Abstract
Polycyclic aromatic hydrocarbons (PAH) are believed to be causative agents for various types of cancers in humans. Benzo[a]pyrene (BaP) is a prototypic carcinogenic PAH, which requires metabolic activation to elicit its detrimental effects. The major end product of its diol epoxide metabolic activation pathway is r-7,t-8,9,c-10-tetrahydroxy-7,8,9,10-tetrahydrobenzo[a]pyrene (trans, anti-BaPT). Individual differences in exposure to, and metabolic activation of, carcinogenic PAH may influence cancer risk. Measurement of PAH metabolites in human urine could provide a direct way to assess individual differences in susceptibility to PAH-related cancer. In this paper, we describe a sensitive and reliable method for quantitation of trans, anti-BaPT in human urine using gas chromatography-negative ion chemical ionization-tandem mass spectrometry (GC-NICI-MS/MS). [13C6] trans, anti-BaPT was used as the internal standard. The urine was treated with β-glucuronidase and sulfatase, and then trans, anti-BaPT was enriched by solid-phase extraction with polymeric reversed phase and phenylboronic acid cartridges. The sample was silylated and analyzed by GC-NICI-MS/MS with selected reaction monitoring (SRM) for the trimethylsilyl (TMS) derivatives of trans, anti-BaPT (m/z 446→ m/z 255) and [13C6]trans, anti-BaPT (m/z 452→ m/z 261). The mean assay recovery was 44%. The instrumental on-column detection limit was about 20 amol of trans, anti-BaPT (as BaPT-TMS). trans, anti-BaPT was readily detected in all urine samples analyzed including 30 smokers (0.71 ± 0.64 fmol/mg creatinine) and 30 non-smokers (0.34 ± 0.2 fmol/mg creatinine) (P = 0.0018). The results of this study demonstrate a highly sensitive and selective method for quantitation of trans, anti-BaPT in human urine. This is to our knowledge the first study to show that smokers have significantly higher levels of trans, anti-BaPT in their urine than do non-smokers. This method may be useful as a direct phenotyping approach to assess individual differences in uptake and metabolic activation of carcinogenic PAH.
Keywords: benzo[a]pyrene metabolites, biomarker, metabolic activation, human urine
Introduction
Polycyclic aromatic hydrocarbons (PAHs) are a large class of compounds mainly formed by incomplete combustion of organic matter. They are commonly found in polluted air and water, tobacco smoke, broiled and smoked foods, and in certain occupational environments such as coke production from coal and other processes involving soots and tars (1–7). Humans readily absorb PAH into the body through the lung, gastrointestinal tract, and skin. Many PAHs are carcinogens, and are believed to be causative agents for various types of human cancers, including lung cancer in smokers (6,8–10). Benzo[a]pyrene (BaP, Scheme 1), a prototypic PAH, is rated as carcinogenic to humans by the International Agency for Research on Cancer (10). This five-ring PAH is present in virtually all PAH mixtures, and is one of the most carcinogenic of those commonly detected. It has been conclusively demonstrated in laboratory animal studies that BaP is a powerful carcinogen, which readily induces tumors in various tissues such as lung and skin at relatively low doses (2,8,10–12).
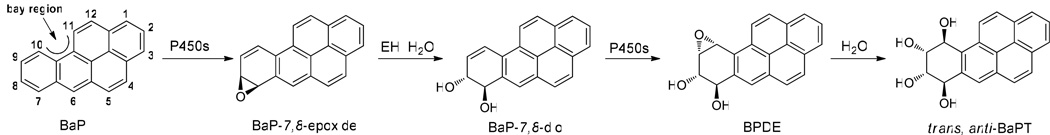
Scheme 1.
Metabolism of benzo[a]pyrene (BaP) to trans, anti-BaPT via the bay region diol epoxide BPDE. P450, cytochrome P450; EH, epoxide hydrolase.
PAHs including BaP are procarcinogens that require metabolic activation to elicit their carcinogenic effects (10,13). Considerable evidence supports the view that these carcinogens act as tumor initiators after metabolic oxidation to reactive electrophiles by the bay region diol epoxide pathway (Scheme 1) (13–15). This pathway starts with an initial cytochrome P450-scatalyzed epoxide formation at the 7,8-position followed by epoxide hydrolase -catalyzed hydration (16–18). Subsequent epoxidation at the 9,10-position then predominantly generates BaP-(7R,8S)-diol-(9S,10R)-epoxide (BPDE) (Scheme 1) (13–15,19,20). BPDE is considered to be a major ultimate carcinogen of BaP because it is carcinogenic and readily reacts with DNA to produce the same adducts as observed in biological systems exposed to BaP (13–15,19,20). The major reaction of BPDE in biological systems is hydrolysis producing predominantly BaP-(7R,8S,9R,10S)-tetraol, which is one enantiomer of r-7,t-8,9,c-10-tetrahydroxy-7,8,9,10-tetrahydrobenzo[a]pyrene (trans, anti-BaPT). Competing with metabolic activation, detoxification pathways of BaP include phenol formation, glutathione conjugation, glucuronidation, and sulfation (8,13,14).
Approximately 11–24% of lifelong smokers develop lung cancer over their lifetimes (6). It would be important to identify individuals in this group with apparently higher cancer susceptibility. Our working hypothesis is that people who metabolically activate tobacco smoke carcinogens more extensively should have higher cancer risk. Large inter-individual differences in the metabolic activation of carcinogenic PAHs have been demonstrated (21–24). Multiple studies have used genotyping methods to examine the relationship between lung cancer risk and polymorphisms in genes such as CYP1A1 and GSTM1 which code for enzymes involved in PAH metabolism (25–33). But the results remain varied and inconsistent, likely because of the complexity of PAH metabolism (34). We propose that a phenotyping strategy, with direct measurement of metabolites generated by the metabolic activation pathways, can provide more comprehensive and reliable information on an individual’s susceptibility to cancer (9,35). With this goal in mind, we developed and optimized a gas chromatography-negative ion chemical ionization-tandem mass spectrometry (GC-NICI-MS/MS) method to quantify racemic trans, anti-BaPT in human urine.
Materials and Methods
Chemicals, Enzymes and Apparatus
All BaP metabolites used in this study were racemic. r-7,t-8,9,c-10-tetrahydroxy-7,8,9,10-tetrahydrobenzo[a]pyrene (trans, anti-BaPT), r-7,t-8,9,10-tetrahydroxy-7,8,9,10-tetrahydrobenzo[a]pyrene (cis, anti-BaPT), r-7,t-8,,c-9,10-tetrahydroxy-7,8,9,10-tetrahydrobenzo[a]pyrene (cis, syn-BaPT), r-7,t-8,c-9,t-10-tetrahydroxy-7,8,9,10-tetrahydrobenzo[a]pyrene (trans, syn-BaPT), trans-4,5-dihydro-4,5-dihydroxybenzo[a]pyrene (BaP-4,5-diol), trans-7,8-dihydro-7,8-dihydroxybenzo[a]pyrene (BaP-7,8-diol) and trans-9,10-dihydro-9,10-dihydroxybenzo[a]pyrene (BaP-9,10-diol) were obtained from the National Cancer Institute Chemical Carcinogen Reference Standard Repository, Midwest Research Institute (Kansas City, MO). The internal standard, [13C6]trans, anti-BaPT was purchased from Cambridge Isotope Laboratories, Inc (Andover, MA). Purities of these standards were >99%, as determined by HPLC analysis. β-Glucuronidase and arylsulfatase (from Helix pomatia) were obtained from Roche Diagnostics Corp.(Indianapolis, IN). Strata-X polymeric reversed phase SPE cartridges (200 mg/6 mL, #8B-S10-FCH) were procured from Phenomenex (Torrance, CA). Bond Elute phenylboronic acid SPE cartridges (100 mg/1 mL, # 12102018) were from Varian, Inc (Palo Alto, CA). bis-Trimethylsilyltrifluoroacetamide (BSTFA) was purchased from Regis Technologies (Morton Grove, IL). Removal of solvents was carried out with an SVC-200 Speedvac (Thermo Savant, Holbrook, NY). Gas chromatography-negative ion chemical ionization-tandem mass spectrometry (GC-NICI-MS/MS) was carried out with a TSQ Quantum instrument (Thermo Scientific).
Urine Samples
This study was approved by the University of Minnesota Institutional Review Board. Urine samples from 30 smokers (11 females) were first morning voids obtained from ongoing studies at the University of Minnesota Tobacco Use Research Center. Current smoking status was confirmed by CO and cotinine levels. Urine samples from thirty non-smokers (16 females) were spot samples taken at similar times of day from laboratory personnel. All urine samples were stored in a −20 °C freezer before analysis.
Determination of urinary creatinine
The creatinine content in urine was determined at the University of Minnesota Medical Center, Fairview, Diagnostic Laboratories, using Vitros CREA slides.
Analysis of Urine for trans, anti-BaPT
A 1.5 mL aliquot of urine was placed in a 10 mL centrifuge tube containing 780 µL of 0.5M NaOAc buffer, pH 5, β-glucuronidase (3,500 units) and arylsulfatase (28,000 units). [13C6]trans, anti-BaPT (20 fmol) in 2 µL CH3CN was added as internal standard. The mixture was incubated in a water bath overnight with shaking at 37 °C. A Strata-X cartridge was preconditioned with 5 mL of CH3OH, then with 5 mL of H2O. The urine sample was applied to the cartridge slowly, along with two 1 mL H2O washings of the urine sample tubes. The cartridge was washed with 5 mL of 1% NH4OH in 50% CH3OH, then with 0.5 mL CH3OH. trans, anti-BaPT was then eluted with 2 mL CH3OH. This fraction contained the analyte and internal standard. Solvents were removed by overnight concentration on a Speedvac. The residue was dissolved in 1 mL of 30% aq CH3OH with sonication and loaded onto a 100 mg/1 mL phenylboronic acid cartridge, which had been preconditioned with 1 mL of CH3OH and then with 1 mL of H2O. The cartridge was then washed with 100 µL of 30% CH3OH in H2O, placed under vacuum overnight to remove residual H2O, and then washed twice with 1 mL acetone which had been dried with Na2SO4. trans, anti-BaPT was eluted with 1 mL of 80% CH3OH in H2O. The solution was concentrated to dryness. The residue was dissolved in 200 µL of CH3OH with sonication and vortexing, transferred to an insert vial, dried in a speed vacuum apparatus, and dissolved in 10 µL of BSTFA. The samples were heated at 60 °C for 60 min with periodic mixing by vortexing, and 2.5 µL was injected into the GC-NICI-MS/MS system.
GC-NICI-MS/MS Analysis
This was modified from that described previously (36). The GC was fitted with a 0.25 mm (inside diameter) × 30 m, 0.15-µm film thickness, DB-17MS column (Agilent Technologies, Palo Alto, CA), and a 0.53 mm (inside diameter) × 2 m deactivated fused silica precolumn. The oven temperature program was as follows: 80 °C for 1 min, then 80 to 200 °C at 35 °C/min, then 200 to 215 °C at 3 °C/min, then 215 to 320 °C at 35 °C/min, then hold for 3 min. The injection port temperature was 250 °C, and the MS transfer line temperature was 320 °C. The flow rate was 1 mL/min He. The injector was operated in the splitless mode; the evaporation temperature was 80 °C for 2 min, then 80 to 260 °C at 5 °C/sec, then hold for 1 min. The NICI-MS/MS conditions were as follows: CI gas, methane at 2.0 mL/min; source temperature, 200 °C; emission current, 500 µA. Selected reaction monitoring (SRM) with a collision energy of 18 eV, electron energy of −150 eV, and Ar collision gas pressure of 1.0 mTorr was used to detect trans, anti-BaPT-tetratrimethylsilyl ether (trans, anti-BaPT-TMS) and [13C6]trans, anti-BaPT-TMS at m/z 446 → m/z 255 and m/z 452 → m/z 261, respectively.
Hepatocyte Incubations
Primary human hepatocytes were purchased from Cellzdirect (St. Louis, MO), and prepared as described before (37). Hepatocytes (approximately 0.12 mg protein per well) were incubated with 10 µM of BaP-4,5-diol, BaP-7,8-diol or BaP-9,10-diol, or H2O as a control. Each diol was dissolved in 20 µL DMSO and added to the 2 mL incubation mixtures. One mL of media was collected at 24 hrs. Samples were stored at −80 °C until analysis.
Statistical Analysis
The hypothesis that the level of trans, anti-BaPT in the urine of smokers (n=30) was significantly higher than that in non-smokers (n=30) was tested using the two-sided two-sample t test.
Results
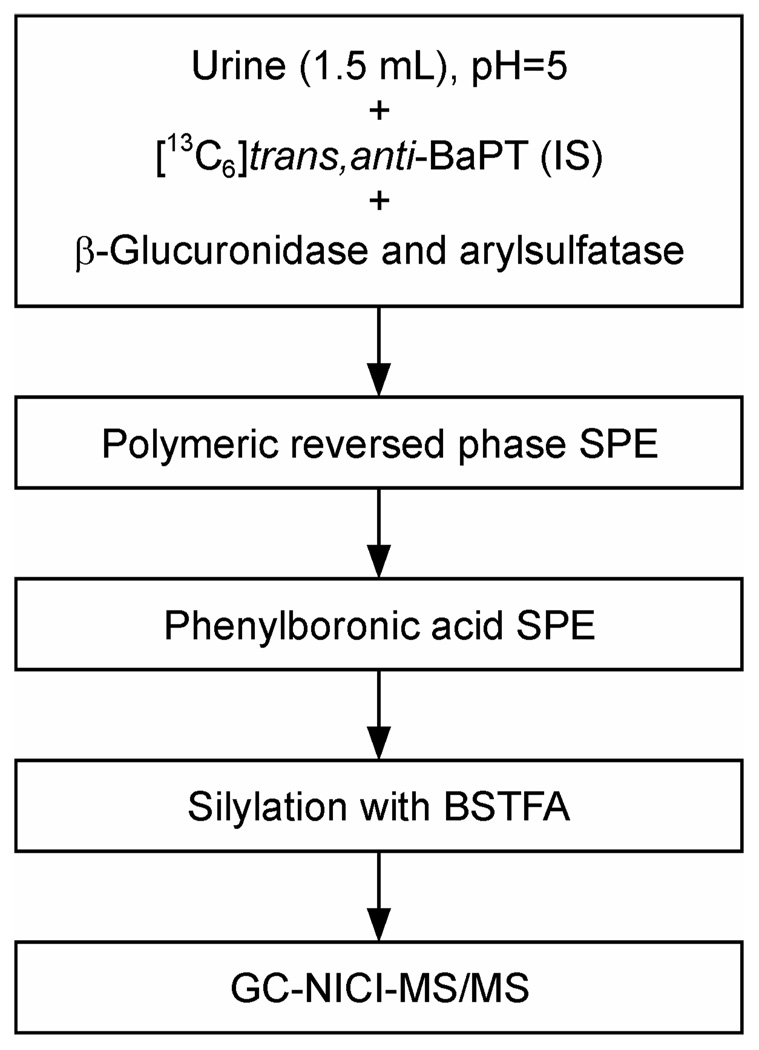
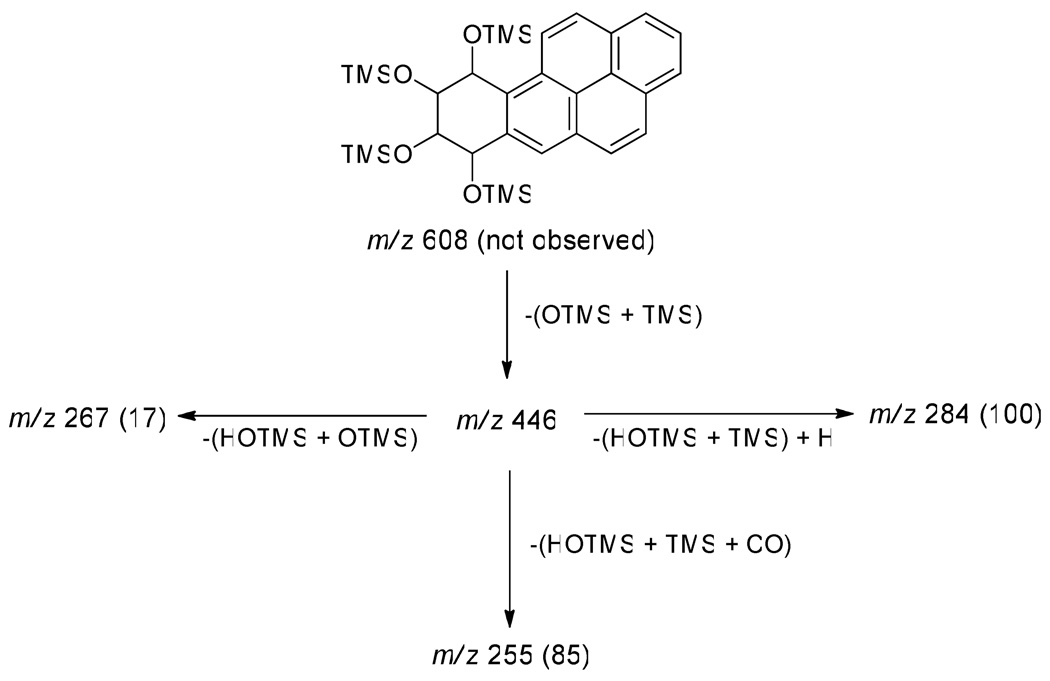
The analytical method is outlined in Scheme 2. After the addition of [13C6] trans, anti-BaPT as the internal standard to a 1.5 mL urine sample, the mixture was incubated with β-glucuronidase and aryl sulfatase to release conjugated BaPT. The samples were purified by solid-phase extraction using polymeric reversed phase cartridges, and then phenylboronic acid cartridges which retain cis-hydroxy groups with high specificity (36). trans, anti-BaPT was derivatized with BSTFA to produce the corresponding tetra-TMS ethers. A full scan NICI-MS of BaPT-TMS showed a base peak at m/z 446 [M − (OSi(CH3)3 + Si(CH3)3)]− with little or no molecular ion (m/z 608). We used GC-NICI-MS/MS-SRM to monitor the transition m/z 446 → m/z 255 for trans, anti-BaPT-TMS, and m/z 452 → m/z 261 for the internal standard [13C6]trans, anti-BaPT-TMS. The daughter ion peak at m/z 255 corresponds to the loss of [HOSi(CH3)3 + (CH3)3Si + CO] from m/z 446 (Scheme 3).
Scheme 2.
Analytical scheme for quantitation of trans, anti-BaPT in human urine. IS, internal standard; SPE, solid phase extraction; BSTFA, bis-trimethylsilyltrifluoroacetamide; GC-NICI-MS/MS, gas chromatography-negative ion chemical ionization-tandem mass spectrometry.
Scheme 3.
Fragmentation pattern and relative intensities (in parentheses) in the daughter ion spectrum of m/z 446 of trans, anti-BaPT-TMS, obtained by GC-NICI-MS/MS. TMS = (CH3)3Si
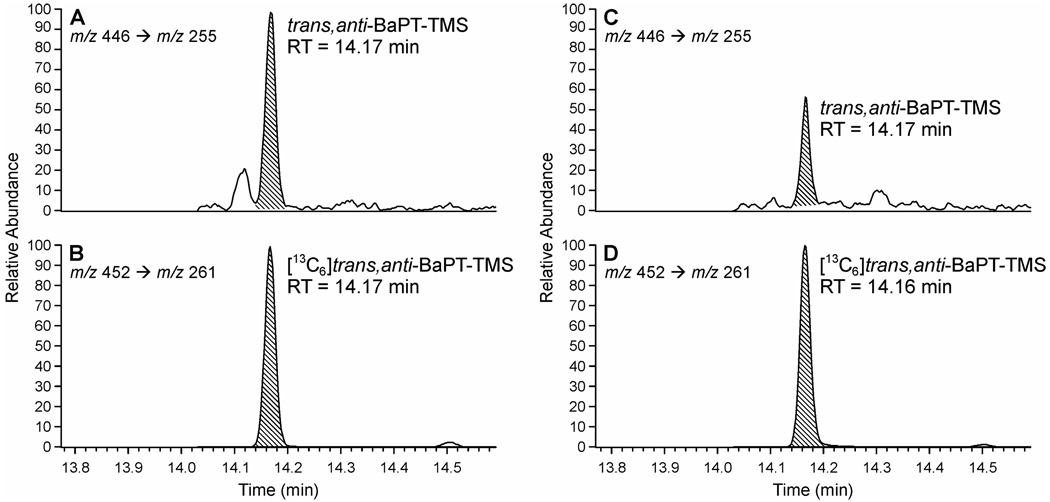
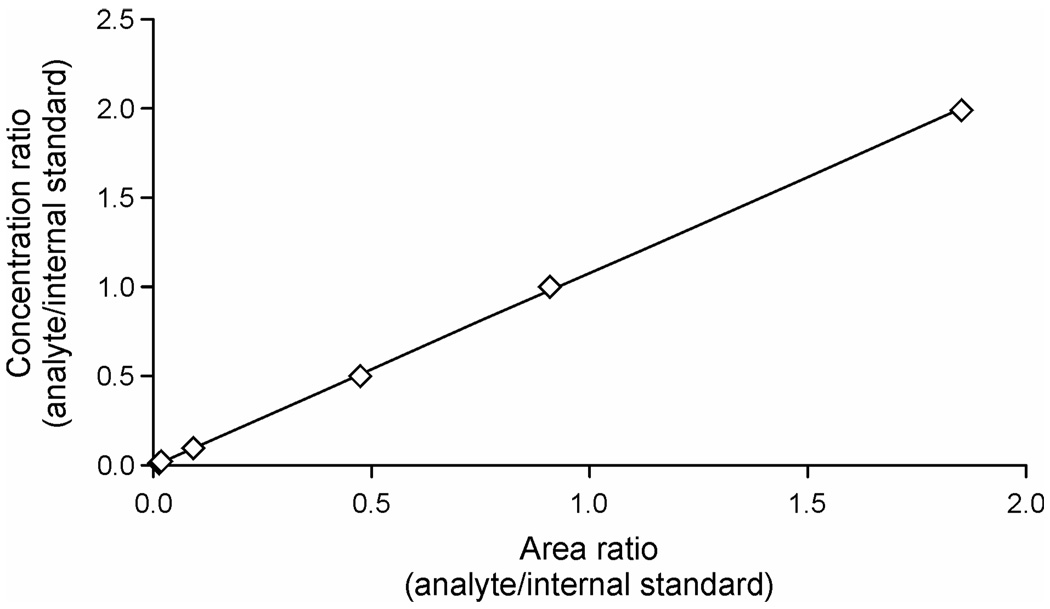
Typical GC-NICI-MS/MS chromatograms obtained upon analysis of trans, anti-BaPT in the urine of a smoker and a non-smoker are shown in Figure 1. The internal standard [13C6]trans, anti-BaPT-TMS eluted at the same retention time as trans, anti-BaPT-TMS. The identity of trans, anti-BaPT-TMS was verified by co-injection with a standard. To further confirm the identity of the analyte peak in the urine samples, three different transitions, from m/z 446 to daughter ions of m/z 255, m/z 267, and m/z 284 were monitored and compared to standards (Scheme 3). As shown in Table 1, the ratios of the integrated peaks from monitoring the three transitions in a urine sample were very close to those in the standards. The GC-NICI-MS/MS method proved to be highly sensitive. The on-column detection limit was about 20 amol of trans, anti-BaPT-TMS. The limit of quantitation of the assay was less than 0.05 fmol trans, anti-BaPT per mL urine. A calibration curve demonstrating linearity when 0.05 – 10 fmol of trans, anti-BaPT was derivatized is illustrated in Figure 2.
Figure 1.
Chromatograms from GC-NICI-MS/MS SRM analysis of trans, anti-BaPT in urine of a smoker (A and B) and a non-smoker (C and D). The indicated peaks are TMS derivatives of trans, anti-BaPT (A and C) and internal standard [13C6]trans, anti-BaPT (B and D).
Table 1.
Ratios of major trans, anti-BaPT-TMS fragment ion intensities from analysis of a human urine sample, compared to trans, anti-BaPT-TMS and [13C6]trans, anti-BaPT-TMS standards.
| m/z 446 → m/z 255 | m/z 446 → m/z 267 | m/z 446 → m/z 284 | |
|---|---|---|---|
| trans, anti-BaPT-TMS from analysis of human urine | 1.0 | 0.2 | 1.3 |
| trans, anti-BaPT-TMS standard | 1.0 | 0.2 | 1.1 |
| m/z 452 → m/z 261 | m/z 452 → m/z 273 | m/z 452 → m/z 290 | |
| [13C6]trans, anti-BaPT-TMS | 1.0 | 0.2 | 1.2 |
Figure 2.
Calibration curve for trans, anti-BaPT-TMS. The amount of standard trans, anti-BaPT was increased from 0.05 fmol to 0.1, 0.5, 2.5, 5, and 10 fmol, with a constant amount of internal standard [13C6]trans, anti-BaPT (20 fmol). The calibration curve shows the concentration ratio (analyte/internal standard) versus their area ratio.
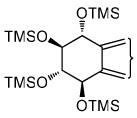
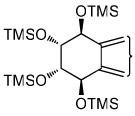
As multiple tetraols could be formed from BaP, we sought further confirmation of the identity of trans, anti-BaPT. Incubation of primary human hepatocytes with BaP-7,8-diol produced four isomers of BaPT, as summarized in Table 2. Each had a retention time identical to those of the TMS derivatives of the corresponding standards - trans, syn-BaPT, trans, anti-BaPT, cis, anti-BaPT, and cis, syn-BaPT. trans, anti-BaPT was the predominant tetraol formed. Incubation of BaP-9,10-diol with human hepatocytes produced two tetraols which co-eluted with standards – trans, syn-BaPT and trans, anti-BaPT. There were also two minor products which did not co-elute with cis, anti-BaPT and cis, syn-BaPT, which was expected since the 9,10-hydroxyl groups in these compounds are cis-, not trans- as in BaP-9,10-diol. The two minor products probably resulted from cis- ring opening of the syn- and anti-9,10-diol-7,8-epoxides, but this was not pursued. A small peak at the same retention time as trans, anti-BaPT was also observed in the incubations of BaP-4,5-diol with human hepatocytes, but the yield of this product was at least 10,000 times lower than those from the two other diols. No tetraol peaks were observed in control incubations.
Table 2.
Summary of retention time (min) and yield (pmol/mL media) of BaPT isomers from incubation of three BaP-diols (10 µM) with human hepatocytes, and analyzed as their TMS derivatives by GC-NICI-MS/MS.
|
trans, syn- BaPT-TMS |
trans, anti- BaPT-TMS |
cis, anti- BaPT-TMS |
cis, syn- BaPT-TMS |
[13C6]trans,anti- BaPT-TMS |
|
|---|---|---|---|---|---|
 |
 |
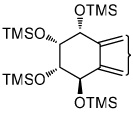 |
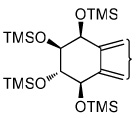 |
||
| tetraol standards | 13.72 | 13.93 | 14.25 | 14.51 | 13.93 |
| tetraols from BaP-7,8-diol | 13.72 (0.4)a | 13.93 (18.1) | 14.25 (1.2) | 14.51 (0.8) | 13.93 |
| tetraols from BaP-9,10-diol | 13.72 (5.6) | 13.93 (7.0) | NDb | ND | 13.93 |
| tetraols from BaP-4,5-diol | 13.93 (0.7×10−3) | 13.93 | |||
retention time (yield)
ND, not detected. Two minor peaks from BaP-9,10-diol were not identified.
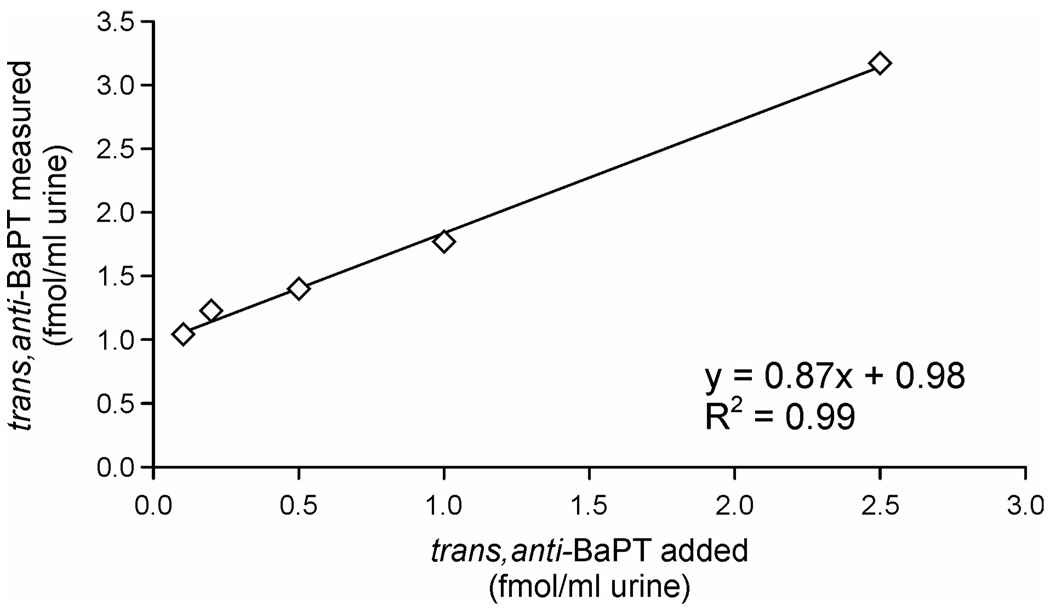
The intraday precision of the assay was determined by analyzing seven aliquots of a smoker’s urine. The results were 1.01 ± 0.05 fmol trans, anti-BaPT/mL urine (relative SD, 4.8%). The interday precision based on analyses of a smoker’s urine (2 per set as positive controls in four sets of assays) was 15.2% (relative SD). Assay accuracy was assessed by the standard addition method. One mL of the positive control urine, which contained 1.01 fmol/mL trans, anti-BaPT, was enriched with 0.1, 0.2, 0.5, 1, and 2.5 fmol trans, anti-BaPT. The results of this experiment are presented in Figure 3. The added and measured levels of trans, anti-BaPT were highly correlated (r = 0.99) and the y intercept was 0.98 fmol/mL urine, in excellent agreement with the amount of trans, anti-BaPT in the untreated sample. Recoveries of internal standard were generally good, averaging 44 ± 19% (n = 80).
Figure 3.
Relationship between levels of trans, anti-BaPT added to a urine sample and levels measured (r=0.998). Points, mean of duplicate determinations.
The method was applied to the analysis of urine samples from 30 non-smokers and 30 smokers. The results are summarized in Table 3. The mean level of trans, anti-BaPT (0.71 ± 0.64 fmol/mg creatinine, n = 30) in the urine of smokers was significantly higher (P = 0.0018) than that in non-smokers (0.34 ± 0.20 fmol/mg creatinine, n = 30). No gender differences in the amounts of this analyte were found among either smokers or non-smokers.
Table 3.
| a. Urinary trans, anti-BaPT concentrations in non-smokers. | ||||
|---|---|---|---|---|
| Non-smokers | trans, anti-BaPT | |||
| Subject no. | age | gender | fmol / ml urine | fmol / mg creatinine |
| 1 | 28 | F | 0.37 | 0.38 |
| 2 | 23 | F | 0.31 | 0.25 |
| 3 | 25 | F | 0.21 | 0.21 |
| 4 | 22 | M | 0.23 | 0.45 |
| 5 | 25 | M | 0.27 | 0.99 |
| 6 | 24 | M | 0.24 | 0.31 |
| 7 | 28 | F | 0.21 | 0.63 |
| 8 | 22 | M | 0.22 | 0.26 |
| 9 | 24 | M | 0.22 | 0.13 |
| 10 | 25 | F | 0.22 | 0.75 |
| 11 | 22 | M | 0.28 | 0.20 |
| 12 | 24 | F | 0.22 | 0.27 |
| 13 | 60 | M | 0.28 | 0.18 |
| 14 | 27 | F | 0.26 | 0.35 |
| 15 | 25 | F | 0.26 | 0.55 |
| 16 | 57 | M | 0.24 | 0.19 |
| 17 | 41 | M | 0.34 | 0.51 |
| 18 | 29 | F | 0.21 | 0.24 |
| 19 | 24 | F | 0.26 | 0.44 |
| 20 | 50 | F | 0.20 | 0.26 |
| 21 | 24 | F | 0.24 | 0.20 |
| 22 | 52 | M | 0.28 | 0.14 |
| 23 | 66 | M | 0.25 | 0.39 |
| 24 | 39 | M | 0.36 | 0.36 |
| 25 | 28 | F | 0.26 | 0.31 |
| 26 | 23 | M | 0.25 | 0.10 |
| 27 | 23 | M | 0.25 | 0.21 |
| 28 | 28 | M | 0.25 | 0.21 |
| 29 | 29 | F | 0.24 | 0.31 |
| 30 | 75 | M | 0.24 | 0.57 |
| Mean ± SD Range |
0.26 ± 0.04 0.20 – 0.37 |
0.34 ± 0.20 0.10 – 0.99 |
||
| 3b. Urinary trans, anti-BaPT concentrations in smokers. | |||||
|---|---|---|---|---|---|
| Smokers (n=30) | trans, anti-BaPT | ||||
| Subject no. | age | gender | cigs/day | fmol / ml urine | fmol / mg creatinine |
| 1 | 24 | F | 14 | 0.61 | 1.38 |
| 2 | 20 | F | 12 | 0.60 | 0.69 |
| 3 | 53 | M | 15 | 0.51 | 0.65 |
| 4 | 24 | M | 16 | 0.50 | 0.74 |
| 5 | 35 | F | 12 | 1.20 | 1.14 |
| 6 | 44 | M | 20 | 0.73 | 0.50 |
| 7 | 66 | M | 20 | 0.41 | 0.35 |
| 8 | 57 | M | 30 | 0.62 | 0.28 |
| 9 | 43 | F | 15 | 1.31 | 0.60 |
| 10 | 56 | M | 10 | 0.93 | 0.33 |
| 11 | 24 | F | 20 | 0.56 | 0.34 |
| 12 | 28 | M | 24 | 0.40 | 0.57 |
| 13 | 20 | M | 20 | 0.47 | 0.47 |
| 14 | 56 | M | 20 | 0.62 | 0.56 |
| 15 | 53 | M | 25 | 0.62 | 0.23 |
| 16 | 49 | M | 8 | 0.55 | 0.33 |
| 17 | 55 | M | 40 | 0.36 | 0.41 |
| 18 | 50 | F | 20 | 0.33 | 0.33 |
| 19 | 43 | M | 30 | 0.27 | 0.34 |
| 20 | 30 | F | 20 | 0.45 | 0.31 |
| 21 | 42 | F | 20 | 0.29 | 0.23 |
| 22 | 54 | F | 20 | 0.29 | 0.93 |
| 23 | 42 | M | 10 | 0.64 | 0.86 |
| 24 | 27 | M | 40 | 0.36 | 1.07 |
| 25 | 38 | M | 12 | 0.38 | 0.42 |
| 26 | 30 | M | 20 | 0.28 | 0.73 |
| 27 | 43 | F | 15 | 1.61 | 0.86 |
| 28 | 40 | F | 15 | 1.38 | 1.74 |
| 29 | 47 | M | 23 | 0.28 | 0.63 |
| 30 | 38 | F | 14 | 0.98 | 3.62 |
| Mean ± SD Range |
0.64 ± 0.34 0.27 – 1.61 |
0.71 ± 0.64 0.23 – 3.63 |
|||
Discussion
We present a highly selective and sensitive GC-NICI-MS/MS-SRM method for quantitation of trans, anti-BaPT in human urine. The method is precise, accurate, yet relatively simple with only two SPE purification steps. The GC-NICI-MS/MS-SRM traces illustrated in Figure 1 are quite clean. The sensitivity of the method, with a quantitation limit of 0.05 fmol of trans, anti-BaPT/mL urine, was essential for its success. Due to its relative simplicity, this analytical method might be applicable to large numbers of samples in epidemiology studies in the future. A potentially significant advantage of this method is that it measures exposure plus metabolic activation of BaP, and therefore may be related to cancer risk.
A major challenge for quantitation of BaP metabolites in human urine is that their levels are extremely low compared to those of smaller PAH such as phenanthrene. The urinary levels of trans, anti-BaPT are, for example, approximately 10,000 times lower than those of the corresponding metabolite of phenanthrene, r-1,t-2,3-c-4-tetrahydroxy-1,2,3,4-tetrahydrophenanthrene (trans, anti-PheT), which we have previously quantified in the urine of smokers (38,39). The substantially lower levels of trans, anti-BaPT in human urine compared to those of trans, anti-PheT results from differences in exposure, metabolism, and excretion. The level of BaP in mainstream cigarette smoke is about 30 times lower than that of phenanthrene (5–10 ng vs. 150–300 ng/cigarette) (4,40). BaP metabolites are mainly excreted in feces while phenanthrene metabolites are excreted mainly in urine, based on studies in laboratory animals (41–44). Metabolism of phenanthrene to trans, anti-PheT may exceed metabolism of BaP to trans, anti-BaPT, but further studies are required on this point.
Only a few studies have previously described methods for the analysis of BaP metabolites in human urine. 3-Hydroxy BaP was quantified as a biomarker of exposure in industrial workers (45,46). Weston et al. and Bowman et al. analyzed BaPT in human urine using immuno-affinity chromatography for sample purification and synchronous fluorescence spectroscopy for quantitation (47,48). They reported BaPT levels of 240–3120 fmol/mL in the urine of four individuals who were highly exposed to PAHs in their diet, and 150 fmol/mL BaPT in psoriasis patients receiving coal tar therapy. Our laboratory determined urinary trans, anti-BaPT by GC-NICI-MS-SIM following three steps of SPE purification, and reported 16 fmol/mL trans, anti-BaPT from psoriasis patients and 0.5 fmol/mL from 9 of 21 smokers, with an on-column limit of detection of 1 fmol (39). The lower sensitivity in that study was due to the lower sensitivity of the MS system available at that time. As shown in this paper, with the modified SPE preparation and high performance GC-NICI-MS/MS-SRM, we were able to achieve an ultra low detection limit of 20 amol, at least 50 times greater than our previous method for trans, anti-BaPT analysis. Thus, in contrast to our previous study, we were able to detect trans, anti-BaPT in 100% of the urine samples from smokers and non-smokers. In addition, the urinary trans, anti-BaPT level in smokers we reported here (mean 0.64 fmol/mL urine, or 0.71 fmol/mg creatinine, n = 30) is quite consistent with our previous data for the samples in which quantitation was possible (mean 0.5 fmol/mL urine, n = 9).
Our results clearly demonstrate, apparently for the first time, that cigarette smokers have significantly higher levels of trans, anti-BaPT, the end-product of the carcinogenic diol epoxide metabolic activation pathway of BaP, in their urine than do non-smokers. Besides higher BaP exposure in smokers from cigarettes (5–10 ng/cigarette, and mean = 19 cigarettes per day per person, in this study) (4,40), this difference may also result from the induction of P450s 1A1, 1A2, and 1B1, known to occur in smokers (49), as these enzymes are involved in producing trans, anti-BaPT. Non-smokers are exposed to BaP probably from polluted air and food. Specifically, our results have shown that smokers excreted approximately twice as much trans, anti-BaPT as did non-smokers. This is quite consistent with previous results comparing urinary metabolites from two other PAH components - pyrene and phenanthrene -between smokers and nonsmokers. Levels of 1-hydroxypyrene, a urinary metabolite of pyrene, are about twice as high in smokers' urine as in non-smokers urine, in most studies (35,50,51). Our laboratory has reported that smokers have about three times higher levels of urinary trans, anti-PheT than do non-smokers (38).
In this study, we quantified racemic trans, anti-BaPT. One enantiomer of this tetraol would be expected to arise from hydrolysis of BPDE, based on known stereoselectivity in the metabolic formation of BPDE, as illustrated in Scheme 1 and summarized previously (36). The opposite enantiomer would be expected from hydrolysis of the reverse diol epoxide, BaP-(9S,10R)-diol-(7R,8S)-epoxide. Thus, racemic trans, anti-BaPT measured here could result from both pathways. We have previously shown that, in the urine of creosote workers exposed to relatively high levels of BaP, 78% of trans, anti-BaPT results from hydrolysis of BPDE. Whether that is also the case in smokers and non-smokers, as in this study, requires further investigation.
Measurement of BPDE-DNA adducts in humans may be a more direct approach to examine BaP metabolic activation than that described here. However, methods such as immunoassays and 32P postlabelling for BaP-DNA adduct have limitations, and quantitation can be unreliable (52). Highly sensitive analytical methods such as HPLC-fluorescence and GC-NICI-MS are quantitative, but usually detect BPDE-DNA adducts in only some human tissues and blood samples (53). The urinary metabolite trans, anti-BaPT may be a more practical biomarker because it can be reliably quantified and detected in all samples (35,54).
The relationship of trans, anti-BaPT levels to lung cancer risk in smokers requires further investigation. Extensive studies of the tumorigenicity of cigarette smoke condensate and its subfractions in mouse skin tumor models indicate that PAH in smoke act mainly as tumor intiators and that expression of their carcinogenicity requires the cocarcinogenic or promoting activity of other smoke constituents (55). Futhermore, cigarette smoke contains multiple carcinogens of other types such as tobacco-specific nitrosamines and volatile organic compounds that most likely contribute to lung cancer in smokers (9). Therefore, it is unlikely that trans, anti-BaPT alone could be related to lung cancer risk. trans, anti-BaPT however could become part of a biomarker panel that would encompass multiple tobacco smoke constituents and their metabolites or DNA or protein adducts, levels of which collectively could be related to cancer risk (54).
In summary, we have developed a relatively simple and ultra-sensitive method for the analysis of trans, anti-BaPT in human urine. Average levels of this metabolite were 0.34 fmol/mg creatinine in non-smokers, and 0.71 fmol/mg creatinine in smokers. This biomarker can potentially provide a critical component of a carcinogen metabolite phenotyping model that may eventually be used to determine individual susceptibility to PAH-induced human cancer.
Acknowledgements
This study was supported by grant no. CA-92025 from the National Cancer Institute. Biostatistical analysis was carried out by Scott Jackson, Biostatistics and Informatics Shared Resource, Masonic Cancer Center, supported in part by grant no. CA-77598. We thank Bob Carlson for editorial assistance.
References
- 1.Brandt HC, Watson WP. Monitoring human occupational and environmental exposures to polycyclic aromatic compounds. Ann Occup. Hyg. 2003;47:349–378. doi: 10.1093/annhyg/meg052. [DOI] [PubMed] [Google Scholar]
- 2.U.S. Department of Health and Human Services. Report on Carcinogens. 11th Edition. N.C.: Research Triangle Park; 2004. pp. III-220–III-222. [Google Scholar]
- 3.Luch A. Polycyclic aromatic hydrocarbon-induced carcinogenesis-an introduction. In: Luch A, editor. The carcinogenic effects of polycyclic aromatic hydrocarbons. London: Imperial College Press; 2005. pp. 1–18. [Google Scholar]
- 4.Ding YS, Ashley DL, Watson CH. Determination of 10 carcinogenic polycyclic aromatic hydrocarbons in mainstream cigarette smoke. J Agric. Food Chem. 2007;55:5966–5973. doi: 10.1021/jf070649o. [DOI] [PubMed] [Google Scholar]
- 5.Stepanov I, Villalta PW, Knezevich A, Jensen J, Hatsukami D, Hecht SS. Analysis of 23 polycyclic aromatic hydrocarbons in smokeless tobacco by gas chromatography-mass spectrometry. Chem. Res. Toxicol. 2010;23:66–73. doi: 10.1021/tx900281u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.International Agency for Research on Cancer. IARC Monographs on the Evaluation of Carcinogenic Risks to Humans. vol. 83. Lyon, FR: IARC; 2004. Tobacco Smoke and Involuntary Smoking; pp. 53–1187. [PMC free article] [PubMed] [Google Scholar]
- 7.International Agency for Research on Cancer. IARC Monographs on the Evaluation of the Carcinogenic Risk of Chemicals to Humans. vol. 38. Lyon, FR: IARC; 1986. Tobacco Smoking; pp. 37–385. [Google Scholar]
- 8.Hecht SS. Tobacco smoke carcinogens and lung cancer. J. Natl. Cancer Inst. 1999;91:1194–1210. doi: 10.1093/jnci/91.14.1194. [DOI] [PubMed] [Google Scholar]
- 9.Hecht SS. Tobacco carcinogens, their biomarkers, and tobacco-induced cancer. Nature Rev. Cancer. 2003;3:733–744. doi: 10.1038/nrc1190. [DOI] [PubMed] [Google Scholar]
- 10.International Agency for Research on Cancer. IARC Monographs on the Evaluation of Carcinogenic Risks to Humans. v. 92. Lyon, FR: IARC; 2010. Some Non-Heterocyclic Polycyclic Aromatic Hydrocarbons and Some Related Exposures; pp. 35–818. [PMC free article] [PubMed] [Google Scholar]
- 11.International Agency for Research on Cancer. IARC Monographs on the Evaluation of the Carcinogenic Risk of Chemicals to Humans. v.32. Lyon, FR: IARC; 1983. Polynuclear aromatic compounds, part 1, chemical, environmental, and experimental data; pp. 33–91. [PubMed] [Google Scholar]
- 12.Culp SJ, Gaylor DW, Sheldon WG, Goldstein LS, Beland FA. A comparison of the tumors induced by coal tar and benzo[a]pyrene in a two-year bioassay. Carcinogenesis. 1998;19:117–124. doi: 10.1093/carcin/19.1.117. [DOI] [PubMed] [Google Scholar]
- 13.Luch A, Baird WM. Metabolic activation and detoxification of polycyclic aromatic hydrocarbons. In: Luch A, editor. The carcinogenic effects of polycyclic aromatic hydrocarbons. London: Imperial College Press; 2005. pp. 19–96. [Google Scholar]
- 14.Gelboin HV. Benzo[a]pyrene metabolism, activation and carcinogenesis: role and regulation of mixed function oxides and related enzymes. Physiol. Rev. 1980;60:1107–1166. doi: 10.1152/physrev.1980.60.4.1107. [DOI] [PubMed] [Google Scholar]
- 15.Conney AH, Chang RL, Jerina DM, Wei SJC. Studies on the metabolism of benzo[a]pyrene and dose-dependent differences in the mutagenic profile of its ultimate carcinogenic metabolite. Drug Metabol. Rev. 1994;26:125–163. doi: 10.3109/03602539409029788. [DOI] [PubMed] [Google Scholar]
- 16.Shimada T, Martin MV, Pruess-Schwartz D, Marnett LJ, Guengerich FP. Roles of individual human cytochrome P-450 enzymes in the bioactivation of benzo(a)pyrene, 7,8-dihydroxy-7,8-dihydrobenzo(a)pyrene, and other dihydrodiol derivatives of polycyclic aromatic hydrocarbons. Cancer Res. 1989;49:6304–6312. [PubMed] [Google Scholar]
- 17.Shimada T, Yun CH, Yamazaki H, Gautier JC, Beaune PH, Guengerich FP. Characterization of human lung microsomal cytochrome P-4501A1 and its role in the oxidation of chemical carcinogens. Mol. Pharmacol. 1992;41:856–864. [PubMed] [Google Scholar]
- 18.Shimada T, Gillam EMJ, Oda Y, Tsumara F, Sutter TR, Guengerich FP, Inoue K. Metabolism of benzo[a]pyrene to trans-7,8-dihydroxy-7,8-dihydrobenzo[a]pyrene by recombinant human cytochrome P450 1B1 and purified liver epoxide hydrolase. Chem. Res. Toxicol. 1999;12:623–629. doi: 10.1021/tx990028s. [DOI] [PubMed] [Google Scholar]
- 19.Conney AH. Induction of microsomal enzymes by foreign chemicals and carcinogenesis by polycyclic aromatic hydrocarbons: G.H.A. Clowes Memorial Lecture. Cancer Res. 1982;42:4875–4917. [PubMed] [Google Scholar]
- 20.Pelkonen O, Nebert DW. Metabolism of polycyclic hydrocarbons: etiologic role in carcinogenesis. Pharmacol. Rev. 1982;34:189–222. [PubMed] [Google Scholar]
- 21.Sabadie N, Richter-Reichhelm HB, Saracci R, Mohr U, Bartsch H. Interindividual differences in oxidative benzo(a)pyrene metabolism by normal and tumorous surgical lung specimens from 105 lung cancer patients. Int. J Cancer. 1981;27:417–425. doi: 10.1002/ijc.2910270402. [DOI] [PubMed] [Google Scholar]
- 22.Harris CC, Autrup H, Connor R, Barrett LA, McDowell EM, Trump BF. Interindividual variation in binding of benzo[a]pyrene to DNA in cultured human bronchi. Science. 1976;194:1067–1069. doi: 10.1126/science.982061. [DOI] [PubMed] [Google Scholar]
- 23.McLemore TL, Adelberg S, Liu MC, McMahon NA, Yu SJ, Hubbard WC, Czerwinski M, Wood TG, Storeng R, Lubet RA, Eggleston JC, Boyd MR, Hines RN. Expression of CYP1A1 gene in patients with lung cancer: evidence for cigarette smoke-induced gene expression in normal lung tissue and for altered gene regulation in primary pulmonary carcinomas. J. Natl. Cancer Inst. 1990;82:1333–1339. doi: 10.1093/jnci/82.16.1333. [DOI] [PubMed] [Google Scholar]
- 24.Nebert DW. Drug-metabolizing enzymes, polymorphisms and interindividual response to environmental toxicants. Clin. Chem Lab Med. 2000;38:857–861. doi: 10.1515/CCLM.2000.124. [DOI] [PubMed] [Google Scholar]
- 25.Bartsch H, Nair U, Risch A, Rojas M, Wikman H, Alexandrov K. Genetic polymorphism of CYP genes, alone or in combination, as a risk modifier of tobacco-related cancers. Cancer Epidemiol Biomarkers & Prev. 2000;9:3–28. [PubMed] [Google Scholar]
- 26.Alexandrov K, Cascorbi I, Rojas M, Bouvier G, Kriek E, Bartsch H. CYP1A1 and GSTM1 genotypes affect benzo[a]pyrene DNA adducts in smokers' lung: comparison with aromatic/hydrophobic adduct formation. Carcinogenesis. 2002;23:1969–1977. doi: 10.1093/carcin/23.12.1969. [DOI] [PubMed] [Google Scholar]
- 27.Smith GB, Harper PA, Wong JM, Lam MS, Reid KR, Petsikas D, Massey TE. Human lung microsomal cytochrome P4501A1 (CYP1A1) activities: impact of smoking status and CYP1A1, aryl hydrocarbon receptor, and glutathione S-transferase M1 genetic polymorphisms. Cancer Epidemiol Biomarkers & Prev. 2001;10:839–853. [PubMed] [Google Scholar]
- 28.Lee WJ, Brennan P, Boffetta P, London SJ, Benhamou S, Rannug A, To-Figueras J, Ingelman-Sundberg M, Shields P, Gaspari L, Taioli E. Microsomal epoxide hydrolase polymorphisms and lung cancer risk: a quantitative review. Biomarkers. 2002;7:230–241. doi: 10.1080/13547500210121882. [DOI] [PubMed] [Google Scholar]
- 29.Benhamou S, Lee WJ, Alexandrie AK, Boffetta P, Bouchardy C, Butkiewicz D, Brockmoller J, Clapper ML, Daly A, Dolzan V, Ford J, Gaspari L, Haugen A, Hirvonen A, Husgafvel-Pursiainen K, Ingelman-Sundberg M, Kalina I, Kihara M, Kremers P, Le Marchand L, London SJ, Nazar-Stewart V, Onon-Kihara M, Rannug A, Romkes M, Ryberg D, Seidegard J, Shields P, Strange RC, Stucker I, To-Figueras J, Brennan P, Taioli E. Meta- and pooled analyses of the effects of glutathione S-transferase M1 polymorphisms and smoking on lung cancer risk. Carcinogenesis. 2002;23:1343–1350. doi: 10.1093/carcin/23.8.1343. [DOI] [PubMed] [Google Scholar]
- 30.Veglia F, Matullo G, Vineis P. Bulky DNA adducts and risk of cancer: a meta-analysis. Cancer Epidemiol. Biomarkers & Prev. 2003;12:157–160. [PubMed] [Google Scholar]
- 31.Raimondi S, Paracchini V, Autrup H, Barros-Dios JM, Benhamou S, Boffetta P, Cote ML, Dialyna IA, Dolzan V, Filiberti R, Garte S, Hirvonen A, Husgafvel-Pursiainen K, Imyanitov EN, Kalina I, Kang D, Kiyohara C, Kohno T, Kremers P, Lan Q, London S, Povey AC, Rannug A, Reszka E, Risch A, Romkes M, Schneider J, Seow A, Shields PG, Sobti RC, Sorensen M, Spinola M, Spitz MR, Strange RC, Stucker I, Sugimura H, To-Figueras J, Tokudome S, Yang P, Yuan JM, Warholm M, Taioli E. Meta- and pooled analysis of GSTT1 and lung cancer: a HuGE-GSEC review. Am J Epidemiol. 2006;164:1027–1042. doi: 10.1093/aje/kwj321. [DOI] [PubMed] [Google Scholar]
- 32.Carlsten C, Sagoo GS, Frodsham AJ, Burke W, Higgins JP. Glutathione S-transferase M1 (GSTM1) polymorphisms and lung cancer: a literature-based systematic HuGE review and meta-analysis. Am J Epidemiol. 2008;167:759–774. doi: 10.1093/aje/kwm383. [DOI] [PubMed] [Google Scholar]
- 33.Hung RJ, Boffetta P, Brockmoller J, Butkiewicz D, Cascorbi I, Clapper ML, Garte S, Haugen A, Hirvonen A, Anttila S, Kalina I, Le Marchand L, London SJ, Rannug A, Romkes M, Salagovic J, Schoket B, Gaspari L, Taioli E. CYP1A1 and GSTM1 genetic polymorphisms and lung cancer risk in Caucasian nonsmokers: a pooled analysis. Carcinogenesis. 2003;24:875–882. doi: 10.1093/carcin/bgg026. [DOI] [PubMed] [Google Scholar]
- 34.Hecht SS, Carmella SG, Yoder A, Chen M, Li Z, Le C, Jensen J, Hatsukami DK. Comparison of polymorphisms in genes involved in polycyclic aromatic hydrocarbon metabolism with urinary phenanthrene metabolite ratios in smokers. Cancer Epidemiol. Biomarkers & Prev. 2006;15:1805–1811. doi: 10.1158/1055-9965.EPI-06-0173. [DOI] [PubMed] [Google Scholar]
- 35.Hecht SS. Human urinary carcinogen metabolites: biomarkers for investigating tobacco and cancer. Carcinogenesis. 2002;23:907–922. doi: 10.1093/carcin/23.6.907. [DOI] [PubMed] [Google Scholar]
- 36.Hecht SS, Carmella SG, Villalta PW, Hochalter JB. Analysis of phenanthrene and benzo[a]pyrene tetraol enantiomers in human urine: relevance to the bay region diol epoxide hypothesis of benzo[a]pyrene carcinogenesis and to biomarker studies. Chem. Res. Toxicol. 2010;23:900–908. doi: 10.1021/tx9004538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Upadhyaya P, Hochalter JB, Balbo S, McIntee EJ, Hecht SS. Preferential glutathione conjugation of a reverse diol epoxide compared with a bay region diol epoxide of benzo[a]pyrene in human hepatocytes. Drug Metab Dispos. 2010;38:1397–1402. doi: 10.1124/dmd.110.034181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hecht SS, Chen M, Yagi H, Jerina DM, Carmella SG. r-1,t-2,3,c-4-Tetrahydroxy-1,2,3,4-tetrahydrophenanthrene in human urine: a potential biomarker for assessing polycyclic aromatic hydrocarbon metabolic activation. Cancer Epidemiol. Biomarkers & Prev. 2003;12:1501–1508. [PubMed] [Google Scholar]
- 39.Simpson CD, Wu MT, Christiani DC, Santella RM, Carmella SG, Hecht SS. Determination of r-7,t-8,9,c-10-tetrahydroxy-7,8,9,10-tetrahydrobenzo[a]pyrene in human urine by gas chromatography-negative ion chemical ionization-mass spectrometry. Chem. Res. Toxicol. 2000;13:271–280. doi: 10.1021/tx990202c. [DOI] [PubMed] [Google Scholar]
- 40.Ding YS, Trommel JS, Yan XJ, Ashley D, Watson CH. Determination of 14 polycyclic aromatic hydrocarbons in mainstream smoke from domestic cigarettes. Environ. Sci. Technol. 2005;39:471–478. doi: 10.1021/es048690k. [DOI] [PubMed] [Google Scholar]
- 41.Kotin P, Falk HL, Busser R. Distribution, retention, and elimination of C14-3,4-benzpyrene after administration to mice and rats. J. Natl. Cancer Inst. 1959;23:541–555. [Google Scholar]
- 42.Hecht SS, Grabowski W, Groth K. Analysis of faeces for benzo[a]pyrene after consumption of charcoal-broiled beef by rats and humans. Food Cosmet. Toxicol. 1979;17:223–227. doi: 10.1016/0015-6264(79)90284-0. [DOI] [PubMed] [Google Scholar]
- 43.Heidelberger C, Weiss SM. The distribution of radioactivity in mice following administration of 3,4-benzpyrene-5-C14 and 1,2,5,6-dibenzanthracene-9,10-C14. Cancer Res. 1951;11:885–891. [PubMed] [Google Scholar]
- 44.Chu I, Ng KM, Benoit FM, Moir D. Comparative metabolism of phenanthrene in the rat and guinea pig. J. Environ. Sci. Health B. 1992;27:729–749. doi: 10.1080/03601239209372809. [DOI] [PubMed] [Google Scholar]
- 45.Gundel J, Angerer J. High-performance liquid chromatographic method with fluorescence detection for the determination of 3-hydroxybenzo[a]pyrene and 3-hydroxybenz[a]anthracene in the urine of polycyclic aromatic hydrocarbon-exposed workers. J. Chromatogr. B Biomed. Sci. Appl. 2000;738:47–55. doi: 10.1016/s0378-4347(99)00499-5. [DOI] [PubMed] [Google Scholar]
- 46.Forster K, Preuss R, Rossbach B, Bruning T, Angerer J, Simon P. 3-Hydroxybenzo[a]pyrene in the urine of workers with occupational exposure to polycyclic aromatic hydrocarbons in different industries. Occup. Environ. Med. 2008;65:224–229. doi: 10.1136/oem.2006.030809. [DOI] [PubMed] [Google Scholar]
- 47.Weston A, Bowman ED, Carr P, Rothman N, Strickland PT. Detection of metabolites of polycyclic aromatic hydrocarbons in human urine. Carcinogenesis. 1993;14:1053–1055. doi: 10.1093/carcin/14.5.1053. [DOI] [PubMed] [Google Scholar]
- 48.Bowman ED, Rothman N, Hackl C, Santella RM, Weston A. Interindividual variation in the levels of certain urinary polycyclic aromatic hydrocarbon metabolites following medicinal exposure to coal tar ointment. Biomarkers. 1997;2:321–327. doi: 10.1080/135475097231553. [DOI] [PubMed] [Google Scholar]
- 49.Nebert DW, Dalton TP, Okey AB, Gonzalez FJ. Role of aryl hydrocarbon receptor-mediated induction of the CYP1 enzymes in environmental toxicity and cancer. J Biol. Chem. 2004;279:23847–23850. doi: 10.1074/jbc.R400004200. [DOI] [PubMed] [Google Scholar]
- 50.van Delft JH, Steenwinkel MS, van Asten JG, de Vogel N, Bruijntjes-Rozier TC, Schouten T, Cramers P, Maas L, Van Herwijnen MH, van Schooten F, Hopmans PM. Biological monitoring the exposure to polycyclic aromatic hydrocarbons of coke oven workers in relation to smoking and genetic polymorphisms for GSTM1 and GSTT1. Ann. Occup. Hyg. 2001;45:395–408. [PubMed] [Google Scholar]
- 51.Nerurkar PV, Okinaka L, Aoki C, Seifried A, Lum-Jones A, Wilkens LR, Le Marchand L. CYP1A1, GSTM1, and GSTP1 genetic polymorphisms and urinary 1-hydroxypyrene excretion in non-occupationally exposed individuals. Cancer Epidemiol. Biomarkers & Prev. 2000;9:1119–1122. [PubMed] [Google Scholar]
- 52.Poirier MC, Santella RM, Weston A. Carcinogen macromolecular adducts and their measurement. Carcinogenesis. 2000;21:353–359. doi: 10.1093/carcin/21.3.353. [DOI] [PubMed] [Google Scholar]
- 53.Boysen G, Hecht SS. Analysis of DNA and protein adducts of benzo[a]pyrene in human tissues using structure-specific methods. Mutation Res. 2003;543:17–30. doi: 10.1016/s1383-5742(02)00068-6. [DOI] [PubMed] [Google Scholar]
- 54.Hecht SS, Yuan J-M, Hatsukami DK. Applying tobacco carcinogen and toxicant biomarkers in product regulation and cancer prevention. Chem. Res. Toxicol. 2010;23:1001–1008. doi: 10.1021/tx100056m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hoffmann D, Schmeltz I, Hecht SS, Wynder EL. Tobacco carcinogenesis. In: Gelboin H, Ts'o POP, editors. Polycyclic Hydrocarbons and Cancer. New York: Academic Press; 1978. pp. 85–117. [Google Scholar]