Abstract
Many tRNA synthetases are homodimers that are catalytically inactive as monomers. An example is the 528-amino acid human tyrosyl-tRNA synthetase, which is made up of an N-terminal catalytic unit (TyrRSMini) and a 164-amino acid C-domain. Although native TyrRS has no known cytokine functions, natural proteolysis of secreted TyrRS releases TyrRSMini, which not only has the same aminoacylation activity as native TyrRS but also has strong activity for stimulating migration of polymorphonuclear leukocytes. The migration-stimulating activity is dependent on an ELR tripeptide motif, similar to that in CXC cytokines like IL-8, and also has the familiar bell-shaped concentration dependence seen for CXC cytokines. Here we show that in contrast to IL-8, where the bell-shaped dependence arises from the effects of CXCR1/2 receptor internalization, TyrRSMini does not induce internalization of CXCR1/2. A rationally designed non-associating monomer and a non-dissociating dimer were constructed. With these constructs, the bell-shaped concentration dependence of leukocyte migration was shown to arise from the agonist (for migration) activity of the catalytically inactive monomer and the antagonist activity of the catalytically active dimer. Thus, the dissociating quaternary structure of TyrRSMini regulates two opposing cytokine activities and suggests the possibility of dissociating quaternary structures regulating novel functions of other tRNA synthetases.
Keywords: Aminoacyl tRNA Synthetase, Cell Migration, Cell Surface Receptor, Protein Domains, Protein Structure, Signal Transduction, Cell-signaling Functions of aaRS
Introduction
Aminoacyl-tRNA synthetases are a family of ancient enzymes essential for decoding genetic information in translation (1). Surprisingly, many tRNA synthetases and synthetase-binding proteins have been expropriated for alternative functions in biological pathways not directly connected to translation. These include roles in angiogenesis (2–5), the inflammatory response (6–8), and immunomodulation (9), involve interactions with cell surface receptors, mRNAs, and nuclear partners (10), and are conferred or regulated by novel domains and sequence motifs added progressively during the evolution of eukaryotes (11). Mechanisms for activation of these expanded functions include post-translational modification, e.g. phosphorylation (7, 10), alternative splicing, or natural proteolysis (4, 5, 12). The variety of disease-associations with tRNA synthetases that have been annotated (13–15), including the identification of casual mutations (16, 17), is thought to reflect, at least in part, the connections of tRNA synthetases to these alternative functions (11).
Tyrosyl-tRNA synthetase (TyrRS)3 is a homodimer throughout evolution. Although the catalytic site for attachment of activated tyrosine to the 3′-end of tRNATyr is entirely contained within the monomer unit, the dimeric state is required for amino acid activation (and therefore for aminoacylation activity) (18, 19). This requirement is due partially to a conformational change across the dimer interface (20). The overall design of TyrRS is different as the tree of life is ascended (21). For example, human TyrRS has a C-terminal domain not found in orthologs of lower eukaryotes, archaebacteria, or prokaryotes (see Fig. 1) (11, 22). Previous work showed that although native human TyrRS is inactive as a cytokine, under inflammatory conditions, it can be secreted and split by leukocyte elastase into two fragments having distinct cytokine activities (4). The C-terminal domain, which is the structural homolog of the mature form of human endothelial monocyte-activating polypeptide II (EMAP II), has potent leukocyte and monocyte chemotaxis activity and stimulates production of myeloperoxidase, tumor necrosis factor-α, and tissue factor (4). On the other hand, the N-terminal catalytic domain TyrRSMini functions in part as an interleukin-8 (IL-8)-like cytokine that stimulates migration of polymorphonuclear (PMN) cells through a mechanism that requires an ELR tripeptide motif like that found in CXC cytokines (4, 5, 23). This tripeptide motif is masked in TyrRS but is exposed when the C-domain is removed to release TyrRSMini. The stimulating effect of TyrRSMini on PMN cell migration has a bell-shaped concentration dependence (4, 24, 25), similar to that seen with IL-8 family members. In the case of IL-8, increasing concentrations are associated with desensitization through internalization of its CXCR1/2 receptors (26–28).
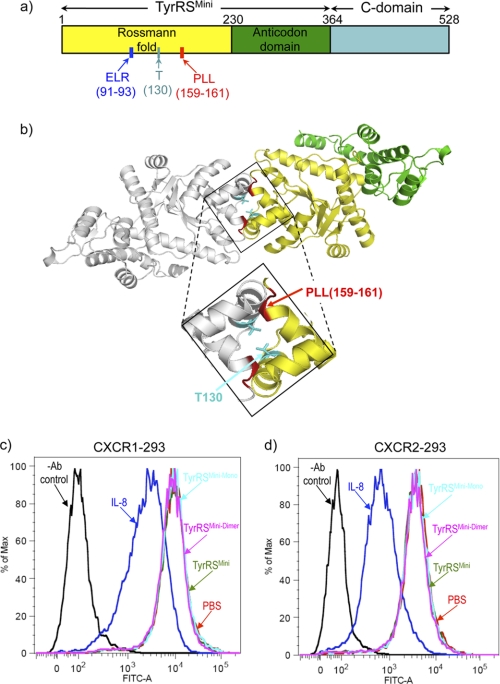
FIGURE 1.
a, schematic of the structural organization of human TyrRS, showing the N-terminal TyrRSMini domain that is orthologous to TyrRSs of lower eukaryotes and bacteria, the C-terminal EMAP II-like domain, and specific landmarks of the structure such as the critical (for cytokine signaling) ELR motif and the engineered residues used in this study. b, a rationally designed non-associating monomeric TyrRSMini and a disulfide-linked non-dissociating dimeric TyrRSMini. The structure of TyrRSMini dimer with individual monomers is shown in yellow and light gray (Protein Data Bank (PDB) 1q11), and the structure of the dimer interface with the location of a three-residue deletion (Pro-159, Leu-160, Leu-161) that disrupts TyrRSMini dimerization is highlighted in red. The Thr-130 that was replaced with Cys to create a disulfide-linked dimer is highlighted in turquoise. c and d, FACS analysis to probe CXCR1 (c) or CXCR2 (d) internalization upon treatment with TyrRSMini or with IL-8. CXCR1- and CXCR2-transfected HEK 293 cells were incubated with 100 nm TyrRSMini or IL-8 at 37 °C for 2 h. The cells were washed and stained with fluorescein-conjugated anti-α-CXCR1 or anti-α-CXCR2 antibodies and subjected to FACS analysis. The untreated cells were stained with either α-CXCR1 or α-CXCR1 (without TyrRSMini or IL-8 (labeled as PBS)). Control cells were not stained with α-CXCR1 or α-CXCR2 (−Ab control).
In exploratory experiments (see below), we found that although receptor internalization could be demonstrated for CXCR1- or CXCR2-expressing HEK 293 cells treated with IL-8, no such internalization was seen when the same cells were treated with TyrRSMini. To understand this observation, we considered the possibility that TyrRSMini was a monomer at concentrations (10 nm) where it acted as an agonist for PMN cell migration. Considering that the Kd for the monomer-dimer equilibrium of Neurospora crassa mitochondrial TyrRS (which is orthologous to TyrRSMini) is ∼100 nm (29) and considering the smaller dimer interface seen in our high-resolution (1.18 Å) structure of human TyrRSMini (22) as compared with that of N. crassa mitochondrial TyrRS (30), we inferred that the Kd of TyrRSMini would be ≥100 nm. These considerations led us to hypothesize that monomeric TyrRSMini is the active cytokine. However, this hypothesis alone does not explain the bell-shaped concentration dependence, and for that reason, we were also interested in exploring the role of the dimer that is formed at higher concentrations. For this purpose, we set out to determine the Kd for the monomer-dimer equilibrium and also to investigate the activities of rationally designed non-associating monomers and non-dissociating dimers. The results of these investigations support the view that the monomer-dimer equilibrium is a critical regulator of the cytokine function of human TyrRS.
EXPERIMENTAL PROCEDURES
Details of experimental protocols are given in the supplemental material. The receptor internalization assay was done with HEK 293 cells that stably express CXCR1 or CXCR2 (a gift from Dr. Adit Ben-Baruch at Tel Aviv University). The plasmid encoding wild-type (WT) human TyrRSMini was been cloned previously (4) and was used to generate TyrRSMini variants by site-directed mutagenesis using the QuikChangeTM mutagenesis kit from Stratagene (La Jolla, CA). Circular dichroism (CD) spectra were obtained with an Aviv model 400 CD spectrometer (Aviv Biomedical, Inc. Lakewood, NJ). Analytical gel chromatography was done by injecting each protein sample (200 μl of 10 μm) onto a Superdex 200 chromatography column (GE Healthcare, 10/300GL) in PBS containing 5 mm β-mercaptoethanol. Amino acid activation assays were performed at 25 °C as described previously (31), with some modifications. Receptor binding assays were done with CXCR1- and CXCR2-transfected HEK 293 cells that were incubated with purified His6-tagged WT TyrRSMini or TyrRSMini-Mono. The Transwell cell migration assay was done with human PMN cells that were prepared from heparin-treated whole blood obtained from healthy volunteers using the RosetteSep human granulocyte enrichment kit (StemCell Technologies, Vancouver, BC, Canada).
RESULTS
Testing for Receptor Internalization
Several laboratories provided evidence that internalization of CXCR1 and CXCR2 receptors upon treatment of CXCR1- or CXCR2-expressing HEK 293 cells with IL-8 accounts for the bell-shaped response of the migration of these cells to the concentration of exogenously added IL-8 (26, 27). We explored receptor internalization by using the same HEK 293 cells transfected with genes expressing either CXCR1 or CXCR2. Using FACS analysis with both the CXCR1 and the CXCR2 cell lines, we observed a strong reduction of the fluorescent signal from fluorescein-labeled α-CXCR1 or α-CXCR2 antibodies bound to cells treated with IL-8 (Fig. 1, c and d). This reduction corresponds to the change associated with receptor internalization. In contrast, treatment of the same cells with TyrRSMini induced no change, suggesting that TyrRSMini does not promote receptor internalization.
Rationally Designed Trapped Monomeric and Dimeric TyrRSMini
The Rossmann fold of all class I tRNA synthetases is split by an insertion known as connective polypeptide 1 (CP1) (32). This insertion makes important contacts for formation of the dimer interface of human TyrRSMini. In particular, our three-dimensional structure showed that Pro-159, Leu-160, and Leu-161 in CP1 are involved in backbone hydrogen-bonding interactions that stabilize the two subunits (22) (Fig. 1b). Previously, monomeric TyrRSMini (designated here as TyrRSMini-Mono) was generated by a Δ159–161 deletion, which resulted in a stable recombinant protein that could be expressed and purified in Escherichia coli (24). To generate a non-dissociating dimer, a disulfide trap strategy was employed. This strategy has been successfully used to create a non-dissociating IL-8 dimer (33–35). With this method, an exposed cysteine is introduced at the dimer interface of each individual monomer so that the -SH groups are spatially proximal in the dimer. Upon oxidation, a single disulfide link can form across the dimer interface.
Inspection of the structure of TyrRSMini suggested that the Thr-130 side chain OHs of each subunit were separated by only 3.5 Å and therefore could be tried as sites to introduce cysteine replacements (Fig. 1b). Recombinant T130C TyrRSMini was created and expressed in and purified from E. coli. Subsequent I2 oxidation led to the formation of a disulfide bond between the two subunits to give a stable dimer designated as TyrRSMini-Dimer (supplemental Fig. 1a).
As expected (18, 19, 21), TyrRSMini-Mono was inactive for aminoacylation. TyrRSMini-Dimer was also inactive. However, the uncross-linked T130C TyrRSMini was fully active, suggesting that flexibility of the dimer interface was needed for catalysis (supplemental Fig. 1b). Far-UV CD measurements (to monitor secondary structure) of TyrRSMini-Mono, TyrRSMini-Dimer, and TyrRSMini were similar (supplemental Fig. 1c), and the thermal melting profile showed no significant difference in the thermal stability of TyrRSMini-Mono as compared with TyrRSMini (supplemental Fig. 1d). Interestingly, TyrRSMini-Dimer had a melting curve with the same shape as those of TyrRSMini-Mono as compared with TyrRSMini but shifted 7.5 °C to higher temperatures, as expected for the extra stabilization provided by the covalent intersubunit linkage (supplemental Fig. 1d). These results collectively suggested that all three proteins were properly folded.
Investigation of Monomer-Dimer Equilibrium by Gel Filtration
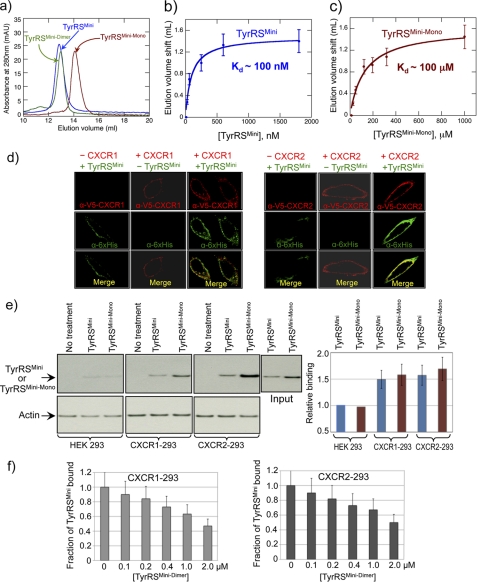
Analytical gel filtration chromatography, at a concentration of 10 μm, showed that as expected, TyrRSMini and TyrRSMini-Dimer eluted as dimers, whereas TyrRSMini-Mono migrated mostly as a monomer (Fig. 2a). By varying the protein concentration, the apparent dissociation constant for the dimer-monomer equilibrium of native TyrRSMini was estimated as a Kd value of ∼100 nm (Fig. 2b) In contrast, the apparent dissociation constant for TyrRSMini-Mono was estimated as ∼100 μm (Fig. 2c), ∼1000-fold higher than that of TyrRSMini.
FIGURE 2.
Determination of dimer-monomer equilibrium dissociation constants for WT and Δ159–161 TyrRSMini. a, analytical gel chromatography of WT, Δ159–161, and T130COX His6-tagged TyrRSMini. WT and T130COX peaks correspond to dimeric TyrRSMini (named TyrRSMini-Dimer), and Δ159–161 peak corresponds to monomeric TyrRSMini (named TyrRSMini-Mono). maU, milliabsorbance units. b and c, estimation of dimer-monomer equilibrium dissociation constant for WT TyrRSMini (b) or TyrRSMini-Mono (c) by gel filtration chromatography in combination with immunoblotting. Error bars correspond to mean ± S.D. from three independent experiments. d, TyrRSMini binds to CXCR1 and CXCR2 receptors transiently expressed in HeLa cells with V5-tagged CXCR1/2. Cells were fixed and doubly stained with anti-His6 (green) and anti-V5 (red) antibodies to detect TyrRSMini and CXCR1/2 receptors, respectively. e, TyrRSMini and TyrRSMini-Mono bind to CXCR1 and CXCR2 receptors expressed in HEK 293 cells. The bar graph was generated by normalizing binding to the input of TyrRSs and amount of actin (as a measure of cell concentration) and is shown as mean ± S.D. from three independent experiments. f, TyrRSMini-Dimer competes with WT TyrRSMini for binding to CXCR1 and CXCR2 receptors. CXCR1-293 and CXCR2-293 cells were incubated with 100 nm WT His6-tagged TyrRSMini or TyrRSMini-Mono at 4 °C for 1 h and then analyzed as described in the supplemental material. Data in bar graph format are shown as mean ± S.D. (n = 3 for each concentration of TyrRSMini-Dimer).
TyrRSMini-Mono and TyrRSMini-Dimer Bind to CXCR1 and CXCR2 Receptors
CXCR1 was initially identified as a receptor for TyrRSMini in PMN cells (4). Subsequent work established CXCR2 as a second receptor. For this purpose, we used confocal microscopy with HeLa cells transiently expressing CXCR1 or CXCR2. We chose HeLa cells because their morphology made them particularly amenable to visualizations by confocal microscopy. We generated HeLa cell lines transiently expressing CXCR1 or CXCR2 receptors. Twenty-four hours after cells were transfected, they were treated with purified His6-TyrRSMini for 1 h at 4 °C. After treatment, cells were washed twice with PBS and then fixed for immunofluorescence staining using anti-His6- and anti-V5-antibodies for TyrRSMini and CXCR1/2 receptors, respectively. Fig. 2d shows that cells expressing CXCR1 or CXCR2 (red staining) have a much higher density of binding TyrRSMini (green staining) than do parental HeLa cells. In further work, we investigated stably transfected HEK 293 cells that were stably transfected with each receptor. (The morphology on plates of HEK 293 cells makes them less amenable to visualization techniques using confocal microscopy.) HEK 293/CXCR1 or HEK 293/CXCR2 cells were incubated with 100 nm of purified His6-tagged TyrRSMini or TyrRSMini-Mono. The binding of exogenously added TyrRSs was detected with α-His6 antibodies. Fig. 2e shows that after treatment with 100 nm of either TyrRSMini or TyrRSMini-Mono and using Western blots (with α-His6 antibodies) of PAGE gels that resolved proteins bound to cells expressing either CXCR1 or CXCR2, TyrRSMini and TyrRSMini-Mono each bound to cells expressing either receptor. (Note that in these experiments, the input of TyrRSMini is less than that of TyrRSMini-Mono. After normalization to the input, the binding of TyrRSMini and TyrRSMini-Mono was closely similar.) In contrast, no binding was observed to the non-receptor-expressing parental HEK 293 cell line (Fig. 2e).
The α-His6 antibodies used in these studies only weakly reacted with TyrRSMini-Dimer, and for that reason, reliable data at 100 nm were not obtained and are omitted. As a way to study binding of TyrRSMini-Dimer, we investigated whether TyrRSMini-Dimer can compete with TyrRSMini for binding to the two receptors. We found that increasing concentrations (above 100 nm) of TyrRSMini-Dimer displaced binding of TyrRSMini (Fig. 2f).
Monomer Is an Agonist and Dimer Is an Antagonist of Induced PMN Cell Migration
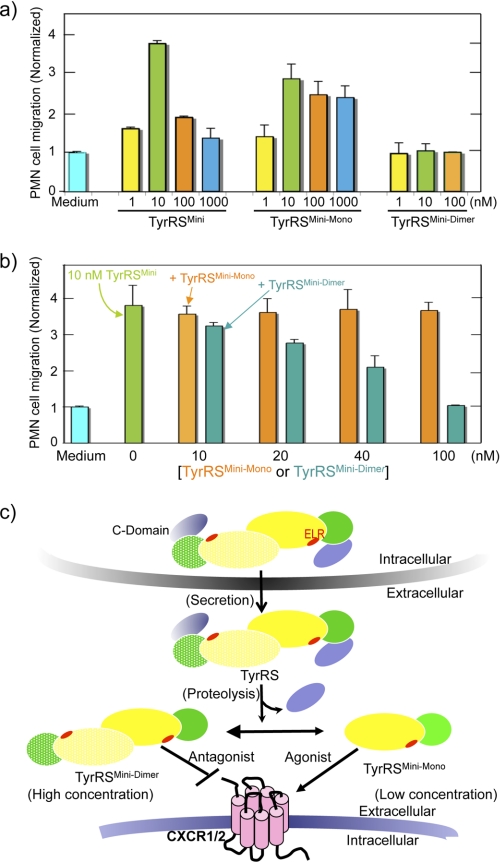
TyrRSMini has potent activity for inducing the migration of human PMN leukocytes (4, 24, 25). We investigated the PMN cell migration activity of TyrRSMini-Mono and TyrRSMini-Dimer. At low concentrations, TyrRSMini-Mono and TyrRSMini stimulated migration to roughly the same degree (compare Fig. 3a at 1 and at 10 nm). (Because the apparent Kd for the dimer-monomer equilibrium of TyrRSMini is ∼100 nm, TyrRSMini is predominantly a monomer at low nanomolar concentrations.) At higher concentrations, TyrRSMini showed the characteristic bell-shaped dependence of migration on concentration. In contrast, TyrRSMini-Mono, at concentrations up to 1 μm, did not show a large diminution in stimulation of cell migration. In contrast, no PMN cell migration activity was observed when TyrRSMini-Dimer was used. Collectively, these results showed that the monomer of TyrRSMini is the active and the dimer is the inactive ligand for PMN cell migration.
FIGURE 3.
Effects of TyrRSMini, TyrRSMini-Mono, and TyrRSMini-Dimer on PMN cell migration activity. a, TyrRSMini-Dimer inhibits WT TyrRSMini-induced PMN cell migration. b, human PMN cell migration was performed using a Transwell permeable support with 5-μm pores as described in the supplemental material. Data from at least four experiments were collected, and mean ± S.D. is shown. c, a schematic diagram illustrating a proposed model for how TyrRSMini mediates and regulates cytokine signaling. In this model, the dissociated monomer form of homodimeric TyrRSMini is the active cytokine, and the dimer is a non-functional antagonist. Dimeric TyrRSMini is labeled with individual monomers shown in lemon and banana, and the ELR motif is labeled in red.
The decrease in cell migration activity of TyrRSMini as the concentration was raised coincides with the concomitant increase in the dimer-to-monomer ratio of TyrRSMini. Because TyrRSMini-Dimer is not active for stimulation of cell migration and because it competes with TyrRSMini for binding to CXCR1 or CXCR2, we speculated that the dimer is a non-functional receptor-binding form that, at high concentrations, blocks binding of monomeric TyrRSMini. Because it can block monomeric TyrRSMini, the cell migration activity is reduced and, at the limit of high concentrations, there should be little or no migration. To test this hypothesis, we performed competitive cell migration assays in which we pretreated PMN cells with progressive increases in the concentration of TyrRSMini-Mono or TyrRSMini-Dimer. Consistent with the bell-shaped response of PMN cell migration to the concentration of TyrRSMini being caused by the antagonist activity of the dimer, TyrRSMini, but not TyrRSMini-Mono, inhibited cell migration in a dose-dependent manner (Fig. 3b).
Lastly, the lack of receptor internalization seen with TyrRSMini was also seen with TyrRSMini-Mono and TyrRSMini-Dimer (Fig. 1, c and d). Thus, other than the difference of agonist versus antagonist activity, the rationally designed TyrRSMini-Mono and TyrRSMini-Dimer behave like TyrRSMini.
DISCUSSION
Fig. 3c summarizes our results showing that dimer dissociation switches secreted TyrRSMini between two opposing activities for cell signaling. Thus, the present work supports an emerging theme of a role for alternative quaternary structures in the novel functions of human tRNA synthetases. For example, mutations in homodimeric glycyl-tRNA synthetase (GlyRS) are casually linked to Charcot-Marie-Tooth disease, the most common peripheral neuropathy (16). A detailed analysis in the light of a high-resolution structure showed that these mutations are in and around the dimer interface (36). In another example, homodimeric human lysyl-tRNA synthetase (LysRS), which is packaged into the HIV virion with the tRNALys3 primer (for reverse transcription) and the Gag protein, is proposed to dissociate into a monomer that bridges between tRNALys and Gag (37). However, in contrast to these examples where the role of the monomer-dimer equilibrium is inferred, we provide here direct evidence for the functional significance of this equilibrium.
The use of a monomer-dimer equilibrium to regulate cytokine function is seen with other CXCR1/2-acting cytokines such as IL-8 and NAP-2 (neutrophil-activating peptide 2). In the case of these cytokines, whereas the monomer-dimer equilibrium affects binding and signaling, an agonist/antagonist relationship has not yet been worked out (34, 38, 39). It will be of interest to investigate whether this agonist/antagonist relationship for two quaternary forms of TyrRSMini is also mirrored in the alternative forms of IL-8 and NAP-2.
From an evolutionary perspective, because the dimeric form of TyrRS is universal and therefore was present before the emergence of higher eukaryotes, the cytokine function per se was not the selective pressure for dimer formation. Instead, a pre-existing monomer-dimer equilibrium was adopted for a regulatory role, at the time of insects, when the critical (for cytokine signaling) ELR motif and EMAP II-like C-domain were simultaneously incorporated into TyrRS(11). As stated above, the dimer interface of human TyrRS is looser than that of a bacterial ortholog. Our calculated buried surface area from formation of the homodimer is 1129 and 1392 Å2 for human (22) and Bacillus stearothermophilus TyrRS (40), respectively.4 Possibly, the development of a looser dimer interface that enabled a more facile monomer-dimer equilibrium may have occurred at the same time as the adoption of the ELR motif and EMAP II-like C-domain.
By analogy with kcat and Km parameters for catalysis of aminoacylation, the antagonist activity of the TyrRSMini dimer suggests that the synthetase has kcat (for signaling) and Km (for binding) parameters for its interaction with the CXCR1/2 receptors. The concentration dependence of the inhibition of the monomer-induced cell migration by the dimer suggests that the apparent Km values for the monomer and dimer are similar (about 10–50 nm (Fig. 3b)). In contrast, to achieve the apparent complete inactivity of the dimer in the cell migration assay (Fig. 3a), we estimate that the kcat for signaling by the dimer must be reduced at least 10-fold as compared with that of the monomer to make it an operational antagonist. Possibly, the dimer interface has determinants that block a conformational change needed for activation of signaling after TyrRSMini is bound to CXCR1/2.
Supplementary Material
This work was supported, in whole or in part, by National Institutes of Health Grants CA92577 (from the NCI) and GM23562 (to P. S.). This work was also supported by a fellowship from the National Foundation for Cancer Research.

The on-line version of this article (available at http://www.jbc.org) contains supplemental Fig. 1 and supplemental Experimental Procedures.
X. L. Yang, unpublished results.
- TyrRS
- tyrosyl-tRNA synthetase
- EMAP II
- endothelial monocyte-activating polypeptide II
- PMN
- polymorphonuclear.
REFERENCES
- 1. Ibba M., Soll D. (2000) Annu. Rev. Biochem. 69, 617–650 [DOI] [PubMed] [Google Scholar]
- 2. Ewalt K. L., Schimmel P. (2002) Biochemistry 41, 13344–13349 [DOI] [PubMed] [Google Scholar]
- 3. Otani A., Slike B. M., Dorrell M. I., Hood J., Kinder K., Ewalt K. L., Cheresh D., Schimmel P., Friedlander M. (2002) Proc. Natl. Acad. Sci. U.S.A. 99, 178–183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Wakasugi K., Schimmel P. (1999) Science 284, 147–151 [DOI] [PubMed] [Google Scholar]
- 5. Wakasugi K., Schimmel P. (1999) J. Biol. Chem. 274, 23155–23159 [DOI] [PubMed] [Google Scholar]
- 6. Park S. G., Kim H. J., Min Y. H., Choi E. C., Shin Y. K., Park B. J., Lee S. W., Kim S. (2005) Proc. Natl. Acad. Sci. U.S.A. 102, 6356–6361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Sampath P., Mazumder B., Seshadri V., Gerber C. A., Chavatte L., Kinter M., Ting S. M., Dignam J. D., Kim S., Driscoll D. M., Fox P. L. (2004) Cell 119, 195–208 [DOI] [PubMed] [Google Scholar]
- 8. Mukhopadhyay R., Jia J., Arif A., Ray P. S., Fox P. L. (2009) Trends Biochem. Sci. 34, 324–331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Levine S. M., Rosen A., Casciola-Rosen L. A. (2003) Curr. Opin. Rheumatol. 15, 708–713 [DOI] [PubMed] [Google Scholar]
- 10. Yannay-Cohen N., Carmi-Levy I., Kay G., Yang C. M., Han J. M., Kemeny D. M., Kim S., Nechushtan H., Razin E. (2009) Mol. Cell 34, 603–611 [DOI] [PubMed] [Google Scholar]
- 11. Guo M., Yang X. L., Schimmel P. (2010) Nat. Rev. Mol. Cell Biol. 11, 668–674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Wakasugi K., Slike B. M., Hood J., Ewalt K. L., Cheresh D. A., Schimmel P. (2002) J. Biol. Chem. 277, 20124–20126 [DOI] [PubMed] [Google Scholar]
- 13. Antonellis A., Green E. D. (2008) Annu. Rev. Genomics Hum. Genet 9, 87–107 [DOI] [PubMed] [Google Scholar]
- 14. Park S. G., Schimmel P., Kim S. (2008) Proc. Natl. Acad. Sci. U.S.A. 105, 11043–11049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Schimmel P. (2008) Protein Sci. 17, 1643–1652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Antonellis A., Ellsworth R. E., Sambuughin N., Puls I., Abel A., Lee-Lin S. Q., Jordanova A., Kremensky I., Christodoulou K., Middleton L. T., Sivakumar K., Ionasescu V., Funalot B., Vance J. M., Goldfarb L. G., Fischbeck K. H., Green E. D. (2003) Am. J. Hum. Genet. 72, 1293–1299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Jordanova A., Irobi J., Thomas F. P., Van Dijck P., Meerschaert K., Dewil M., Dierick I., Jacobs A., De Vriendt E., Guergueltcheva V., Rao C. V., Tournev I., Gondim F. A., D'Hooghe M., Van Gerwen V., Callaerts P., Van Den Bosch L., Timmermans J. P., Robberecht W., Gettemans J., Thevelein J. M., De Jonghe P., Kremensky I., Timmerman V. (2006) Nat. Genet. 38, 197–202 [DOI] [PubMed] [Google Scholar]
- 18. Ward W. H., Fersht A. R. (1988) Biochemistry 27, 5525–5530 [DOI] [PubMed] [Google Scholar]
- 19. Jones D. H., McMillan A. J., Fersht A. R., Winter G. (1985) Biochemistry 24, 5852–5857 [DOI] [PubMed] [Google Scholar]
- 20. Yaremchuk A., Kriklivyi I., Tukalo M., Cusack S. (2002) EMBO J. 21, 3829–3840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Bonnefond L., Giegé R., Rudinger-Thirion J. (2005) Biochimie 87, 873–883 [DOI] [PubMed] [Google Scholar]
- 22. Yang X. L., Skene R. J., McRee D. E., Schimmel P. (2002) Proc. Natl. Acad. Sci. U.S.A. 99, 15369–15374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Liu J., Yang X. L., Ewalt K. L., Schimmel P. (2002) Biochemistry 41, 14232–14237 [DOI] [PubMed] [Google Scholar]
- 24. Yang X. L., Kapoor M., Otero F. J., Slike B. M., Tsuruta H., Frausto R., Bates A., Ewalt K. L., Cheresh D. A., Schimmel P. (2007) Chem. Biol. 14, 1323–1333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kapoor M., Otero F. J., Slike B. M., Ewalt K. L., Yang X. L. (2009) Chem. Biol. 16, 531–539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Feniger-Barish R., Ran M., Zaslaver A., Ben-Baruch A. (1999) Cytokine 11, 996–1009 [DOI] [PubMed] [Google Scholar]
- 27. Richardson R. M., Pridgen B. C., Haribabu B., Ali H., Snyderman R. (1998) J. Biol. Chem. 273, 23830–23836 [DOI] [PubMed] [Google Scholar]
- 28. Rose J. J., Foley J. F., Murphy P. M., Venkatesan S. (2004) J. Biol. Chem. 279, 24372–24386 [DOI] [PubMed] [Google Scholar]
- 29. Saldanha R. J., Patel S. S., Surendran R., Lee J. C., Lambowitz A. M. (1995) Biochemistry 34, 1275–1287 [DOI] [PubMed] [Google Scholar]
- 30. Paukstelis P. J., Chen J. H., Chase E., Lambowitz A. M., Golden B. L. (2008) Nature 451, 94–97 [DOI] [PubMed] [Google Scholar]
- 31. Chong Y. E., Yang X. L., Schimmel P. (2008) J. Biol. Chem. 283, 30073–30078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Starzyk R. M., Webster T. A., Schimmel P. (1987) Science 237, 1614–1618 [DOI] [PubMed] [Google Scholar]
- 33. Clark-Lewis I., Kim K. S., Rajarathnam K., Gong J. H., Dewald B., Moser B., Baggiolini M., Sykes B. D. (1995) J. Leukoc. Biol. 57, 703–711 [DOI] [PubMed] [Google Scholar]
- 34. Leong S. R., Lowman H. B., Liu J., Shire S., Deforge L. E., Gillece-Castro B. L., McDowell R., Hébert C. A. (1997) Protein Sci. 6, 609–617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Rajarathnam K., Prado G. N., Fernando H., Clark-Lewis I., Navarro J. (2006) Biochemistry 45, 7882–7888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Nangle L. A., Zhang W., Xie W., Yang X. L., Schimmel P. (2007) Proc. Natl. Acad. Sci. U.S.A. 104, 11239–11244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Guo M., Shapiro R., Morris G. M., Yang X. L., Schimmel P. (2010) J. Phys. Chem. B 114, 16273–16279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Das S. T., Rajagopalan L., Guerrero-Plata A., Sai J., Richmond A., Garofalo R. P., Rajarathnam K. (2010) PLoS One 5, e11754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Nasser M. W., Raghuwanshi S. K., Grant D. J., Jala V. R., Rajarathnam K., Richardson R. M. (2009) J. Immunol. 183, 3425–3432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Brick P., Bhat T. N., Blow D. M. (1989) J. Mol. Biol. 208, 83–98 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.