Abstract
Rationale
The exact etiology of sporadic AD is unclear but it is interesting that several cardiovascular risk factors are associated with higher incidence of AD. The link between these risk factors and AD has yet to be identified; however, a common feature is endothelial dysfunction, specifically, decreased bioavailability of nitric oxide (NO).
Objective
To determine the relationship between endothelial derived NO and the expression and processing of amyloid precursor protein (APP).
Methods and Results
We utilized human brain microvascular endothelial cells (BMECs) to examine the role of NO in modulating APP expression and processing in vitro. Inhibition of endothelial nitric oxide synthase (eNOS) with the specific NOS inhibitor, N(G)-Nitro-L-Arginine Methyl Ester (L-NAME), led to increased APP and β-site APP cleaving enzyme 1 (BACE1) protein levels as well as increased secretion of the amyloidogenic, amyloid beta (Aβ), peptide (control 10.93 ± 0.70 pg/mL; L-NAME 168.21 ± 27.38 pg/mL, P<0.001). To examine the role of NO in modulation of APP expression and processing in vivo, we utilized brain and cerebral microvessels from the eNOS−/− mice. Brain tissue from eNOS deficient (eNOS−/−) mice had statistically higher APP and BACE1 protein levels as well as increased BACE1 enzyme activity and Aβ (Aβ1–42 wild-type control 0.737pg/mg; eNOS−/− 1.475pg/mg, P<0.05), compared to wild-type controls (n=6–8 animals per background). Brain microvessels from eNOS−/− mice also showed statistically higher BACE1 protein levels as compared to wild-type control.
Conclusions
Our data suggest that endothelial NO plays an important role in modulating APP expression and processing within the brain and cerebrovasculature. The NO/cGMP pathway may be an important therapeutic target in preventing and treating mild cognitive impairment as well as AD.
Keywords: Endothelium, Amyloid precursor protein, Alzheimer’s disease, cerebrovascular biology, beta amyloid
Alzheimer’s disease (AD) is a chronic neurodegenerative disease affecting over 5 million persons in the US and over 20 million worldwide1. AD is characterized by progressive loss of neurons, cognitive decline and two defining histopathologies: extracellular amyloid plaques and intracellular tangles composed primarily of amyloid beta (Aβ) peptide and hyperphosphorylated tau, respectively2. Furthermore, AD is often accompanied by cerebrovascular dysfunction as well as amyloid deposition within the cerebral vessels, termed cerebral amyloid angiopathy (CAA)3.
Aβ is generated from sequential cleavages of its parent molecule, the amyloid precursor protein (APP), by the activities of beta-site APP-cleaving enzyme 1 (BACE1) and gamma secretase4, 5. Importantly, Aβ, has been shown to exert a plethora of effects on endothelial phenotype, including: angiogenesis, proliferation, adhesion, and responsiveness to vasoactive molecules6, 7. Moreover, it has been suggested that APP has functional roles in coagulation, adhesion and inflammation8.
The exact etiology of sporadic AD is unclear but it is interesting that cardiovascular risk factors including hypertension, hypercholesterolemia, diabetes mellitus, aging and sedentary life style are associated with higher incidence of AD9. The link between cardiovascular risk factors and AD has yet to be identified; however, a common feature is endothelial dysfunction, specifically, decreased bioavailability of nitric oxide (NO)10. In the cerebral circulation, endothelial NO is generated by endothelial nitric oxide synthase (eNOS) which under basal conditions is expressed exclusively in endothelial cells11. NO is an extremely important signaling molecule responsible for maintaining vascular homeostasis by promoting vasodilatation, inhibiting platelet aggregation and leukocyte adhesion12. Taken together these data suggest that NO availability may be a common link between cardiovascular risk factors and the development of AD, therefore, we examine here the role of endothelial-derived NO in modulating brain and microvascular APP expression and processing and generation of the amyloidogenic fragment Aβ.
Methods
A detailed methods section is provided online at http://circres.ahajournals.org in the Online Supplement and includes detailed methods regarding: animals, tissue collection, cerebral microvessel isolation, human brain microvascular endothelial cells (BMECs) culture, confocal microscopy, eNOS, guanylyl cylcase and phosphodiesterase 5 inhibition, small interfering (si)RNA transfection, Western blotting, ELISA for Aβ, NOx measurements, BACE1 enzyme activity assay, and statistical analysis.
Results
eNOS-derived NO and APP expression and processing in human BMECs
Inhibition of eNOS led to increased APP and BACE1 protein levels after 3 days of treatment in BMECs (Fig. 1A–B). Addition of L-Arginine, the stereoisomer specific substrate of eNOS, was able to reverse the L-NAME-induced increases in APP and BACE1 protein levels (Fig. 1B). Furthermore, genetic knockdown of eNOS led to similar increases in APP and BACE1 protein levels (Fig. 1D). Levels of the amyloidogenic peptide, Aβ, were significantly higher following eNOS inhibition and attenuated with the addition of L-Arginine (P<0.001 from control).
Figure 1.
Inhibitory effect of eNOS-derived NO on APP and BACE1 protein expression and Aβ generation in human BMEC. A, BMEC were cultured in the absence or presence of 0.3 mM L-NAME for 1, 3, and 5 days; medium was changed daily. Representative image from 3–5 independent experiments and densitometric analysis is shown. Data is presented as mean ±SD (*P<0.05 from control, **P<0.01 from control). BMEC were cultured in the absence or presence of 1 mM L-Arginine or D-Arginine and 0.3 mM L-NAME for 4 days; medium was changed daily. B, Representative image from 3–5 independent experiments and densitometric analysis shown. Data is presented as mean ±SD (*P<0.05 from control, &P<0.05 from L-NAME, $P<0.01 from L-NAME). C, Cell supernatant was collected and secreted Aβ1–40 and Aβ1–42 was measured using a commercially available colorimetric ELISA. Data is represented as mean ± SD (***P<0.001 from control, #P<0.001 from L-NAME, $P<0.01 from L-NAME, &P<0.05 from L-NAME). D, BMEC were transfected with 30 nM of eNOS or Control siRNA. Cells were lysed 3 days after transfections and used for Western analyses. Representative image from 3–5 independent experiments and densitometric analysis is shown. Data is presented as mean ±SD (*P<0.05 from untreated, &P<0.05 from Control siRNA). Statistical analysis was performed using one-way ANOVA and Tukey-Kramer post hoc comparison.
cGMP pathway and APP expression and processing
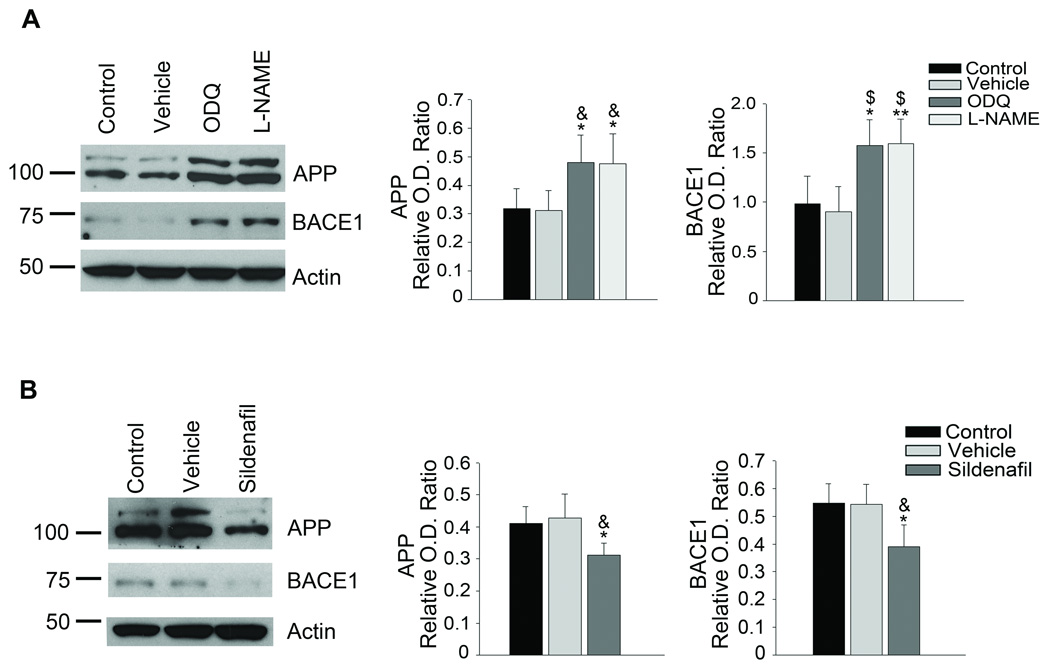
The effects of NO are commonly mediated by activation of guanylyl cyclase which then generates cyclic guanosine monophosphate (cGMP)13. BMECs treated with1H-[1,2,4]Oxadiazolo[4,3-a]quinoxalin-1-one (ODQ), highly selective inhibitor of soluble guanylyl cyclase had statistically higher protein levels of APP and BACE1 (Fig. 2A). Furthermore, when we treated BMECs with Sildenafil, a selective inhibitor of cGMP specific phosphodiesterase type 5 (PDE5), to increase cGMP levels, APP and BACE1 protein levels were significantly reduced (Fig. 2B). Taken together, these data suggest that basal NO levels, provided by the activity of eNOS, suppress APP and BACE1 protein levels via the guanylyl cyclase pathway.
Figure 2.
NO-mediated suppression of APP and BACE1 protein expression is mediated by the guanylyl cyclase/cGMP pathway. A, BMEC were cultured in the absence or presence of DMSO vehicle or 1 µM ODQ, a soluble guanylyl cyclase inhibitor, for 4 days, medium was changed daily. Representative image from 3–5 independent experiments and densitometric analysis is shown. Data is presented as mean ±SD (*P<0.05 from control, **P<0.01 from control, &P<0.05 from vehicle, $P<0.01 from vehicle). B, BMEC were cultured in the absence or presence of 1 µM sildenafil citrate, for 4 days, medium was changed daily. Representative image from 3–5 independent experiments and densitometric analysis is shown. Data is presented as mean ±SD (*P<0.05 from control, &P<0.05 from vehicle).
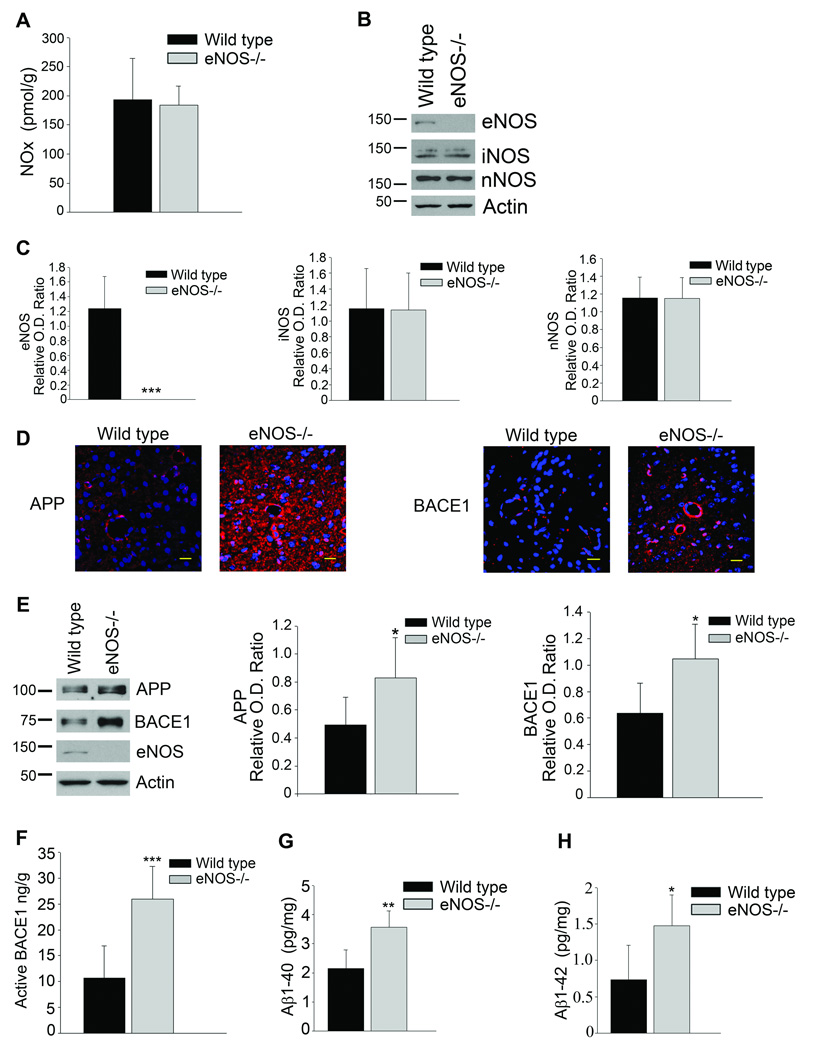
NOx and BACE1 levels in cerebral microvessels from eNOS−/− mice
Microvessels from eNOS−/− mice displayed an approximately 50% reduction in the levels of nitrate/nitrite (NOx) as compared to wild type controls (Fig 3A). While overall NOx levels were decreased in the microvessels of eNOS−/− mice protein expression of inducible NOS (iNOS) were unchanged and neuronal NOS (nNOS) undetectable in the microvessels from both mice (Fig 3B).
Figure 3.
Levels of NO were decreased while BACE1 protein levels were increased in the micovessels of eNOS−/− mice as compared to age-matched wild type control animals. A, Brain microvascular tissue from eNOS−/− and wild type animals was collected and analyzed for levels of NOx using a commercially available kit. Data is presented as mean NOx levels expressed in pmol/g brain tissue (6–8 animals per background; *P<0.05 from wild type control). B, Brain microvascular tissue from eNOS−/− and wild type control animals was Western blotted using anti-eNOS, anti-iNOS, anti-nNOS and anti-Actin (loading control) antibodies or C, anti-APP, anti-BACE1, anti-eNOS, and anti-Actin (loading control) antibodies. Representative Western blot and densitometric analysis is shown (6–8 animals). Data is presented as relative mean O.D. ± SD (*P<0.05, ***P<0.001 from wild type control).
APP and BACE1 protein levels from the microvascular fraction of both wild type and eNOS−/− animals. While APP protein levels were not significantly different between the two, BACE1 levels were statistically higher in the microvascular fraction from the eNOS−/− animals as compared to wild type (Fig. 3C). As the majority of Aβ produced by the endothelial cells would have been secreted into the vessel lumen, we were unable to detect Aβ levels from the microvascular fraction.
We did not detect any alterations in other key enzymes involved in APP processing or Aβ degredation14 (Supplemental Fig. I and II).
APP, BACE1 and Aβ in the brains of eNOS−/− mice
Levels of brain NOx were unchanged between eNOS−/− and wild type control mice (Fig 4A). Furthermore, protein levels of iNOS and nNOS were unaltered in the brains of eNOS−/− mice as compared to wild type control (Fig 2B–C). These results suggest that the deficiency in eNOS-derived NO is restricted to the microvessels.
Figure 4.
Levels of NO were unaltered while APP, BACE1 and Aβ levels were increased in the brains of eNOS−/− mice as compared to age-matched wild type control animals. A, Brain tissue from eNOS−/− and wild type animals was analyzed for levels of NOx using a commercially available kit. Data is presented as mean NOx levels expressed in pmol/g brain tissue (6–8 animals per background). B, C, Brain tissue from eNOS−/− and wild-type animals was Western blotted using anti-eNOS, anti-iNOS, anti-nNOS, and anti-Actin (loading control) antibodies. Representative image and densitometric analysis is shown. Data is presented as mean ±SD (***P<0.001 from wild type control). D, Fixed tissue sections from the brains of wild type and eNOS−/− animals were immunolabeled with anti-APP or anti-BACE1 antibodies. Representative images of the cortex are shown. Maginifcation 40× bar is representative of 20 µm. E, Brain tissue from eNOS−/− and wild-type animals was Western blotted using anti-APP, anti-BACE1, anti-eNOS, and anti-Actin (loading control) antibodies. Representative blot and densitometric analysis is shown (6–8 animals). Data is presented as relative mean O.D. ± SD (*P<0.05). F, BACE1 activity was measured from the brain tissue of eNOS−/− and wild type animals (6–8 animals per background) using a commercially available BACE1 activity kit. Data is represented as mean active BACE1 enzyme (ng/g protein) ±SD (***P<0.001 from wild type control). G, Aβ1–40 and Aβ1–42 levels from 6–8 individual brain lysates (500 μg) from eNOS−/− and wild type control were analyzed via commercially available ELISA kits. Data is represented as mean ± SD (**P<0.01, *P<0.05 from wild type control).
APP and BACE1 protein levels were significantly higher in the brains of eNOS−/− animals as compared to control (Fig 4D–E). Levels of active BACE1 enzyme were also statistically higher in the brains of eNOS−/− animals as compared to wild type control mice (Fig 4F, P<0.001). Furthermore, brain lysates from eNOS−/− animals demonstrated significantly higher levels of both Aβ1–40 and Aβ1–42 as compared to wild type (Fig. 4G–H, P<0.01, P<0.05, respectively). Importantly, this demonstrates that loss of endothelial NO is sufficient to increase protein levels of APP, BACE1, and the amyloidogenic Aβ peptide in brain tissue.
We observed no alteration in the alpha or gamma secretase proteins or Aβ degradation enzymes (Supplemental Fig. III-IV).
Discussion
Our results support the concept that the relationship between endothelial dysfunction and the development of AD is at least in part mediated by the loss of basal eNOS-generated NO, and subsequent upregulation of expression and amyloidogenic processing of APP. We have observed this both in vitro and in vivo. Our in vitro data suggests that this NO-dependent mechanism is mediated by activation of guanylyl cyclase leading to increases in cGMP levels as inhibition of guanylyl cyclase also led to increases in APP and BACE1 protein expression while PDE5 lowered APP and BACE1 expression. Consistent with our observations, Pak et al15 demonstrated that in human neuroblastoma cells, NO downregulated β secretase suggesting that NO might suppress Aβ levels in the brain. Our in vivo observations demonstrate that endothelial derived-NO modulates APP and BACE1 expression, not only within the vessels themselves, but also in brain tissue. Most importantly, endothelial NO also seems to inhibit Aβ generation and secretion. Our findings also suggest that the effect of NO is selective for BACE1 because we did not detect alterations in expression of α-secretases, γ-secretases or Aβ degradation enzymes.
It is important to note that phenotypically, eNOS−/− animals have a higher resting mean arterial blood pressure and are prone to insulin resistance as compared to wild type animals16. While additional studies are required to determine the exact contribution of hypertension and insulin resistance to the elevation of Aβ our in vitro data indicate that loss of endothelial NO is sufficient to increase APP, BACE1 and Aβ levels in the absence of any hemodynamic forces or insulin resistance. In addition, existing evidence suggests that hypoperfusion can lead to alterations in brain APP and Aβ levels; however, prior reports demonstrated that cerebral blood flow is normal in eNOS−/− animals17, 18.
The results from the present study demonstrate that the NO/cGMP pathway plays an important role in maintaining APP, BACE1 and Aβ levels within the brain and cerebral microvasculature and that this may provide a therapeutic target in treating and preventing mild cognitive impairment as well as AD. Indeed, in humans, physical exercise has been shown to be protective against developing AD19. Moreover, elevated shear stress imposed on the endothelium by exercise-induced increased blood flow is known to upregulate eNOS20. Taken together, our findings offer novel insights into previously unrecognized mechanisms underlying the beneficial effects of eNOS-derived NO on prevention of Aβ elevation in cerebrovascular and neuronal tissue.
Novelty and Significance
What Is Known?
Amyloid precursor protein (APP) is the parent molecule that when sequentially cleaved by β-site APP cleaving enzyme 1 (BACE1) and γ-secretase generates the amyloid beta (Aβ) peptide which is the primary component of Alzheimer’s disease (AD) plaques.
Cardiovascular risk factors such as hypertension, hypercholoersolemia, diabetes mellitus, aging and a sedentary lifestyle are associated with a higher incidence of AD.
Endothelial dysfunction caused by decreased bioavailability of nitric oxide (NO) is a common feature among cardiovascular risk factors.
What New Information Does This Article Contribute?
Endothelial-derived NO suppresses APP, BACE1 and Aβ by increasing cyclic guanosine monophosphate (cGMP) in brain microvascular endothelial cells (BMECs).
Endothelial nitric oxide synthase (eNOS) deficient (eNOS−/−) mice display increased APP, BACE1, and Aβ peptide in brain tissue and increased BACE1 protein levels in the cerebral microvasculature when compared with wild-type control mice.
NO-mediated effects were specific to APP and BACE1 as there were no differences in the other secretase enzymes or Aβ-degradation enzymes between eNOS−/− and wild-type I mice.
Summary
Several cardiovascular risk factors are associated with a higher incidence of AD. We show for the first time that loss of endothelial derived NO generated by eNOS leads to an increase in APP, BACE1 and Aβ peptide both in cultured BMECs and in the brains of eNOS−/− mice. Our data suggest that endothelial NO plays an important role in suppressing APP, BACE1 and Aβ levels within the brain and cerebral vasculature. These findings identify a previously unrecognized mechanism linking endothelial dysfunction with amyloidogenic processing of APP. Our results suggest that preservation of the NO/cGMP signaling pathway may be an important therapeutic strategy for preventing and treating mild cognitive impairment as well as AD.
Supplementary Material
Acknowledgments
Sources of Funding
This work was supported by National Institutes of Health grants HL-53524 and HL-91867, the Mayo Alzheimer’s Disease Research Center (Z.S.K.), AHA scientist development grant (0835436N, A.V.S.), Clinical Pharmacology Training Grant (T32 GM08685) (Trainee S.A.A.) and the Mayo Foundation.
Non-standard Abbreviations and Acronyms
- Aβ
amyloid beta
- AD
Alzheimer’s disease
- APP
amyloid precursor protein
- NO
nitric oxide
- BMECs
brain microvascular endothelial cells
- NOS
nitric oxide synthase
- eNOS
endothelial nitric oxide synthase
- iNOS
inducible nitric oxide synthase
- nNOS
neuronal nitric oxide synthase
- L-NAME
N(G)-Nitro-L-Arginine Methyl Ester
- eNOS−/−
eNOS deficient
- BACE1
β-site APP cleaving enzyme 1
- cGMP
cyclic guanosine monophosphate
- CAA
cerebral amyloid angiopathy
- siRNA
small interfering RNA
- ODQ
[1,2,4]Oxadiazolo[4,3-a]quinoxalin-1-one
- PDE5
phosphodiesterase type 5
- NOx
nitrate/nitrite
- ADAM
a disintegrin and metalloprotease
- Aph1
anterior defective-1
- Pen2
presenilin enhancer 2
- PS
presenilin
- ECE
endothelin converting enzyme
- IDE
insulin degrading enzyme
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures
None.
References
- 1.Ferri CP, Prince M, Brayne C, Brodaty H, Fratiglioni L, Ganguli M, Hall K, Hasegawa K, Hendrie H, Huang Y, Jorm A, Mathers C, Menezes PR, Rimmer E, Scazufca M. Global prevalence of dementia: a Delphi consensus study. Lancet. 2005;366:2112–2117. doi: 10.1016/S0140-6736(05)67889-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gorevic PD, Goni F, Pons-Estel B, Alvarez F, Peress NS, Frangione B. Isolation and partial characterization of neurofibrillary tangles and amyloid plaque core in Alzheimer' disease: immunohistological studies. J Neuropathol Exp Neurol. 1986;45:647–664. doi: 10.1097/00005072-198611000-00004. [DOI] [PubMed] [Google Scholar]
- 3.Glenner GG, Wong CW. Alzheimer's disease: initial report of the purification and characterization of a novel cerebrovascular amyloid protein. Biochem Biophys Res Commun. 1984;120:885–890. doi: 10.1016/s0006-291x(84)80190-4. [DOI] [PubMed] [Google Scholar]
- 4.Hussain I, Powell D, Howlett DR, Tew DG, Meek TD, Chapman C, Gloger IS, Murphy KE, Southan CD, Ryan DM, Smith TS, Simmons DL, Walsh FS, Dingwall C, Christie G. Identification of a novel aspartic protease (Asp 2) as beta-secretase. Mol Cell Neurosci. 1999;14:419–427. doi: 10.1006/mcne.1999.0811. [DOI] [PubMed] [Google Scholar]
- 5.Xia W, Zhang J, Perez R, Koo EH, Selkoe DJ. Interaction between amyloid precursor protein and presenilins in mammalian cells: implications for the pathogenesis of Alzheimer disease. Proc Natl Acad Sci U S A. 1997;94:8208–8213. doi: 10.1073/pnas.94.15.8208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Folin M, Baiguera S, Tommasini M, Guidolin D, Conconi MT, De Carlo E, Nussdorfer GG, Parnigotto PP. Effects of beta-amyloid on rat neuromicrovascular endothelial cells cultured in vitro. Int J Mol Med. 2005;15:929–935. [PubMed] [Google Scholar]
- 7.Niwa K, Carlson GA, Iadecola C. Exogenous A beta 1–40 reproduces cerebrovascular alterations resulting from amyloid precursor protein overexpression in mice. J Cereb Blood Flow Metab. 2000;20:1659–1668. doi: 10.1097/00004647-200012000-00005. [DOI] [PubMed] [Google Scholar]
- 8.Austin SA, Sens MA, Combs CK. Amyloid precursor protein mediates a tyrosine kinase-dependent activation response in endothelial cells. J Neurosci. 2009;29:14451–14462. doi: 10.1523/JNEUROSCI.3107-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Purnell C, Gao S, Callahan CM, Hendrie HC. Cardiovascular risk factors and incident Alzheimer disease: a systematic review of the literature. Alzheimer Dis Assoc Disord. 2009;23:1–10. doi: 10.1097/WAD.0b013e318187541c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dudzinski DM, Igarashi J, Greif D, Michel T. The regulation and pharmacology of endothelial nitric oxide synthase. Annu Rev Pharmacol Toxicol. 2006;46:235–276. doi: 10.1146/annurev.pharmtox.44.101802.121844. [DOI] [PubMed] [Google Scholar]
- 11.Demas GE, Kriegsfeld LJ, Blackshaw S, Huang P, Gammie SC, Nelson RJ, Snyder SH. Elimination of aggressive behavior in male mice lacking endothelial nitric oxide synthase. J Neurosci. 1999;19:RC30. doi: 10.1523/JNEUROSCI.19-19-j0004.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ignarro LJ. Nitric oxide. A novel signal transduction mechanism for transcellular communication. Hypertension. 1990;16:477–483. doi: 10.1161/01.hyp.16.5.477. [DOI] [PubMed] [Google Scholar]
- 13.Murad F, Mittal CK, Arnold WP, Katsuki S, Kimura H. Guanylate cyclase: activation by azide, nitro compounds, nitric oxide, and hydroxyl radical and inhibition by hemoglobin and myoglobin. Adv Cyclic Nucleotide Res. 1978;9:145–158. [PubMed] [Google Scholar]
- 14.De Strooper B. Proteases and proteolysis in Alzheimer disease: a multifactorial view on the disease process. Physiol Rev. 90:465–494. doi: 10.1152/physrev.00023.2009. [DOI] [PubMed] [Google Scholar]
- 15.Pak T, Cadet P, Mantione KJ, Stefano GB. Morphine via nitric oxide modulates beta-amyloid metabolism: a novel protective mechanism for Alzheimer's disease. Med Sci Monit. 2005;11:BR357–BR366. [PubMed] [Google Scholar]
- 16.Huang PL. eNOS, metabolic syndrome and cardiovascular disease. Trends Endocrinol Metab. 2009;20:295–302. doi: 10.1016/j.tem.2009.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Atochin DN, Demchenko IT, Astern J, Boso AE, Piantadosi CA, Huang PL. Contributions of endothelial and neuronal nitric oxide synthases to cerebrovascular responses to hyperoxia. J Cereb Blood Flow Metab. 2003;23:1219–1226. doi: 10.1097/01.WCB.0000089601.87125.E4. [DOI] [PubMed] [Google Scholar]
- 18.Lundblad C, Grande PO, Bentzer P. Hemodynamic and histological effects of traumatic brain injury in eNOS-deficient mice. J Neurotrauma. 2009;26:1953–1962. doi: 10.1089/neu.2009.0955. [DOI] [PubMed] [Google Scholar]
- 19.Scarmeas N, Luchsinger JA, Schupf N, Brickman AM, Cosentino S, Tang MX, Stern Y. Physical activity, diet, and risk of Alzheimer disease. Jama. 2009;302:627–637. doi: 10.1001/jama.2009.1144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Green DJ, Maiorana A, O'Driscoll G, Taylor R. Effect of exercise training on endothelium-derived nitric oxide function in humans. J Physiol. 2004;561:1–25. doi: 10.1113/jphysiol.2004.068197. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.