Abstract
Peroxiredoxin 6 (Prdx6), a bifunctional enzyme with glutathione peroxidase and phospholipase A2 (PLA2) activities, participates in the activation of NADPH oxidase 2 (NOX2) in neutrophils, but the mechanism for this effect is not known. We now demonstrate that Prdx6 is required for agonist-induced NOX2 activation in pulmonary microvascular endothelial cells (PMVEC) and that the effect requires the PLA2 activity of Prdx6. Generation of reactive oxygen species (ROS) in response to angiotensin II (Ang II) or phorbol 12-myristate 13-acetate was markedly reduced in perfused lungs and isolated PMVEC from Prdx6 null mice. Rac1 and p47phox, cytosolic components of NOX2, translocated to the endothelial cell membrane after Ang II treatment in wild-type but not Prdx6 null PMVEC. MJ33, an inhibitor of Prdx6 PLA2 activity, blocked agonist-induced PLA2 activity and ROS generation in PMVEC by >80%, whereas inhibitors of other PLA2s were ineffective. Transfection of Prx6 null cells with wild-type and C47S mutant Prdx6, but not with mutants of the PLA2 active site (S32A, H26A, and D140A), “rescued” Ang II-induced PLA2 activity and ROS generation. Ang II treatment of wild-type cells resulted in phosphorylation of Prdx6 and its subsequent translocation from the cytosol to the cell membrane. Phosphorylation as well as PLA2 activity and ROS generation were markedly reduced by the MAPK inhibitor, U0126. Thus, agonist-induced MAPK activation leads to Prdx6 phosphorylation and translocation to the cell membrane, where its PLA2 activity facilitates assembly of the NOX2 complex and activation of the oxidase.
Keywords: MAP Kinases (MAPKs), Perfusion, Peroxidase, Phorbol Esters, Reactive Oxygen Species (ROS), Angiotensin II
Introduction
Reactive oxygen species (ROS)2 comprising O2⨪, H2O2, ·OH, and others, are now regarded as important signaling molecules in biological systems. For example, H2O2 modulates cell growth, apoptosis, and various other endothelial cell functions (1, 2). NADPH oxidases (NOXs) are a family of seven widely distributed enzymes that enzymatically generate O2⨪ and, through dismutation, H2O2 (3). Of these, NOX2 is the canonical NOX responsible for O2⨪ generation by the respiratory burst in neutrophils. NOX2 is also found in other cell types and is a major source of ROS in endothelial cells (1, 2). In the unstimulated state, NOX2 is quiescent, with the intrinsic membrane subunits (cytochrome b558 comprising gp91phox and p22phox) and cytosolic subunits (Rac1, p47phox, p67phox, and p40phox) confined to their respective compartments (4). Activation of the enzyme complex by stimuli such as angiotensin II (Ang II), phorbol esters, or thrombin leads to translocation of the cytosolic components to the plasma membrane, resulting in assembly of the oxidase complex. Cytochrome b558 with its heme, NADPH, and flavin binding sites functions to transfer electrons from NADPH to oxygen, thereby generating O2⨪ in the extracellular space. Dismutation of O2⨪, either catalyzed or spontaneous, generates H2O2, which is regarded as the primary ROS signaling molecule in endothelium (1, 2).
Peroxiredoxins are a family of antioxidants that express peroxidase activity and catalyze the removal of H2O2 and other hydroperoxides (5). Of the six mammalian members of the family, peroxiredoxin 6 (Prdx6) is the only peroxiredoxin with both phospholipase A2 (PLA2) and peroxidase activities (6). The PLA2 activity catalyzes the hydrolysis of the acyl group at the sn-2 position of glycerophospholipids, with special affinity for phosphatidylcholine, to produce free fatty acids and a lysophospholipid; the peroxidase activity uses glutathione as the co-factor for reduction of hydroperoxides, including phospholipid hydroperoxides (6). In a previous study, Prdx6 (therein called p29) was found to participate in the activation of NOX2 in human neutrophils (7). However, the mechanism by which Prdx6 might activate NOX2-mediated ROS generation was not determined. Here, we investigated the role of Prdx6 in NOX2 activation in alveolar macrophages and in the endothelium in situ and in vitro. We found that phosphorylation of Prdx6 through MAPK activity causes its translocation to the cell membrane, and the resultant PLA2 activity leads to assembly of the NOX2 enzyme complex and ROS generation.
EXPERIMENTAL PROCEDURES
Materials
Horseradish peroxidase (HRP) and the fluorescent dyes dihydrodichlorofluorescein (H2DCF) diacetate, Amplex Red, and dihydroethidium (HE) were obtained from Invitrogen. Ang II was from Bachem Bioscience (Torrance, CA). Phorbol 12-myristate 13-acetate (PMA) and non-radioactive lipids were from Sigma; ERK2 was from Upstate (Temecula, CA). 1-Palmitoyl, 2-[3H]palmitoyl, sn-glycero-3-phosphocholine (3H-DPPC) was from American Radiolabeled Chemicals (St. Louis, MO). pGFP-C1 and pIRES2-ZsGreen1 vector plasmids were obtained from Clontech. Inhibitors used were bromoenol lactone (BEL) (Cayman Chemical, Ann Arbor, MI), arachidonyltrifluoromethyl ketone (AACOCF3) (Calbiochem), p-bromophenacyl bromide (pBPB), and 1-hexadecyl-3-trifluoroethylglycero-sn-2-phosphomethanol (MJ33) (Sigma-Aldrich), and MEK inhibitor U0126 (Promega, Madison, WI). Rat recombinant Prdx6 was generated in Escherichia coli and purified by HPLC (8). For phosphorylated Prdx6, the purified protein was incubated in vitro for 90 min with ERK2 MAPK as described previously, resulting in ∼45% phosphorylation of the protein (8).
Antibodies utilized were monoclonal anti-flotillin (BD Transduction Laboratories, Lexington, KY), polyclonal anti-gp91phox (NOX2) (Santa Cruz Biotechnology, Inc., Santa Cruz, CA), polyclonal anti-p47phox and anti-Rac1 (Upstate), monoclonal anti-Rac1 (Abcam, Cambridge, MA), and monoclonal anti-platelet endothelial cell adhesion molecule 1 (PECAM-1) (BD Pharmingen). Polyclonal anti-mouse Prdx6 antibody was produced in rabbits using recombinant, full-length mouse Prdx6 with a C-terminal His tag as the immunogen. The antibody subsequently was affinity-purified using full-length mouse Prdx6 (without the His tag) covalently bound to Sepharose. The rabbit immunizations and serum collection were handled by Covance Research Products (Denver, CO). Unless otherwise indicated, this antibody was used for all studies of Prdx6 immunoreactivity. A polyclonal antibody to phosphorylated Prdx6 was generated using as the antigen a phosphorylated peptide corresponding to the region surrounding threonine 177 (peptide sequence: TGTKPVApTPVDWKKG, where pT represents the phosphothreonine) with a cysteine added at the amino terminus for coupling to a protein carrier. Generation of the peptide, immunization of rabbits, and antibody purification using affinity columns was by Proteintech Group (Chicago, IL). Separate pools of antibody that either recognized phosphorylated Prdx6 or were phosphorylation-indifferent (recognized total Prdx6) were obtained by this purification scheme.
Animals
The use of mice for these studies was approved by the University of Pennsylvania Animal Care and Use Committee. Three types of mice were studied: C57Bl/6 wild type, Prdx6 null, and gp91phox (NOX2) null. Wild-type mice and NOX2 null breeder pairs were obtained from the Jackson Laboratory (Bar Harbor, ME). The generation of Prdx6 null mice has been described previously (9); these mice have been fully backcrossed to the C57Bl/6 background (10). NOX2 and Prdx6 null mice were bred in our animal facility.
Isolated Lung Perfusion
The isolated perfused mouse lung technique has been described previously (11). Briefly, mice were anesthetized with 50 mg/kg intraperitoneal sodium pentobarbital, and the lungs were cleared of blood and then removed from the thorax and placed in a perfusion chamber. Lungs were continuously ventilated through a tracheal cannula with 5% CO2 in air (BOC, Murray Hill, NJ) and perfused with recirculating Krebs-Ringer bicarbonate solution supplemented with 10 mm glucose and 3% bovine serum albumin. H2O2 generation was measured by the addition of Amplex Red (50 μm) plus HRP (50 μg/ml) to the perfusate; this fluorophore does not permeate the cell membrane and thus detects extracellular H2O2. Ang II (50 μm) was added to the lung perfusate as a NOX2 agonist. Aliquots of the perfusate were removed during 30-min intervals, and fluorescence intensity was measured (excitation/emission, 545/610) using a spectrofluorimeter (Photon Technology International, Inc., Birmingham, NJ) and expressed as arbitrary fluorescence units. ROS generation was determined for wild-type and Prdx6 null lungs using NOX2 null lungs as a negative (no ROS) control.
Isolation of Cells and Experimental Design
Microvascular endothelial cells (PMVEC) were isolated from lungs of wild-type, Prdx6 null, and NOX2 null mice as reported previously (12, 13). Briefly, minced lungs were treated with collagenase (3 mg/ml), the digest was forced through an 18-gauge needle and centrifuged, binding buffer (6.5 mm sodium phosphate, pH 7.4) was added to the pellet, and the cell suspension was incubated with anti-PECAM antibody followed by incubation with prewashed Dynabeads® (Dynal, Oslo, Norway) coated with sheep anti-rat IgG (beads coated with anti-mouse IgG were not available). Isolated cells were cultured on tissue culture plastic plates. Cell islands were obtained in 1–2 weeks. Non-endothelial cells, consisting mainly of fibroblasts, could be identified by their irregular shape, and these were scraped off and discarded. A second round of immunoselection was carried out after an additional 2 weeks of culture by sorting cells labeled with anti-PECAM FITC-labeled antibody using a fluorescence-activated cell sorter and FACSDiva software (BD Biosciences). The endothelial phenotype of the preparation was confirmed by evaluating cellular uptake of the endothelium-specific marker DiIAcLDL (DiI-acetylated low density lipoprotein) and immunostaining for PECAM, von Willebrand factor, vascular endothelial cadherin, Flt-1 (vascular endothelial growth factor receptor-1, VEGFR-1), and Flk-2 (VEGFR-2) (12). Isolated cells were used to study ROS production, PLA2 activity, and translocation of cytoplasmic components to the cell membrane following stimulation with Ang II (10 μm) or PMA (10 nm).
Alveolar macrophages were isolated by centrifugation of material obtained by lung lavage and used to study ROS production following stimulation with N-formyl-Met-Leu-Phe peptide (fMLF; 1 μm) or concanavalin A (250 μg/ml).
Assays for ROS Production by Cells
ROS production by PMVEC was measured by fluorescence microscopy after loading cells for 10–15 min with one of the ROS-sensitive dyes, HE or H2DCF diacetate. The agonist was added, and cells were harvested after 30 min. Cells were kept in the dark during the dye loading and agonist exposure periods. Fluorescence of the oxidized HE product was monitored by excitation/emission of 495/585 nm and of DCF at excitation/emission of 490/530 using an epifluorescence microscope fitted with a ×20 objective (Nikon Diaphot TMD). For quantification, endothelial cells were randomly selected from the phase images, and the fluorescence intensity of those cells was then measured using Metamorph software (Molecular Devices, Sunnyvale, CA). For each field, the intensity of 6–10 cells was averaged, and 4–5 fields were analyzed for each condition. An increase in DCF fluorescence reflects oxidation of deacetylated H2DCF by H2O2, and the fluorescent HE product reflects oxidation by O2⨪, although neither fluorophore is totally specific for the indicated species of ROS.
ROS generation by PMVEC also was evaluated by fluorescence spectrometry of the incubation medium using Amplex Red in the presence of HRP. Cells grown to 80% confluence in Petri dishes were washed with medium free of serum and phenol red and incubated with the detector plus agonist (Ang II or PMA) for 30 min. The medium was collected and centrifuged, and the fluorescence of the supernatant was measured as described above for isolated lung studies. The protein content of scraped cells was measured by the Coomassie Blue binding assay (Bio-Rad) using bovine γ-globulin as the standard. Authentic H2O2 was used to generate a standard curve for Amplex Red, and ROS generation was expressed as pmol/30 min/mg of protein.
ROS production by alveolar macrophages was measured by fluorescence microscopy in cells loaded with H2DCF, as described above for PMVEC. ROS production (specifically O2⨪) also was determined by the cytochrome c method using a previously described protocol (14). This method measures the difference in cytochrome c reduction in the presence and absence of superoxide dismutase. Absorbance of cytochrome c (20 μm) was measured at 550 nm. Macrophages obtained from three mice were pooled and used at ∼5 × 105 cells/assay.
Plasmid Construction, Site-directed Mutagenesis, and Transfection of Prdx6 Null Cells
Plasmid constructs expressing wild-type Prdx6 were generated in pGFP-C1 and pIRES2-ZsGreen 1 vectors. Construction of the pGFP-C1 fusion with the rat Prdx6 cDNA has been described (15). For the pIRES2-ZsGreen1 construct, mouse Prdx6 cDNA was cloned by PCR after reverse transcription of RNA from C57Bl/6J mouse liver using the Transcriptor First Strand cDNA synthesis kit (Roche Applied Science) The PCR primers used were: 5′-CCATGCCCGGAGGGTTGCTTCTC-3′ and 5′-CTTAAGGCTGGGGTGTATAACGGAGG-3′. The underlined bases represent the start and termination codons. The conditions used for the PCR were 95 °C for 5 min and then 30 cycles of 95 °C denaturation (1 min each), 66 °C annealing (1.5 min each), and 72 °C elongation (2 min each). Subsequently, the reaction was incubated for 10 min at 72 °C and then placed at 4 °C. PCR products were purified using the Qiaquick PCR purification kit (Qiagen, Valencia, CA) and cloned into pCR2.1-Topo cloning vector (Invitrogen). The identity of the cloned PCR product was confirmed by DNA sequencing. Subsequently, the mouse Prdx6 cDNA was excised with EcoRI using the EcoRI sites that flank the cloning site and recloned into the EcoRI site of the pIRES2-ZsGreen1 vector. Mutations in the mouse Prdx6 cDNA in the pIRES2-ZsGreen1 vector were made by site-directed mutagenesis, using the QuikChange site-directed mutagenesis kit (Stratagene, La Jolla, CA). Separate constructs were generated for the His26, Ser32, Asp140, or Thr177 mutations of Prdx6 substituted by Ala and the Cys47 mutation substituted by Ser. Forward primers used for mutagenesis were as follows (reverse primers were the complements of the forward primers; underlined nucleotides were mutated): H26A, 5′-CGGCCGCATCCGCTTCGCCGATTTCCTGGGAGATTC-3′; S32A, 5′-CCACGATTTCCTGGGAGATGCATGGGGCATTCTCTTTTCC-3′; C47S, 5′-GGGACTTTACCCCAGTGAGCACCACAGAACTTGGCAGAGC-3′; D140A, 5′-GTGGTGTTCATTTTTGGCCCTGCCAAGAAACTGAAGCTGTCTATCC-3′; T177A, 5′-CAGGCACAAAGCCGGTTGCCGCCCCAGTTGACTGGAAGAAGGG-3′.
For transfection experiments, PMVEC derived from lungs of Prdx6 null mice were grown to 50–60% confluence, trypsinized from the dish, pelleted by centrifugation, and resuspended in Amaxa Nucleofector endothelial kit solution (Amaxa, Gaithersburg, MD). Cells were transfected using plasmid DNA (3–4 μg) that co-expressed Prdx6 or mutants and either ZsGreen or GFP. Transfection was performed by electroporation using program T-023 with the Amaxa Nucleofactor system. Cells were evaluated for transfection efficiency and ROS generation at 48 h after transfection by confocal fluorescence microscopy using the sequential scanning mode at red and green wavelengths to obviate possible overlap of the individual signals.
Isolation of Endothelial Cell Membranes and Immunoblots
A plasma membrane enriched fraction was isolated from PMVEC as described previously (16). Cells in culture dishes were untreated or treated with MJ33 (1 mol % in liposomes) or U0126 (10 μm) for 30 min followed by Ang II (10 μm) for an additional 30 min. Cells were dissociated from the plate with trypsin, sonicated, and centrifuged, and the resuspended pellet was layered on a sucrose gradient. After centrifugation, the plasma membrane layer was removed, subjected to PAGE, and immunoblotted for Rac1, p47phox, or Prdx6. In order to visualize two proteins of different sizes, the blots were cut horizontally, and the separate halves were reacted with different antibodies. Immunoblotting was performed by the two-color Odyssey (Li-Cor, Omaha, NE) Western blot analysis technique. Secondary dual labeled antibodies were IRDyeTM 800 goat anti-rabbit for the green channel and Alexa 680 green anti-mouse for the red channel. Blots were scanned by placing the membrane on the Odyssey two-color scanner, and the scanned images were converted to grayscale. All manipulations of contrast were done for the entire gel, although in some cases entire lanes were removed for clarity of presentation. The individual bands were quantified using Odyssey software.
Cellular Immunofluorescence
To evaluate Prdx6 phosphorylation in intact endothelial cells, PMVEC were fixed in 4% paraformaldehyde for 10 min at room temperature, washed three times, permeabilized with 1% Triton X-100 for 10 min, blocked, and treated with primary antibody followed by Alexa488-labeled secondary antibody. Cells were imaged on an epifluorescence microscope.
Co-localization of Proteins by the Duolink Procedure
This assay allows for visualization of protein-protein co-localization by fluorescence microscopy (Duolink, Olink, Uppsala, Sweden) using primary antibodies for each protein obtained from different species (17). The present studies used polyclonal anti-Prdx6 antibody generated in rabbits and a monoclonal antibody to the other protein of interest (flotillin or Rac1) generated in mice. Cells were fixed with 4% paraformaldehyde and treated with a blocking reagent followed by the primary antibodies. The Duolink kit contained secondary antibodies to rabbit and mouse IgG, each attached to a unique synthetic oligonucleotide; if the two proteins are in proximity (<40 nm), ligation causes the two oligonucleotides to hybridize, allowing DNA replication and amplification of a fluorescence signal. The resulting signal was imaged by epifluorescence or confocal (Radiance 2000, Bio-Rad) microscopy.
PLA2 Activity
PLA2 activity was measured in PMVEC that were disrupted by sonication and incubated with radiolabeled liposomes as described previously (18, 19). Unilamellar liposomes (∼100 μm) consisting of 3H-DPPC, egg phosphatidylcholine, phosphatidylglycerol, and cholesterol (0.5, 0.25, 0.1, and 0.15 mol fraction) were prepared by extrusion through a membrane under pressure. Lysates were incubated in Ca2+-free buffer (50 mm Tris-HCl, 1 mm EGTA) at pH 7.0 for 1 h. The radiolabeled free fatty acid product was extracted, resolved by thin layer chromatography using hexane/ether/acetic acid, and analyzed by scintillation counting (18, 19). To evaluate inhibitors, MJ33 was added to the liposomes at 1 mol %; other inhibitors were added to the incubation medium in aqueous solution.
Phospholipase activity also was measured in intact cells. To prelabel phospholipids, ∼106 PMVEC attached to the culture dish were incubated for 24 h with 1 μCi of [methyl-3H]choline Cl. Cells were then washed and incubated with or without an inhibitor (MJ33 or U01026, both at 10 μm) for 30 min, followed by the addition or not of Ang II (10 μm) for 1 h. Cells before and after the Ang II incubation were lysed by sonication, extracted with CH3/MeOH, and analyzed by thin layer chromatography using CHCl3/MeOH/NH4OH as the solvent system, and dpm in the [lyso-3H]PC band was measured by scintillation counting as described previously (18, 19). Results are the dpm recovered after the 1-h incubation period minus the “zero time” control and are expressed as dpm/mg of protein in 1 h. Because positional specificity of the lyso-PC product was not determined, this assay reflects both PLA1 and PLA2 activities and is called PLA activity.
Statistical Analysis
Values are shown as mean ± S.E. Statistical significance was assessed with SigmaStat software (Jandel Scientific, San Jose, CA). Group differences were evaluated by one-way analysis of variance or by Student's t test as appropriate. Differences between mean values were considered statistically significant at p < 0.05.
RESULTS
ROS Production with Ang II Treatment of Lung Endothelium in Situ and Endothelial Cells in Vitro
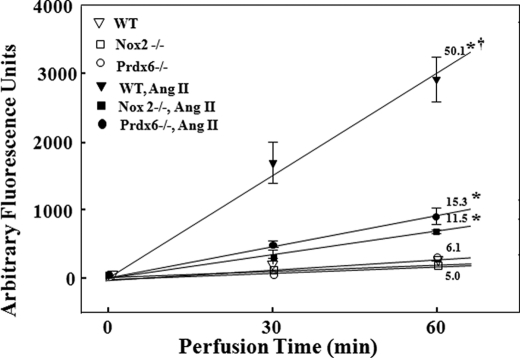
ROS production by the isolated mouse lung as indicated by oxidation of Amplex Red in the presence of HRP was measured at 30 and 60 min of recirculating perfusion (Fig. 1). There was essentially no ROS production in the absence of added agonist (basal) for any of the three types of lungs that were studied (wild-type, NOX2 null, and Prdx6 null). With Ang II added to the perfusate, there was a linear increase in oxidized Amplex Red with time that was 10-fold greater than the basal rate in wild-type lungs, indicating a constant rate of H2O2 production. The Ang II-stimulated rate of H2O2 production was markedly diminished in Prdx6 null compared with wild-type lungs. H2O2 production was similarly diminished in NOX2 null lungs, although it was greater than the basal level, suggesting a minor contribution to ROS production from non-NOX2 sources. The rate of Amplex Red oxidation following Ang II was not statistically different (p > 0.05) between the NOX2 null and Prdx6 null lungs.
FIGURE 1.
ROS production following Ang II stimulation is markedly decreased in isolated perfused mouse lungs from Prdx6 null mice. Lungs from WT, Prdx6 null, and NOX2 null were isolated and perfused in a recirculating system containing Amplex Red (25 μm) and HRP (25 μg/ml). ROS generation was measured in arbitrary fluorescence units by Amplex Red oxidation at 30 and 60 min of perfusion. Ang II when present was 50 μm. The numbers above or below the lines indicate the slope (change in arbitrary fluorescence units/min) calculated by least mean squares. *, p < 0.05 versus the corresponding control (no Ang II); †, p < 0.05 versus other Ang II-stimulated conditions. Error bars, S.E.
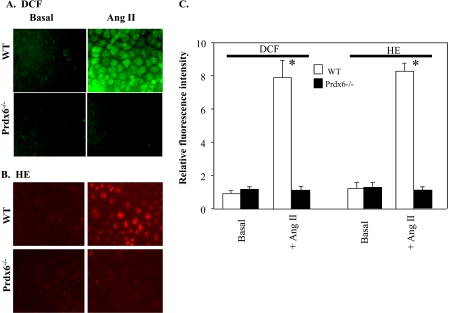
ROS production in isolated mouse PMVEC was measured by change in fluorescence of two different ROS-sensitive dyes. In wild-type cells, Ang II resulted in a significant increase (6–8-fold) in both DCF (Fig. 2A) and oxidized HE fluorescence (Fig. 2B). However, there was no appreciable change in fluorescence following Ang II stimulation of Prdx6 null cells. H2O2 production in isolated cells was quantitated by the Amplex Red method during a 30-min incubation in the presence of Ang II or PMA (Table 1). Agonist stimulation resulted in an ∼25-fold increase in ROS generation with wild-type cells, whereas Prdx6 null cells showed no change from the basal (control) value.
FIGURE 2.
Prdx6 is required for ROS production by PMVEC in response to Ang II treatment. PMVEC derived from WT or Prdx6 null mice were monitored by fluorescence microscopy for ROS production with and without the addition of Ang II (10 μm) by dihydrochlorofluorescein oxidation to DCF (green) (A) or by HE oxidation (red) (B). C, to quantify fluorescence intensity, 8–10 PMVEC in each of five fields were randomly selected in the phase images, and then fluorescence of these cells in arbitrary units was quantified using Metamorph software. Results are expressed as mean ± S.E. (error bars) for n = 8. *, p < 0.05 versus basal (no Ang II).
TABLE 1.
ROS generation in PMVEC derived from wild-type or Prdx6 null mouse lungs
Wild-type or Prdx6 null mouse PMVEC were incubated with Ang II (10 μm) or PMA (10 nm) for 30 min, during which ROS generation was measured by the Amplex Red assay. Wild-type cells stimulated with Ang II or PMA also were co-incubated with U0126 (10 μm).
| ROS generation |
|||
|---|---|---|---|
| WT | Prdx6 null | WT + U0126 | |
| pmol/mg protein/30 min | |||
| Basal | 5.8 ± 0.33 | 6.2 ± 0.2 | |
| With Ang II | 134 ± 3.6a | 6.3 ± 0.4b | 20.7 ± 1.1b |
| With PMA | 149 ± 2.6a | 5.2 ± 0.3b | 17.3 ± 0.4b |
a p < 0.05 versus the corresponding basal condition.
b p < 0.05 versus the corresponding agonist-stimulated wild-type cells.
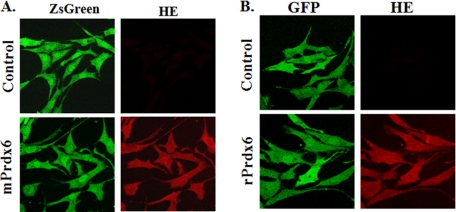
Rescue experiments were carried out to confirm that deletion of Prdx6 was responsible for the lack of response to Ang II in the null cells. Two different expression vectors were used for this purpose in order to be certain that the vector itself had no influence on the results. Expression of a green fluorophore (either ZsGreen1 or GFP) was used as a transfection efficiency control, and hydroethidine (red) was used to detect ROS. The pIRES-ZsGreen vector encoded mouse Prdx6, whereas the GFP-C1 vector encoded rat Prdx6; the deduced Prdx6 protein sequences for the two species show 94% amino acid identity (20). Transfection efficiency in these cells using the Amaxa electroporation system was ∼70%. Transfection of Prdx6 null cells with wild-type Prdx6 constructs in either vector rescued the ability of Ang II to stimulate ROS production as indicated by HE fluorescence (Fig. 3). Note the relative absence of red fluorescence in the control cells, confirming that the demonstrated HE oxidation is not an artifact due to “bleed-through” from the green channel.
FIGURE 3.
Transfection of Prdx6 null mouse PMVEC with a vector expressing Prdx6 restores Ang II-induced ROS generation. PMVEC isolated from Prdx6 null mice were transfected with vector only or vector expressing either pIRES2-ZsGreen1 vector (Control) or mouse Prdx6:pIRES2-ZsGreen1 (mPrdx6) (A) or pGFP-C1 (Control) or rat Prdx6:GFP-C1 (rPrdx6) (B). Cells were evaluated by fluorescence microscopy. Green fluorescence (ZsGreen or GFP) indicates transfection. Transfected cells were treated with Ang II (10 μm) for 30 min and monitored for ROS generation (red) by oxidation of HE.
Phospholipase A2 Activity of Prdx6 Is Required for NOX2 Activation
The results in Figs. 1–3 and Table 1 indicate that Prdx6 is necessary for the Ang II- or PMA-mediated activation of ROS generation by lungs or PMVEC. In order to determine whether the PLA2 activity of Prdx6 is required for activation, the effect of Ang II on PLA2 activity of PMVEC was determined. Assays were carried out by determining formation of labeled free fatty acid from exogenous 3H-DPPC by lysed cells (Table 2) and labeled lyso-PC from [3H]choline-labeled endogenous phospholipids by intact cells (Table 3). For the lysed cell assays, wild-type PMVEC showed some PLA2 activity under resting conditions that was stimulated 4-fold in the presence of Ang II (Table 2). Prdx6 null cells in the resting state showed a trivial level of PLA2 activity that presumably reflects enzymatic activity unrelated to Prdx6. Unlike the wild-type, there was no significant stimulation of PLA2 activity by Ang II in Prdx6 null cells. Similar results were observed for PLA activity for intact cells; wild-type cells showed a 2.8-fold stimulation of PLA activity by Ang II, but there was no significant response to Ang II by Prdx6 null PMVEC (Table 3).
TABLE 2.
PLA2 activity of lysates of mouse PMVEC
PMVEC were incubated in the absence (basal) or presence of 10 μm Ang II and/or 10 μm U0126 for 1 h. PLA2 activity of the cell lysate was measured at pH 7.0 using 3H-DPPC in mixed unilamellar liposomes as substrate. Values are mean ± S.E. (n = 3) for wild type and Prdx6 null and mean ± range (n = 2) for wild type + U0126.
| PLA2 activity |
|||
|---|---|---|---|
| WT | Prdx6 null | WT + U0126 | |
| nmol/h/mg protein | |||
| Basal | 4.4 ± 0.14 | 0.09 ± 0.02 | 3.9 ± 0.08 |
| With Ang II | 16.9 ± 0.46a | 0.2 ± 0.01 | 3.8 ± 0.15 |
a p < 0.05 vs. corresponding basal condition.
TABLE 3.
PLA activity of intact mouse PMVEC
PMVEC were prelabeled by incubation for 24 h with [methyl-3H]choline and then pretreated for 30 min with or without MJ33 (10 μm) or U0126 (10 μm) followed by 60 min with or without Ang II (10 μm). The [3H]lyso-PC fraction was isolated, and dpm measured. PLA activity is the dpm at the end minus dpm at the start of the 1-h incubation period (zero time). The zero time values were 612 for wild type, 437 for wild type + MJ33, and 593 for wild type + U0126. Values are mean ± S.E. (n = 3).
| PLA activity |
||||
|---|---|---|---|---|
| WT | Prdx6 null | WT + MJ33 | WT + U0126 | |
| dpm/h/mg protein | ||||
| Basal | 1880 ± 17 | 320 ± 26 | 355 ± 20 | 1780 ± 63 |
| With Ang II | 5220 ± 153a | 340 ± 8 | 318 ± 19 | 1720 ± 40 |
a p < 0.05 versus corresponding basal condition.
The role of PLA2 was further investigated by rescue experiments. Prdx6 null cells were transfected with constructs that do or do not result in protein that expresses PLA2 activity. Transfection of Prdx6 null PMVEC with the wild-type mouse Prdx6 construct rescued the response to Ang II for both PLA2 activity and ROS generation (Table 4). Transfection of the Prdx6 null cells with the C47S mutant also was evaluated because Cys47 is the known catalytic center for the peroxidatic function in the protein but does not influence the PLA2 activity (21); these cells do not express Prdx6-dependent peroxidase activity (not shown). Expression of the C47S Prdx6 mutant partially rescued (∼50%) both the agonist-induced PLA2 activity and ROS generation (Table 4). On the other hand, there was no significant rescue of either PLA2 activity or ROS generation in endothelial cells transfected with constructs that express S32A, H26A, or D140A mutant Prdx6; these 3 amino acids constitute the Prdx6 PLA2 catalytic triad, and each is necessary for PLA2 activity but not for H2O2 peroxidase activity (21, 22). These results for “rescue” show that the PLA2 activity of Prdx6 is required for Ang II-mediated activation of NOX2 in mouse PMVEC.
TABLE 4.
PLA2 activity and ROS generation of Prdx6 null mouse PMVEC transfected with wild-type or mutant mouse Prdx6 constructs
Transfection utilized the pIRES vector encoding wild-type or mutant mouse Prdx6. PMVEC were incubated under basal conditions (no additions) or in the presence of Ang II (10 μm) for 30 min. ROS generation during incubation was measured by the Amplex Red oxidation method. PLA2 activity of lysed cells was measured at the end of a parallel incubation in the absence of Amplex Red. Values are mean ± S.E. for n = 3 or meant ± range (n = 2) for basal ROS. Δ, Ang II minus basal.
| Transfection construct | PLA2 activity |
ROS generation |
||||
|---|---|---|---|---|---|---|
| Basal | +Ang II | Δ | Basal | +Ang II | Δ | |
| nmol/h/mg protein | pmol/mg protein/30 min | |||||
| None | 1.0 ± 0.08 | 6.0 ± 0.08 | 5 | |||
| Vector only | 0.08 ± 0.02 | 0.15 ± 0.06 | 0.1 | 1.0 ± 0.2 | 6.0 ± 0.1 | 5 |
| Wild type | 3.9 ± 0.05a | 15.9 ± 0.3a | 12 | 1.1 ± 0.1 | 107 ± 3.8a | 106 |
| C47S | 3.5 ± 0.4a | 9.0 ± 0.3a | 5.5 | 0.8 ± 0.1 | 66 ± 2.9a | 65 |
| S32A | 0.18 ± 0.01 | 0.20 ± 0.10 | 0.0 | 1.1 ± 0.04 | 6.2 ± 0.4 | 5 |
| D140A | 0.19 ± 0.05 | 0.17 ± 0.04 | 0.0 | 1.0 ± 0.2 | 3.9 ± 0.3 | 3 |
| H26A | 0.14 ± 0.01 | 0.17 ± 0.04 | 0.0 | 1.1 ± 0.3 | 5.2 ± 0.2 | 4 |
| T177A | 2.1 ± 0.2 | 2.2 ± 0.4 | 0.1 | 1.0 ± 0.2 | 6.2 ± 0.9 | 5 |
a p < 0.05 versus transfection with vector only.
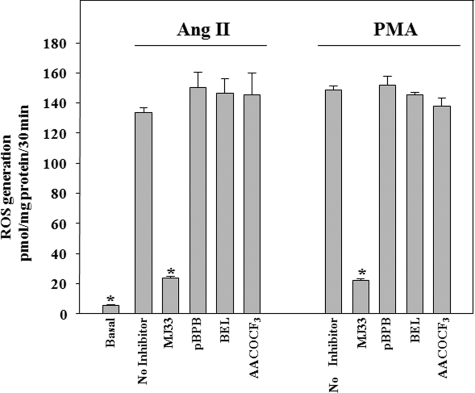
We then asked whether the PLA2 requirement for activation of endothelial NOX2 is specific for Prdx6 PLA2 activity or whether other PLA2 enzymes can substitute. To test this, we used chemical inhibitors that have been shown previously to have some (although not total) specificity for the different PLA2s; concentrations of inhibitors were chosen based on previous reports (18, 23). MJ33 inhibits Prdx6 PLA2, pBPB is an inhibitor of secreted PLA2, BEL inhibits intracellular calcium-independent PLA2, and AACOCF3 inhibits cytosolic PLA2. The agonist-induced PLA2 activity of lysed PMVEC was markedly inhibited by MJ33, but there was no significant effect of pBPB, BEL, or AACOCF3 (Table 5). MJ33 also inhibited Ang II-stimulated PLA activity in the intact cell assay (Table 3). The effect of these inhibitors on ROS generation as determined with the Amplex Red assay in mouse PMVEC treated with either Ang II or PMA also was tested. Similar to PLA2 activity, agonist-stimulated ROS generation was markedly inhibited by the presence of MJ33, whereas pBPB, BEL, and AACOCF3 had no significant effect (Fig. 4). These results indicate that PLA2 activity associated with non-Prdx6 sources does not play a role in NOX2 activation. This conclusion is consistent with the lack of change in PLA2 activity in Ang II-stimulated Prdx6 null PMVEC (Tables 2 and 3). Thus, activation of NOX2 by Ang II or PMA specifically requires the activity of Prdx6 PLA2.
TABLE 5.
Effect of inhibitors on PLA2 activity of lysed PMVEC
PLA2 was measured at pH 7.0 in Ca2+-free medium. Basal was in the absence of added Ang II or PMA. Ang II (10 μm) or PMA (10 nm) was added for 30 min before assay of PLA2 activity. The inhibitor was added 10 min before the addition of agonist. Values are mean ± S.E. for n = 6 for basal and control and n = 3 for inhibitors.
| PLA2 activity |
||
|---|---|---|
| Ang II | PMA | |
| nmol/h/mg protein | ||
| Basal (no agonist) | 4.0 ± 0.3a | |
| Control (no inhibitor) | 11.5 ± 0.4 | 13.7 ± 0.6 |
| With MJ33 (1 mol %) | 4.0 ± 0.1a | 4.1 ± 0.1a |
| With pBPB (20 μm) | 11.6 ± 0.5 | 11.9 ± 0.4 |
| With BEL (100 μm) | 11.6 ± 0.2 | 11.6 ± 0.3 |
| With AACOCF3 (100 μm) | 12.4 ± 0.7 | 11.6 ± 0.2 |
a p < 0.05 versus the corresponding control.
FIGURE 4.
ROS generation with Ang II or PMA treatment is blocked by an inhibitor of Prdx6 PLA2 activity but not by other PLA2 inhibitors. PMVEC were pretreated with inhibitor for 10 min and then incubated with Ang II (10 μm) or PMA (10 nm). ROS production was measured during the incubation with agonist by the Amplex Red oxidation method. Inhibitors were MJ33 (1 mol % in liposomes), pBPB (20 μm), BEL (100 μm), and AACOCF3 (100 μm). Values are mean ± S.E. (error bars) for n = 3. *, p < 0.05 versus Ang II or PMA alone. Basal (no additions) was the same for both Ang II and PMA.
Translocation of Cytosolic Components of NOX2 to the Plasma Membrane
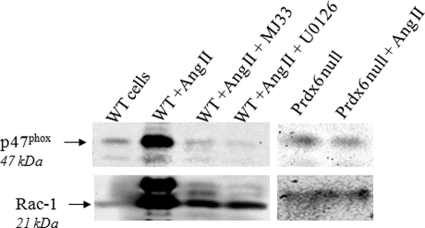
NOX2 activation requires the translocation of several cytosolic components of the enzyme complex to the cell membrane (4). We monitored translocation of two of those components, Rac1 and p47phox, following Ang II stimulation of wild-type and Prdx6 null PMVEC by isolation of a plasma membrane fraction (Fig. 5 and Table 6). Immunoblot of the membranes isolated from cells under basal conditions showed weak signals for Rac1 and for p47phox in wild-type cell membranes and stronger signals for both proteins following Ang II stimulation. There was no change in the association of Rac1 or p47phox with the plasma membrane following Ang II stimulation of Prdx6 null cells, consistent with the lack of ROS generation under those conditions. Translocation of Rac1 and p47phox was markedly inhibited by pretreatment of cells with the Prdx6 PLA2 inhibitor, MJ33, consistent with the results for the Prdx6 null cells (Fig. 5 and Table 6).
FIGURE 5.
NOX2 cytosolic components (Rac1 and p47phox) translocate to the cell membrane upon Ang II treatment in wild-type but not Prdx6 null PMVEC. PMVEC from WT or Prdx6 null mice were treated for 30 min with 10 μm Ang II. Where indicated, wild-type cells also were pretreated with MJ33 (1 mol % in liposomes) or U0126 (10 μm) for 30 min before the addition of Ang II. Endothelial cell membranes were isolated by subcellular fractionation and immunoblotted using antibodies to Rac1 or p47phox. Rac1 appears as a doublet as described previously (41); the lower band was used for quantitation (Table 6). Results were obtained with separate gels for wild-type and Prdx6 null cells. Gels were cut and processed for two different proteins as described under “Experimental Procedures.”
TABLE 6.
Quantitation by densitometry of immunoblots showing translocation of Rac1 and p47phox to the cell membrane following Ang II stimulation
Values are mean ± S.E. for n = 3.
| Rac1 | p47phox | |
|---|---|---|
| arbitrary unitsa | ||
| Wild type (basal) | 53 ± 16b | 166 ± 12b |
| With Ang II | 873 ± 16 | 689 ± 12 |
| With Ang II + MJ33 | 231 ± 22b | 117 ± 8b |
| With Ang II + U0126 | 233 ± 37b | 149 ± 19b |
| Prdx6 null (basal) | 55 ± 14 | 63 ± 9 |
| With Ang II | 38 ± 6 | 85 ± 4 |
a Arbitrary units are pixels × 10−2.
b p < 0.05 versus the corresponding Ang II (alone) value.
Translocation of Prdx6 to the Plasma Membrane
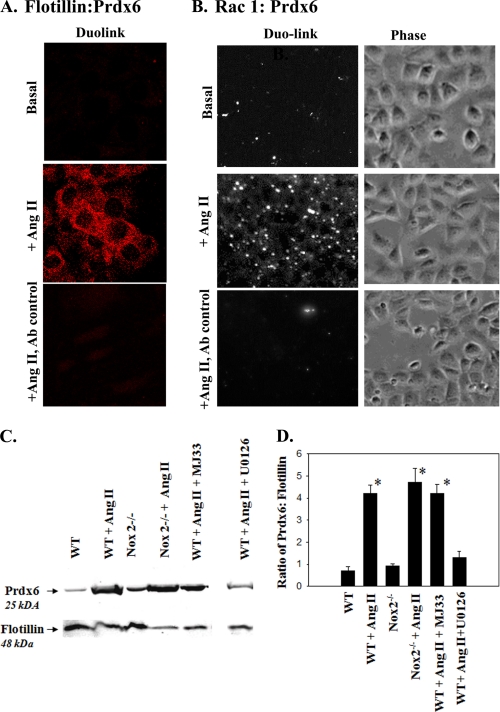
Although Prdx6 is present in lysosomes and secretory organelles, it is also a cytoplasmic enzyme (6, 18, 24), consistent with its function in antioxidant defense. Presumably, cell membrane phospholipids are the substrate for Prdx6 PLA2 activity associated with activation of NOX2. We therefore used the Duolink procedure to evaluate possible translocation of Prdx6 to the plasma membrane upon agonist stimulation by determining its co-localization with the intrinsic plasma membrane protein flotillin (Fig. 6A) or with translocated Rac1 (Fig. 6B). Prdx6 was shown to be in close proximity to both proteins only after Ang II treatment compatible with its agonist-induced translocation to the cell membrane. A similar result was obtained by Western blot of a PMVEC plasma membrane fraction isolated from wild-type cells, showing that Prdx6 had translocated to the cell membrane following Ang II treatment (Fig. 6, C and D). Plasma membranes from NOX2 null cells showed translocation of Prdx6 similar to wild type cells (Fig. 6, C and D). Likewise, MJ33 pretreatment of wild-type cells had no effect on Prdx6 translocation to the cell membrane associated with Ang II exposure (Fig. 6, C and D). For these experiments, flotillin (molecular mass 48 kDa) was used as a control, and densitometric data for the Prdx6 lanes were normalized to the respective flotillin expression in each lane. Because NOX2 null and MJ33-treated cells do not generate ROS following Ang II treatment, Prdx6 translocation is independent and presumably upstream of the NOX2 activation pathway.
FIGURE 6.
Prdx6 associates with the plasma membrane upon Ang II treatment. Duolink procedure to evaluate co-localization of Prdx6 in PMVEC with flotillin (A), an integral plasma membrane protein, and Rac1 (B), a cytoplasmic protein that translocates to the plasma membrane upon stimulation. Fluorescence indicates proximity (<40 nm) of the two proteins; phase images in B show cell density. Upper panels, basal (no Ang II); middle and lower panels, Ang II (10 μm). Upper and middle panels were treated with anti-Prdx6 (polyclonal Ab) and either anti-flotillin or anti-Rac1 (mAb); lower panels are the Ab control (IgG from rabbit and mouse). Images were obtained similarly for A and B, but positive results are shown as red fluorescence (A) or converted to black and white (B). The phase image is shown in B because the cell borders are not apparent in the fluorescence image. C, immunoblots of the cell membrane fraction isolated by gradient centrifugation from mouse WT and NOX2 null PMVEC with and without Ang II (10 μm) treatment using anti-Prdx6 and anti-flotillin antibodies. MJ33 (1 mol % in liposomes) was used as an inhibitor of Prdx6 PLA2 activity, and U0126 (10 μm) was used as a MAPK inhibitor. The gap indicates where several non-relevant lanes were removed. The blot was cut horizontally for simultaneous analysis of both proteins as described under “Experimental Procedures.” D, densitometric quantitation (mean ± S.E. (error bars) for n = 3) of the bands on immunoblot in arbitrary units using ImageJ software; results are expressed as the ratio of Prdx6 to flotillin. *, p < 0.05 versus wild-type control (no Ang II).
Phosphorylation of Prdx6 Is Required for Its Translocation
Our previous studies with recombinant Prdx6 in vitro have shown that at pH 7 (i.e. cytoplasmic pH), Prdx6 does not bind appreciably to phospholipids (unless they are oxidized) and demonstrates little PLA2 activity (25). Thus, translocation of cytoplasmic Prdx6 to the plasma membrane reflecting membrane phospholipid binding would not be expected in the absence of oxidative stress. However, phospholipid binding and PLA2 activity in vitro at this pH are greatly increased if Prdx6 has been phosphorylated by incubation with a MAPK (ERK, p38) (8, 25). In the present studies, incubation of cells with an inhibitor of ERK activation (U0126) prevented Ang II-induced translocation of Prdx6 to the plasma membrane (Fig. 6, C and D) and increased PLA (Table 3) and PLA2 activities (Table 2). In the intact cell studies, there was a basal level of PLA activity in the presence of U0126 that was inhibited by MJ33 and was not present in the Prdx6 null cells. This basal activity presumably represents Prdx PLA2 activity that is independent of agonist-induced phosphorylation, whereas the response to Ang II is MAPK-dependent.
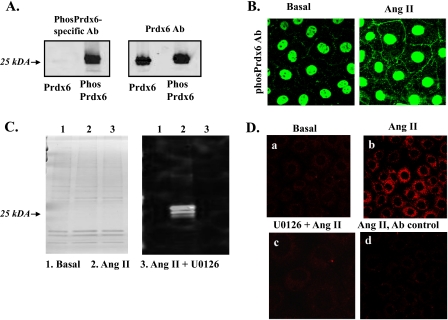
The antibody to phosphorylated Prdx6 (anti-phosPrdx6) was used to evaluate phosphorylation of Prdx6 in PMVEC in response to Ang II stimulation. This antibody recognized recombinant mouse Prdx6 that had been phosphorylated in vitro by incubation with ERK2 MAPK but did not recognize the native (non-phosphorylated) form (Fig. 7A). The phosphorylation-indifferent polyclonal anti-Prdx6 peptide antibody (total Prdx6 Ab) recognized both phosphorylated and non-phosphorylated Prdx6 (Fig. 7A). By microscopy, PMVEC under basal (unstimulated) conditions showed nuclear staining after incubation with the anti-phosPrdx6 antibody and faint immunofluorescence at the cell borders (Fig. 7B). After treatment with Ang II, there was a marked increase in immunofluorescence both in the cell cytoplasm and at the cell borders compatible with phosphorylation and translocation of Prdx6. Immunoblot of plasma membranes from PMVEC using the anti-phosPrdx6 antibody indicated no band under basal conditions but showed a marked increase of phosphorylated Prdx6 following treatment with Ang II (Fig. 7C). This result was confirmed by the Duolink procedure, which indicated that phosphorylated Prdx6 co-localizes with the plasma membrane marker protein, flotillin (Fig. 7D). The presence of phosphorylated Prdx6 on the isolated plasma membrane and its co-localization with flotillin in the presence of Ang II was abolished by pretreatment of PMVEC with the MAPK inhibitor U0126 (Fig. 7, C and D). Thus, MAPK activity is required for phosphorylation of Prdx6 and its translocation to the cell membrane.
FIGURE 7.
Prdx6 phosphorylation upon Ang II treatment. A, immunoblot to show specificity of the polyclonal Ab to phosphorylated Prdx6. Recombinant mouse Prdx6 was phosphorylated in vitro by incubation with ERK2 MAPK. The Ab to the phosphorylated peptide reacts with phosphorylated Prdx6 but does not recognize non-phosphorylated Prdx6, whereas the anti-Prdx6 peptide antibody reacts to both (total Prdx6). B, immunofluorescence of PMVEC using the phospho-specific Prdx6 Ab, without (basal) and with Ang II stimulation. The basis for the nuclear staining is not known. C, Coomassie Blue-stained PAGE (left) and immunoblot using anti-phosphorylated Prdx6 (Phos Prdx6) antibody (right) of isolated PMVEC cell membrane preparation before (basal) and following incubation with Ang II with or without U0126. Protein loading was 3 μg/lane. D, Duolink with polyclonal anti-phospho-Prdx6 and monoclonal anti-flotillin antibodies. a, no treatment (basal); b, Ang II treatment; c, pretreatment with MAPK inhibitor U0126, followed by Ang II; d, Ang II-stimulated cells treated with rabbit and mouse IgG as an antibody control. For B–D, Ang II treatment was 10 μm for 30 min; U0126 (10 μm) when added was 30 min before Ang II.
Further evidence that phosphorylation of Prdx6 is required for Ang II-mediated activation of NOX2 was obtained by transfection of Prdx6 null cells with a T177A mutant (rescue experiment). We have shown previously that Thr177 is the sole site of phosphorylation following in vitro treatment with MAPKs (8). Unlike transfection of Prdx6 null PMVEC with the wild-type construct, transfection with T177A failed to rescue the Ang II-mediated increase in either PLA2 activity or ROS generation (Table 4).
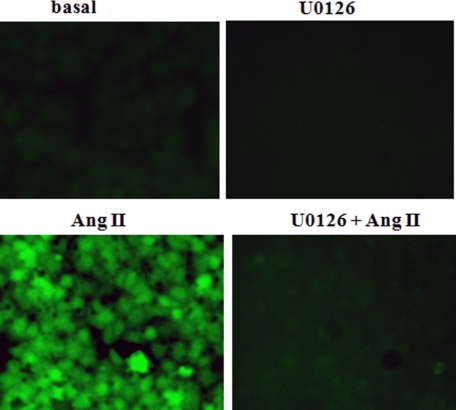
A role for MAPKs in the activation of NOX2 in polymorphonuclear leukocyte (PMN) macrophages and endothelial cells as well as other cell types has been known for some time (26–29). This role was confirmed in mouse PMVEC by measuring the effect of U0126 on translocation of Rac1/p47phox and ROS production following Ang II treatment. The MAPK inhibitor blocked translocation of the cytoplasmic components of NOX2 to the plasma membrane (Fig. 5 and Table 6) and markedly suppressed agonist-induced cellular ROS production, as indicated by DCF fluorescence (Fig. 8) or Amplex Red oxidation (Table 1). The inhibition of ROS production by U0126 is consistent with an inhibition of Prdx6 phosphorylation and translocation in response to Ang II, although MAPK could be responsible in addition for other phosphorylation events in the signaling cascade leading to NOX2 activation.
FIGURE 8.
Pretreatment with MAPK inhibitor U0126 blocks Ang II-induced ROS generation. Ang II (10 μm) was added to mouse PMVEC in the absence of or following a 10-min pretreatment with U0126 (10 μm). ROS generation was evaluated by fluorescence microscopy; increased fluorescence indicates oxidation of H2DCF to DCF.
ROS Generation by Alveolar Macrophages
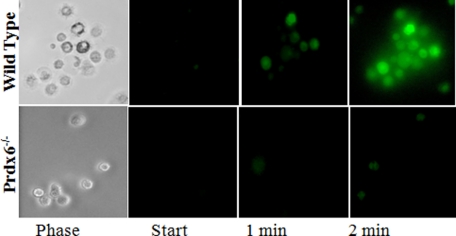
The cytochrome c assay was used to measure the response of alveolar macrophages to concanavalin A, a classic stimulant for NOX2-dependent ROS production by these cells (14). Concanavalin A-stimulated ROS production by macrophages was ∼62 pmol/min/105 cells or about 0.8 nmol/min/mg of protein, assuming that 105 macrophages represent 80 μg of protein (Table 7). This rate of ROS generation is ∼200 times greater than the ROS generation by Ang II-stimulated PMVEC. ROS generation in stimulated macrophages was decreased by 77% in Prdx6 null cells and by 96% in NOX2 null cells (Table 7). ROS production also was measured by fluorescence microscopy following stimulation with fMLF. There was a marked increase in fluorescence in the wild type cells but a minimal response in the Prdx6 null AM (Fig. 9 and Table 7). Thus, the alveolar macrophage, a phagocytic cell, is similar to the endothelial cell in its Prdx6 requirement for agonist-mediated generation of ROS.
TABLE 7.
Effect of Prdx6 deletion on agonist-induced ROS generation by alveolar macrophages
O2⨪ generation in cells stimulated with 250 μg/ml concanavalin A was measured by SOD-inhibitable cytochrome c reduction. For DCF fluorescence, cells were stimulated with 1 μm fMLF. DCF fluorescence of cells was measured in arbitrary units using the Metamorph program as the difference between zero time and 2 min images as shown in Fig. 9. Values are mean ± S.E. for n = 3.
| Cell type | O2⨪ generation | DCF fluorescence |
|---|---|---|
| pmol/min/105 cells | arbitrary units/min/cell | |
| Wild type | 62.0 ± 3.6 | 2030 ± 103 |
| Prdx6 null | 14.1 ± 2.1a | 206 ± 28.2a |
| NOX2 null | 2.4 ± 1.1a | — |
a p < 0.05 versus wild type.
FIGURE 9.
ROS production in alveolar macrophages. Cells were obtained from wild type and Prdx6 null mice by lung lavage. ROS generation is shown by fluorescence imaging of H2DCF oxidation (increased fluorescence) at 1 and 2 min after the addition of 1 μm fMLF. The phase images show that all cells in that field of wild type cells were stimulated to produce ROS.
DISCUSSION
NOX2 has been well established as the major source of ROS generation in PMN and macrophages (3). Although NOX2 was originally considered as a protein complex exclusive to these phagocytic cells, there is compelling evidence to indicate that NOX2 expression is widely distributed, and it is now considered as the major enzyme system for ROS generation in vascular endothelial cells (1, 2). NOX2 consists of membrane and cytosolic components that, in response to a wide variety of agonists, assemble at the plasma membrane, thereby activating the enzyme to produce O2⨪. However, the exact mechanisms that drive this assembly are not known. What is clear is that NOX2 assembly can be initiated by pathways involving either a receptor- or non-receptor-mediated process (1, 4). An example of the former is Ang II that works through its receptor subtypes AT1 and AT2 to activate downstream signaling pathways, whereas non-receptor-mediated agonists include the phorbol esters (e.g. PMA) that directly stimulate PKC. In this study, we used both Ang II and PMA to activate NOX2-dependent ROS production by intact perfused lungs or isolated PMVEC. With either agonist, NOX2 activation did not occur in the absence of Prdx6 and was rescued by transfection of Prdx6 null cells with a construct expressing wild-type Prdx6. Thus, we conclude that Prdx6 is essential for NOX2 activation and ROS production in endothelial cells. A similar Prdx6 requirement was demonstrated for agonist-mediated ROS production by alveolar macrophages.
A similar conclusion regarding a role for Prdx6 in NOX2 activation was reached in a previous in vitro study of PMN in which ROS production was approximately doubled by the presence of recombinant Prdx6 (also known as p29); this latter study used a reconstituted system consisting of isolated plasma membrane from PMN and recombinant cytosolic proteins (7). Although the recombinant Prdx6 (p29) exhibited both PLA2 and peroxidase activities, neither activity could be conclusively associated with NOX2 activation, which raised the possibility that enhancement of NOX2 activity by Prdx6 was unrelated to either enzymatic activity. However, the present study using PMVEC shows conclusively that Prdx6 facilitates NOX2 assembly and activation through its PLA2 activity. Evidence to support this mechanism is provided by the following findings: 1) activation of PLA2 activity with agonist (Ang II, PMA) stimulation in wild-type but not Prdx6 null cells; 2) the rescue of Ang II response by transfection of Prdx6 null cells with constructs leading to the expression of Prdx6 with PLA2 activity, whereas constructs that did not lead to PLA2 activity were ineffective; and 3) the inhibition of activation in the presence of the PLA2 inhibitor, MJ33. Thus, we conclude that the essential role of Prdx6 is associated with its PLA2 activity.
Our previous studies have demonstrated that Prdx6 has its peak PLA2 activity at pH 4 with minimal activity at cytosolic pH (20, 21). This finding correlated with the ability of Prdx6 to bind to native phospholipid substrate (liposomes) at acidic but not neutral pH (22, 25). However, lipid binding at pH 7 and subsequent PLA2 activity were markedly enhanced by phosphorylation of the protein (8). Prdx6 phosphorylation was shown in lung epithelial cells in response to PMA as determined by immunoprecipitation and was blocked by inhibition of MAPKs (8). In the present study, Prdx6 phosphorylation and its association with the endothelial cell plasma membrane (presumably as a result of binding to membrane lipids) were demonstrated subsequent to treatment of PMVEC with Ang II. The plasma membrane association was shown by immunofluorescence, immunoblot, and the Duolink procedure using an antibody to phosphorylated Prdx6. Further, Prdx6 translocation, PLA2 activity, and activation of NOX2 were blocked in the presence of U0126, an inhibitor of ERK MAPK activation and shown previously to inhibit Prdx6 phosphorylation (8). The requirement for phosphorylation of Prdx6 was confirmed by transfection with a construct expressing a Thr177 mutant into Prdx6 null cells. Thr177 is the unique site of protein phosphorylation when recombinant protein is treated in vitro with MAPKs (ERK, p38) (8). In distinction from transfection with the wild-type construct, transfection with a construct expressing the T177A mutant, which cannot be phosphorylated (8), was unable to rescue Ang II activation of NOX2 despite exhibiting basal PLA2 activity that was about 50% of the wild-type activity. Thus, we conclude that Prdx6 phosphorylation is required for NOX2 activation.
This study has not investigated which product of PLA2 activity is responsible for NOX2 activation. PLA2 liberates both a free fatty acid and lyso-PC from phosphatidylcholine substrate, and previous studies have provided evidence that either metabolite might be directly or indirectly responsible for initiating the events leading to activation of the NOX2 pathway (30–32). Of note, Prdx6 does not show a preference for arachidonate-containing phospholipids so that arachidonic acid would not be preferentially liberated by Prdx6 PLA2 activity (24). Thus, the specific product of PLA2 activity that mediates NOX2 activation remains unresolved.
Previous studies have provided evidence that PLA2 activity is important for NOX2 activation in PMN and possibly endothelial and other cell types (30, 33–35). However, assessment of the specific PLA2 involved in activation has been hampered by the relative non-specificity of inhibitors used in many of the earlier studies. Although the member of the PLA2 family that might be responsible for NOX2 activation was not identified precisely, suspicion has focused on cytoplasmic PLA2 (cPLA2) (36, 37). cPLA2 is ubiquitously expressed in cells and demonstrates a preference for arachidonate-containing phospholipids as the substrate; release of arachidonic acid can give rise to the broad spectrum of eicosanoid metabolites. cPLA2 can be phosphorylated by MAPKs and translocates to the cell membrane, similar to the pathway we have described herein for Prdx6 (36, 38). Phosphorylation of cPLA2 and release of arachidonic acid were demonstrated in vascular smooth muscle cells stimulated with Ang II, although this was not directly linked to NOX2 activation (33). ROS production by endothelial cells activated with low density lipoprotein was inhibited by the presence of the cPLA2 inhibitor, AACOCF3 (35). The possible role for cPLA2 in NOX2 activation in PMN and monocytes has been inferred by its presence on the plasma membrane following stimulation and by decreased ROS production when expression of cPLA2 was “knocked down” with antisense oligodeoxyribonucleotides (31, 36, 37). On the other hand, the effects of AACOCF3 on ROS production with PMN stimulated by PMA were unimpressive (30). Further, deletion of cPLA2 by gene targeting did not alter NOX2 activation in PMN or peritoneal macrophages treated with various agonists, including opsonized zymosan, PMA, and formyl-MLF peptide (39, 40). These latter results indicate that a role for cPLA2 in activation of NOX2 in phagocytes is unlikely. In the present study, AACOCF3 did not block the ROS response to Ang II or PMA in PMVEC. Thus, cPLA2 is not involved in Ang II- or PMA-mediated NOX2 activation in endothelial cells, although a role for cPLA2 in response to other agonists cannot be excluded.
In summary, this study has shown that Prdx6 is required for activation of NOX2 in mouse PMVEC stimulated with Ang II or PMA and alveolar macrophages stimulated with concanavalin A or fMLF. Exposure of PMVEC to agonists results in MAPK-mediated phosphorylation of Prdx6, its translocation to the cell membrane, and activation of phospholipase A2 activity that leads to translocation of NOX2 cytoplasmic components and activation of the enzyme complex.
Acknowledgments
We thank Dr. Mahendra Jain for constructive criticism; the Abramson Cancer Center Flow Cytometry and Cell Sorting Shared Resource for assistance with flow cytometry; Daniel Gonder, Ling Gao, and Alex Knihnicky for technical support; and Victoria Brown for typing the manuscript.
This work was supported, in whole or in part, by National Institutes of Health Grant HL105509. This work was presented in part at the 2010 Experimental Biology meeting in Anaheim, CA.
- ROS
- reactive oxygen species
- AACOCF3
- arachidonyltrifluoromethyl ketone
- Ang II
- angiotensin II
- BEL
- bromoenol lactone
- DCF
- dichlorofluoroscein
- H2DCF
- dihydrodichlorofluorescein
- DPPC
- 1-palmitoyl, 2-palmitoyl, sn-glycero-3-phosphocholine
- fMLF
- N-formyl-Met-Leu-Phe peptide
- HE
- hydroethidium
- MJ33
- 1-hexadecyl-3-trifluoroethylglycero-sn-2-phosphomethanol
- NOX
- NADPH oxidase
- pBPB
- p-bromophenacyl bromide
- PLA2
- phospholipase A2
- cPLA2
- cytoplasmic PLA2
- PMA
- phorbol 12-myristate 13-acetate
- PMVEC
- pulmonary microvascular endothelial cell(s)
- Prdx
- peroxiredoxin
- H2DCF
- dihydrodichlorofluorescein
- PECAM
- platelet endothelial cell adhesion molecule 1
- PC
- phosphatidylcholine
- Ab
- antibody
- PMN
- polymorphonuclear leukocyte(s).
REFERENCES
- 1. Frey R. S., Ushio-Fukai M., Malik A. B. (2009) Antioxid. Redox Signal. 11, 791–810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Lassegue B., Griendling K. K. (2010) Arterioscler. Thromb. Vasc. Biol. 30, 653–661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Lambeth J. D. (2002) Curr. Opin. Hematol. 9, 11–17 [DOI] [PubMed] [Google Scholar]
- 4. Pendyala S., Usatyuk P. V., Gorshkova I. A., Garcia J. G., Natarajan V. (2009) Antioxid. Redox Signal. 11, 841–860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Rhee S. G., Chae H. Z., Kim K. (2005) Free Radic. Biol. Med. 38, 1543–1552 [DOI] [PubMed] [Google Scholar]
- 6. Fisher A. B. (2011) Antioxid. Redox Signal., in press [Google Scholar]
- 7. Leavey P. J., Gonzalez-Aller C., Thurman G., Kleinberg M., Rinckel L., Ambruso D. W., Freeman S., Kuypers F. A., Ambruso D. R. (2002) J. Biol. Chem. 277, 45181–45187 [DOI] [PubMed] [Google Scholar]
- 8. Wu Y., Feinstein S. I., Manevich Y., Chowdhury I., Pak J. H., Kazi A., Dodia C., Speicher D. W., Fisher A. B. (2009) Biochem. J. 419, 669–679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Mo Y., Feinstein S. I., Manevich Y., Zhang Q., Lu L., Ho Y. S., Fisher A. B. (2003) FEBS Lett. 555, 192–198 [DOI] [PubMed] [Google Scholar]
- 10. Liu G., Feinstein S. I., Wang Y., Dodia C., Fisher D., Yu K., Ho Y. S., Fisher A. B. (2010) Free Radic. Biol. Med. 49, 1172–1181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Zhang Q., Matsuzaki I., Chatterjee S., Fisher A. B. (2005) Am. J. Physiol. Lung Cell. Mol. Physiol. 289, L954–L961 [DOI] [PubMed] [Google Scholar]
- 12. Milovanova T., Chatterjee S., Manevich Y., Kotelnikova I., Debolt K., Madesh M., Moore J. S., Fisher A. B. (2006) Am. J. Physiol. Cell. Physiol. 290, C66–C76 [DOI] [PubMed] [Google Scholar]
- 13. Milovanova T., Chatterjee S., Hawkins B. J., Hong N., Sorokina E. M., Debolt K., Moore J. S., Madesh M., Fisher A. B. (2008) Biochim. Biophys. Acta. 1783, 1866–1875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Forman H. J., Nelson J., Fisher A. B. (1980) J. Biol. Chem. 255, 9879–9883 [PubMed] [Google Scholar]
- 15. Manevich Y., Sweitzer T., Pak J. H., Feinstein S. I., Muzykantov V., Fisher A. B. (2002) Proc. Natl. Acad. Sci. U.S.A. 99, 11599–11604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Zhang Q., Chatterjee S., Wei Z., Liu W. D., Fisher A. B. (2008) Antioxid. Redox Signal. 10, 679–689 [DOI] [PubMed] [Google Scholar]
- 17. Söderberg O., Gullberg M., Jarvius M., Ridderstråle K., Leuchowius K. J., Jarvius J., Wester K., Hydbring P., Bahram F., Larsson L. G., Landegren U. (2006) Nat. Methods 3, 995–1000 [DOI] [PubMed] [Google Scholar]
- 18. Fisher A. B., Dodia C. (1996) J. Lipid Res. 37, 1057–1064 [PubMed] [Google Scholar]
- 19. Fisher A. B., Dodia C., Feinstein S. I., Ho Y. S. (2005) J. Lipid Res. 46, 1248–1256 [DOI] [PubMed] [Google Scholar]
- 20. Kim T. S., Dodia C., Chen X., Hennigan B. B., Jain M., Feinstein S. I., Fisher A. B. (1998) Am. J. Physiol. 274, L750–L761 [DOI] [PubMed] [Google Scholar]
- 21. Chen J. W., Dodia C., Feinstein S. I., Jain M. K., Fisher A. B. (2000) J. Biol. Chem. 275, 28421–28427 [DOI] [PubMed] [Google Scholar]
- 22. Manevich Y., Reddy K. S., Shuvaeva T., Feinstein S. I., Fisher A. B. (2007) J. Lipid Res. 48, 2306–2318 [DOI] [PubMed] [Google Scholar]
- 23. Ackermann E. J., Conde-Frieboes K., Dennis E. A. (1995) J. Biol. Chem. 270, 445–450 [DOI] [PubMed] [Google Scholar]
- 24. Akiba S., Dodia C., Chen X., Fisher A. B. (1998) Comp. Biochem. Physiol. B Biochem. Mol. Biol. 120, 393–404 [DOI] [PubMed] [Google Scholar]
- 25. Manevich Y., Shuvaeva T., Dodia C., Kazi A., Feinstein S. I., Fisher A. B. (2009) Arch. Biochem. Biophys. 485, 139–149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Lu D. J., Furuya W., Grinstein S. (1993) Blood Cells 19, 343–349; discussion 349–351 [PubMed] [Google Scholar]
- 27. Dusi S., Donini M., Rossi F. (1994) Biochem. J. 304, 243–250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Torres M., Forman H. J. (1999) Arch. Biochem. Biophys. 366, 231–239 [DOI] [PubMed] [Google Scholar]
- 29. Yu G., Peng T., Feng Q., Tyml K. (2007) Microcirculation 14, 125–136 [DOI] [PubMed] [Google Scholar]
- 30. Bostan M., Galatiuc C., Hirt M., Constantin M. C., Brasoveanu L. I., Iordachescu D. (2003) J. Cell. Mol. Med. 7, 57–66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Dana R., Leto T. L., Malech H. L., Levy R. (1998) J. Biol. Chem. 273, 441–445 [DOI] [PubMed] [Google Scholar]
- 32. Silliman C. C., Elzi D. J., Ambruso D. R., Musters R. J., Hamiel C., Harbeck R. J., Paterson A. J., Bjornsen A. J., Wyman T. H., Kelher M., England K. M., McLaughlin-Malaxecheberria N., Barnett C. C., Aiboshi J., Bannerjee A. (2003) J. Leukoc. Biol. 73, 511–524 [DOI] [PubMed] [Google Scholar]
- 33. Rao G. N., Lassègue B., Alexander R. W., Griendling K. K. (1994) Biochem. J. 299, 197–201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Dana R., Malech H. L., Levy R. (1994) Biochem. J. 297, 217–223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. O'Donnell R. W., Johnson D. K., Ziegler L. M., DiMattina A. J., Stone R. I., Holland J. A. (2003) Endothelium 10, 291–297 [DOI] [PubMed] [Google Scholar]
- 36. Shmelzer Z., Haddad N., Admon E., Pessach I., Leto T. L., Eitan-Hazan Z., Hershfinkel M., Levy R. (2003) J. Cell. Biol. 162, 683–692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Zhao X., Bey E. A., Wientjes F. B., Cathcart M. K. (2002) J. Biol. Chem. 277, 25385–25392 [DOI] [PubMed] [Google Scholar]
- 38. Hazan I., Dana R., Granot Y., Levy R. (1997) Biochem. J. 326, 867–876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Rubin B. B., Downey G. P., Koh A., Degousee N., Ghomashchi F., Nallan L., Stefanski E., Harkin D. W., Sun C., Smart B. P., Lindsay T. F., Cherepanov V., Vachon E., Kelvin D., Sadilek M., Brown G. E., Yaffe M. B., Plumb J., Grinstein S., Glogauer M., Gelb M. H. (2005) J. Biol. Chem. 280, 7519–7529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Gijón M. A., Spencer D. M., Siddiqi A. R., Bonventre J. V., Leslie C. C. (2000) J. Biol. Chem. 275, 20146–20156 [DOI] [PubMed] [Google Scholar]
- 41. García Arguinzonis M. I., Galler A. B., Walter U., Reinhard M., Simm A. (2002) J. Biol. Chem. 277, 45604–45610 [DOI] [PubMed] [Google Scholar]