Abstract
The feasibility of using carbohydrate-based vaccines for the immunotherapy of cancer is being actively explored at the present time. Although a number of clinical trials have already been conducted with glycoconjugate vaccines, the optimal design and composition of the vaccines has yet to be determined. Among the candidate antigens being examined is Lewisy (Ley), a blood group-related antigen that is overexpressed on the majority of human carcinomas. Using Ley as a model for specificity, we have examined the role of epitope clustering, carrier structure, and adjuvant on the immunogenicity of Ley conjugates in mice. A glycolipopeptide containing a cluster of three contiguous Ley-serine epitopes and the Pam3Cys immunostimulating moiety was found to be superior to a similar construct containing only one Ley-serine epitope in eliciting antitumor cell antibodies. Because only IgM antibodies were produced by this vaccine, the effect on immunogenicity of coupling the glycopeptide to keyhole limpet hemocyanin was examined; although both IgM and IgG antibodies were formed, the antibodies reacted only with the immunizing structure. Reexamination of the clustered Ley-serine Pam3Cys conjugate with the adjuvant QS-21 resulted in the identification of both IgG and IgM antibodies reacting with tumor cells, thus demonstrating the feasibility of an entirely synthetic carbohydrate-based anticancer vaccine in an animal model.
A long-time goal of tumor immunology has been to co-opt the potentially formidable capacities of the human immune system to attack cancer. With this end in mind, specific immunotherapy has been widely attempted with the passive administration of antitumor monoclonal antibodies (1). Recently, attention has also turned to active immunization approaches using a variety of antigens, including whole cells, cell extracts, and defined antigens. Included in the last category are various carbohydrate structures that have been identified as being preferentially expressed in cancers. Examples include gangliosides, e.g., GM2 (2), GD3 (3), and fucosyl GM1 (4), and blood group-related specificities, e.g., Lewisy (Ley) (5) and globo H (6–8), and mucin-core structures, e.g., T-F (9), and sTn (9, 10). Preclinical studies in mice and clinical studies in patients have demonstrated the ability of vaccines containing these structures, usually as carbohydrate-protein conjugates, to induce specific antitumor cell antibody responses.
Ley is a carbohydrate specificity belonging to the A, B, H, Lewis blood group family that is overexpressed on the majority of carcinomas, including ovary, pancreas, prostate, breast, colon, and non-small cell lung cancers (11). In ovarian cancers, Ley is preferentially expressed in serous and endometrial cancers (12). Based on this pattern of expression and the results of a preclinical study in mice demonstrating the immunogenicity of a Ley oligosaccharide-keyhole limpet hemocyanin (KLH) conjugate (13), we recently conducted a Phase I clinical trial in ovarian cancer patients using this conjugate together with the immunological adjuvant QS21 (5). This trial was successful in that the vaccine induced antibody responses in the majority of the patients (18/24) and that the vaccine was well tolerated with no adverse effects related to autoimmunity being observed. However, most of the antibody responses were modest and were mainly restricted to immunoglobulins of the IgM class. With the goal of improving on these results, we have examined the effect of varying epitope clustering, carrier structure, and adjuvant on IgM and IgG production in mice. The rationale for studying epitope clustering is based on the observation that in mucins, which express Ley (and many other carbohydrate structures), the epitopes are carried on adjacent serine or threonine residues in blocks or clusters (14, 15) and that such clusters are often the preferred targets of antitumor cell monoclonal antibodies (16, 17). This study shows that all three parameters influence the immunogenicity of Ley-containing vaccines.
Materials and Methods
Antigens.
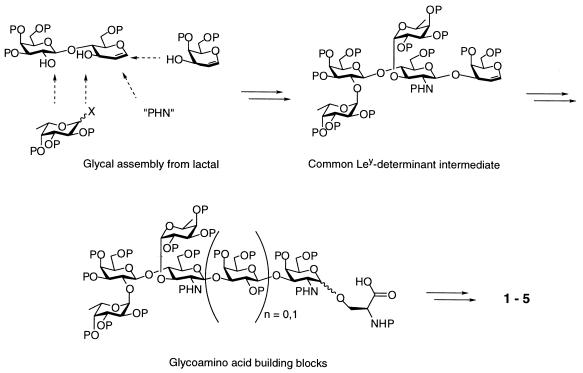
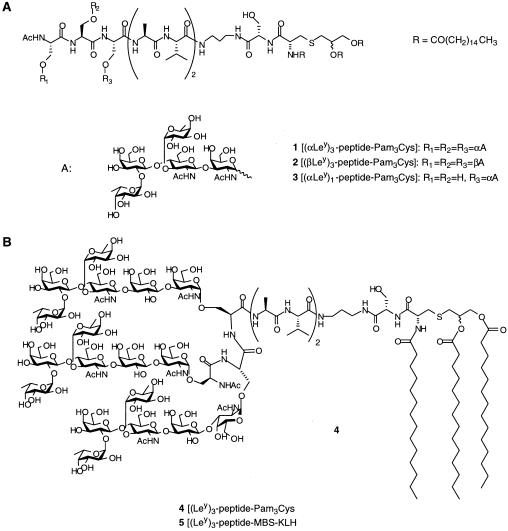
Fig. 1 illustrates the general approach to the synthesis of the glycopeptides used in this study, in which a protected oligosaccharyl-serine moiety is prepared and used to synthesize compounds 1-5. Structures 1–3 (Fig. 2A), consisting of three (1 and 2) or one (3) Ley pentasaccharide attached to serine residues of a heptapeptide terminated with a Pam3Cys moiety, were synthesized via glycal assembly (18–21). In compounds 1 and 3, the pentasaccharide was linked to the serine of the peptide through an α-linkage, whereas compound 2 contained a β-linkage. The synthesis of compound 4 (an analog of compound 1 with three hexasaccharides attached to the peptide; Fig. 2B) has been reported (20, 21). The conjugate 5 (Fig. 2B) in which the (Ley)3-substituted peptide was attached to KLH was synthesized from a thiol-terminated version of the glycopeptide using maleimidobenzoyl-N-hydroxysuccinimide ester (MBS) as reported (22). This compound reacted with an anti-Ley monoclonal antibody (3S193), showing that the Ley epitope was retained (data not shown). The conjugate was found to contain an average of 111 mol of glycopeptide/mol of KLH. Ley-Cer (13), the Ley-expressing mucin (23), and Ley-BSA (13) have been described.
Figure 1.
Schematic showing general route to synthesis of glycopeptides. P, protecting groups.
Figure 2.
Structures of Ley vaccines used in this study. (A) Structures of three glycolipopeptide constructs bearing three (1 and 2) or one (3) Ley pentasaccharide. These structures are abbreviated as (αLey)3-peptide-Pam3Cys (1), (βLey)3-peptide-Pam3Cys (2), and (αLey)1-peptide-Pam3Cys (3), respectively. (B) Structures of clustered Ley construct bearing three Ley hexasaccharides linked to Pam3Cys (4) or KLH (5).
Animals and Vaccinations.
Groups of five or eight mice (female; BALB/c for immunization with compounds 1, 2, and 3 or C57/BL6 for immunization with compounds 4 and 5) were immunized subcutaneously in two sites with amounts of the constructs containing 10 μg of carbohydrate at 0, 1, 2, and 3 weeks. Mice were bled 10 days after the final immunizations. QS21 (10 μg) was included in some of the vaccines, as indicated in Results.
ELISA.
ELISA was performed as described previously (5, 13). The data reported in Fig. 3 used p-nitrophenylphosphate as the substrate and detection of the product at 405 nm, whereas the remainder of the experiments used fluorescein diphosphate (Molecular Probes) as the substrate and detection in a Wallac (Gaithersburg, MD) plate-reading fluorimeter (model 1420). For determination of the Ig class of the responses, anti-IgG (γ-chain) or anti-IgM (μ-chain specific) reagents from Southern Biotechnology Associates were used as the second antibody.
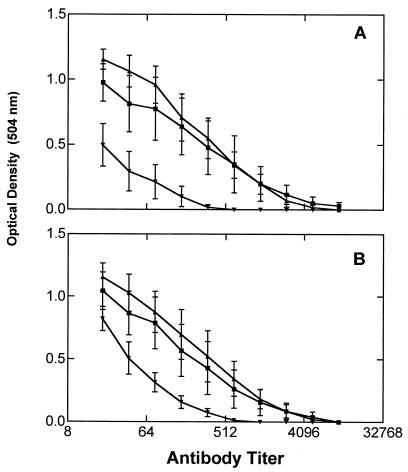
Figure 3.
Reactivity of sera from mice immunized with clustered or nonclustered Ley-Pam3Cys constructs tested by ELISA. (A) Tested with Ley-Cer. (B) Tested with Ley mucin. ▴, Average values of five mice immunized with construct 1; ■, five mice immunized with construct 2; ▾, average values of five mice immunized with construct 3.
Flow Cytometry.
Reactivity of sera with a tumor cell line (OVCAR-3) was assayed by fluorescence-activated cell sorting as described (5). Anti-mouse Ig (H and L chain), and γ- or μ-specific antibodies were used as the second reagent.
Results
Comparison of Lipoglycopeptide Conjugates with Clustered and Nonclustered Ley Epitopes.
We first compared the immunogenicity of three different Ley conjugates having either clustered (1 and 2) or single Ley (3) oligosaccharides attached to a Ser-Ser-Ser-Ala-Val-Ala-Val peptide that was in turn linked to a B-cell stimulating moiety, termed Pam3Cys. Because Pam3Cys is an immunostimulatory structure (24, 25), we omitted QS21 or other adjuvants from this vaccine. Sera from groups of five mice were examined for antibody production by ELISA tests with Ley-ceramide, a Ley-containing mucin, and various Ley-protein conjugates and by fluorescence-activated cell sorting analysis for reactivity with Ley-positive tumor cells.
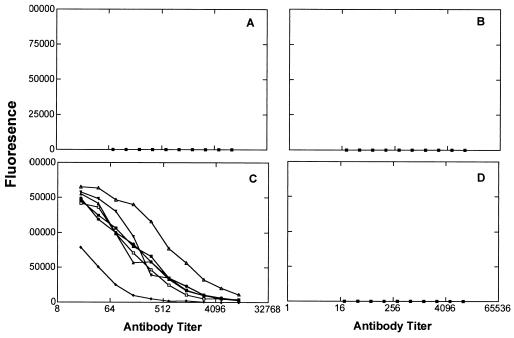
As shown in Fig. 3, mice immunized with the (Ley)3 construct in either the α (1) or β (2) configuration produced substantial titers of antibodies reacting with both Ley-Cer (Fig. 3A) or Ley-mucin (Fig. 3B). Mice immunized with the (Ley)1-bearing construct (3) (Fig, 3 A and B), however, responded much more poorly. The average titers for Ley-Cer were 1:4,096, 1:4,096, and 1:512, respectively. When tested against Ley-positive tumor cells (ovarian cancer cell line OVCAR-3), the same trend was observed, with the mice immunized with the clustered-epitope constructs (groups A and B) producing antibodies reacting more strongly with the cancer cell line (P = 0.03; Table 1). We also tested the mice sera against synthetic Ley-protein conjugates by ELISA (Fig. 4). Surprisingly, all three groups of mice reacted better with Ley-BSA (in which a single Ley oligosaccharide was coupled directly to BSA) than with a conjugate in which the (αLey)3 peptide was coupled to BSA. With the Ley-BSA target, the mice immunized with trivalent 1 had higher titers than those immunized with monovalent 3. In all three groups of mice, tested against the various conjugates, only IgM antibodies were observed.
Table 1.
Reactivities of sera from mice immunized with (Ley)3-peptide-Pam3Cys and (Ley)3-peptide-KLH conjugates with OVCAR-3 ovarian cancer cells analyzed by flow cytometry
| Mice | Immunogen | Ig class tested | Mean fluorescence | % Positive cells |
|---|---|---|---|---|
| Group A | (αLey)3-Pam3Cys (1) | IgM | 446.2 ± 109 | 72.5 ± 4.5 |
| IgG | <10.0 | <5.0 | ||
| Group B | (βLey)3-Pam3Cys (2) | IgM | 518.2 ± 69.7 | 73.7 ± 2.7 |
| IgG | <10.0 | <5.0 | ||
| Group C | (αLey)1-Pam3Cys (3) | IgM | 267.4 ± 20.5 | 57.4 ± 10.8 |
| IgG | <10.0 | <5.0 | ||
| Group D | (αLey)3-Pam3Cys (4) + QS21 | IgM | 170.1 ± 30.0 | 77.8 ± 4.9 |
| IgG | 37.1 ± 6.7 | 49.8 ± 19.0 | ||
| Group E | (αLey)3-KLH (5) + QS21 | IgM | ||
| IgG | 72.6 ± 7.6 | 23.3 ± 14.6 |
Groups A vs. C (P = 0.03) and B vs. C (P = 0.03) were compared by using a two-sample t-test.
Figure 4.
Reactivity of sera of mice immunized with clustered or nonclustered Ley-Pam3Cys constructs tested on by ELISA Ley-protein conjugates. (A) Tested on (Ley)3-peptide-BSA. (B) Tested by ELISA on Ley-KLH. See legend to Fig. 3 for symbols.
Immunogenicity of a Clustered Ley-KLH Conjugate.
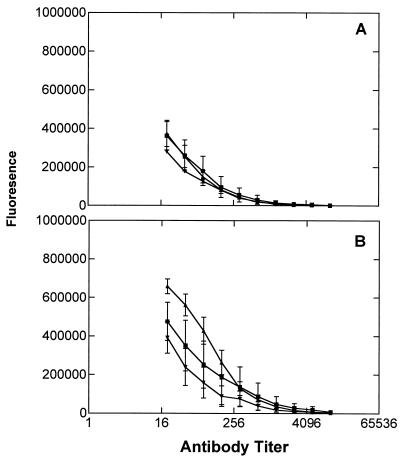
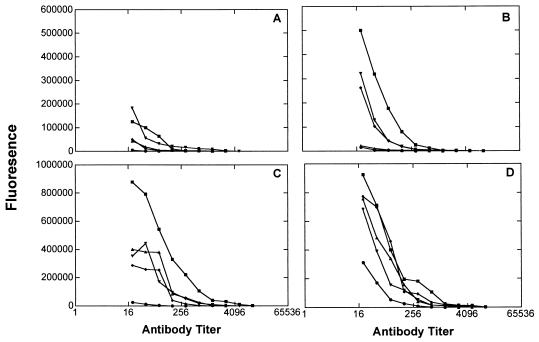
In an attempt to produce IgG as well as IgM responses to the (Ley)3 clustered epitope, we synthesized a conjugate in which the (Ley)3-bearing peptide was conjugated to the KLH protein carrier (5). Its immunogenicity in mice was compared with a Ley-KLH conjugate, which was previously studied (13). Unexpectedly, mice immunized with the (Ley)3-peptide-KLH conjugate responded by producing antibodies reacting only with Ley3-peptide epitope (coupled to BSA). No antibodies were observed to be reactive with Ley-Cer, Ley-mucin, Ley-BSA, or even with 1 (Fig. 5). This result could not be explained by problems with the conjugation, as compound 5 reacted with an anti-Ley antibody, showing that an intact Ley epitope was still present. Both IgG and IgM responses were observed, with the IgG responses being stronger than IgM responses (data not shown). As expected, the mice immunized with Ley directly coupled to KLH produced antibodies (both IgG and IgM) capable of recognizing Ley-Cer and Ley-mucin (data not shown), as we had observed previously (13). Thus, although coupling the (Ley)3-peptide to the KLH carrier protein resulted in a vaccine capable of eliciting both IgG and IgM responses, the specificity of the response was apparently restricted to the immunizing epitope as indicated by ELISA. Surprisingly, some reactivity with OVCAR-3 cells was noted by flow cytometry (group E in Table 1), although the level of reactivity was moderate (23.3% positive cells).
Figure 5.
Reactivity of sera of mice immunized with (Ley)3-peptide-KLH conjugate-tested by ELISA. (A) Tested on Ley-Cer. (B) Tested on Ley-mucin. (C) Tested on (Ley)3-peptide-BSA. (D) Tested on Ley-BSA target. Results for total Ig from eight immunized mice are shown.
Effect of Including QS21 Adjuvant in the (Ley)3–Peptide-Pam3Cys Vaccine.
As an alternative to using the KLH carrier to increase immunogenicity of the (Ley)3-peptide construct, we examined the effect of including QS21 adjuvant with the clustered glycolipopeptide 1. This approach was successful in that both IgG and IgM antibodies reactive with natural forms of Ley, i.e., Ley-Cer and Ley-mucin, were formed, although IgM responses (Fig. 6 C and D) generally appeared greater than IgG responses (Fig. 6 A and B). When tested against synthetic glycoconjugates, we found that the antibodies reacted better with Ley-BSA than with (Ley)3-BSA, in a similar manner to the antibodies induced without QS21 (data not shown). The antibodies produced in these mice were also capable of reacting with Ley-positive tumor cells, as tested by flow cytometry (group D in Table 1). Both IgM and IgG cell-reactive antibodies were detected, although, as noted previously, the intensity of staining appeared greater for IgM antibodies than IgG antibodies (Table 1).
Figure 6.
Reactivity of sera of mice immunized with (Ley)3-peptide-Pam3Cys in the presence of QS21 adjuvant. (A and C) Tested by ELISA with Ley-Cer. (B and D) Tested by ELISA with Ley-mucin. (A and B) IgG antibodies. (C and D) IgM antibodies. Results from five mice are shown.
Discussion
In designing conjugate anticancer vaccines, the initial goals are (i) to elicit the production of antibodies that react with natural forms of the antigen, i.e., glycoproteins and glycolipids, and with antigen-positive tumor cells, and (ii) to elicit a wide spectrum of immunoglobulins, including both IgG and IgM antibodies. An additional goal in our studies was to produce entirely synthetic constructs that could be produced reproducibly and in large quantities and meet regulatory guidelines. The present studies addressed all three points by using Ley as a model antigen.
The first point we examined was the possible advantage of using, as the immunogen, a short peptide construct bearing a clustered moiety of three Ley oligosaccharides substituted on three contiguous serine residues. The rationale for using this motif is that it would closely mimic the natural form of Ley as found on mucins, and possibly in glycolipid rafts, on the cell surface. Moreover, it is well known that some monoclonal antibodies produced to tumor cells or mucins preferentially recognize clustered carbohydrate epitopes (16, 17). The best studied example is probably the recognition of a clustered Tn structure, i.e., (α-O-GalNAc), Ser(α-O-GalNAc)Ser(α-O-GalNAc)Ser by mouse monoclonal antibody MLS 102 (16). The present study demonstrates that the clustered Ley structure, 1, was more efficient at eliciting antibodies reacting with natural forms of Ley and with tumor cells than a construct substituted with a single Ley oligosaccharide (3). Importantly, this is the first time that such an effect has been shown by actually vaccinating with various synthetic clustered and nonclustered structures. Earlier studies compared selected monoclonal antibodies with clustered-epitope immunogens rather than analyzing the total antibody response or were not informative on this point (26, 27). Although the benefits of multivalency is well established for both antibody and lectin binding (16, 17, 28, 29), the molecular mechanism underlying this phenomenon is poorly understood. Presumably the effect is not attributable to recognition of a combined epitope encompassing three or more sugar chains as such a structure would exceed the size of antibody combining sites. Whiteside and coworkers (30) have suggested that the increased affinity attributable to multivalency arises mainly from decrease in entropy following protein-ligand interactions. Whatever the mechanism involved, our study clearly shows that immunization with a clustered structure induces antibodies reacting selectively with clustered antigens and cells on which they are expressed.
As a part of this study on clustered epitopes, we compared the immunogenicity of constructs in which the oligosaccharides were linked to the peptide through the natural α-O- GalNAc Ser linkage (1) with those linked through a unnatural β-O-GalNAc Ser linkage (2). Perhaps not surprisingly, both antigen species were equally efficient at producing antibodies to natural forms of Ley and cells. One possible explanation of this result is that in mucins and cells Ley epitopes may be carried on longer glycan chains than used in the immunogen such that the β-linked GalNAc may substitute for the β-linked GlcNAc or Gal that would be found in these long chains (e.g., Fucα1 → 2Galβ1 → 4 [Fucα1 → 3]GlcNAcβ1 → 3Galβ1 →). Alternatively, it could be that the anomeric linkage is not a part of the immunogenic epitope.
With respect to the failure of the Pam3Cys-substituted glycolipopeptides to elicit IgG antibodies, this finding is perhaps not unexpected in that this construct would function as a T-cell independent antigen. Such antigens poorly stimulate T-cell help and do not generally induce class-switching or affinity maturation of B lymphocytes. More surprising is the failure of the (Ley)3-KLH construct (5), in which an immunogenic protein carrier is substituted for Pam3Cys, to induce antibodies reacting with natural forms of Ley. From ELISA analysis, it appears that this more potent antigenic construct produced antibodies, particularly IgG, that are highly specific for the immunizing structure and have little or no reactivity with related structures, such as Ley-Cer and Ley-mucin. However, the induced antibodies were able to react with cancer cells expressing Ley, and therefore further clinical evaluation need not be ruled out. Indeed, in addition to inherent differences between animal and human response to vaccination, these results may be indicative of subtle differences in the way that Ley is displayed on a cell surface in contrast to display in a plastic surface assay.
In view of our goal to design completely synthetic vaccines capable of producing a full spectrum of antibody responses, we examined whether including an adjuvant with the glycolipopeptide vaccine, previously shown to produce only an IgM responses, would be effective in this regard. We were encouraged to find that including QS21 adjuvant with the clustered glycolipopeptide (4) resulted in the elicitation of IgG as well as IgM antibodies. Previous studies on protein and carbohydrate-protein conjugates have also shown the effectiveness of QS21 in this regard (31). Although IgM antibodies still predominated in our study, the results show that the addition of adjuvant induced class-switching of B lymphocytes to produce IgG antibodies. Thus, although Pam3Cys alone may serve as a B cell stimulating moiety (23, 24), it seems that other signals, e.g., from QS21, are needed to fully implement an antibody response. T-cell independent antigens that need additional stimulation through bacterial or other structures, such as our vaccine, are referred to as TI-1 antigens. The mechanism probably involves the release of cytokines (e.g., IL-4) from natural killer or other cells, which promote B cell class-switching (32).
The major conclusions of this study, in practical terms, are (i) glycolipopeptide with clustered Ley epitopes are more effective than related structures with single Ley epitopes in producing antitumor cell antibodies; (ii) antibody responses to the clustered Ley-structure conjugated to KLH were skewed toward the immunizing carbohydrate structure; and (iii) totally synthetic constructs can be effective immunogens in conjunction with a suitable adjuvant, the effect of which is to bypass the need for specific T-cell help to stimulate IgG as well as IgM antibodies. The development of a totally synthetic vaccine, in contrast to carbohydrate-protein conjugate vaccines, would greatly facilitate the production of the vaccine for large scale clinical trials and could simplify regulatory approval.
Acknowledgments
We thank Ms. Claudette Bryant for skillful secretarial assistance. This work was supported by the National Institutes of Health (Grants CA 71506, CA 28824, and CA 08748), and by the Pepsico Foundation. Postdoctoral Fellowship support is gratefully acknowledged by P.G. (American Cancer Society, PF98026) and L.J.W. (the Selma and Lawrence Ruben Foundation). We gratefully acknowledge Dr. George Sukenick (NIH CA 08748) of the SKI NMR Core Facility for mass spectral and NMR analyses.
Abbreviations
- Ley
Lewisy
- KLH
keyhole limpet hemocyanin
References
- 1.Scott A M, Welt S. Curr Opin Immunol. 1997;9:717–722. doi: 10.1016/s0952-7915(97)80054-4. [DOI] [PubMed] [Google Scholar]
- 2.Livingston P O, Wong G Y C, Adluri S, Tao Y, Padavan M, Parente R, Hanlon C, Calves M J, Helling F, Ritter G, et al. J Clin Oncol. 1994;12:1036–1044. doi: 10.1200/JCO.1994.12.5.1036. [DOI] [PubMed] [Google Scholar]
- 3.Ragupathi G, Meyers M, Adluri S, Howard L, Musselli C, Livingston P O. Int J Cancer. 2000;85:659–666. doi: 10.1002/(sici)1097-0215(20000301)85:5<659::aid-ijc11>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 4.Dickler M N, Ragupathi G, Liu N X, Musselli C, Martino D J, Miller V A, Kris M G, Brezicka F T, Livingston P O, Grant S C. Clin Cancer Res. 1999;10:2773–2779. [PubMed] [Google Scholar]
- 5.Sabbatini P, Kudryashov V, Ragupathi G, Danishefsky S J, Livingston P O, Bornmann W, Spassova M, Zatorski A, Spriggs D, Aghajanian C, et al. Int J Cancer. 2000;87:79–85. [PubMed] [Google Scholar]
- 6.Ragupathi G, Slovin S F, Adluri S, Sames D, Kim I J, Kim H M, Spassova M, Bornmann W G, Lloyd K O, Scher H I, et al. Angew Chem Int Ed Engl. 1999;38:563–566. doi: 10.1002/(SICI)1521-3773(19990215)38:4<563::AID-ANIE563>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 7.Slovin S F, Ragupathi G, Adluri S, Ungers G, Terry K, Kim S, Spassova M, Bornmann W G, Fazzari M, Dantis L, et al. Proc Natl Acad Sci USA. 1999;96:5710–5715. doi: 10.1073/pnas.96.10.5710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang Z G, Williams L J, Zhang X F, Zatorski A, Kudryashov V, Ragupathi G, Spassova M, Bornmann W, Slovin S F, Scher, et al. Proc Natl Acad Sci USA. 2000;97:2719–2724. doi: 10.1073/pnas.97.6.2719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Adluri S, Helling F, Ogata S, Zhang S, Itzkowitz S H, Lloyd K O, Livingston P O. Cancer Immunol Immunother. 1995;41:185–192. doi: 10.1007/BF01521345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.MacLean G D, Reddish M, Koganty R R, Wong T, Gandhi S, Smolenski M, Samuel J, Nabholtz J M, Longenecker B M. Cancer Immunol Immunother. 1993;36:215–222. doi: 10.1007/BF01740902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hellstrom I, Garrigues H J, Garrigues U, Hellstrom K E. Cancer Res. 1990;50:2183–2190. [PubMed] [Google Scholar]
- 12.Federici M F, Kudryashov V, Saigo P E, Finstad C L, Lloyd K O. Int J Cancer. 1999;81:193–198. doi: 10.1002/(sici)1097-0215(19990412)81:2<193::aid-ijc5>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 13.Kudryashov V, Kim H M, Ragupathi G, Danishefsky S J, Livingston P O, Lloyd K O. Cancer Immunol Immunother. 1998;45:281–286. doi: 10.1007/s002620050444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kim Y S, Gum J, Jr, Brockhausen I. Glycoconjugate J. 1996;13:693–707. doi: 10.1007/BF00702333. [DOI] [PubMed] [Google Scholar]
- 15.Muller S, Goletz S, Packer N, Gooley A, Lawson A M, Hanisch F G. J Biol Chem. 1997;272:24780–24793. doi: 10.1074/jbc.272.40.24780. [DOI] [PubMed] [Google Scholar]
- 16.Nakada H, Inoue M, Numata Y, Tanaka N, Funakoshi I, Fukui S, Mellors A, Yamashina I. Proc Natl Acad Sci USA. 1993;90:2495–2499. doi: 10.1073/pnas.90.6.2495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang S, Walberg L A, Ogata S, Itzkowitz S H, Koganty R R, Reddish M, Gandhi S S, Longenecker B M, Lloyd K O, Livingston P O. Cancer Res. 1995;55:3364–3368. [PubMed] [Google Scholar]
- 18.Seeberger P H, Danishefsky S J. Accounts Chem Res. 1998;31:685–695. [Google Scholar]
- 19.Danishefsky S J, Behar V, Randolph J T, Lloyd K O. J Am Chem Soc. 1995;117:5701–5711. [Google Scholar]
- 20.Glunz P W, Hintermann S, Schwarz J B, Kuduk S D, Chen X T, Williams L J, Sames D, Danishefsky S J, Kudryashov V, Lloyd K O. J Am Chem Soc. 1999;121:10636–10637. [Google Scholar]
- 21.Glunz P W, Hintermann S, Williams L J, Schwarz J B, Kuduk S D, Kudryshov V, Lloyd K O, Danishefsky S J. J Am Chem Soc. 2000;122:7273–7279. [Google Scholar]
- 22.Zhang S, Graeber L A, Helling F, Ragupathi G, Adluri S, Lloyd K O, Livingston P O. Cancer Res. 1996;56:3315–3319. [PubMed] [Google Scholar]
- 23.Lloyd K O, Kabat E A. Proc Natl Acad Sci USA. 1968;61:1470–1477. doi: 10.1073/pnas.61.4.1470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bessler W G, Cox M, Lex A, Suhr B, Wiesmuller K H, Jung G. J Immunol. 1985;135:1900–1905. [PubMed] [Google Scholar]
- 25.Toyokuni T, Dean B, Cai S, Boivin D, Hakomori S, Singhal A K. J Am Chem Soc. 1994;116:395–396. [Google Scholar]
- 26.Reddish M A, Jackson L, Koganty R R, Qiu D, Hong W, Longenecker B M. Glycoconjugate J. 1997;14:549–560. doi: 10.1023/a:1018576224062. [DOI] [PubMed] [Google Scholar]
- 27.Ragupathi G, Howard M, Cappello S, Koganty R R, Qiu D, Longenecker B M, Reddish M A, Lloyd K O, Livingston P O. Cancer Immunol Immunother. 1999;48:1–8. doi: 10.1007/s002620050542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Irimura T, Denda K, Iida S, Takeuchi H, Kato K. Biochem (Tokyo) 1999;126:975–985. doi: 10.1093/oxfordjournals.jbchem.a022565. [DOI] [PubMed] [Google Scholar]
- 29.Lee Y C, Lee R T. In: Glycoconjugates: Composition, Structure and Function. Allan H J, Kisailus E C, editors. New York: Dekker; 1992. pp. 148–153. [Google Scholar]
- 30.Mammen M, Choi S-K, Whitesides G M. Angew Chem Int Ed Engl. 1998;37:2755–2794. doi: 10.1002/(SICI)1521-3773(19981102)37:20<2754::AID-ANIE2754>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 31.Helling F, Shang A, Calves M, Zhang S, Ren S, Yu R K, Oettgen H F, Livingston P O. Cancer Res. 1994;54:197–203. [PubMed] [Google Scholar]
- 32.Singh M, O'Hagan D. Nat Biotechnol. 1999;17:1075–1081. doi: 10.1038/15058. [DOI] [PubMed] [Google Scholar]