. (1999) Mol. Microbiol. 32, 741–751 [DOI] [PubMed] [Google Scholar]
- 4. Shen X., Mizuguchi G., Hamiche A., Wu C. (2000) Nature 406, 541–544 [DOI] [PubMed] [Google Scholar]
- 5. Jin J., Cai Y., Yao T., Gottschalk A. J., Florens L., Swanson S. K., Gutiérrez J. L., Coleman M. K., Workman J. L., Mushegian A., Washburn M. P., Conaway R. C., Conaway J. W. (2005) J. Biol. Chem. 280, 41207–41212 [DOI] [PubMed] [Google Scholar]
- 6. Cai Y., Jin J., Yao T., Gottschalk A. J., Swanson S. K., Wu S., Shi Y., Washburn M. P., Florens L., Conaway R. C., Conaway J. W. (2007) Nat. Struct. Mol. Biol. 14, 872–874 [DOI] [PubMed] [Google Scholar]
- 7. Yao T., Song L., Jin J., Cai Y., Takahashi H., Swanson S. K., Washburn M. P., Florens L., Conaway R. C., Cohen R. E., Conaway J. W. (2008) Mol. Cell 31, 909–917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Wu S., Shi Y., Mulligan P., Gay F., Landry J., Liu H., Lu J., Qi H. H., Wang W., Nickoloff J. A., Wu C., Shi Y. (2007) Nat. Struct. Mol. Biol. 14, 1165–1172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hur S. K., Park E. J., Han J. E., Kim Y. A., Kim J. D., Kang D., Kwon J. (2010) Cell. Mol. Life Sci. 67, 2283–2296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Park E. J., Hur S. K., Kwon J. (2010) Biochem. J. 431, 179–187 [DOI] [PubMed] [Google Scholar]
- 11. Jiang Y., Wang X., Bao S., Guo R., Johnson D. G., Shen X., Li L. (2010) Proc. Natl. Acad. Sci. U.S.A. 107, 17274–17279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Klymenko T., Papp B., Fischle W., Köcher T., Schelder M., Fritsch C., Wild B., Wilm M., Müller J. (2006) Genes Dev. 20, 1110–1122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hogan C. J., Aligianni S., Durand-Dubief M., Persson J., Will W. R., Webster J., Wheeler L., Mathews C. K., Elderkin S., Oxley D., Ekwall K., Varga-Weisz P. D. (2010) Mol. Cell. Biol. 30, 657–674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Flaus A., Martin D. M., Barton G. J., Owen-Hughes T. (2006) Nucleic Acids Res. 34, 2887–2905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Wu W. H., Alami S., Luk E., Wu C. H., Sen S., Mizuguchi G., Wei D., Wu C. (2005) Nat. Struct. Mol. Biol. 12, 1064–1071 [DOI] [PubMed] [Google Scholar]
- 16. Jónsson Z. O., Jha S., Wohlschlegel J. A., Dutta A. (2004) Mol. Cell 16, 465–477 [DOI] [PubMed] [Google Scholar]
- 17. Szerlong H., Hinata K., Viswanathan R., Erdjument-Bromage H., Tempst P., Cairns B. R. (2008) Nat. Struct. Mol. Biol. 15, 469–476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Shen X., Ranallo R., Choi E., Wu C. (2003) Mol. Cell 12, 147–155 [DOI] [PubMed] [Google Scholar]
- 19. Marsden R. L., McGuffin L. J., Jones D. T. (2002) Protein Sci. 11, 2814–2824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Cai Y., Jin J., Gottschalk A. J., Yao T., Conaway J. W., Conaway R. C. (2006) Methods 40, 312–317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Dignam J. D., Lebovitz R. M., Roeder R. G. (1983) Nucleic Acids Res. 11, 1475–1489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Washburn M. P., Wolters D., Yates J. R., 3rd (2001) Nat. Biotechnol. 19, 242–247 [DOI] [PubMed] [Google Scholar]
- 23. Florens L., Washburn M. P. (2006) Methods Mol. Biol. 328, 159–175 [DOI] [PubMed] [Google Scholar]
- 24. Eng J. K., McCormack A. L., Yates J. R., 3rd (1994) J. Am. Soc. Mass Spectrom. 5, 976–989 [DOI] [PubMed] [Google Scholar]
- 25. Tabb D. L., McDonald W. H., Yates J. R., 3rd (2002) J. Proteome Res. 1, 21–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Florens L., Carozza M. J., Swanson S. K., Fournier M., Coleman M. K., Workman J. L., Washburn M. P. (2006) Methods 40, 303–311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Paoletti A. C., Parmely T. J., Tomomori-Sato C., Sato S., Zhu D., Conaway R. C., Conaway J. W., Florens L., Washburn M. P. (2006) Proc. Natl. Acad. Sci. U.S.A. 103, 18928–18933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Zybailov B., Mosley A. L., Sardiu M. E., Coleman M. K., Florens L., Washburn M. P. (2006) J. Proteome Res. 5, 2339–2347 [DOI] [PubMed] [Google Scholar]
- 29. Zhang Y., Wen Z., Washburn M. P., Florens L. (2010) Anal. Chem. 82, 2272–2281 [DOI] [PubMed] [Google Scholar]
- 30. Lowary P. T., Widom J. (1998) J. Mol. Biol. 276, 19–42 [DOI] [PubMed] [Google Scholar]
- 31. Owen-Hughes T., Utley R. T., Steger D. J., West J. M., John S., Côté J., Havas K. M., Workman J. L. (1999) Methods Mol. Biol. 119, 319–331 [DOI] [PubMed] [Google Scholar]
- 32. Dion V., Shimada K., Gasser S. M. (2010) Curr. Opin. Cell Biol. 22, 383–391 [DOI] [PubMed] [Google Scholar]
- 33. Harata M., Oma Y., Mizuno S., Jiang Y. W., Stillman D. J., Wintersberger U. (1999) Mol. Biol. Cell 10, 2595–2605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Dephoure N., Zhou C., Villén J., Beausoleil S. A., Bakalarski C. E., Elledge S. J., Gygi S. P. (2008) Proc. Natl. Acad. Sci. U.S.A. 105, 10762–10767 [DOI] [PMC free article] [PubMed] [Google Scholar]