Abstract
Resveratrol is a polyphenolic phytoestrogen that has been shown to exhibit potent anti-oxidant, anti-inflammatory, and anti-catabolic properties. Increased osteoclastic and decreased osteoblastic activities result in bone resorption and loss of bone mass. These changes have been implicated in pathological processes in rheumatoid arthritis and osteoporosis. Receptor activator of NF-κB ligand (RANKL), a member of the TNF superfamily, is a major mediator of bone loss. In this study, we investigated the effects of resveratrol on RANKL during bone morphogenesis in high density bone cultures in vitro. Untreated bone-derived cell cultures produced well organized bone-like structures with a bone-specific matrix. Treatment with RANKL induced formation of tartrate-resistant acid phosphatase-positive multinucleated cells that exhibited morphological features of osteoclasts. RANKL induced NF-κB activation, whereas pretreatment with resveratrol completely inhibited this activation and suppressed the activation of IκBα kinase and IκBα phosphorylation and degradation. RANKL up-regulated p300 (a histone acetyltransferase) expression, which, in turn, promoted acetylation of NF-κB. Resveratrol inhibited RANKL-induced acetylation and nuclear translocation of NF-κB in a time- and concentration-dependent manner. In addition, activation of Sirt-1 (a histone deacetylase) by resveratrol induced Sirt-1-p300 association in bone-derived and preosteoblastic cells, leading to deacetylation of RANKL-induced NF-κB, inhibition of NF-κB transcriptional activation, and osteoclastogenesis. Co-treatment with resveratrol activated the bone transcription factors Cbfa-1 and Sirt-1 and induced the formation of Sirt-1-Cbfa-1 complexes. Overall, these results demonstrate that resveratrol-activated Sirt-1 plays pivotal roles in regulating the balance between the osteoclastic versus osteoblastic activity result in bone formation in vitro thereby highlighting its therapeutic potential for treating osteoporosis and rheumatoid arthritis-related bone loss.
Keywords: NF-kappa B, p300, Resveratrol, SIRT, Stem Cell, Cbfa-1, RANKL, Osteoclastogenesis, Osteogenesis
Introduction
Bone connective tissue has a highly specific architecture, two-thirds of which is composed of inorganic material and the remainder is organic material, including extracellular matrix proteins and the cells forming the bone-specific extracellular matrix and water (1). It is very important in bone tissue to maintain the architectural stability, morphology, and composition of the calcified structures. Bone contains three principal cell types that are responsible for maintaining the following functions: bone-synthesizing osteoblasts, bone-resident osteocytes, and bone-resorbing osteoclasts and undifferentiated mesenchymal cells. Remodeling and maintenance of bone tissue are regulated through bone extracellular matrix synthesis by osteoblasts and extracellular matrix resorption by osteoclasts (2). Under physiological conditions, a delicate equilibrium exists between new bone synthesis and old bone resorption. However, in pathological conditions such as rheumatoid arthritis and osteoporosis, this fine balance between synthesis and degradation goes astray resulting in excessive loss of bone mineral density and extracellular matrix degradation exacerbating osteoporosis and rheumatoid arthritis-related bone loss (3).
Osteoclasts are multinucleated cells formed by the fusion of hematopoietic lineage-derived monocytes-macrophages in bone marrow (4). They are phenotypically characterized by high production of tartrate-resistant acid phosphatase (TRAP)3 and cathepsin K (5). Numerous factors have been reported to induce osteoclast activation, including receptor activator of nuclear factor κB ligand (RANKL), tumor necrosis factor-α (TNF-α), interleukin-1β (IL-1β), and macrophage colony-stimulating factor (M-CSF) (6–9). RANKL is a member of the TNF superfamily and is produced by osteoblasts and bone marrow stromal cells (10–12). Its receptor RANK is expressed on the surface of preosteoclasts and plays important roles during osteoclastogenesis (13). RANK acts as a receptor for RANKL and osteoprotegerin, a soluble receptor, produced by osteoblasts. RANK and osteoprotegerin can bind RANKL and control osteoclast formation (14–17). It has been shown that RANKL knock-out mice possess an osteopetrotic phenotype with dentition defects, cartilage destruction, and bone erosion and exhibit a deficiency of osteoclasts (18). Furthermore, RANKL is able to stimulate osteoclasts and can cause the resorption of bone mass in vivo by activating preosteoclasts (10, 11). RANKL is essential for bone remodeling and plays an important role in mature osteoclast survival (10).
The transcriptional co-activator p300 is a histone acetyltransferase (19, 20). It serves to integrate signaling pathways involved in diverse cellular functions (21). This protein promotes histone acetylation and regulates promoter activity by removing chromatin-dependent repression (22). Furthermore, it acetylates a number of transcriptional factors (e.g. p53, FOXO-1, E2F, and HMG I) resulting in transcriptional regulation (23–25). It has been reported that p300 acetyltransferase acetylates NF-κB-p65 (at lysine 310) (26) that, in turn, activates NF-κB-specific transcriptional activity and up-regulates the expression of the anti-apoptotic genes of the Bcl-2 family, such as Bcl-xL (27, 28).
The bone transcription factor Cbfa-1 (core binding factor α-1) is one of the earliest and most specific markers of osteogenesis (29). Cbfa-1 acts as an activator of transcription and can induce osteoblast-specific gene expression in vitro (29). Mechanical signals can regulate Cbfa-1 activation favoring osteoblast differentiation through the activation of the MAPK signal transduction pathway and Ras/Raf-dependent Erk1/2 activation, independent of p38 MAPK signaling (30).
The polyphenolic and phytoestrogenic plant resveratrol (trans-3,4′-trihydroxystilbene) naturally occurs in the skin of red grapes, vines, various other fruits, peanuts, and root extracts of the weed Polygonum cuspidatum (31). In plants, resveratrol protects against fungal infections and exhibits antiprotozoal activity (32). Resveratrol also has potent anti-inflammatory, anti-tumor, immunomodulatory, cardioprotective, anti-oxidative, and chemopreventive properties (33, 34). However, its effects on osteoclast differentiation have not been evaluated thus far. Resveratrol can inhibit NF-κB activation and down-regulate the proinflammatory gene products COX-2, IL-1β, and IL-6, which play important roles in various forms of arthritis (35, 36). We have recently shown that resveratrol exerts anti-apoptotic, anti-oxidative, anti-tumor suppressor protein p53, and anti-inflammatory functions in chondrocytes (37–39). These chemopreventive properties of resveratrol have been associated with the inhibition of NF-κB. Furthermore, resveratrol is a potent activator of the Sirt-1/Sir2 (silent information regulator 2) family of NAD-dependent histone deacetylases (40). These deacetylases remove the acetyl group from acetyl-lysine in histones and non-histone substrates, such as transcription factors and co-activators of transcription and cytoplasmic proteins (41), subsequently causing widespread effects on cell function (42). Several studies have shown that many of the beneficial effects of resveratrol are due to activation of Sirt-1, including stress resistance and life span extension (40). Although resveratrol is a potent inhibitor of NF-κB, its effects on osteoblasts and osteoclasts have not yet been fully investigated at the cellular or molecular levels. Because phytoestrogens are known to affect the biology of osteoblasts and osteoclasts, and osteoblasts regulate osteoclast activity through the expression of RANKL, we evaluated the effects of resveratrol on RANKL-stimulated signaling and osteoclastogenesis.
EXPERIMENTAL PROCEDURES
Antibodies
Antibodies to β-actin were obtained from Sigma. Antibodies against p65, pan-IκBα, and TRAP were obtained from Santa Cruz Biotechnology (Santa Cruz, CA). Antibodies against phospho-specific IκBα (Ser-32/36) and anti-phospho-specific p65 (Ser-536) were obtained from Cell Technology (Beverly, MA). Anti-IκBα kinase (IKK)-α and anti-IKK-β antibodies were obtained from Imgenex (Germany). Monoclonal anti-Sirt-1 antibody (Ab 12193) was purchased from Abcam. Polyclonal anti-Cbfa-1 (CBFA11-A) was purchased from Alpha Diagnostic International Inc., San Antonio, TX. Monoclonal anti-poly(ADP-ribose) polymerase antibody was purchased from BD Biosciences. Acetylated lysine (Ac-K-103) antibody was purchased from Cell Signaling Technology (Danvers, MA). Anti-p300 antibody was purchased from Millipore. All antibodies were used at concentrations and dilutions recommended by the manufacturer (dilutions ranged from 1:100 for immunomorphological experiments to 1:10,000 for Western blot analysis).
Growth Media, Chemicals, and Cytokines
Growth medium (Ham's F-12/Dulbecco's modified Eagle's medium (50:50) containing 10% fetal calf serum (FCS), 25 μg/ml ascorbic acid, 50 IU/ml streptomycin, 50 IU/ml penicillin, 2.5 μg/ml amphotericin B, essential amino acids and l-glutamine) was obtained from Seromed (Munich, Germany). Trypsin/EDTA (EC 3.4.21.4) was purchased from Sigma. Epon was obtained from Plano (Marburg, Germany). RANKL was purchased from R&D Systems (Abingdon, UK).
Resveratrol with purity greater than 98% was purchased from Sigma. A 100 mm stock solution of resveratrol (molecular weight, 228.2) was prepared in ethanol and further diluted in cell culture medium for working concentrations. The maximum final concentration of ethanol in culture was less than 0.1% and had no cytotoxic effects.
IL-1β was obtained from Acris Antibodies GmbH (Herold, Germany). Peptide aldehydes and a specific proteasome inhibitor N-Ac-Leu-Leu-norleucinal were obtained from Roche Applied Science. For osteoclast differentiation, bone-derived cells were suspended in growth medium supplemented with 10% FCS and 100 ng/ml (5 nm) RANKL.
Primary Bone-derived Cell Isolation and Culture
Canine bone fragments were cut from femoral heads obtained during total hip replacement surgery with fully informed owner consent and ethical project approval from the ethical review committee of Ludwig-Maximilians-University, Munich, Germany. Bone-derived cells used in the experiments described were always from the same animal. The experiments were performed a total of three times, and samples from three different donors were used in each experiment. Donor ages ranged from 5 to 7 years. Care was taken to isolate bone fragments only from the inner trabecular bone to avoid contamination with soft or other fibrous tissues. The 5–10 bone fragments of ∼3–4-mm were plated per 35-mm Petri dish in 10 ml of cell culture medium and incubated at 37 °C, 5% CO2. After 1–2 weeks, bone cells migrated from the bone fragments and adhered to the cell culture dish. After colonies reached confluency, the cells were trypsinized. Primary isolated bone tissue cells were passaged 2–3 times to gain sufficient cells for the preparation of high density cultures.
Preosteoblastic Cell Line Culture
The mouse preosteoblastic MC3T3-E1 (DSMZ, Braunschweig, Germany) was selected as an in vitro model of preosteoblastic cells. The cells were cultured in α-minimal essential medium containing 10% FBS, 100 units/ml penicillin, and 100 mg/ml streptomycin. The cells were maintained in a humidified, 95% air, 5% CO2 atmosphere at 37 °C. All experiments were performed with third passage MC3T3-E1 cells.
Experimental Design
High Density Cultures
Primary isolated bone tissue cells were cultivated in high density cultures. The cells were either left untreated, treated with 10 nm RANKL alone for the indicated time periods, or pretreated with 5 μm resveratrol for 4 h followed by co-treatment with 10 nm RANKL and 5 μm resveratrol for the indicated time periods. High density cultures were performed as described previously (43). Briefly, high density cultures were established by adding the cells onto a nitrocellulose filter, which was placed on a steel net bridge. Approximately 8 μl of cell suspension, containing ∼1 million cells, and after 1 day in culture, this aggregated into a cell pellet. High density cultures were incubated with osteogenic induction medium. The osteogenic induction medium was prepared as described previously (44); it consisted of DMEM as a base medium, 10% FCS, penicillin/streptomycin solution (10,000 IU/10,000 IU/100 ml), 10−7 m dexamethasone (Sigma, catalog no. D-8893), 10 mm β-glycerophosphate (Sigma, catalog no. G-9891), and 50 μm ascorbate 2-phosphate (Sigma, catalog no. A-8960). Cells were nurtured through diffusion at the filter medium interface and evaluated after 3, 7, 10, and 14 days.
Monolayer Cultures
For investigation of NF-κB translocation and IκBα phosphorylation, primary bone tissue cell monolayer cultures were washed three times with serum-starved medium and incubated for 1 h with serum-starved medium (3% FCS) either treated with 5 μm resveratrol or 10 nm RANKL or co-treated with 10 nm RANKL and 5 μm resveratrol for 0, 10, 20, 30, 40, and 60 min, and nuclear and cytoplasmic extracts were prepared. These experiments were performed in triplicate, and the results are provided as mean values from three independent experiments.
For induction of the osteoblast phenotype, the MC3T3-E1 cells at 80% confluence were cultured in differentiation medium (α-minimal essential medium containing 10% FBS, 10 mm β-glycerol phosphate, and 50 mg/ml l-ascorbic acid) for 3 days (45). For investigation of resveratrol-promoted osteogenesis, the MC3T3-E1 cells were either left untreated or treated with 5 μm resveratrol or 10 nm RANKL for 1 h or pretreated with 5 μm resveratrol for 1 h followed by 3 days co-treatment with 10 nm RANKL and 5 μm resveratrol.
Immunofluorescence Microscopy
Immunofluorescence studies of primary bone tissue cells were carried out as described previously (38). Briefly, bone cells were seeded on glass plates and incubated with osteogenic medium. After 24 h, the cells were treated with or without RANKL (10 mm) or pretreated with resveratrol (5 μm) for 4 h and co-treated with RANKL (10 mm) for the indicated time. Cultures were immunolabeled against TRAP and examined under a fluorescent microscope (Axiophot 100, Zeiss, Germany).
Transmission Electron Microscopy
Samples were fixed 1 h with Karnovsky's fixative followed by post-fixation in 1% OsO4 solution (0.1 m phosphate buffer). Monolayer cell pellets were rinsed and dehydrated in an ascending alcohol series before being embedded in Epon and cut on a Reichert-Jung Ultracut E (Germany). Ultrathin sections were contrasted with 2% uranyl acetate/lead citrate. A transmission electron microscope (TEM 10, Zeiss, Jena, Germany) was used to examine the cultures.
Quantification of Multinucleated Osteoclast Cells
To quantify the multinucleated osteoclasts, the number of cells exhibiting typical morphological features of multinucleated cells was determined by scoring 100 cells from 30 different microscopic fields per culture, and the number of multinucleated cells was expressed as an indicator of osteoclastogenesis.
Immunoelectron Microscopy (Post-embedding Technique)
Immunoelectron microscopy was performed as described previously in detail (46). Briefly, cultures were fixed for 1 h in 3% freshly prepared formaldehyde (paraformaldehyde plus 0.25% glutaraldehyde), washed three times with 0.1 m PBS, dehydrated in an ascending series of alcohol baths, and embedded in LR-white (Plano, Marburg, Germany). Ultrathin sections of the cultures were prepared and treated as follows: 1% BSA at ambient temperature (AT) for 30 min; testicular chondroitinase (5000 units/ml) for 5 min to unmask antigen epitopes; 1% PBS/BSA and 0.5% Tween 20 two times for 5 min at AT; primary antibodies (diluted 1:50 in 1% PBS/BSA) overnight at 4 °C; 1% PBS/BSA two times for 5 min at AT; secondary antibodies conjugated with goat anti-rabbit immunoglobulin with 10-nm gold particles (1:50 for 30 min) at AT; and after rinsing two times for 5 min at AT, contrasting was carried out with 1% tannic acid for 20 min at AT, with 4% osmium tetroxide for 10 min and with 2% uranyl acetate for 30 min. Finally, the sections were rinsed and examined under a transmission electron microscope (Zeiss, Germany).
Preparation of Nuclear Extracts from Bone Tissue Cells
Cells were trypsinized and washed twice in 1 ml of ice-cold PBS. The supernatant was carefully removed. The cell pellet was resuspended in 400 μl of hypotonic lysis buffer containing protease inhibitors and incubated on ice for 15 min. 12.5 μl of 10% Nonidet P-40 were added, and the cell suspension was vigorously mixed for 15 s. The extracts were centrifuged for 1.5 min. The supernatants (cytoplasmic extracts) were frozen at −70 °C. 25 μl of ice-cold nuclear extraction buffer was added to the pellets and incubated for 30 min with intermittent mixing. Extracts were centrifuged, and the supernatant (nuclear extracts) was transferred to prechilled tubes for storage at −70 °C.
Immune Complex Kinase Assay
To test the effect of resveratrol on RANKL-induced IKK activation, immune complex kinase assays were performed. Immune complex kinase assays were performed as described previously (47). The IKK complex was immunoprecipitated from whole cell lysates with antibodies against IKK-α and IKK-β and subsequently incubated with protein A/G-agarose beads (Pierce). After 2 h of incubation, the beads were washed with lysis buffer and resuspended in a kinase assay solution containing 50 mm HEPES (pH 7.4), 20 mm MgCl2, 2 mm dithiothreitol, 10 μm unlabeled ATP, and 2 mg of substrate GST-IκBα (amino acid 1–54) and incubated at 30 °C for 30 min. This was followed by boiling in SDS-PAGE sample buffer for 5 min. The proteins were transferred to a nitrocellulose membrane after SDS-PAGE under reducing conditions as described above. Phosphorylation of GST-IκBα was assessed using a specific antibody against phospho-specific IκBα (Ser-32/36). To demonstrate the total amounts of IKK-α and IKK-β in each sample, whole cell lysates were transferred to a nitrocellulose membrane after SDS-PAGE under reducing conditions as described above. Detection of IKK-α and IKK-β was performed by immunoblotting with either anti-IKK-α or anti-IKK-β antibodies.
Immunoprecipitation and Immunoblotting
A detailed description of the technique used for the following experiments has been previously published (39, 48). Briefly, HD cultures were rinsed in PBS and the proteins extracted with lysis buffer (50 mm Tris-HCl (pH 7.2), 150 mm NaCl, l% (v/v) Triton X-100, 1 mm sodium orthovanadate, 50 mm sodium pyrophosphate, 100 mm sodium fluoride, 0.01% (v/v) aprotinin, pepstatin A (4 μg/ml), leupeptin (10 μg/ml), and 1 mm phenylmethylsulfonyl fluoride (PMSF)) for 30 min on ice. After adjusting the total protein concentration, samples were separated by SDS-PAGE (5, 7.5, or 10% gels) under reducing conditions. For immunoprecipitation, the extracts were precleared by incubating them first with 25 μl of either normal rabbit IgG serum or normal mouse IgG serum and Staphylococcus aureus cells, then with primary antibodies diluted in wash buffer (0.1% Tween 20, 150 mm NaCl, 50 mm Tris-HCl (pH 7.2), 1 mm CaCl2, 1 mm MgCl2, and 1 mm PMSF) for 2 h at 4 °C, and finally with S. aureus cells for 1 h at 4 °C. Control immunoprecipitations were performed by incubating the samples with nonimmune rabbit anti-mouse IgG alone. S. aureus cells were washed five times with wash buffer and once with 50 mm Tris-HCl (pH 7.2) and then boiled in SDS-PAGE sample buffer. Separated proteins were transferred to nitrocellulose membranes and incubated in blocking buffer (5% (w/v) skimmed milk powder in PBS, 0.1% Tween 20) for 1 h at AT. Membranes were incubated with the primary antibodies (Cbfa-1, TRAP, Sirt-1, p65, IκBα, p-IκBα, and β-actin, and diluted in blocking buffer, overnight at 4 °C), washed three times with blocking buffer, and then incubated with the secondary antibody conjugated with alkaline phosphatase for 90 min at AT. Membranes were rinsed with blocking buffer and then washed three times in 0.1 m Tris (pH 9.5) containing 0.05 m MgCl2 and 0.1 m NaCl. Specific antigen-antibody complexes were rendered visible using nitro blue tetrazolium and 5-bromo-4-chloro-3-indolyl phosphate (p-toluidine salt; Pierce) as the substrates for alkaline phosphatase. Total protein concentration was determined according to the bicinchoninic acid system (Pierce) using bovine serum albumin as a standard. Specific binding was quantified by densitometry using “Quantity One” (Bio-Rad).
p65 Acetylation Assay
p65 lysine acetylation was analyzed by immunoprecipitation of p65 followed by Western blotting using acetyl-lysine antibodies. Cells were treated with 5 μm resveratrol for 4 h and then exposed to 10 nm RANKL for the indicated times. Whole cell extracts were prepared, immunoprecipitated with an anti-p65 antibody, and subjected to Western blot analysis using an anti-acetyl-lysine antibody.
Statistical Analysis
Numerical data are expressed as mean values (±S.D.) for a representative experiment performed in triplicate. The means were compared using Student's t test assuming equal variances. Differences were considered to be statistically significant if the p value was less than 0.05.
RESULTS
Cell Culture
Explants of canine bone (about 3–5 mm) were cultured in a flask with growth medium. After 1–2 weeks, bone tissue cells migrated from the bone tissue, adhered to the culture flask, and formed a monolayer (data not shown). This monolayer was passaged 2–3 times to gain sufficient cells for the preparation of high density cultures. Using a well established model of osteogenesis (high density culture), the effects of resveratrol on RANKL-induced NF-κB signaling pathway on bone-derived cells were determined.
Resveratrol Inhibits RANKL-induced Osteoclastogenesis
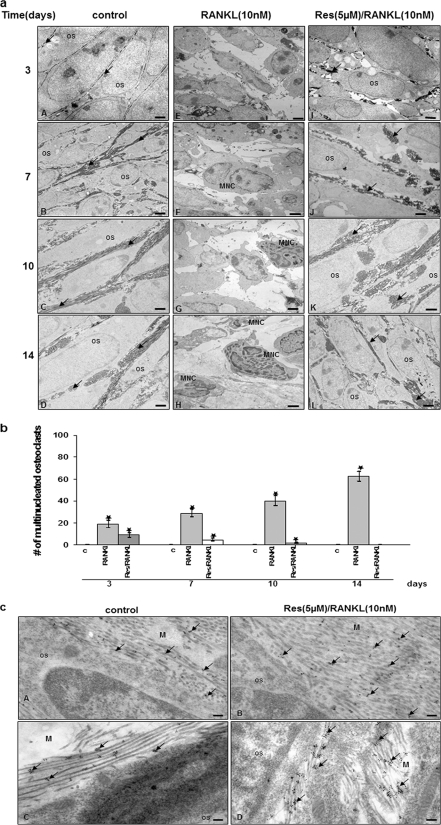
To observe the osteogenic potential of the bone-derived cells in an environment as close to the in vivo situation as possible, primary osteoblasts were transferred to three-dimensional surroundings in high density culture. Transmission electron microscopy was used to obtain high resolution images from the high density cultures after 14 days to evaluate cellular ultrastructure and extracellular matrix formation (Fig. 1).
FIGURE 1.
Cultivation of primary isolated bone tissue cells in high density culture. Resveratrol inhibited RANKL-induced osteoclastogenesis. a, bone-derived cells (1 × 106 cells) were incubated in high density culture with or without RANKL (10 nm) and with or without 5 μm resveratrol for 14 days before evaluation by transmission electron microscopy. Os, osteoblast; MNC, multinucleated cells; arrow, extracellular matrix. Magnification, ×5000; bar = 1 μm. b, multinucleated osteoclasts (those containing 2–3 nuclei) were counted. c, cells exposed to medium alone (control). c, immunoelectron microscopy. Collagen type I and osteocalcin in the matrix of untreated (panel A) and pretreated primary isolated bone tissue cells cultures (panel B) abundant amounts of collagen type I were detected. Secondary gold particle-labeled antibody was observed directly on the bundles of thick extracellular matrix fibrils. In the matrix of untreated (panel C) and pretreated primary isolated bone tissue cells cultures (panel D), abundant amounts of osteocalcin were confirmed. Secondary gold particle-labeled antibodies clustered together indicating the existence of osteocalcin between the mesh of the collagen fibers. The quantity and distribution of gold particles was similar between untreated and precultures. Magnification ×24,000; bar, 0.25 μm; Os, osteoblast.
In the untreated high density cultures, osteogenesis was observed in primary bone-derived cell cultures (Fig. 1a, panels A–D). Cells exhibited high levels of euchromatin in their nuclei, a large number of morphologically normal cellular organelles (mitochondria, rough ER, and Golgi apparatus), numerous cell-cell processes, and large quantities of thick fibrils of well organized extracellular matrix.
On the 1st day in culture, bone-derived cells made intimate contact and exhibited large, mostly euchromatic nuclei and a rounded shape. Cell organelles such as rough ER, mitochondria, granules, and vesicles became visible (data not shown). On day 3, cells exhibited a more spindle-like shape and increasingly detached from each other (Fig. 1a, panel A). Cells had an irregular, elongated, and darkly stained nucleus and intercellular spaces widened. Cell organelles were clearly visible, i.e. rough ER, free ribosomes, Golgi apparatus, glycogen granules, and vesicles. Cells developed many microvilli-like and sheet-like cytoplasmic processes. By means of these processes, cells remained in contact with neighboring cells. The first indication of an extracellular matrix was in the immediate pericellular space near the cell membrane (Fig. 1a, panel A). On day 7, many bundles of extracellular matrix fibrils were clearly visible in the newly formed extracellular matrix (Fig. 1a, panel B). Extracellular matrix fibrils formed bundles of parallel oriented fibrils. Fibril bundles made contact with cell membranes, and fibrils increased more and more from day 10 onward (Fig. 1a, panel C). On day 14, intercellular spaces widened further and contained much more matrix. Typically cross-striated collagen fibrils, which had tight contact to the cell membrane, were visible, some of them arranged in bundles along the osteocytes others oriented more transversally (Fig. 1a, panel D).
To investigate the effect of RANKL on osteoclastogenesis, we used a culture system where bone-derived cells were cultured in a three-dimensional high density system in the presence of RANKL and/or resveratrol. In contrast to untreated cultures, high density cultures of bone tissue cells treated with RANKL were completely differentiated to osteoclasts-like cells (Fig. 1a, panels E–H). After 3 days of treatment, an extensive breakdown of bone matrix was observed, whereby the cell membrane detached from the matrix and a space developed between cell membrane and matrix. The bone-derived cells themselves responded to RANKL treatment with certain changes in their shape. Some cells became flat, initially assumed a polygonal shape and later took on an elongated shape, showed some processes, and some of them developed rounded cells (Fig. 1a, panel E). After 4–7 days, multinucleated osteoclasts were observed. Depending on its localization, the matrix was differently affected. Initially, the pericellular matrix was degraded; later on the entire matrix was affected (Fig. 1a, panel F). After 10 and 14 days of RANKL treatment in high density cultures, the number of bone-derived cells showing the above-mentioned changes had increased, and completely degraded matrix could be found (Fig. 1a, panels G and H).
Because RANKL signaling is critical for osteoclastogenesis, resveratrol effect on RANKL-induced osteoclastogenesis in high density cultures was examined. Bone-isolated cells were incubated with resveratrol (5 μm) for 4 h and then co-treated with RANKL (10 nm) and 5 μm resveratrol for the indicated time. As shown in Fig. 1a, panels I–L, resveratrol significantly inhibited RANKL-induced differentiation of osteoclasts. Pretreatment with resveratrol resulted in well developed osteoblast cultures with viable cells and well developed and organized cell organelles. The cells formed a dense and regular extracellular matrix (Fig. 1a, panels I–L). In addition, the number of osteoclasts decreased with increasing incubation time with resveratrol (Fig. 1b).
Demonstration of Collagen Type I and Osteocalcin in High Density Cultures Treated with or without RANKL by Immunoelectron Microscopy
To further characterize the quality of the extracellular matrix produced, immunoelectron microscopy was employed, and cultures were immunolabeled with antibodies against collagen type I (Fig. 1c, panels A and B), which is the most abundant collagen type found in bone matrix and osteocalcin, a bone matrix-specific proteoglycan (Fig. 1c, panels C and D). High levels of collagen type I expression were confirmed in untreated high density cultures and cultures pretreated with resveratrol and then co-treated with RANKL after 7 days (Fig. 1c, panels A and B); abundant quantities of gold particles could be observed directly on the bundles of thick extracellular matrix fibrils. Furthermore, strong labeling for osteocalcin was observed in untreated isolated bone tissue cultures and cultures pretreated with resveratrol and then co-treated with RANKL (Fig. 1c, panels C and D). As expected for osteocalcin, gold particles were clustered together indicating existence of osteocalcin between the mesh of the collagen fibers as is typical for proteoglycan distribution in extracellular matrix. Labeling of RANKL-treated cultures was not performed as it had occurred without matrix formation.
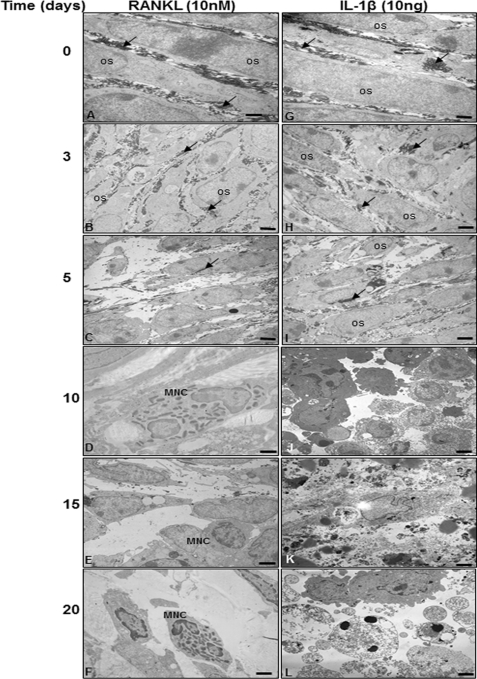
In High Density Culture of Bone-isolated Cells, RANKL Induced Osteoclastogenesis, but IL-1β Induced Apoptosis
To test and compare the results performed with RANKL-treated bone-isolated cells, the same investigations were performed in high density culture with IL-1β-treated bone-isolated cells as a negative control and finally analyzed with electron microscopy. Treatment with RANKL resulted in well developed multinucleated osteoclast-like cells with viable cells and well developed and organized cell organelles (Fig. 2, A–F). As further shown in Fig. 2, bone-derived cells underwent apoptosis when treated with IL-1β (10 ng/ml) for 10 days (Fig. 2J). IL-1β-treated bone-derived cells showed degenerative changes such as swelling of rough ER and clustering of swollen mitochondria and degeneration of other cell organelles. After longer incubation periods (15–20 days), more severe features of cellular degeneration were seen in response to IL-1β treatment (Fig. 2, K and L). These include areas of condensed heterochromatin in the cell nuclei. The bone-derived cells became more and more rounded, lost their microvilli-like processes, produced no matrix, and underwent apoptosis, the characteristic morphological features of this process being membrane blebbing, disintegration of the nucleus, chromatin condensation along the nuclear envelope, and the presence of apoptotic bodies (Fig. 2, K and L).
FIGURE 2.
RANKL-induced osteoclastogenesis in bone cells but IL-1β-induced apoptosis. Bone-derived cells (1 × 106 cells) were incubated in high density culture with RANKL (10 nm) or with IL-1β (10 ng/ml) for 20 days and then evaluated by transmission electron microscopy. Os, osteoblast; MNC, multinucleated cells; arrow, extracellular matrix. Magnification ×5000; bar, 1 μm.
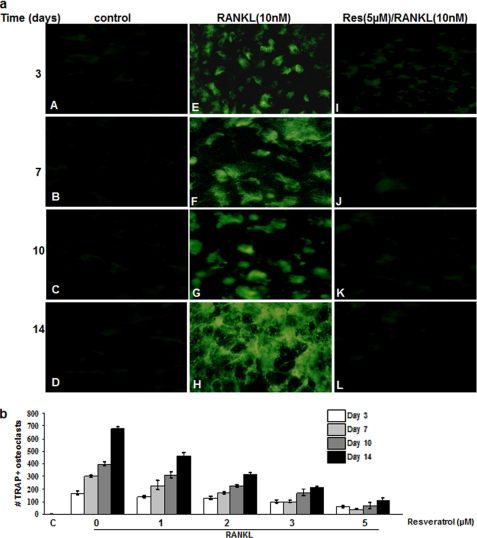
Resveratrol Inhibits RANKL-induced Osteoclastogenesis
Next, we examined the effects of resveratrol on RANKL-induced osteoclastic differentiation of bone-derived cells. The bone-derived cells cultured in the absence of RANKL failed to differentiate into TRAP-positive osteoclasts (Fig. 3a, panels A–D), but 3 days of treatment with 5 nm RANKL dramatically changed the morphology of cells and resulted in the appearance of TRAP-positive osteoclast-like cells (Fig. 3a, panels E–H). TRAP immunofluorescence staining at day 3 of RANKL stimulation revealed the presence of multinucleated TRAP-positive osteoclast-like cells (Fig. 3a, panels E–H), whereas unstimulated control cells (Fig. 3a, panels A–D) and pretreated cells (Fig. 3a, panels I–L) with resveratrol remained mononuclear and TRAP-negative. Statistical analysis demonstrated that RANKL-stimulated cultures displayed significantly higher TRAP activity 3, 7, 10, and 14 days of culture compared with control cells (Fig. 3b). In contrast, resveratrol reduced the formation of TRAP-positive osteoclasts in a concentration- and time-dependent manner (Fig. 3b). As little as 1 μm resveratrol had a significant effect on RANKL-induced TRAP-positive osteoclast formation. Complete inhibition of osteoclastogenesis was observed at 5 μm resveratrol concentration. Nonetheless, the viability of cells under these conditions was not affected at the concentrations used in this study.
FIGURE 3.
Resveratrol inhibits RANKL-induced osteoclastogenesis. a, bone-derived cells (1 × 106 cells) were incubated in high density culture with or without RANKL (10 nm) and with or without the indicated concentrations of resveratrol for 14 days and then immunostained for TRAP expression. Control was for cells exposed to medium alone (control). TRAP-positive cells were photographed (original magnification, ×40). b, TRAP-positive multinucleated osteoclasts were counted. Data represent the mean of three measurements. C, control.
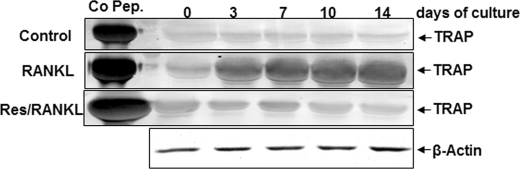
Resveratrol Suppresses RANKL-induced Expression of Osteoclastic Marker (TRAP) in High Density Cultures
To confirm the immunohistological results, we examined the expression of TRAP, as it is an early marker for osteoclastic lineage commitment (49), by immunoblotting assay. In untreated cultures of bone-derived cells, TRAP was not detected in high density cultures (Fig. 4). In contrast, in bone-derived cell cultures treated with RANKL, TRAP expression was significantly increased in a time-dependent manner (Fig. 4). Furthermore, in pretreated cultures with resveratrol and then co-treated with RANKL, TRAP was not detectable (Fig. 4).
FIGURE 4.
Resveratrol inhibits RANKL-dependent expression of TRAP in high density cultures. Bone-derived cells (1 × 106 cells) in high density culture were incubated either alone (control) or treated with RANKL (10 nm) for different times alone or pretreated with resveratrol (5 μm) for 4 h and then co-treated with 10 nm RANKL for the same time period indicated above. Whole cell extracts were prepared, and cell lysates were resolved by SDS-PAGE, electrotransferred to nitrocellulose membrane, and then probed for TRAP expression by Western blot analysis using antibodies to this protein. β-Actin served as an internal control. TRAP control peptide (Co Pep) was used as a control.
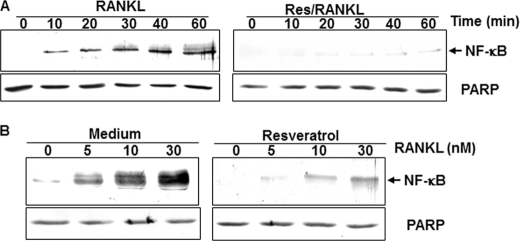
Resveratrol Suppresses RANKL-induced NF-κB Activation
To determine the effect of resveratrol on RANKL-induced NF-κB activation in bone-derived cells, we investigated the NF-κB signaling pathway. Cells were either pretreated with resveratrol for 4 h or left untreated, followed by 5 nm RANKL for 0, 10, 20, 30, 40, and 60 min in monolayer cultures. Whole cell lysates, cytoplasmic extracts, and nuclear extracts were taken at various time points and evaluated by Western blotting. As demonstrated in Fig. 5A, RANKL clearly induced the translocation of NF-κB in the nucleus in a time-dependent manner. As shown, the activation of NF-κB by RANKL was time-dependent. Furthermore, the activation of NF-κB induced by RANKL was clearly suppressed in resveratrol-pretreated cells (Fig. 5A). To determine whether resveratrol blocks RANKL-induced activation of NF-κB in a concentration-dependent manner by higher concentrations of RANKL, resveratrol-pretreated and -untreated cells were co-treated with different concentrations of RANKL for 50 min.
FIGURE 5.
Resveratrol suppresses RANKL-induced NF-κB activation in a time- and dose-dependent manner. A, bone-derived cells (1 × 106 cells) in monolayer culture were either untreated controls (left panel) or treated with resveratrol (5 μm) for 4 h and with 10 nm RANKL for the indicated times (right panel). Nuclear extracts were prepared and assayed for NF-κB activation by Western blot analysis as described under “Experimental Procedures.” B, bone cells (1 × 106 cells) in monolayer cultures either alone (left panel) or preincubated with the 5 μm resveratrol for 4 h were treated with the indicated concentrations of RANKL for 50 min. Nuclear extracts were prepared and assayed for NF-κB activation by Western blot analysis as described under “Experimental Procedures.” Synthesis of poly(ADP-ribose) polymerase (PARP) remained unaffected in nuclear extracts.
Nuclear extracts were prepared and analyzed for NF-κB activation by Western blotting. At a concentration of 30 nm, RANKL caused the maximum NF-κB activation in bone-derived cells. Resveratrol pretreatment, however, significantly suppressed the activation induced by either concentration of RANKL (Fig. 5B). Unlike Fig. 5A, however, the suppression of NF-κB activation induced by 10 nm RANKL was not complete.
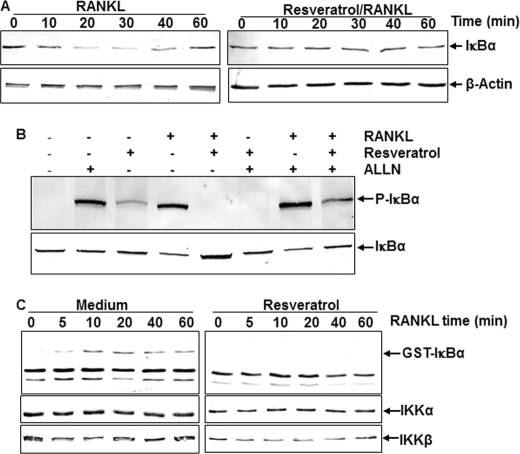
Resveratrol Suppresses RANKL-induced IκBα Degradation in Bone-derived Cells in Vitro
It is known that activation of NF-κB requires degradation of its inhibitory subunit IκBα (50). Bone-derived cells were incubated with RANKL (10 nm) either alone or in the presence of resveratrol (5 μm) for the indicated times. Cytoplasmic extracts were prepared, fractionated by 10% SDS-PAGE, and transferred to nitrocellulose membranes. To identify the mechanism by which resveratrol is involved in the inhibition of RANKL-induced NF-κB activation, IκBα expression was analyzed by Western blot analysis. In untreated control cells, IκBα expression decreased within 10 min after treatment with RANKL but returned to normal expression again within 60 min (Fig. 6A, left panel). Opposite to that, RANKL-induced IκBα degradation was completely suppressed in cells pretreated with resveratrol (Fig. 6A, right panel). Synthesis of the housekeeping protein β-actin remained unaffected, indicating that the effect was specific (Fig. 6A).
FIGURE 6.
Resveratrol suppresses RANKL-induced IkBα degradation (A) and phosphorylation (B) through inhibition of IKK activity (C). A, bone-derived cells (1 × 106 cells) in monolayer culture were incubated with RANKL (10 nm) either alone (left panel) or in the presence of resveratrol (5 μm) (right panel) for the indicated times. Cytoplasmic extracts were prepared, fractionated by SDS-PAGE, and electrotransferred to nitrocellulose membranes. Western blot analysis was performed with anti-IκBα. B, bone cells (1 × 106 cells) in monolayer culture were preincubated with 100 μm N-Ac-Leu-Leu-norleucinal (ALLN) for 30 min, resveratrol (5 μm) for 4 h, RANKL (10 nm) for 30 min, or the indicated combinations. Some cultures were left untreated and evaluated after 12 h. Cytoplasmic extracts (500 ng of protein per lane) were fractionated and subjected to Western blotting with phospho-specific IκBα antibody. The same membrane was re-blotted with antibodies to β-actin. C, bone cells (1 × 106 cells) in monolayer culture were incubated with RANKL (10 nm) either alone (left panel) or in the presence of resveratrol (5 μm) (right panel) for the indicated times. To examine the effect of resveratrol on the expression level of IKK proteins, whole cell extracts were immunoprecipitated with an antibody against IκB kinase (IKK)-α and analyzed by an immune complex kinase assay as described under “Experimental Procedures.” To examine the effect of resveratrol on the level of IKK proteins, whole cell extracts (500 ng of protein per lane) were fractionated by SDS-PAGE and examined using Western blotting with anti-IKK-α and anti-IKK-β antibodies. Data shown are representative of three independent experiments.
Resveratrol Suppresses RANKL-induced IκBα Phosphorylation
To test whether resveratrol is able to block the RANKL-induced phosphorylation of IκBα, serum-starved bone-derived cells were treated with RANKL for 1 h and examined by Western blot analysis using an antibody that is able to recognize the phosphorylated form of IκBα. It is known that phosphorylation of IκBα leads to its degradation (50), and the phosphorylation and degradation of IκBα were inhibited by a specific proteosome inhibitor N-Ac-Leu-Leu-norleucinal (39). As shown by Western blot analysis in Fig. 6B, RANKL was able to phosphorylate IκBα in cells pretreated with the inhibitor; the phosphorylation of IκBα was significantly higher compared with control cells. Interestingly, resveratrol was able to inhibit the phosphorylation of IκBα induced by RANKL in the presence or absence of the inhibitor (Fig. 6B).
Resveratrol Suppresses RANKL-induced IKK Activation
Resveratrol sustained RANKL-induced phosphorylation of IκBα by inhibition of proteasome function. Therefore, we determined the effect of resveratrol on RANKL-induced IKK activation, which is required for phosphorylation of IκBα. Immunocomplex kinase assays of bone-derived cells treated with RANKL clearly showed an IKK activity, as indicated by the phosphorylation of GST-IκBα within 5 min. In contrast, as shown in Fig. 6C, cells pretreated with resveratrol completely suppressed RANKL-induced activation of IKK. To see whether the decrease of IKK activity was because of the decrease of IKK protein expression, the levels of the IKK subunits IKK-α and IKK-β were analyzed by Western blotting. RANKL or resveratrol had no direct effect on the expression of IKK proteins (Fig. 6C).
RANKL-mediated p300 Expression
Transcriptional co-activators such as p300 (acetyltransferase) play critical roles in transcriptional activation (19, 20). However, the effect of RANKL on p300 function during osteoclastogenesis has not been investigated. We investigated whether the effects of RANKL on NF-κB are mediated by p300. It is now commonly accepted that p300 protein displays acetyltransferase activity that transfers an acetyl group to the ϵ-amino group of lysine residues located in histone or non-histone proteins (20).
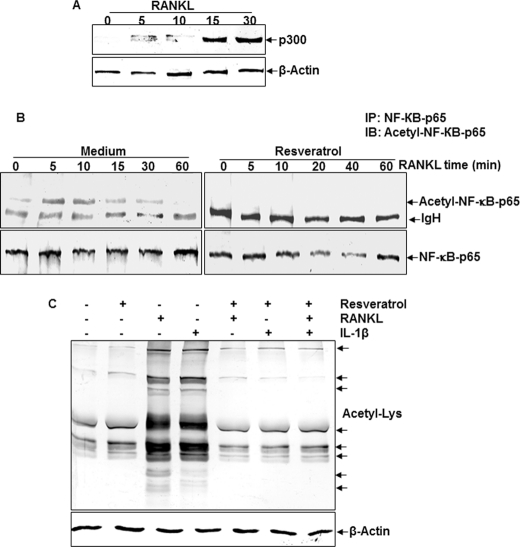
To assess the involvement of the RANKL in p300 signaling, we used an anti-p300 antibody in immunoblot experiments to identify p300 in RANKL-treated bone-derived cells. In bone-derived cell cultures treated with RANKL, p300 expression was significantly increased in a time-dependent manner (Fig. 7A).
FIGURE 7.
Analysis of RANKL-mediated p300 expression and resveratrol-mediated inhibition of RANKL-dependent p65 acetylation in bone-derived cells. A, detection of p300 expression. Bone-derived cells (1 × 106 cells) in monolayer culture were incubated with RANKL (10 nm) for the indicated times and immunoblotted (IB) with anti-p300 antibody or anti-actin antibody (control). B, effect of resveratrol on RANKL-induced acetylation of p65. Cells were treated with 5 μm resveratrol for 4 h and then exposed to 10 nm RANKL. Whole cell extracts were prepared, immunoprecipitated (IP) with an anti-p65 antibody, and subjected to Western blot analysis using an anti-acetyl-lysine antibody. The same blots were reprobed with an antibody to anti-p65. C, effect of resveratrol on RANKL- or IL-1β-induced protein acetylation. Cells were treated with 5 μm resveratrol for 4 h and then exposed to 10 nm RANK or 10 ng/ml IL-1β for 20 min. Whole cell extracts were prepared and subjected to Western blot analysis using an anti-acetyl-lysine antibody.
Resveratrol Inhibited Acetylation of p65
To test whether resveratrol can inhibit acetylation of p65, Western blot analysis provided evidence that RANKL induces the acetylation of p65, and furthermore, resveratrol inhibits the RANKL-induced acetylation (Fig. 7B). To determine whether resveratrol is able to block the RANKL-induced acetylation of several proteins, whole cell lysates from cells treated with RANKL, IL-1β, resveratrol, or a combination of both of them were analyzed by Western blot using anti-acetyl-lysine antibody. As shown in Fig. 7C, RANKL and IL-1β induced acetylation of several proteins, whereas resveratrol suppressed the acetylation of these proteins.
Resveratrol-activated SIRT-1 (Deacetylase) Inhibits p300 Acetyltransferase and NF-κB Signaling
SIRT-1 is a member of the sirtuin protein family of NAD-dependent deacetylases that mediates down-regulation of transcriptional activity and promotes cell survival (40–42). Resveratrol has been shown to activate SIRT-1 deacetylase activity (40). Phosphorylation of NF-κB-p65 is required for its transcriptional regulation, and acetylation of NF-κB-p65 by p300 acetyltransferase plays an essential role in regulating NF-κB signaling (26). Resveratrol-activated SIRT-1 deacetylase promotes SIRT-1-p300 complex formation resulting in inactivation of p300 acetyltransferase and a reduction in the acetylation of NF-κB-p65 (51).
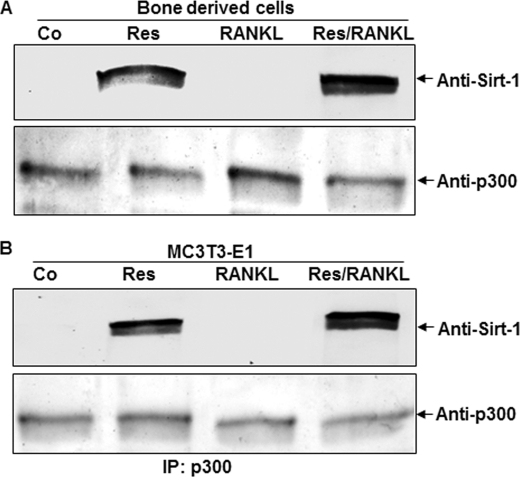
To examine the functional impact of SIRT-1 regulation of RANKL-mediated p300 signaling events (e.g. NF-κB pathways), cells were cultured in the presence or absence of resveratrol and in the presence of RANKL, or we pretreated bone-derived cells with resveratrol and then co-treated them with RANKL. Treatment of the bone-derived cells with resveratrol alone and pretreatment of bone-derived cells with resveratrol and co-treatment with RANKL markedly enhanced the formation of Sirt-1 complexes with p300 (Fig. 8A). To see whether this event occurs transiently during osteogenesis, we performed the same experiment with the preosteoblastic cell line (MC3T3-E1). The results obtained were similar to those with bone-derived cells (Fig. 8B). This may cause, in part, a significant reduction in RANKL-induced p300 acetyltransferase activity. It is noted that RANKL-p300-induced acetylation of NF-κB-p65 (Fig. 7B) is also inhibited by resveratrol. Consequently, transcriptional activities (mediated by NF-κB-p65) are attenuated (Fig. 7). These findings suggest that resveratrol-mediated activation of Sirt-1 plays an important role in inhibiting RANKL-activated p300 acetyltransferase and impairing the NF-κB-p65 signaling cascades during osteoclastogenesis.
FIGURE 8.
Analysis of resveratrol-mediated Sirt-1-p300 interaction in bone-derived cells and preosteoblastic MC3T3-E1 cells. Bone-derived cells (1 × 106 cells) and the preosteoblastic cell line (1 × 106 cells) in monolayer culture were either left untreated or treated with 5 μm resveratrol (Res) alone or treated with 10 mm RANKL alone for 3 days or pretreated with 5 μm resveratrol for 4 h and then exposed to 10 nm RANKL for 3 days. Cell lysates were immunoprecipitated (IP) with anti-p300 antibody followed by immunoblotting with anti-SIRT-1 antibody or reblotted with anti-p300 antibody (as a loading control (Co)).
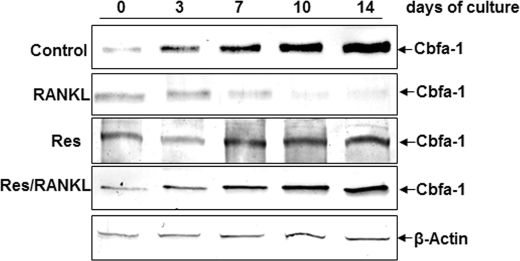
Resveratrol Suppresses RANKL-induced Inhibition of Specific Osteogenic Transcription Factor Cbfa-1 in High Density Cultures
Next, we examined the osteogenic transcription factor Cbfa-1 as it is an early marker for osteogenic lineage commitment (29). In untreated or with resveratrol alone-treated cultures of bone-derived cells, high quantities of Cbfa-1 expression were detected during osteogenesis in high density cultures (Fig. 9). In contrast to this, in bone-derived cell cultures treated with RANKL, Cbfa-1 expression was significantly decreased in a time-dependent manner (Fig. 9). Furthermore, in pretreated cultures with resveratrol and then co-treated with RANKL, Cbfa-1 was detected with enhanced expression (Fig. 9).
FIGURE 9.
Effects of resveratrol on RANKL-induced inhibition of Cbfa-1 production in bone cells. High density bone-derived cell cultures were either untreated or treated with 10 nm RANKL alone or with 5 μm resveratrol (Res) alone or pretreated with 5 μm resveratrol for 4 h and then cotreated with 10 nm RANKL for the indicated times. To evaluate the effects of resveratrol on RANKL-stimulated osteogenic inhibition in cultures, whole cell lysates (500 ng of protein per lane) were fractionated and then subjected to Western blotting with antibodies to the specific osteogenic transcription factor Cbfa-1. Synthesis of the housekeeping protein β-actin was unaffected.
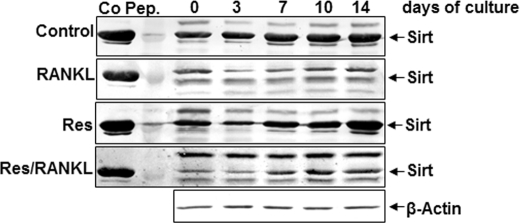
Resveratrol Suppresses RANKL-induced Inhibition of Sirt-1 in High Density Cultures
To show the possible mechanism for Cbfa-1 activation by resveratrol, cells were cultured in the presence or absence of resveratrol and in the presence of RANKL, or we pretreated bone-derived cells with resveratrol and then co-treated them with RANKL. As shown in Fig. 10, in untreated cells and with resveratrol-treated cells, the expression of Sirt increased in high density cultures. In contrast, when cells were treated with 10 nm RANKL, the expression of endogenous Sirt-1 was decreased. Interestingly, resveratrol pretreatment caused significant Sirt-1 up-regulation (Fig. 10).
FIGURE 10.
Effects of resveratrol on RANKL-induced inhibition of Sirt-1 production in bone tissue cells. High density bone cell cultures were either untreated or treated with 10 nm RANKL alone or with 5 μm resveratrol alone or pretreated with 5 μm resveratrol for 4 h and then cotreated with 10 nm RANKL for the indicated time periods. To evaluate the effects of resveratrol on RANKL-stimulated osteogenic inhibition in cultures, whole cell lysates (500 ng of protein per lane) were fractionated and subjected to Western blotting with antibodies to Sirt-1. Synthesis of the housekeeping protein β-actin was unaffected. Sirt-1 control peptide (Co Pep) was used as a control.
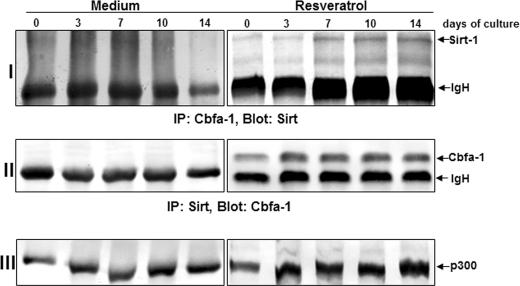
Association of Sirt-1 Proteins with the Early Osteogenic Transcription Factor Cbfa-1 by Resveratrol in Osteoblasts
To determine possible downstream signaling proteins in osteoblasts, we examined whether Sirt-1 associates with the early osteogenic transcription factor Cbfa-1 and subsequently activates the pathway that stimulates osteogenesis. To this end, cells were cultured in the presence or absence of resveratrol, lysed, and immunoprecipitated with anti-Sirt-1 or with anti-Cbfa-1 antibodies. The anti-Sirt-1 immunoprecipitates were then immunoblotted with anti-Cbfa-1 antibodies, and the anti-Cbfa-1 immunoprecipitates were then immunoblotted with anti-Sirt-1 antibodies. Interestingly, anti-Sirt-1 and anti-Cbfa-1 immunoprecipitates from resveratrol-stimulated but not from unstimulated cultures revealed that the Sirt-1 protein co-immunoprecipitated with the osteogenic transcription factor Cbfa-1 protein (Fig. 11, I and II). Moreover, the same immunoprecipitates were reblotted with anti-p300 antibody and showed a complex formation with p300-Sirt-1-Cbfa-1 (Fig. 11, III). Taken together, these results indicate that during osteogenesis resveratrol activates Sirt-1 and induces Sirt-Cbfa-1 complex formation, which activates the osteogenic pathway.
FIGURE 11.
Interaction of Sirt-1 with Cbfa-1 and p300 by resveratrol, as revealed by a co-immunoprecipitation assay. Cells were cultured in the presence or absence of resveratrol, lysed, and immunoprecipitated (IP) with anti-Sirt-1 or with anti-Cbfa-1 antibody. The immunoprecipitates were separated by SDS-PAGE and analyzed by immunoblotting using anti-Sirt-1 and anti-Cbfa-1 antibodies or reblotted with anti-p300 antibody (as a loading control). Results shown are representative of three independent experiments. IgH, immunoglobulin heavy chain.
DISCUSSION
The purpose of this study was to investigate the effects of resveratrol on RANKL-induced NF-κB activation and on osteoclastogenesis in primary isolated bone cells. Using the well established high density culture system for osteoclastogenesis and osteogenesis, we found that resveratrol inhibited RANKL-induced NF-κB activation through the suppression of IκBα phosphorylation, IκBα degradation, and IκBα kinase activity, thereby preventing multinucleated osteoclast formation. Moreover, we also found that resveratrol activates the bone tissue cells to osteoblast and osteogenesis through activation of the osteogenic transcription factor Cbfa-1 and NAD-dependent acetylase enzyme Sirt-1. More interestingly, we found that Sirt-1 associated with the Cbfa-1, enhancing transcription of bone specific collagen type I in a Cbfa-1-dependent fashion.
Resveratrol is a naturally occurring polyphenol, and it has been shown to exhibit anti-inflammatory and anti-oxidant effects in various cell types and has been suggested to have therapeutic potential for treatment of various diseases, including osteoporosis (52). Osteoporosis is characterized by a negative balance of bone metabolism, which leads to a decreased bone mass with subsequent thinning of the trabecular and cortical bone and a significantly greater risk of bone fractures (3). Therefore, it is of critical interest to study the mechanism by which resveratrol activates osteoblasts. It has been reported that resveratrol acts as a phytoestrogen and may exhibit variable degrees of estrogen receptor agonism (52). The similarity in structure between resveratrol and the synthetic estrogen diethylstilbestrol encouraged the investigators, and it was the basic hypothesis of this study to see whether resveratrol might exhibit estrogenic activity, a property that is known to produce a protective benefit in osteoporosis (53).
In this study, we investigated the effects of resveratrol on osteogenesis and bone metabolism and therefore we employed monolayer and high density in vitro models of bone tissue consisting of three different cell types as follows: osteoblasts and osteocytes, preosteoclasts, and undifferentiated mesenchymal cells. This cell population of bone tissue showed the direct effect of RANKL on osteoclast formation and allowed us to focus on the process of RANKL signaling in preosteoclasts.
The results indicate that RANKL induces NF-κB activation in osteoclastic cells through the activation of IKK and the subsequent phosphorylation and degradation of IκBα, a finding that is in complete agreement with previous in vitro studies (54). Furthermore, our results indicate that resveratrol abolishes the RANKL-induced IKK activation that is required for the phosphorylation of IκBα (50). Indeed, it has been recently shown that IKKs are major regulators of cytokine-induced osteoclastogenesis and inflammatory arthritis (55).
It has already been shown that NF-κB signaling plays an important role in osteoclastogenesis (56). RANKL activates NF-κB through the cell surface receptor RANK during osteoclastogenesis. Indeed, in double knock-out mice with NF-κB p50−/− and p52−/−, heavy osteopetrosis was caused by the failure of osteoclast formation (57, 58). This is an example that shows NF-κB p50 and p52 expression are necessary for the differentiation of RANK-expressing preosteoclast or osteoclast precursors into TRAP-positive osteoclasts in response to RANKL and other osteoclastogenic cytokines (59). It is known that NF-κB is activated after interaction between RANKL and RANK. Furthermore, osteoclast differentiation is inhibited by osteoprotegerin, which binds to RANKL and thereby prevents its interaction with RANK (60).
Several studies have already reported that NF-κB is activated by RANKL in different cell types (16, 61) and that it is required for osteoclast formation in vivo (57). Therefore, suppression of NF-κB activation might play an essential role during osteoclastogenesis. Interestingly, in this study, we note that suppression of NF-κB activation by resveratrol correlated with inhibition of osteoclastogenesis indicating that NF-κB activation is critical for RANKL-induced osteoclastogenesis.
As a phytoestrogen, resveratrol, is a potent activator of Sirt-1 deacetylase activity. In this study, we found that both p300 acetyltransferase and Sirt-1 deacetylase are expressed in bone-derived cells. In addition, we have shown that RANKL activates p300 acetyltransferase activity. Our results also demonstrate that RANKL-activated p300 acetyltransferase serves as a potent transcriptional regulator by acetylating NF-κB-p65. In fact, it has been reported that acetylation of RelA (p65) at lysine 310 is regulated by prior phosphorylation of serine 276 and 536. Furthermore, the phosphorylated and acetylated RelA displays enhanced transcriptional activity (26). Our results indicate that resveratrol may suppress RANKL-induced p65 acetylation through inhibition of histone acetyltransferase activity. In contrast, resveratrol-activated Sirt-1 deacetylase promoted Sirt-1-p300 complex formation during osteogenesis resulting in inactivation of p300 acetyltransferase and a reduction in the acetylation of NF-κB-p65 (Fig. 7B). These results highlight a novel functional interaction between RANKL-activated p300 acetyltransferase and phytoestrogen resveratrol-activated Sirt-1 deacetylase during the regulation of acetylation versus deacetylation states of NF-κB-p65 in osteogenesis/osteoclastogenesis. Consequently, whether acetylation (or deacetylation) of NF-κB modulates their functions during osteoclastogenesis/osteogenesis is a very important issue.
We also observed activation of the specific osteogenic transcription factor Cbfa-1 as a marker for osteogenic activity. Cbfa-1 is the earliest and most specific marker of osteogenesis (29). It has been shown that Cbfa-1 acts as an activator of transcription and can induce osteoblast-specific gene expression in fibroblasts and myoblasts in culture (29). Therefore, we examined the effect of resveratrol on the Cbfa-1 activity of bone-derived cells. In this study, Cbfa-1 activation was observed in control cells and resveratrol-treated primary isolated bone cells. Resveratrol dose-dependently increased Cbfa-1 activity and seemed to stimulate the proliferation and differentiation of osteoblasts.
In contrast, RANKL blocked the expression of Cbfa-1 in bone cells, and this was clearly inhibited by the presence of resveratrol. Therefore, these results suggest that resveratrol increases bone formation, at least in part, by Cbfa-1 activation. The inhibitory effect of resveratrol on RANKL-induced Cbfa-1 activation in osteoblastic cells may lead to the inhibition or differentiation from preosteoclastic cells and stem cells to osteoclasts and the stimulation of mineralization.
Finally, resveratrol is one of the most potent Sirt-1 activators. By binding to an as yet undefined binding site, it induces a conformational change in Sirt-1, inducing the acetylated substrate and NAD, resulting in an increased enzymatic activity (40). Furthermore, many Sirt-1 substrates are transcription factors and key regulators known to participate in embryonic growth and in neoplasia (i.e. the tumor suppressor gene p53, the nuclear factor-κB (NF-κB), the DNA repair factor Ku70, and the longevity-associated forkhead transcription factors (FoxOs) (62)). However, the relationship between Sirt-1 activity and osteogenesis is not fully elucidated and is still open to debate. Our results demonstrate clearly that the Sirt-1 protein is expressed in bone cells and is further stimulated in cultures treated with resveratrol. Sirt-1 has no inherent DNA binding ability, and it was thought that its effect is mediated through the Cbfa-1 transcription factor, which regulates expression of many bone-specific genes (29). In our study, co-immunoprecipitation experiments demonstrated that the two proteins associate in vitro. Consistent with this observation, Sirt-1 appears to deacetylate Cbfa-1 and thus Sirt-1 contributes to the maintenance of the osteoblast phenotype by direct regulation of factors such as Cbfa-1. Therefore, Cbfa-1 appears similar to a growing number of other transcription factors, such as p53, NF-κB, MyoD, and FOXO, in that it can be modified by acetylation/deacetylation.
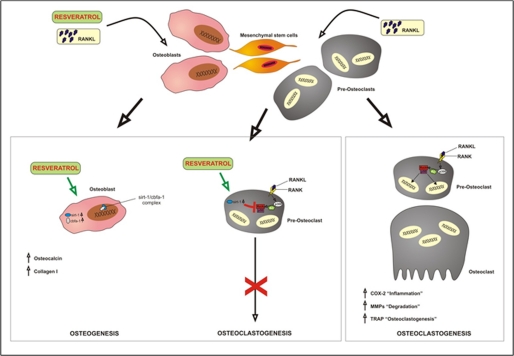
In conclusion, this study has provided important insights into the mechanisms by which RANKL-activated p300 functions and modulates NF-κB signaling, as well as how their target genes regulate osteoclastogenesis. We have further demonstrated that resveratrol can exert anti-osteoclastogenic effects, which is most likely mediated through the suppression of RANKL-p300-NF-κB activation (see the illustration in Fig. 12). These results provide the theoretical basis for a novel approach to treating osteoporosis and rheumatoid-related bone lesions using resveratrol and structurally related compounds. However, further studies are needed to understand the mechanistic details. For example, the cellular mechanism of action of Sirt-1 in resveratrol-mediated osteogenesis demands further investigation using appropriate in vivo models. Resveratrol may become a useful adjunct in the prevention and treatment of osteoporosis.
FIGURE 12.
A schematic model illustrating the effects of resveratrol on bone-derived cells and the mechanisms by which RANKL regulates osteoclast differentiation and function.
Acknowledgments
We thank Christina Pfaff and Ursula Schwikowski for excellent technical assistance and Marlene Eggeret and Azadeh Montaseri for additional support. We thank Madura Batuwangala for the artwork in Fig. 12.
Footnotes
- TRAP
- tartrate-resistant acid phosphatase
- RANKL
- receptor activator of NF-κB ligand
- AT
- ambient temperature
- IKK
- IκBα kinase
- ER
- endoplasmic reticulum.
REFERENCES
- 1. Robinson R. A. (1979) Johns Hopkins Med. J. 145, 10–24 [PubMed] [Google Scholar]
- 2. Raggatt L. J., Partridge N. C. (2010) J. Biol. Chem. 285, 25103–25108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Clarke B. L., Khosla S. (2010) Radiol. Clin. North Am. 48, 483–495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Suda T., Takahashi N., Martin T. J. (1992) Endocr. Rev. 13, 66–80 [DOI] [PubMed] [Google Scholar]
- 5. Drake F. H., Dodds R. A., James I. E., Connor J. R., Debouck C., Richardson S., Lee-Rykaczewski E., Coleman L., Rieman D., Barthlow R., Hastings G., Gowen M. (1996) J. Biol. Chem. 271, 12511–12516 [DOI] [PubMed] [Google Scholar]
- 6. Boyle W. J., Simonet W. S., Lacey D. L. (2003) Nature 423, 337–342 [DOI] [PubMed] [Google Scholar]
- 7. Janowska-Wieczorek A., Belch A. R., Jacobs A., Bowen D., Padua R. A., Paietta E., Stanley E. R. (1991) Blood 77, 1796–1803 [PubMed] [Google Scholar]
- 8. Manolagas S. C., Jilka R. L. (1995) N. Engl. J. Med. 332, 305–311 [DOI] [PubMed] [Google Scholar]
- 9. Pacifici R. (1998) Endocrinology 139, 2659–2661 [DOI] [PubMed] [Google Scholar]
- 10. Katagiri T., Takahashi N. (2002) Oral Dis. 8, 147–159 [DOI] [PubMed] [Google Scholar]
- 11. Kong Y. Y., Yoshida H., Sarosi I., Tan H. L., Timms E., Capparelli C., Morony S., Oliveira-dos-Santos A. J., Van G., Itie A., Khoo W., Wakeham A., Dunstan C. R., Lacey D. L., Mak T. W., Boyle W. J., Penninger J. M. (1999) Nature 397, 315–323 [DOI] [PubMed] [Google Scholar]
- 12. Yasuda H., Shima N., Nakagawa N., Yamaguchi K., Kinosaki M., Mochizuki S., Tomoyasu A., Yano K., Goto M., Murakami A., Tsuda E., Morinaga T., Higashio K., Udagawa N., Takahashi N., Suda T. (1998) Proc. Natl. Acad. Sci. U.S.A. 95, 3597–3602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Teitelbaum S. L. (2000) Science 289, 1504–1508 [DOI] [PubMed] [Google Scholar]
- 14. Bucay N., Sarosi I., Dunstan C. R., Morony S., Tarpley J., Capparelli C., Scully S., Tan H. L., Xu W., Lacey D. L., Boyle W. J., Simonet W. S. (1998) Genes Dev. 12, 1260–1268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Cao J., Venton L., Sakata T., Halloran B. P. (2003) J. Bone Miner. Res. 18, 270–277 [DOI] [PubMed] [Google Scholar]
- 16. Lacey D. L., Timms E., Tan H. L., Kelley M. J., Dunstan C. R., Burgess T., Elliott R., Colombero A., Elliott G., Scully S., Hsu H., Sullivan J., Hawkins N., Davy E., Capparelli C., Eli A., Qian Y. X., Kaufman S., Sarosi I., Shalhoub V., Senaldi G., Guo J., Delaney J., Boyle W. J. (1998) Cell 93, 165–176 [DOI] [PubMed] [Google Scholar]
- 17. Simonet W. S., Lacey D. L., Dunstan C. R., Kelley M., Chang M. S., Lüthy R., Nguyen H. Q., Wooden S., Bennett L., Boone T., Shimamoto G., DeRose M., Elliott R., Colombero A., Tan H. L., Trail G., Sullivan J., Davy E., Bucay N., Renshaw-Gegg L., Hughes T. M., Hill D., Pattison W., Campbell P., Sander S., Van G., Tarpley J., Derby P., Lee R., Boyle W. J. (1997) Cell 89, 309–319 [DOI] [PubMed] [Google Scholar]
- 18. Pettit A. R., Ji H., von Stechow D., Müller R., Goldring S. R., Choi Y., Benoist C., Gravallese E. M. (2001) Am. J. Pathol. 159, 1689–1699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Bannister A. J., Kouzarides T. (1996) Nature 384, 641–643 [DOI] [PubMed] [Google Scholar]
- 20. Ogryzko V. V., Schiltz R. L., Russanova V., Howard B. H., Nakatani Y. (1996) Cell 87, 953–959 [DOI] [PubMed] [Google Scholar]
- 21. Janknecht R., Hunter T. (1996) Nature 383, 22–23 [DOI] [PubMed] [Google Scholar]
- 22. Sterner D. E., Berger S. L. (2000) Microbiol. Mol. Biol. Rev. 64, 435–459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kiernan R. E., Vanhulle C., Schiltz L., Adam E., Xiao H., Maudoux F., Calomme C., Burny A., Nakatani Y., Jeang K. T., Benkirane M., Van Lint C. (1999) EMBO J. 18, 6106–6118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Gu W., Roeder R. G. (1997) Cell 90, 595–606 [DOI] [PubMed] [Google Scholar]
- 25. Martínez-Balbás M. A., Bauer U. M., Nielsen S. J., Brehm A., Kouzarides T. (2000) EMBO J. 19, 662–671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Chen Lf., Fischle W., Verdin E., Greene W. C. (2001) Science 293, 1653–1657 [DOI] [PubMed] [Google Scholar]
- 27. Graham B., Gibson S. B. (2005) Cell Cycle 4, 1342–1345 [DOI] [PubMed] [Google Scholar]
- 28. Rayet B., Gélinas C. (1999) Oncogene 18, 6938–6947 [DOI] [PubMed] [Google Scholar]
- 29. Ducy P. (2000) Dev. Dyn. 219, 461–471 [DOI] [PubMed] [Google Scholar]
- 30. Kanno T., Takahashi T., Tsujisawa T., Ariyoshi W., Nishihara T. (2007) J. Cell. Biochem. 101, 1266–1277 [DOI] [PubMed] [Google Scholar]
- 31. Baolin L., Inami Y., Tanaka H., Inagaki N., Iinuma M., Nagai H. (2004) Planta Med. 70, 305–309 [DOI] [PubMed] [Google Scholar]
- 32. Leiro J., Arranz J. A., Paramá A., Alvarez M. F., Sanmartín M. L. (2004) Dis. Aquat. Organ. 59, 171–174 [DOI] [PubMed] [Google Scholar]
- 33. Dong Z. (2003) Mutat. Res. 523, 145–150 [DOI] [PubMed] [Google Scholar]
- 34. Gusman J., Malonne H., Atassi G. (2001) Carcinogenesis 22, 1111–1117 [DOI] [PubMed] [Google Scholar]
- 35. Aggarwal B. B., Shishodia S. (2004) Ann. N.Y. Acad. Sci. 1030, 434–441 [DOI] [PubMed] [Google Scholar]
- 36. Manna S. K., Mukhopadhyay A., Aggarwal B. B. (2000) J. Immunol. 164, 6509–6519 [DOI] [PubMed] [Google Scholar]
- 37. Csaki C., Keshishzadeh N., Fischer K., Shakibaei M. (2008) Biochem. Pharmacol. 75, 677–687 [DOI] [PubMed] [Google Scholar]
- 38. Shakibaei M., John T., Seifarth C., Mobasheri A. (2007) Ann. N.Y. Acad. Sci. 1095, 554–563 [DOI] [PubMed] [Google Scholar]
- 39. Shakibaei M., Csaki C., Nebrich S., Mobasheri A. (2008) Biochem. Pharmacol. 76, 1426–1439 [DOI] [PubMed] [Google Scholar]
- 40. Howitz K. T., Bitterman K. J., Cohen H. Y., Lamming D. W., Lavu S., Wood J. G., Zipkin R. E., Chung P., Kisielewski A., Zhang L. L., Scherer B., Sinclair D. A. (2003) Nature 425, 191–196 [DOI] [PubMed] [Google Scholar]
- 41. McKinsey T. A., Zhang C. L., Olson E. N. (2002) Curr. Opin. Cell Biol. 14, 763–772 [DOI] [PubMed] [Google Scholar]
- 42. Lin S. J., Ford E., Haigis M., Liszt G., Guarente L. (2004) Genes Dev. 18, 12–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Shakibaei M., Schröter-Kermani C., Merker H. J. (1993) Histol. Histopathol. 8, 463–470 [PubMed] [Google Scholar]
- 44. Pittenger M. F., Mackay A. M., Beck S. C., Jaiswal R. K., Douglas R., Mosca J. D., Moorman M. A., Simonetti D. W., Craig S., Marshak D. R. (1999) Science 284, 143–147 [DOI] [PubMed] [Google Scholar]
- 45. Fatokun A. A., Stone T. W., Smith R. A. (2006) Bone 39, 542–551 [DOI] [PubMed] [Google Scholar]
- 46. Shakibaei M. (1998) Exp. Cell Res. 240, 95–106 [DOI] [PubMed] [Google Scholar]
- 47. Csaki C., Mobasheri A., Shakibaei M. (2009) Arthritis Res. Ther. 11, R165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Shakibaei M., John T., De Souza P., Rahmanzadeh R., Merker H. J. (1999) Biochem. J. 342, 615–623 [PMC free article] [PubMed] [Google Scholar]
- 49. Minkin C. (1982) Calcif. Tissue Int. 34, 285–290 [DOI] [PubMed] [Google Scholar]
- 50. Ghosh S., Karin M. (2002) Cell 109, S81–S96 [DOI] [PubMed] [Google Scholar]
- 51. Bourguignon L. Y., Spevak C. C., Wong G., Xia W., Gilad E. (2009) J. Biol. Chem. 284, 26533–26546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Baur J. A., Sinclair D. A. (2006) Nat. Rev. Drug. Discov. 5, 493–506 [DOI] [PubMed] [Google Scholar]
- 53. Lobo R. A. (1995) Am. J. Obstet. Gynecol. 173, 982–989 [DOI] [PubMed] [Google Scholar]
- 54. Wei S., Teitelbaum S. L., Wang M. W., Ross F. P. (2001) Endocrinology 142, 1290–1295 [DOI] [PubMed] [Google Scholar]
- 55. Dai S., Hirayama T., Abbas S., Abu-Amer Y. (2004) J. Biol. Chem. 279, 37219–37222 [DOI] [PubMed] [Google Scholar]
- 56. Boyce B. F., Xing L., Franzoso G., Siebenlist U. (1999) Bone 25, 137–139 [DOI] [PubMed] [Google Scholar]
- 57. Franzoso G., Carlson L., Xing L., Poljak L., Shores E. W., Brown K. D., Leonardi A., Tran T., Boyce B. F., Siebenlist U. (1997) Genes Dev. 11, 3482–3496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Iotsova V., Caamaño J., Loy J., Yang Y., Lewin A., Bravo R. (1997) Nat. Med. 3, 1285–1289 [DOI] [PubMed] [Google Scholar]
- 59. Xing L., Bushnell T. P., Carlson L., Tai Z., Tondravi M., Siebenlist U., Young F., Boyce B. F. (2002) J. Bone Miner. Res. 17, 1200–1210 [DOI] [PubMed] [Google Scholar]
- 60. Hsu H., Lacey D. L., Dunstan C. R., Solovyev I., Colombero A., Timms E., Tan H. L., Elliott G., Kelley M. J., Sarosi I., Wang L., Xia X. Z., Elliott R., Chiu L., Black T., Scully S., Capparelli C., Morony S., Shimamoto G., Bass M. B., Boyle W. J. (1999) Proc. Natl. Acad. Sci. U.S.A. 96, 3540–3545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Jimi E., Akiyama S., Tsurukai T., Okahashi N., Kobayashi K., Udagawa N., Nishihara T., Takahashi N., Suda T. (1999) J. Immunol. 163, 434–442 [PubMed] [Google Scholar]
- 62. Saunders L. R., Verdin E. (2007) Oncogene 26, 5489–5504 [DOI] [PubMed] [Google Scholar]