Abstract
Studies suggest that diabetes may specifically elevate risk of sudden cardiac death in excess of other heart disease outcomes. In this study, we examined the association of type 2 diabetes with incidence of sudden cardiac death as compared to the incidence of non-sudden cardiac death and non-fatal myocardial infarction (MI). We used data from the Atherosclerosis Risk in Communities (ARIC) study to examine incidence of sudden and non-sudden cardiac death and non-fatal MI among persons with and without diabetes in follow-up from the baseline data collection (1987-1989) through December 31, 2001. There were 209 cases of sudden cardiac death, 119 of non-sudden cardiac death, and 739 of non-fatal MI identified in this cohort over an average 12.4 years of follow-up. In analyses adjusted for age, race/ARIC center, gender, and smoking, the Cox proportional hazard ratio of the association of baseline diabetes was 3.77 (95% CI 2.82, 5.05) for sudden cardiac death, 3.78 (95% CI 2.57, 5.53) for non-sudden cardiac death, and 3.20 (95% CI 2.71, 3.78) for non-fatal MI. Elevated risk for each of the three outcomes associated with diabetes was independent of adjustment for measures of blood pressure, lipids, inflammation, hemostasis, and renal function. Among those with diabetes, the risk of cardiac death, but not of non-fatal MI, was similar for men and women. Findings from this prospective, population-based cohort investigation indicate that diabetes does not confer a specific excess risk of sudden cardiac death. Our results suggest that diabetes attenuates gender-differences in risk of fatal cardiac events.
Keywords: sudden cardiac death, myocardial infarction, cohort study, diabetes, coronary heart disease
Introduction
Mortality attributed to sudden cardiac death accounts for more than fifty percent of total coronary heart disease mortality [1]. This high rate underscores the need to identify risk factors specific to sudden cardiac death, characterize populations at elevated risk, and develop population approaches aimed at prevention. Diabetes carries with it a high risk of cardiovascular disease. The risk of primary cardiovascular disease events in persons with diabetes is comparable with the risk of secondary events in those without diabetes [2]. Elevated arrhythmogenic potential, occurring as a result of diabetes-related autonomic neuropathy, may be one of the factors contributing to increased risk of sudden cardiac death among those with diabetes. Few studies have explored whether, among those with diabetes, the risk of fatal events is greater than the risk of non-fatal events.
In this investigation, we used data from the prospective Atherosclerosis Risk in Communities (ARIC) Study to examine the association of baseline and incident type 2 diabetes with the risk of sudden cardiac death as compared to that of non-sudden cardiac death or non-fatal myocardial infarction. Our hypothesis, based on existing studies [3-6], was that type 2 diabetes would be more strongly associated with the risk of sudden cardiac death, as compared to the risk of non-sudden cardiac death, or non-fatal myocardial infarction. Additionally, we examined gender and race differences in the rate of fatal and non-fatal coronary heart disease events in those with diabetes.
Methods
Study design, setting, and population
Atherosclerosis Risk in Communities (ARIC) [7] is a prospective population-based cohort, consisting of 15,792 men and women, 45-64 years of age at baseline (1987-1989), selected as a probability sample from four US communities located in North Carolina, Mississippi, Minnesota, and Maryland. The cohort was evaluated at baseline and at three subsequent triennial examinations through 1998 and by annual telephone follow-up calls. The present study is based on data obtained at baseline and during follow-up through 2001. We excluded from analysis persons whose self-reported race was neither black nor white, persons who were black and residing in the Minnesota or Maryland community (where the percent of blacks was negligibly small), those with baseline coronary heart disease, those missing information on baseline coronary heart disease status, and persons missing baseline information on diabetes and selected analysis covariates: hypertension, systolic blood pressure, anti-hypertension medication, HDL-cholesterol, cigarette smoking status, cigarette years of smoking. The final analysis cohort consisted of 13,978 ARIC participants. All participants signed an informed consent form and the study was approved by the institutional review board of the University of North Carolina.
Baseline measurements
At each clinical visit, ARIC participants had blood drawn from an antecubital vein into tubes containing EDTA or a separating gel [8]. Plasma and serum were separated by centrifugation at 4°C and stored at −70°C. Body mass index was calculated as the ratio of weight in kilograms to the square of height in meters. Sitting blood pressure was measured three times using a random zero sphygmomanometer. Blood pressure calculations were made as an average of the second and third measurement. Hypertension was defined as present based on use of antihypertensive medication within two weeks of baseline data collection or if systolic blood pressure measured at baseline was greater than or equal to 140 mm Hg, or diastolic blood pressure was greater than or equal to 90 mm Hg. Plasma HDL-cholesterol levels were measured using the method of Warnick et al [9]. Total plasma cholesterol levels were determined enzymatically [10] using a Cobas-Bio analyzer with reagents purchased from Boehringer Mannheim Biochemicals, Indianapolis, IN. Serum creatinine levels were determined using the modified kinetic Jaffe method [11] (DART Creatinine Reagent, Coulter Diagnostics, Hialeah, FL) and calibrated using regression to the Cleveland Clinic laboratory by subtraction of 0.24 mg/dl [12]. White blood cell count was determined in the whole blood sample using a Coulter Counter. Plasma levels of fibrinogen, von Willebrand factor and factor VIIIc were determined in the ARIC Central Hemostasis Laboratory using established procedures [13]. Fibrinogen levels were determined using the thrombin time titration method with reagents obtained from General Diagnostics Organon Technica Co., Morris Plains, NJ. Plasma levels of the von Willebrand factor antigen were determined using ELISA kits from American Bioproducts Co. Factor VIII activity was determined on the basis of a coagulation titration curve established using factor VIII–deficient plasma (George King Biochemical Inc., Overland Park, KS).
Baseline diabetes was defined as either a self-reported physician’s diagnosis of diabetes, use of hypoglycemic medications, non-fasting serum glucose levels greater than 200 mg/dL, or fasting (≥ 8 hr) serum glucose level equal to or greater than126 mg/dL. Baseline prediabetes was defined as fasting serum glucose level of 100-126 mg/dL among those not meeting criteria for diabetes. Incident diabetes incorporated serum glucose levels obtained at the post-baseline visits. Case definition of incident diabetes observed at post-baseline visits was the same as at baseline and time to diabetes was estimated by interpolation [14].
Classification of outcomes
Sudden cardiac death
Cases of sudden cardiac death were identified during follow-up from the baseline visit through December 31, 2001. All deaths attributed to heart disease in the main ARIC adjudication were reviewed to ascertain whether the event was due to sudden cardiac death. These cases underwent a separate physician panel review using a uniform abstraction form to determine whether the death was characterized as a sudden, pulseless condition without a known non-cardiac cause. Adjudication was performed independently by two physicians on the basis of data obtained from death certificates, informant interviews, physician questionnaires, coroner reports, or hospital discharge summaries. This review was performed for both in- and out-of- hospital deaths. Inter-reviewer reliability was evaluated in a subset of cases. The kappa coefficient describing the classification of sudden cardiac death between the two physician reviewers was 0.79. Discordant classifications were resolved by a third independent review.
Non-sudden cardiac death
Non-sudden cardiac death was identified during follow-up based on informant interviews, physician and coroner questionnaires, review of medical records and death certificate information. It was defined as fatal myocardial infarction, or definite or possible fatal coronary heart disease that was not identified as sudden cardiac death.
Non-fatal myocardial infarction
Non-fatal myocardial infarction was identified during follow-up from the baseline examination through December 31, 2001, on the basis of community hospital surveillance, annual questionnaires, or at follow-up examinations. It was defined, according to ARIC study criteria, as a definite or probable myocardial infarction that did not result in a fatal outcome within 28 days [15].
Prevalent heart failure
Prevalent heart failure (HF) at baseline was defined as stage 3 HF based on Gothenburg criteria [16], or as use of HF medications in the two weeks preceding the baseline examination.
Statistical analysis
Baseline characteristics were summarized as means and proportions of selected variables. Pairwise t-tests and Pearson’s χ2 coefficients were used to compare means and proportions of baseline characteristics among the exposed (diabetes) and unexposed (no diabetes) groups.
Cox proportional hazard regression of the association of diabetes and incidence of sudden cardiac death, non-sudden cardiac death, or non-fatal MI was performed with evaluation of the following baseline variables for inclusion: age, race/center, body mass index, gender, cigarette years of smoking, cigarette smoking status, systolic blood pressure, use of anti-hypertensive medication, HDL-cholesterol. Inclusion of variables into the model was based on the 10% change-in-estimate criterion [17]. The race/center variable was created as a categorical variable encompassing 5 categories of race and ARIC center. Diabetes was modeled as a time-varying covariate in analyses that evaluated the association of incident, in addition to prevalent, diabetes with the outcomes. For other covariates only baseline values were used.
Multiplicative interaction terms used partial likelihood ratio tests based on nested models and evaluated whether the risk of sudden cardiac death associated with diabetes differed by race, sex, and other covariates. All continuous variables were evaluated for linearity of association with the main outcome using regression analyses with hazard ratios plotted as a function of the exposure categorized into quintiles of distribution. Cox proportional hazard assumptions were examined for all variables individually and for the final model using the Cox test, the ln-ln plots, and plots of scaled Schoenfeld residuals. Sensitivity analyses were performed using multivariate polytomous regression and competing risk analysis. Competing risk of cardiac death for the risk of non-fatal MI analysis was performed according to the method of Allison [18]. All analyses were conducted using STATA 10.0 (College Station, TX) and SAS 9.1 (SAS Institute, Cary, NC).
Results
Baseline characteristics
Characteristics of the sample population according to diabetes status at baseline are presented in Table 1. Member of the ARIC cohort with diabetes were older, more likely to be black, and have hypertension and heart failure than those without baseline diabetes. Their HDL-cholesterol level was on average lower than that of those without diabetes, while their mean total cholesterol level was higher. The prevalence of ever-smokers was lower and the mean serum creatinine level was higher among those with diabetes as compared to those without. Levels of markers of inflammation or hemostasis indicated a higher overall inflammatory burden in those with baseline diabetes as compared to those without.
Table 1.
Baseline characteristics (mean (SD) or percent) according to baseline diabetes status, the ARIC study cohort. p-values based on two-tailed pairwise t-tests and Pearson’s χ2 coefficients of differences between those with and without baseline diabetes.
| Baseline diabetes |
|||
|---|---|---|---|
| Baseline characteristic | Yes (n=1,550) |
No (n=12,428) |
p |
| Gender (% male) | 43.5 | 42.7 | 0.55 |
| Race (% black) | 43.3 | 23.5 | <0.01 |
| Hypertension (%) | 59.2 | 30.2 | <0.01 |
| Prevalent HF (%) | 9.1 | 3.2 | <0.01 |
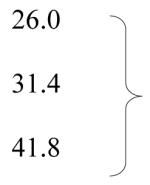
| Smoking status: current | 23.1 |
|
|
| former | 30.1 | <0.01 | |
| never | 45.1 | ||
| Age (years) | 55.5 (5.7) | 53.8 (5.7) | <0.01 |
| HDL-cholesterol (mg/dL) | 45.4 (14.8) | 52.0 (17.1) | <0.01 |
| Total cholesterol (mg/dL) | 220.8 (47.4) | 213.5 (40.8) | <0.01 |
| BMI (kg/m2) | 31.0 (6.0) | 27.2 (5.1) | <0.01 |
| Systolic blood pressure | 129.8 (20.8) | 119.9 (18.1) | <0.01 |
| Cigarette years of smoking (smokers only) | 583.1 (493.2) | 532.1 (426.8) | <0.01 |
| Serum creatinine (mg/dL) | 0.9 (0.8) | 0.9 (0.3) | <0.01 |
| Serum fibrinogen (mg/dL) | 327.2 (75.2) | 299.1 (62.8) | <0.01 |
| Serum albumin (mg/dL) | 3.8 (0.3) | 3.9 (0.30 | <0.01 |
| White blood cell count (x10−6) | 6.7 (2.1) | 6.0 (1.9) | <0.01 |
| Plasma von Willebrand factor (%) | 141.0 (58.9) | 114.6 (45.5) | <0.01 |
| Plasma factor VIIIc (%) | 158.8 (53.0) | 127.5 (35.9) | <0.01 |
Risk of sudden cardiac death in comparison with the risk of non-sudden cardiac death, or non-fatal MI
There were 209 cases of sudden cardiac death, 119 cases of non-sudden cardiac death, and 739 cases of non-fatal myocardial infarction (MI) identified in this cohort during 12.4 (SD 2.7) years of follow-up. The overall incidence rates of sudden cardiac death and non-sudden cardiac death were significantly lower than the incidence rate of non-fatal MI (age-adjusted incidence rates per 10,000 person-years: IRSCD = 11.7 (95% confidence interval (CI): 10.1, 13.3), IRNSCD = 6.7 (95% CI 5.5, 7.9), and IRNFMI= 42.3 (95% CI 39.3, 45.4)). Overall, risk of all three CHD outcomes was greater among men compared to women, and among blacks compared to whites. Diabetes was associated with an elevated risk of all three examined outcomes overall and among subgroups defined by gender and race. The age-adjusted incidence rate of sudden cardiac death was approximately one third that of non-fatal MI, both among the diabetic and the non-diabetic cohort members and across gender and race strata (Table 2). The incidence of sudden cardiac death, non-sudden cardiac death and non-fatal MI was lower among women without diabetes, as compared to men without diabetes. In the presence of diabetes, the gender-differences in the incidence of sudden cardiac death and of non-sudden cardiac death were attenuated, with the incidence in women approximating the incidence in men. Statistical tests for the presence of effect modification of the outcome associations of diabetes, according to gender and race, were not significant.
Table 2.
Age-adjusted incidence rates of sudden cardiac death, or non-sudden cardiac death, or non-fatal MI, according to diabetes status, gender, and race during follow-up from 1987 through 2001, the ARIC study cohort
| Diabetes status |
Sudden cardiac death (n=209) | Non-sudden cardiac death (n=119) | Non-fatal MI (n=739) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Events | p-years | IR (95% CI) (per 10,000 p-years) |
Events | p-years | IR (95% CI) (per 10,000 p-years |
Events | p-years | IR (95% CI) (per 10,000 p-years) |
|
| Baseline diabetes | |||||||||
| No | 140 | 160,403 | 8.9 (7.4, 10.3) | 80 | 160,403 | 5.1 (4.0, 6.2) | 545 | 157,389 | 34.9 (32.0, 37.9) |
| Yes | 69 | 18,360 | 34.3 (26.0, 42.5) | 39 | 18,360 | 19.7 (13.1, 26.2) | 194 | 17,485 | 106.3 (91.1, 121.5) |
| Baseline and incident diabetes | |||||||||
| No | 119 | 142,479 | 8.5 (7.0, 10.0) | 65 | 142,479 | 4.7 (3.5, 5.8) | 488 | 140,194 | 35.2 (32.1, 38.3) |
| Yes | 90 | 36,284 | 23.7 (18.8, 28.6) | 54 | 36,284 | 14.0 (10.3, 17.8) | 254 | 34,898 | 70.4 (61.8, 79.1) |
| Baseline and incident diabetes, women | |||||||||
| No | 44 | 82,058 | 5.6 (3.9, 7.2) | 24 | 82,058 | 2.8 (1.7, 4.0) | 175 | 81,317 | 22.0 (18.7, 25.3) |
| Yes | 47 | 19,872 | 22.6 (16.1, 29.0) | 31 | 19,872 | 13.5 (8.4, 18.5) | 116 | 19,317 | 56.9 (46.5, 67.3) |
| Baseline and incident diabetes, men | |||||||||
| No | 75 | 60,420 | 12.4 (9.6, 15.2) | 39 | 60,420 | 6.9 (4.8, 9.0) | 313 | 58,877 | 53.1 (47.2, 59.0) |
| Yes | 43 | 16,412 | 24.9 (17.9, 32.4) | 23 | 16,412 | 14.4 (8.8, 20.0) | 138 | 15,581 | 85.3 (71.0, 99.6) |
| Baseline and incident diabetes, whites | |||||||||
| No | 65 | 111,945 | 5.8 (4.4, 7.2) | 46 | 111,945 | 4.3 (3.1, 5.5) | 392 | 110,050 | 35.5 (32.0, 39.0) |
| Yes | 41 | 22,494 | 16.8 (11.6, 22.1) | 38 | 22,494 | 15.3 (10.4, 20.3) | 163 | 21,519 | 72.2 (60.9, 83.5) |
| Baseline and incident diabetes, blacks | |||||||||
| No | 54 | 30,534 | 19.8 (14.5, 25.1) | 19 | 30,534 | 7.3 (4.0, 10.6) | 96 | 30,144 | 33.6 (26.7, 40.4) |
| Yes | 49 | 13,791 | 35.8 (25.8, 45.9) | 16 | 13,791 | 11.1 (5.7, 16.6) | 91 | 13,379 | 68.0 (54.1, 82.0) |
In age-adjusted analysis, the hazard ratio for sudden cardiac death associated with baseline diabetes was 3.88 (95% CI 2.90, 5.19), whereas the hazard ratio for non-fatal MI was 3.07 (95% CI 2.60, 3.63) (Table 3). The age-adjusted hazard ratio for non-sudden cardiac death in association with prevalent diabetes was 3.76 (95% CI 2.60, 5.53). Further adjustment for race/ARIC center, gender, smoking status and cigarette years of smoking did not significantly attenuate the hazard ratios for sudden cardiac death, non-sudden cardiac death, or non-fatal MI. Although adjustment for lipids and blood pressure variables further attenuated the estimates for all three outcomes, the hazard ratios remained significantly elevated.
Table 3.
Association of baseline Type 2 diabetes with incident sudden cardiac death, or non-sudden cardiac death, or incident non-fatal myocardial infarction, ARIC study cohort, 1987-2001
| Baseline and Incident diabetes |
||||||
|---|---|---|---|---|---|---|
| Outcome | Baseline diabetes | Total sample |
Gender | Race | ||
| Women | Men | Whites | Blacks | |||
| Sudden cardiac death | ||||||
| Model 1 | 3.88 (2.90, 5.19) | 3.22 (2.44, 4.25) | 4.44 (2.93, 6.71) | 2.46 (1.68, 3.58) | 3.47 (2.34, 5.15) | 2.07 (1.40, 3.05) |
| Model 2 | 3.77 (2.82, 5.05) | 3.06 (2.32, 4.04) | 4.00 (2.63, 6.08) | 2.41 (1.65, 3.52) | 3.19 (2.14, 4.74) | 2.17 (1.47, 3.21) |
| Model 3 | 2.60 (1.91, 3.54) | 2.11 (1.58, 2.83) | 2.06 (1.31, 3.24) | 2.03 (1.37, 2.99) | 2.28 (1.50, 3.46) | 1.66 (1.10, 2.50) |
| Non-sudden cardiac death | ||||||
| Model 1 | 3.76 (2.60, 5.53) | 3.50 (2.44, 5.04) | 5.53 (3.15, 9.70) | 2.49 (1.53, 4.107 | 4.23 (2.74, 6.52) | 2.05 (1.05, 4.00) |
| Model 2 | 3.78 (2.57, 5.53) | 3.36 (2.33, 4.85) | 5.25 (2.96, 9.31) | 2.42 (1.49, 3.99) | 3.88 (2.51, 6.00) | 2.13 (1.08, 4.18) |
| Model 3 | 2.60 (1.73, 3.90) | 2.34 (1.59, 3.44) | 2.91 (1.57, 5.40) | 1.97 (1.19, 3.27) | 2.47 (1.56, 3.93) | 1.96 (0.97, 3.97) |
| Non-fatal MI | ||||||
| Model 1 | 3.07 (2.60, 3.63) | 2.24 (1.93, 2.60) | 2.91 (2.31, 3.68) | 1.83 (1.51, 2.23) | 2.28 (1.91, 2.73) | 2.24 (1.68, 3.00) |
| Model 2 | 3.20 (2.71, 3.78) | 2.24 (1.93, 2.60) | 2.97 (2.34, 3.76) | 1.83 (1.51, 2.23) | 2.11 (1.76, 2.53) | 2.36 (1.76, 3.15) |
| Model 3 | 2.32 (1.95, 2.76) | 2.24 (1.92, 2.60) | 1.93 (1.49, 2.49) | 1.48 (1.21, 1.82) | 1.60 (1.32, 1.94) | 1.71 (1.27, 2.32) |
Model 1: adjustment for age
Model 2: adjustment for age, gender, race/ARIC center, smoking status, and cigarette years of smoking.
Model 3: adjustment for age, gender, race/ARIC center, smoking status, and cigarette years of smoking, systolic blood pressure, anti-hypertensive medication use, gender-by-systolic blood pressure, HDL-cholesterol
Analyses stratified by gender or race do not include gender or race/ARIC center as covariates respectively
There were 1,380 incident cases of type 2 diabetes identified in the sample population during follow-up. As shown in Table 3, use of incident and prevalent diabetes, as compared to only prevalent diabetes, as the exposure in multivariable regression models (adjusted for age, gender, race, and smoking status), attenuated the hazard ratio estimate for sudden cardiac death to 3.06 (95 % CI 2.32, 4.04), for non-sudden cardiac death to 3.36 (95 % CI 2.33, 4.85), and for non-fatal MI to 2.24 (95 % CI 1.93, 2.60)
As with baseline diabetes, inclusion of lipids and blood pressure variables further attenuated the hazard ratio estimates. Additional adjustment of the estimates for serum creatinine levels and for markers of inflammation and hemostasis, as well as cholesterol lowering medication did not appreciably change the hazard ratios. Partial likelihood ratio tests comparing multivariable-adjusted Cox regression models with and without multiplicative diabetesxrace and diabetesxgender interaction terms were not statistically significant for the outcomes examined.
Sensitivity analyses
Of the 209 cases of sudden cardiac death, twenty one were preceded by a myocardial infarction occurring more than 28 days prior to death (non-fatal MI). Seventeen of the 119 cases of non-sudden cardiac death were preceded by a non-fatal MI. We examined the effect of this potential overlap of cases of sudden or non-sudden cardiac death with non-fatal MI on the observed associations with baseline diabetes using the following sensitivity analyses: censoring of those individuals who experienced non-fatal myocardial infarctions prior to cardiac death, analysis of competing risks, and analyses using maximum-likelihood multinomial logit models. Competing risk analysis was performed by sequentially fitting a Cox regression model for each event type while censoring the other event types [18]. In multinomial regression, coronary heart disease events were categorized as follows: non-fatal MI not followed by cardiac death; sudden cardiac death not preceded by a non-fatal MI; non-sudden cardiac death not preceded by a non-fatal MI; sudden cardiac death following a non-fatal MI; or non-sudden cardiac death following a non-fatal MI. Those event types were fitted into one model with the non-cases as the base category. Since the number of sudden and non-sudden cardiac death events preceded by a non-fatal MI was too small for meaningful analysis, we examined only the three independent outcomes: sudden and non-sudden cardiac deaths and non-fatal MI. Results of all three sensitivity analyses confirmed results obtained using separate Cox regression models presented in Table 3.
In analysis with sudden cardiac death defined as out-of-hospital death within one hour of symptom onset (n=71) we found the association to be somewhat weaker than the main estimates reported in Table 3 (HRbaseline diabetes=1.83 (95% CI 1.06, 3.17), HRtotal diabetes=1.78 (95% CI 1.09, 2.92)).
Presence of baseline heart failure (HF) reduced the predictive power of diabetes as a risk factor for non-fatal MI (HR= 1.41 (95% CI 0.72, 2.75)). Due to a small number of cases, we were not able to estimate the hazard ratios of sudden and non-sudden cardiac death in those with prevalent heart failure. Restriction of analysis (adjusted for age, gender race/ARIC center, smoking status, and cigarette years of smoking) to those without prevalent HF resulted in the following hazard ratio estimates for the association of baseline and incident diabetes: HRSCD = 3.08 (95% CI 2.31, 4.10), HRNSCD = 3.24 (95% CI 2.19, 4.78)), HRNFMI = 2.30 (95% CI 1.96, 2.68). Those values were not different from estimates for the entire sample population.
Cohort members whose diagnosis of diabetes was based on their use of diabetes-related medication had a higher risk of both fatal and non-fatal CHD events as compared to those cohort members whose diagnosis of diabetes was based on fasting glucose levels, or on a physician’s report. The differences however were not statistically significant (data not shown).
Pre-diabetes and the risk of sudden cardiac death
In analysis adjusted for age, race/ARIC center, and smoking in which we excluded those on anti-diabetic medication, we observed a gradient of association for sudden cardiac death, non-sudden cardiac death, and non-fatal MI within categories of fasting glucose levels, however none of the associations in the pre-diabetic range were statistically significant.
Discussion
This population-based prospective study of persons initially free of clinical artery disease compliments and extends our understanding of the relationship between diabetes and cardiac outcomes by evaluating the diabetes-specific risk of sudden cardiac death in the context of non-fatal MI and non-sudden cardiac death. We found that for both fatal and non-fatal CHD outcomes, diabetes was a strong, significant, and independent predictor of risk. We did not find evidence that would confirm our initial hypothesis of a specific association of diabetes with the risk of sudden cardiac death. Traditional cardiovascular disease risk factors conferred part of the risk, overall and among the demographic subgroups. Moreover, hemostatic and inflammatory markers, known predictors of increased risk of coronary heart disease in those with diabetes, did not appreciably attenuate the diabetes-specific risk in models adjusted for traditional risk factors.
In this study, we observed an attenuating effect of diabetes on gender-differences in the risk of cardiac death. In the absence of diabetes, the incidence of cardiac death was higher among men than women, an observation that is consistent with existing studies [5, 19, 20]. Presence of diabetes appeared to equalize the absolute risk of sudden and non-sudden cardiac death across gender groups. This effect was not true for non-fatal MI, where the risk was greater for men, as compared to women, regardless of diabetes status. Our findings of risk-equalizing effect of diabetes across gender groups are consistent with previous observations [21, 22] and suggest that women with diabetes, free of existing cardiovascular disease, constitute a population group at a high risk of cardiac death. This is of particular importance as the decline in sudden cardiac death mortality among women is not as strong as it is for men [23], and in the case of younger women, population trends suggest an increase in sudden cardiac death incidence [24].
In our study, we observed a greater incidence of sudden and non-sudden cardiac death among blacks as compared to whites, but no difference in the incidence of non-fatal MI across race strata regardless of diabetes status. These findings suggest that the excess burden of coronary heart disease observed among blacks may be attributable to fatal, rather than non-fatal cardiac events. Taken together, the results indicate that blacks with diabetes are at especially high risk of cardiac mortality and represent a group that can benefit from efforts aimed at prevention of sudden cardiac death.
Our observation of no statistically significant increase in the hazard ratio for sudden cardiac death, non-sudden cardiac death, or non-fatal MI in those ARIC cohort members whose baseline fasting plasma glucose levels were in the pre-diabetic range, are in agreement with a recent study by Pankow et al.[25], which did not find impaired fasting glucose to be associated with an increased risk of incident CHD. However, many patients with pre-diabetes go on to develop diabetes. Longer follow-up may reveal a greater risk as these persons transition to diabetes.
Study limitations
This study had several limitations. Although there were 205 sudden cardiac deaths during the 12.4 years of follow-up, we did not have sufficient statistical power to evaluate some clinical subgroups. The relatively small proportion of blacks in the ARIC cohort limited interpretation of the observed effects of race on the examined associations. Although we did observe significant differences in the incidence of sudden cardiac death between blacks and whites, the hazard ratio estimates were similar. blacks constitute only 27 percent of the ARIC cohort, thus this study may have been underpowered to detect meaningful effect modification by race of the risk of sudden cardiac death in diabetics. In this study we did not have information concerning duration of prevalent diabetes. It is possible that the modest differences we observed in the association of diabetes with non-sudden as compared to sudden cardiac death are a function of the length of time associated with the diabetes exposure.
We explored mechanisms of sudden cardiac death by incorporating serum measures of renal function, hemostatic factors, and inflammatory measures. Other measures of these processes or even different morbidities (i.e. neuropathy) may better explain the mechanisms by which diabetes confers risk.
The focus of this study was the association of diabetes with sudden cardiac death. In an attempt to separate the role of diabetes in the incidence of sudden cardiac death from its effect on overall coronary heart disease mortality, we also examined the association of diabetes with the incidence of non-sudden cardiac death. We did not find differences in the association of diabetes with sudden as compared to non-sudden cardiac death. However, our power to detect meaningful differences in the association of diabetes with the risk of three separate coronary heart disease events was limited by the modest number of cases of sudden and non-sudden cardiac death.
Conclusion
Our results do not suggest that diabetes confers a greater risk of sudden cardiac death, as compared to the risk of non-sudden cardiac death, or non-fatal MI. They do however suggest a gender-equalizing effect of diabetes on the risk of cardiac death and emphasize the need for targeted cardiovascular disease prevention among women with diabetes.
Acknowledgement
The authors thank the staff and participants of the ARIC study for their important contributions.
Funding: The Atherosclerosis Risk in Communities Study is carried out as a collaborative study supported by National Heart, Lung, and Blood Institute contracts N01-HC-55015; N01-HC-55016; N01-HC-55018; N01-HC-55019; N01-HC-55020; N01-HC-55021; and N01-HC-55022. This work was also supported by a National Research Service Award training grant T32 H2-0007055 [to AMK-N].
Footnotes
Conflict of interest: none declared
References
- 1.McGovern PG, Jacobs R, Shahar E, et al. Trends in Acute Coronary Disease Mortality, Morbidity, and Medical Care From 1985 Through 1997. Circulation. 2001;104:19–24. doi: 10.1161/01.cir.104.1.19. [DOI] [PubMed] [Google Scholar]
- 2.Haffner SM, Lehto S, Ronnemaa T, Pyorala K, Laakso M. Mortality from coronary heart disease in subjects with type 2 diabetes and in nondiabetic subjects with and without prior myocardial infarction. N Engl J Med. 1998;339:229–234. doi: 10.1056/NEJM199807233390404. [DOI] [PubMed] [Google Scholar]
- 3.Albert CM, Chae CU, Grodstein F, et al. Prospective study of sudden cardiac death among women in the United States. Circulation. 2003;107:2096–2101. doi: 10.1161/01.CIR.0000065223.21530.11. [DOI] [PubMed] [Google Scholar]
- 4.Jouven X, Desnos M, Guerto C, Ducimetiere P. Predicting sudden death in the population, the Paris Prospective Study I. Circulation. 1999;99:1978–1983. doi: 10.1161/01.cir.99.15.1978. [DOI] [PubMed] [Google Scholar]
- 5.Jouven X, Lemaitre RN, Rea TD, Sotoodehnia N, Empana JP, Siscovick DS. Diabetes, glucose level, and risk of sudden cardiac death. European Heart Journal. 2005;26:2142–2147. doi: 10.1093/eurheartj/ehi376. [DOI] [PubMed] [Google Scholar]
- 6.Balkau B, Jouven X, Ducimetiere P, Eschwege E. Diabetes as a risk factor for sudden death. The Lancet. 1999;354:1968–1969. doi: 10.1016/S0140-6736(99)04383-4. [DOI] [PubMed] [Google Scholar]
- 7.The ARIC Investigators The Atherosclerosis Risk in Communities (ARIC) study: design and objectives. Am J Epidemiol. 1989;129:687–702. [PubMed] [Google Scholar]
- 8.Atherosclerosis Risk in Communities Study Manual E
- 9.Warnick GR, Mayfield C, Benderson J, Chen JS, Albers JJ. HDL cholesterol quantitation by phosphotungstate-Mg2+ and by dextran sulfate-Mn2+-polyethylene glycol precipitation, both with enzymic cholesterol assay compared with the lipid research method. Am J Clin Pathol. 1982;78:718–723. doi: 10.1093/ajcp/78.5.718. [DOI] [PubMed] [Google Scholar]
- 10.Siedel J, Hagele EO, Ziegenhorn J, Wahlefeld AW. Reagent for the enzymatic determination of serum total cholesterol with improved lipolytic efficiency. Clin Chem. 1983;29:1075–1080. [PubMed] [Google Scholar]
- 11.Lustgarten JA, Wenk RE. Simple, rapid, kinetic method for serum creatinine measurement. Clin Chem. 1972;18:1419–1422. [PubMed] [Google Scholar]
- 12.Manjunath G, Tighiouart H, Ibrahim H, et al. Level of kidney function as a risk factor for atherosclerotic cardiovascular outcomes in the community. J Am Coll Cardiol. 2003;41:47–55. doi: 10.1016/s0735-1097(02)02663-3. [DOI] [PubMed] [Google Scholar]
- 13.Papp AC, Hatzakis H, Bracey A, Wu KK. ARIC hemostasis study--I. Development of a blood collection and processing system suitable for multicenter hemostatic studies. Thromb Haemost. 1989;61:15–19. [PubMed] [Google Scholar]
- 14.Duncan BB, Schmidt MI, Pankow JS, et al. Low-grade systemic inflammation and the development of type 2 diabetes - The atherosclerosis risk in communities study. Diabetes. 2003;52:1799–1805. doi: 10.2337/diabetes.52.7.1799. [DOI] [PubMed] [Google Scholar]
- 15.White AD, Rosamond WD, Chambless LE, et al. Sex and race differences in short-term prognosis after acute coronary heart disease events: the Atherosclerosis Risk In Communities (ARIC) study. Am Heart J. 1999;138:540–548. doi: 10.1016/s0002-8703(99)70158-4. [DOI] [PubMed] [Google Scholar]
- 16.Eriksson H, Caidahl K, Larsson B, et al. Cardiac and pulmonary causes of dyspnoea--validation of a scoring test for clinical-epidemiological use: the Study of Men Born in 1913. Eur Heart J. 1987;8:1007–1014. doi: 10.1093/oxfordjournals.eurheartj.a062365. [DOI] [PubMed] [Google Scholar]
- 17.Mickey RM, Greenland S. The Impact of Confounder Selection Criteria on Effect Estimation. Am. J. Epidemiol. 1989;129:125–137. doi: 10.1093/oxfordjournals.aje.a115101. [DOI] [PubMed] [Google Scholar]
- 18.Allison P. Survival Analysis Using SAS, A Practical Guide. SAS Institute; Cary, NC: 1995. [Google Scholar]
- 19.Kannel WB, Schatzkin A. Sudden death: lessons from subsets in population studies. Journal of the American College of Cardiology. 1985;5:141B–149B. doi: 10.1016/s0735-1097(85)80545-3. [DOI] [PubMed] [Google Scholar]
- 20.Escobedo LG, Caspersen CJ. Risk factors for sudden coronary death in the United States. Epidemiology. 1997;8:175–180. doi: 10.1097/00001648-199703000-00009. [DOI] [PubMed] [Google Scholar]
- 21.Dale AC, Nilsen TI, Vatten L, Midthjell K, Wiseth R. Diabetes mellitus and risk of fatal ischaemic heart disease by gender: 18 years follow-up of 74,914 individuals in the HUNT 1 Study. Eur Heart J. 2007;28:2924–2929. doi: 10.1093/eurheartj/ehm447. [DOI] [PubMed] [Google Scholar]
- 22.Juutilainen A, Kortelainen S, Lehto S, Ronnemaa T, Pyorala K, Laakso M. Gender difference in the impact of type 2 diabetes on coronary heart disease risk. Diabetes Care. 2004;27:2898–2904. doi: 10.2337/diacare.27.12.2898. [DOI] [PubMed] [Google Scholar]
- 23.Ford ES, Capewell S. Coronary Heart Disease Mortality Among Young Adults in the U.S. From 1980 Through 2002: Concealed Leveling of Mortality Rates. Journal of the American College of Cardiology. 2007;50:2128–2132. doi: 10.1016/j.jacc.2007.05.056. [DOI] [PubMed] [Google Scholar]
- 24.Zheng Z-J, Croft JB, Giles WH, Mensah GA. Sudden cardiac death in the United States, 1989 to 1998. Circulation. 2001;104:2158–2163. doi: 10.1161/hc4301.098254. [DOI] [PubMed] [Google Scholar]
- 25.Pankow JS, Kwan DK, Duncan BB, et al. Cardiometabolic Risk in Impaired Fasting Glucose and Impaired Glucose Tolerance: The Atherosclerosis Risk in Communities Study. Diabetes Care. 2007;30:325–331. doi: 10.2337/dc06-1457. [DOI] [PubMed] [Google Scholar]


