Abstract
Histone deactylase inhibitors (HDACis) show promise as cancer therapeutics, however, the full scope of their utility remains unknown. Here we report findings that strongly rationalize clinical evaluation of HDACis in malignant peripheral nerve sheath tumors (MPNSTs), a class of highly aggressive, therapeutically resistant and commonly fatal malignancies that occur sporadically or in patients with the inherited neurofibromatosis type 1 (NF1) syndrome. We evaluated the effects of the chemical HDACis PCI-24781, SAHA, and MS-275 on a panel of human NF1-associated and sporadic MPNSTs in vitro and in vivo. A subset of MPNSTs were found to be highly sensitive to HDACis, especially to PCI-24781. All cell-lines in this group were NF1-associated. Significant pro-apoptotic effects were noted in vitro and in vivo and were independent of p53 mutational status. In contrast, as a group the sporadic MPNST cells were markedly resistant to HDACi treatment. HDACis were found to induce productive autophagy in MPNST cells. Genetic and/or pharmacological autophagy blockade resulted in significant HDACi-induced apoptosis in cells defined as resistant or sensitive, leading to abrogated growth of primary tumors and lung metastases in tumor xenograft assays. Among autophagy-associated genes expressed in response to HDACi, the immune related GTPase IRGM was validated as a critical target in mediating HDACi-induced autophagy and enhanced apoptosis. Taken together, our findings strongly support the evaluation of HDACi currently in clinical trials as an important new therapeutic strategy to treat MPNST, including in combination with autophagy blocking combination regimens in particular for patients with sporadic MPNST.
Keywords: MPNST, HDAC inhibitors, Autophagy, Targeted-Therapies, Chloroquine
Introduction
Malignant peripheral nerve sheath tumors (MPNSTs) are rare malignancies originating from Schwann lineage cells and arising proximate to peripheral nerves (1, 2). MPNSTs account for 3–10% of all soft tissue sarcomas (STS) and are a highly aggressive histological-subtype (3–5). >50% occur in patients with the inherited neurofibromatosis type-1 (NF1) syndrome; ~8–12% of NF1 patients will develop an MPNST in their lifetime, commonly arising within a pre-existing deep, plexiform neurofibroma (6, 7); the remainder develop sporadically (6). In adults with NF1, MPNSTs are the most common malignancy, the major source of morbidity, and the leading cause of NF1-related mortality (8, 9). Complete surgical resection, frequently not feasible due to local invasiveness and/or uncontrollable metastases, is the only potentially curative option; radio- and chemotherapy have not demonstrably affected survival, underlying 20–50% five-year survival rates (1, 8, 9). Lack of effective systemic therapies is the major unresolved MPNST clinical problem; new therapeutic approaches are urgently needed.
Recently, attention has focused on potentially reversible alterations in chromatin structure which modulate gene expression during malignant transformation (10). Histone deacetylases (HDACs) play an important role in the epigenetic regulation of gene expression by catalyzing the removal of acetyl groups from histone and non-histone proteins, stimulating chromatin condensation, and promoting transcriptional repression and other molecular processes (11). The emerging delineation o f HDAC-driven alterations that coincide with tumorigenicity and malignant progression has provided impetus for development of HDAC inhibitors (HDACi) as novel cancer therapeutics (12, 13). Such initiatives are prompted by broad growth-inhibitory and cytotoxic HDACi effects observed in cultured cancer cells (with normal cell sparing), and significant in vivo effects seen in human tumor xenograft models (14). To date, >15 early-phase clinical trials have documented HDACi potential efficacy in multiple cancer types (15, 16).
We have recently demonstrated in vitro and in vivo efficacy for broad spectrum hydroxamic acid-based HDACis (SAHA and PCI-24781) against a range of genetically complex STS, especially when administered in combination with doxorubicin (17). MPNSTs were not included in these original investigations; to the best of our knowledge, the effect of HDACi on these tumors has not been assessed. The goal of the current study was to bridge this investigational gap and to evaluate the effects of HDACis on MPNST is a pre-clinical setting.
Materials and Methods
Cell-lines
Human NF1-related MPNST cell-lines ST88-14, T265, and S462 and non-NF1 sporadic human MPNST cell-lines STS26T and MPNST724 were maintained and propagated as previously described (18). Primary cultured normal human Schwann cells served as controls. The NF1-associated cell-line MPNST642 was established by us (Supp data); DNA fingerprinting (STR; Supp data) was conducted for all cell-lines <6mo prior to the conduct of the studies, confirming that no cross contamination has occurred. STS26T and MPNST724 cells were stably transfected to express GFP-LC3; over-expressing cells were FACS-sorted on the basis of GFP expression. HDAC inhibitors included PCI-24781 (Pharmacyclics, Sunnyvale, CA), suberoylanilide hydroxamic acid (SAHA) and MS-275 (Cayman Chemical, Ann Arbor, MI). Bafilomycin and chloroquine were obtained from Sigma (St Louis, MO). Commercially available antibodies were used for immunoblot or immunohistochemical detection of: acetylated H3, acetylated H4 (Millipore, Billerica, MA); acetylated tubulin (Sigma); caspase 3, LC3B (Cell Signaling, Danvers, MA); GFP, beclin, p53, actin (Santa Cruz, Santa Cruz, CA); IRGM, PARP (Abcam, Cambridge, MA); Ki-67 (MIB-1), vim (Dako, Carpenteria, CA); and S-100 (Biogenex, San Ramon, CA).
Cellular assays
MTS, clonogenicity, and soft agar colony formation assays were performed as previously described (19). Doses needed to inhibit growth by 50% (GI50) were determined. Western blot analyses were performed by standard methods (17). Apoptosis was measured using the Apoptosis Detection kit I (BD Biosciences, San Jose, CA) per manufacturer’s recommendations Further information is available as Supp Data.
Transfection procedures
siRNAs and p53 construct transfections procedures are described in Supp Data
Gene Expression Assays
Gene expression profiling was conducted using the Autophagy RT2 Profiler™ PCR Array (SABiosciences, Frederick, MD). RT-PCR and qRTPCR were conducted by standard methods. Additional information and primer sequences are provided in Supp Data.
Transmission Electron Microscopy and Quantification of acidic vesicular organelles
Assays were performed as previously described (20). Additional information is provided in Supp Data.
In vivo animal models
All animal procedures/care was approved by UTMDACC Institutional Animal Care and Usage Committee. Animals received humane care as per the Animal Welfare Act and the NIH "Guide for the Care and Use of Laboratory Animals." Animal models were utilized as previously described (17). Animal models, therapeutic schemas, drug doses, and immunohistochemical procedures are provided in Supp Data.
Statistics
Cell culture-based assays were repeated at least thrice; mean ± SD was calculated. Cell-lines were examined separately. For outcomes that were measuredat a single time point, two-sample t-tests were used to assessthe differences. Differences in xenograft growth (tumor/metastases) in vivo were assessed using a two-tailed Student's t-test. Significance was set at P ≤0.05.
Results
HDACis induce significant apoptosis in a subset of human MPNST
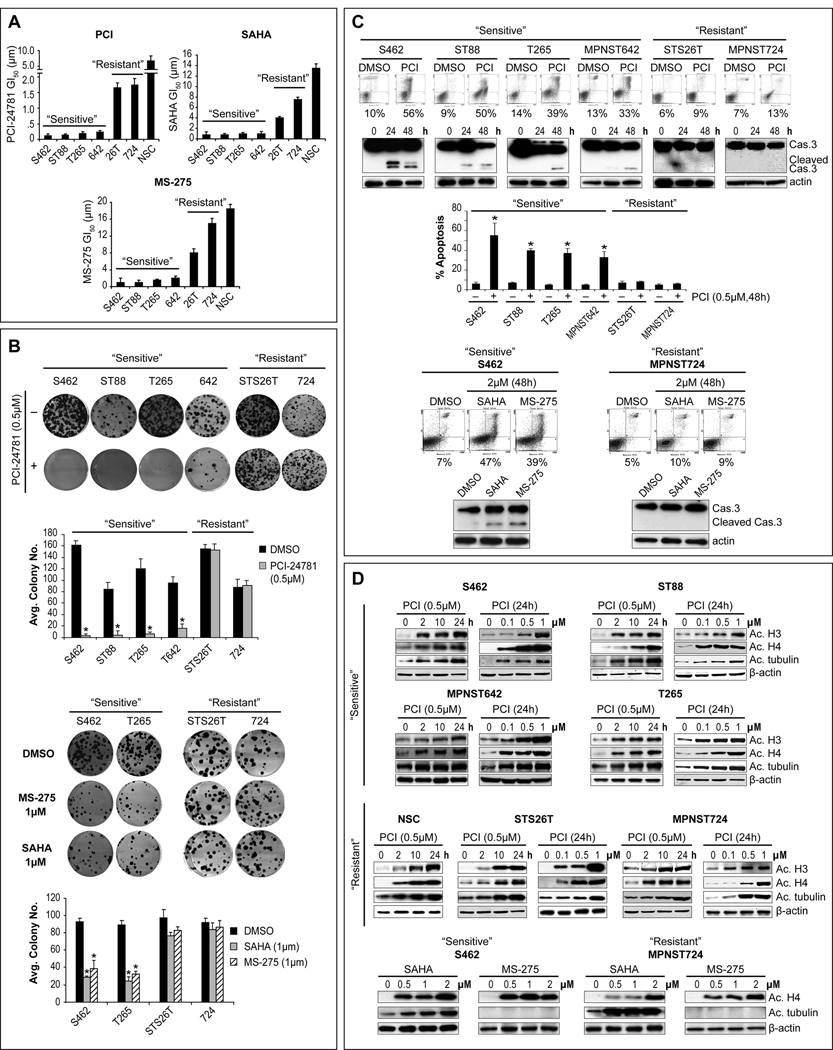
HDACi effects on human MPNST cell growth and clonogenicity were evaluated. Six MPNST cell-lines were used including the NF1-associated MPNST642 cell-line recently established by us (Figure S1); primary cultured normal human Schwann cells (NSCs) served as controls. Three HDACis were tested: PCI-24781, SAHA, and MS-275. PCI-24781 induced a time- and dose-dependent growth inhibition that was most pronounced in a subset of cell-lines tested independent of growth rate (see Supplemental Results). Fig 1A depicts GI50s at 48hr; four MPNST cell-lines were markedly sensitive to PCI-24781, with GI50s ranging between 0.1–0.35µM, while the two additional cell-lines (STS26T and MPNST724) were relatively resistant exhibiting GI50s above the clinically relevant dose (>1µM). NSCs were resistant to PCI-24781 growth inhibitory effects. Higher doses of SAHA and MS-275 were needed to achieve PCI-24781-equivalent MPNST growth-inhibition; however, a similar response pattern was found for all these drugs, enabling MPNST cell designations to “sensitive” and “resistant” cohorts (Fig 1A). A similar pattern of response was noted when the effect of HDACis on colony-forming capacity was evaluated (Fig 1B). This pattern was also reflected in the induction of apoptosis by these compounds (Fig 1C). Marked apoptosis (evaluated by Annexin-V/PI staining FACS analysis) was seen in “sensitive” cell-lines while no significant apoptosis was induced in “resistant” cells. Similarly, an increase in cleaved caspase-3 was seen in “sensitive” but not in “resistant” cells (Fig 1C). A time- and dose-dependent increase in target protein acetylation could be observed in all the cell lines regardless of the growth inhibitory effects (Fig 1D). This aligns with previous published data demonstrating that protein acetylation occurs even in normal cells which are relatively resistant to HDACi effects suggesting that while drugs are delivered into the cells and reach their intended targets, additional molecular mechanisms drive therapeutic sensitivity and resistance (21).
Figure 1. A subset of MPNST cell-lines is highly sensitive to HDACis.
A. Growth-inhibitory effects (48h) were determined via MTS assays, GI50s are depicted. One MPNST cell-line subset was highly sensitive to the effects of all three drugs (highest sensitivity was seen to PCI-24781 [=PCI]), while a second exhibited relative resistance. No significant effect on normal human Schwann cells’ (NSC) growth was noted in clinically relevant therapeutic doses; B. Clonogenic assays demonstrated a similar pattern of response; C. Marked apoptosis was noticed in “sensitive” cell-lines but not in “resistant” cell-lines (Annexin-V (=X axis)/PI (=Y axis) FACS analysis (48h) and cleaved caspase-3 WB); D. A time- and dose-dependent increase in target protein acetylation was demonstrated after treatment with either of the compounds in all cell-lines (including NSC) independent of growth inhibitory response (MS-275 is a selective HDAC1/HDAC2 inhibitor and does not affect tubulin acetylation) [Graphs represent the average of three repeated experiments ±SD; * denotes statistically significant effects (p<0.05)]
Since HDACis have also been shown to exert anti-tumor effects through activation of wild-type p53 (22), and p53 deregulation is a common event in MPNST (23) the p53 mutational status of these cells was evaluated (Fig S2A). These studies indicated that in MPNST, HDACi response is independent of p53 mutation/function. Interestingly, we observed that all MPNST cells in the “sensitive” group are NF1-associated while both “resistant” cell-lines are sporadic (Fig S2B).
HDACi sensitivity/resistance pattern is recapitulated in vivo
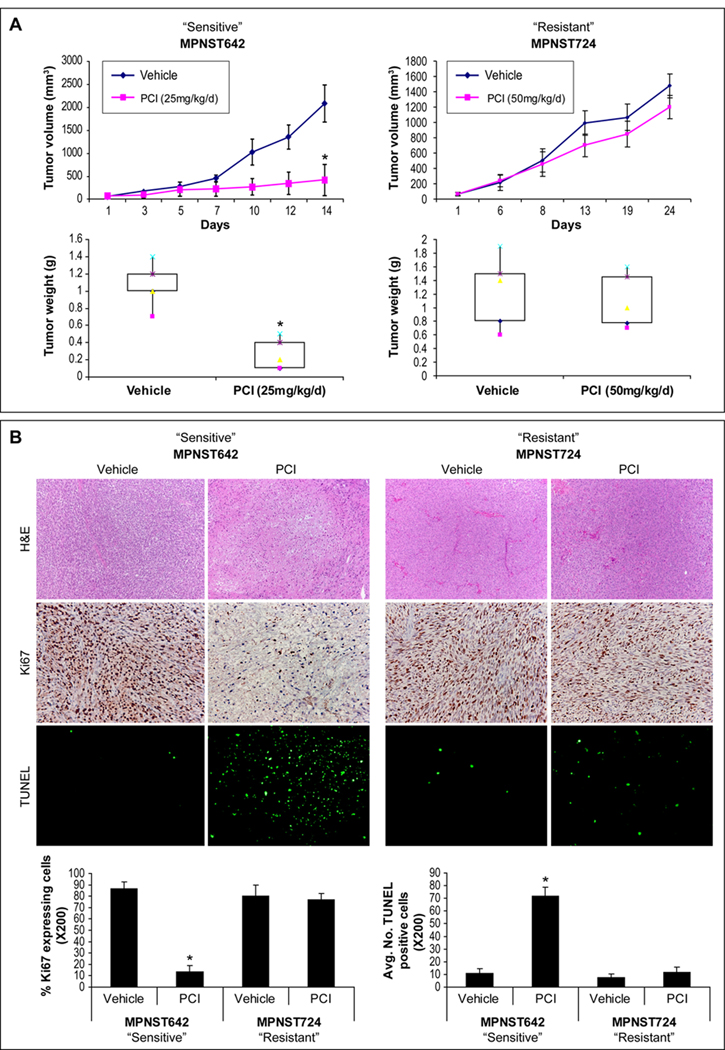
Human MPNST xenografts (Fig S1), including HDACi “sensitive” (MPNST642) and “resistant” (MPNST724 and STS26T) tumors, were used to evaluate the effects of HDACi on tumor growth in vivo. PCI-24781 was selected due to its enhanced efficacy in vitro. As shown in Fig 2A, a relatively low (25mg/kg/d) PCI-24781 dose markedly abrogated the growth of MPNST642 xenografts. A significant decrease in average tumor size and weight of treated tumors as compared to controls was noted at study termination (p=0.00016 and p=0.0004). In contrast, no significant growth inhibitory effects were seen in MPNST724 (Fig 2A) and STS26T (data not shown) xenografts, although a higher dose of PCI-24781 was tested (50mg/kg/d). In accordance, immunohistochemical analysis of tumor sections for cell proliferation (Ki67) and apoptosis (TUNEL) confirmed that PCI-24781 induced anti-proliferative, pro-apoptotic effects in the “sensitive” tumors (p=0.008 and p=0.0008), but not in the MPNST724 xenografts (Fig 2B). Together, these data suggest that a subset of MPNSTs is highly sensitive to HDACi monotherapy in vitro and in vivo. In addition, HDACi “resistance” observed in vitro can be recapitulated in vivo, offering a novel model for further investigation of HDACi tumor response mechanisms.
Figure 2. PCI-24781 inhibits the growth of MPNST xenografts exhibiting significant sensitivity in vitro.
A. SCID mice bearing MPNST642 and MPNST724 xenografts were treated with PCI-24781 (PCI; 25mg/kg/d or 50mg/kg/d, respectively) or vehicle (10mice/group). Tumor growth/weight curves are depicted demonstrating that PCI-24781 abrogated the growth of MPNST642 tumors (p=0.00016 and 0.0004 for tumor size and weight, respectively) but not of MPNST724 tumors although treated with a higher drug dose; B. IHC analysis demonstrated enhanced tumor necrosis in MPNST642 PCI-24781 treated tumors (H+E), decreased tumor proliferation (Ki67; p=0.008) and increased apoptosis (TUNEL; p=0.0008). No significant differences were found for MPNST724 xenografts. (* denotes statistically significant effects; p<0.05)
HDACis induce autophagy in MPNST cells in vitro and in vivo
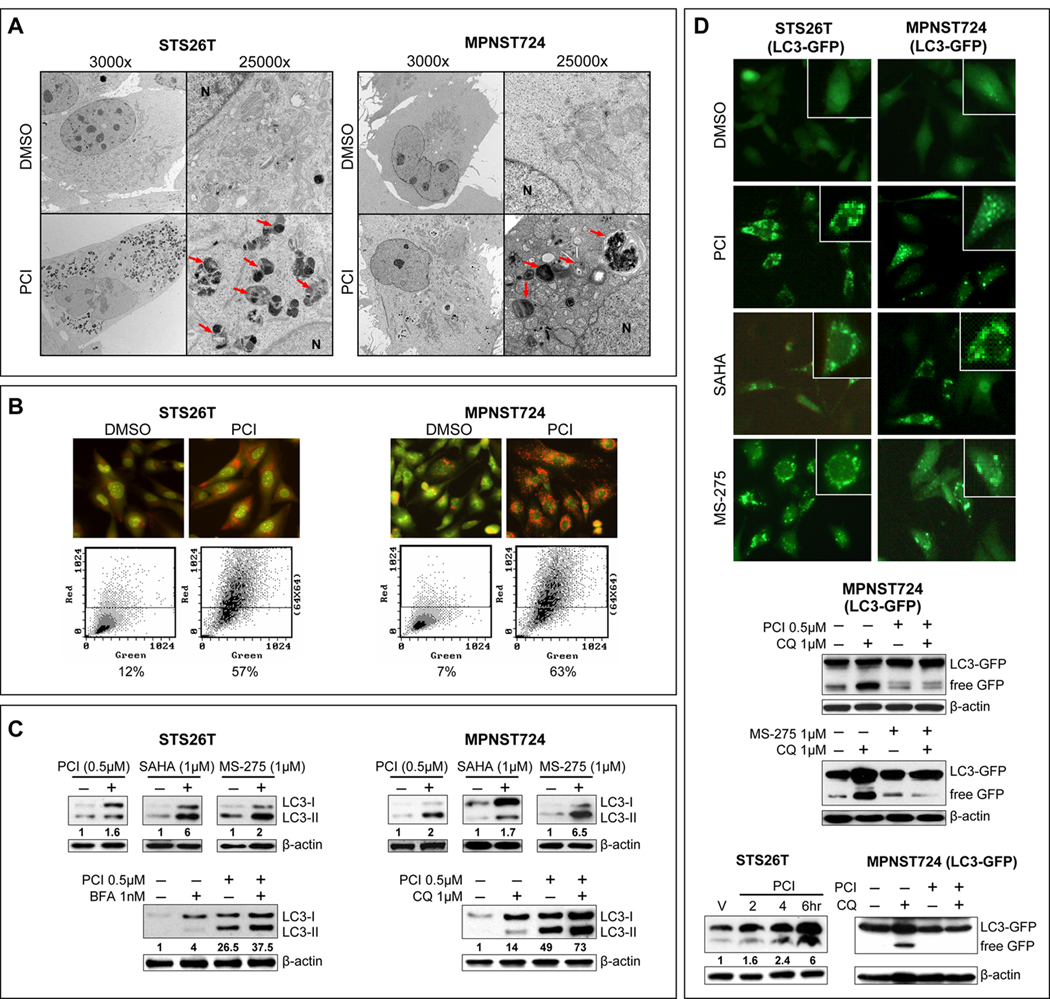
To further evaluate HDACi-induced structural changes in “resistant” cells, transmission electron microscopic (TEM) evaluation was conducted: a large number of cytoplasmic autophagosomes was noticed after treatment (24 [Fig 3A], 48, and 72 hr) but no signs of apoptosis were seen. While the noted autophagosome accumulation suggests drug-induced autophagy, multiple assays are needed to confirm this observation (24). Acridine-orange staining demonstrated increased acidic vesicular organelles (AVOs) in PCI-24781 (0.5µM/24hr) treated compared to control DMSO treated cells, as was confirmed via FACS analysis (Fig 3B). Furthermore, LC3B conversion and LC3B-II expression (normalized to actin) both increased after treatment with all three HDACis tested (Fig 3C). Since the experiments described above may represent either enhanced autophagosome synthesis and productive autophagy or reduced autophagosome turnover due delayed trafficking or reduced autophagosome-lysosome fusion (24), cells were pretreated (1hr) with low doses of the autophagy inhibitors Bafilomycin A1 (1nM) or chloroquine (1µM) prior to PCI-24781 treatment (24hr). PCI-24781 treatment produced increased LC3B-II expression even in the presence of these inhibitors, providing evidence of efficient autophagic flux (Fig 3C). Furthermore, cells stably transduced to express LC3-GFP, exhibited increased GFP puncta in response to HDACi treatment (Fig 3D). WB demonstrated increased GFP cleavage following HDACi treatment that was blocked by pretreatment with chloroquine, further supporting HDACi-induced productive autophagy (Fig 3D). Similarly, HDACi-induced autophagy could be observed in vivo. Animals bearing STS26T tumors were treated with PCI-24781 for four days and on day five tumors were harvested two, four, and six hours after final dose. WB analysis demonstrated a time-dependent increase in LC3B-II expression (Fig 3D). Similarly, MPNST724/GFP-LC3 xenografts were treated with PCI-24781, chloroquine, or their combination (Fig 3D). A marked increase in free GFP expression was noted in response to PCI-24781. Chloroquine blocked HDACi-induced increases in free GFP.
Figure 3. HDACis induce productive autophagy in therapeutic resistant MPNST cells.
A. TEM pictures demonstrating ultrastructural changes in MPNST cells in response to PCI-24781 (0.5µM/24h). A large number of autophagic vesicles were identified (arrows) without evidence of chromatin condensation, nuclear membranes remained intact further supporting lack of apoptotic effect; B. Acridine-orange staining demonstrated increased acidic vesicular organelles (AVOs) in PCI-24781 (0.5µM/24hr) compared to control DMSO treated cells, as was further confirmed via FACS analysis; C. Increased LC3B-II expression (normalized to actin; densitometry values are depicted) was noticed after treatment with any of the HDACis (upper panels). Cells were pretreated (1hr) with low doses of the inhibitors Bafilomycin A1 (1nM) or chloroquine (1µM) prior to PCI-24781 treatment (24hr; lower panels). Additional increased LC3B-II expression in response to PCI-24781 was noticed in the presence of these inhibitors; D. Cells stably transduced to express LC3-GFP exhibited increased GFP puncta in response to HDACis (upper panels). GFP cleavage was found in response to HDACi and was blocked by pretreatment with chloroquine (middle panels). Furthermore, WB of STS26T xenografts’ protein treated with PCI-24781 (50mg/kg/d) harvested two, four, and six hr after final dose demonstrated a time-dependent increase in LC3B-II expression (left lower panel). Similarly, MPNST724 GFP-LC3 xenografts were treated with PCI-24781, chloroquine, or their combination. Free GFP expression was found in response to PCI-24781 treatment. Chloroquine blocked HDACi-induced increases in free GFP (right lower panel).
HDACi-induced autophagy was not exclusive to sporadic MPNST cells and in a series of experiments as depicted above we found autophagy to occur in response to PCI-24781 in all NF1-assciated HDACi “sensitive” cells (Fig S3). However, in contrast to the MPNST ‘resistant’ cells, morphologic features of apoptosis were also present. Together, our data strongly support that HDACis induce productive autophagy in MPNST cells in vitro and in vivo.
Autophagy blockade enhances HDACi pro-apoptotic effects in MPNST cells
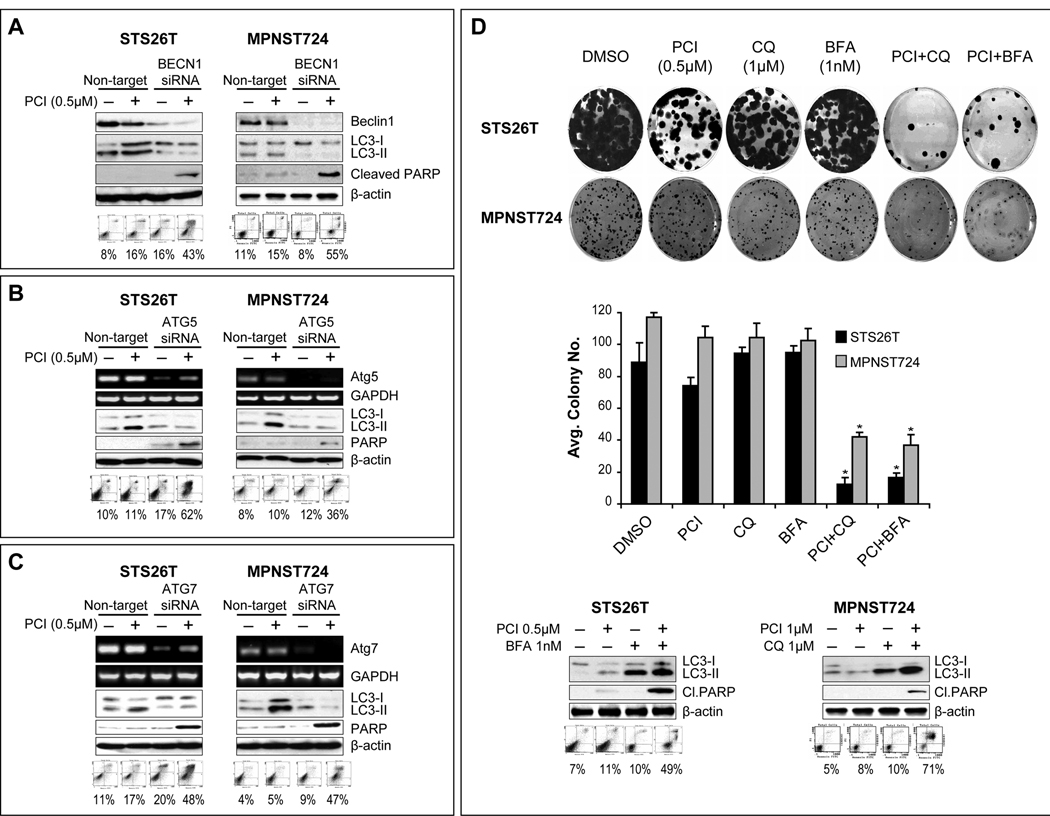
It is currently unclear whether drug-induced autophagy contributes to cell death or possibly represents a mechanism of therapeutic resistance (25, 26). To that end, we evaluated the impact of autophagy-blockade using complementary genetic and pharmacological manipulations on HDACi-induced effects. siRNA knockdown of beclin, ATG5, and ATG7 were conducted (Fig 4A–C). Autophagy blockade was assessed via LC3B-II WB; knockdown of any of the three targets decreased both baseline and PCI-24781-induced LC3B-II expression. Most importantly, enhanced apoptosis in response to PCI-24781 pretreatment of cells was found after target knockdown, as determined via PARP cleavage and Annexin-V/PI FACS analysis (Fig 4A–C). Pharmacologic autophagy inhibition was achieved using bafilomycin and chloroquine (Fig 4D). The clonogenic capacity of STS26T or MPNST724 cells was not affected by any of the compounds when used alone, but was significantly affected by chloroquine or bafilomycin pretreatment of cells (1hr) followed by PCI-24781 for 24hr (p<0.05). The combination also resulted in increased apoptosis. Together, these data suggest that autophagy blockade sensitized “resistant” MPNST cells to the pro-apoptotic effects of HDACi in vitro.
Figure 4. Autophagy blockade sensitizes MPNST cells to the pro-apoptotic effects of HDACis.
A. Anti-Beclin siRNA (20nM pool) knockdown was confirmed via WB and autophagy blockade was validated using LC3B-II WB. Enhanced apoptosis in response to PCI-24781 (0.5µM/24h) was determined via PARP cleavage and Annexin-V/PI FACS analysis; B. Similar effects were noted after ATG5 knockdown. Knockdown was confirmed by RTPCR; C. As above, ATG7 knockdown (confirmed by RTPCR) resulted in increased PCI-24781 sensitivity; D. Pharmacologic autophagy-inhibition was achieved using bafilomycin and chloroquine. None of the compounds alone significantly affected the clonogenic capacity of STS26T or MPNST724; however, significant effects were noticed after chloroquine (1µM) or bafilomycin (1nM) pretreatment of cells (1hr) followed by PCI-24781 for 24hr (upper panels; p<0.05). Markedly increased apoptosis was also identified using these therapeutic combinations (lower panels). (* denotes statistically significant effects; p<0.05)
Next, we evaluated the impact of autophagy blockade on “sensitive” MPNST cell lines where autophagy in response to HDACi occurs in parallel with apoptosis. Using an experimental approach as per above we found that blocking autophagy significantly enhanced apoptosis both when low doses (0.1µM/24h) and high doses (1µM/24h) of PCI24781 were used (Fig S3). These data suggests that even in HDACi sensitive cells autophagy is a potential survival mechanism opposing apoptosis.
Autophagy blockade enhances HDACi pro-apoptotic effects in vivo
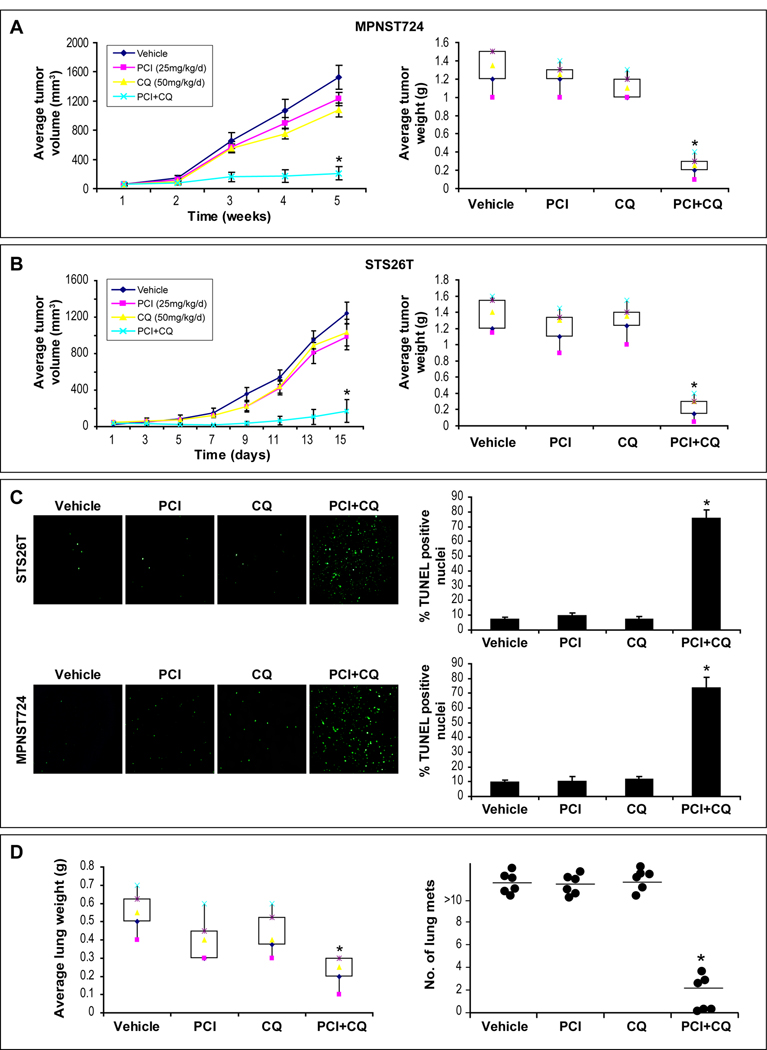
Next, we evaluated the effects of autophagy-blockade on HDACi treatment response in vivo. MPNST724 and STS26T xenografts were used and therapy was initiated when tumors reached a mean diameter of 0.5cm (Fig 5A&B). No major side-effects were noted. No significant growth inhibition was seen with PCI-24781 or choloroquine as monotherapy. However, chloroquine/PCI-24781 combination significantly inhibited tumor growth in both animal models as compared to control and either of the compounds alone (p<0.05). A significant increase in apoptosis was observed in combination-treated tumors as determined by TUNEL staining (p<0.05; Fig 5C).
Figure 5. PCI-24781/chloroquine combination results in superior local and metastatic MPNST growth inhibition in vivo.
A. Tumor growth curves/weight bars demonstrating a significant impact of PCI-24781/choloroquine combination on MPNST724 growth (10 mice/treatment group; p=0.04). No significant effect was noted after either compound alone; B. A similar superior effect of combination therapy was found after the treatment of STS26T xenografts (p=0.03); C. A significant increase in apoptosis (TUNEL staining) was observed in combination-treated xenografts (p<0.05); D. STS26T lung metastases bearing mice were treated with PCI-24781, chloroquine, or their combination. A significant decrease in average lung weight and number of visible metastasis were found in the combination therapy group (p<0.05) but not after treatment with either agent alone. (* denotes statistically significant effects; p<0.05)
Next, an experimental MPNST lung metastasis model (Fig S1) was used; therapy was initiated a week after tail-vein injection and continued for two wks (Fig 5D). All control (n=6), PCI-24781 (n=7) and chloroquine (N=7) treated mice exhibited extensive macroscopic lung metastases at study termination, whereas in 3/7 combination treated mice there were no visible metastases, and <5 metastases could be found in the remaining four mice. A significantly lower average lung weight was found in combination treated mice (p<0.05). Together, these data suggest that autophagy blockade can sensitize MPNST to the pro-apoptotic effects of HDACi in vivo.
HDACi-induced autophagy related gene-expression changes
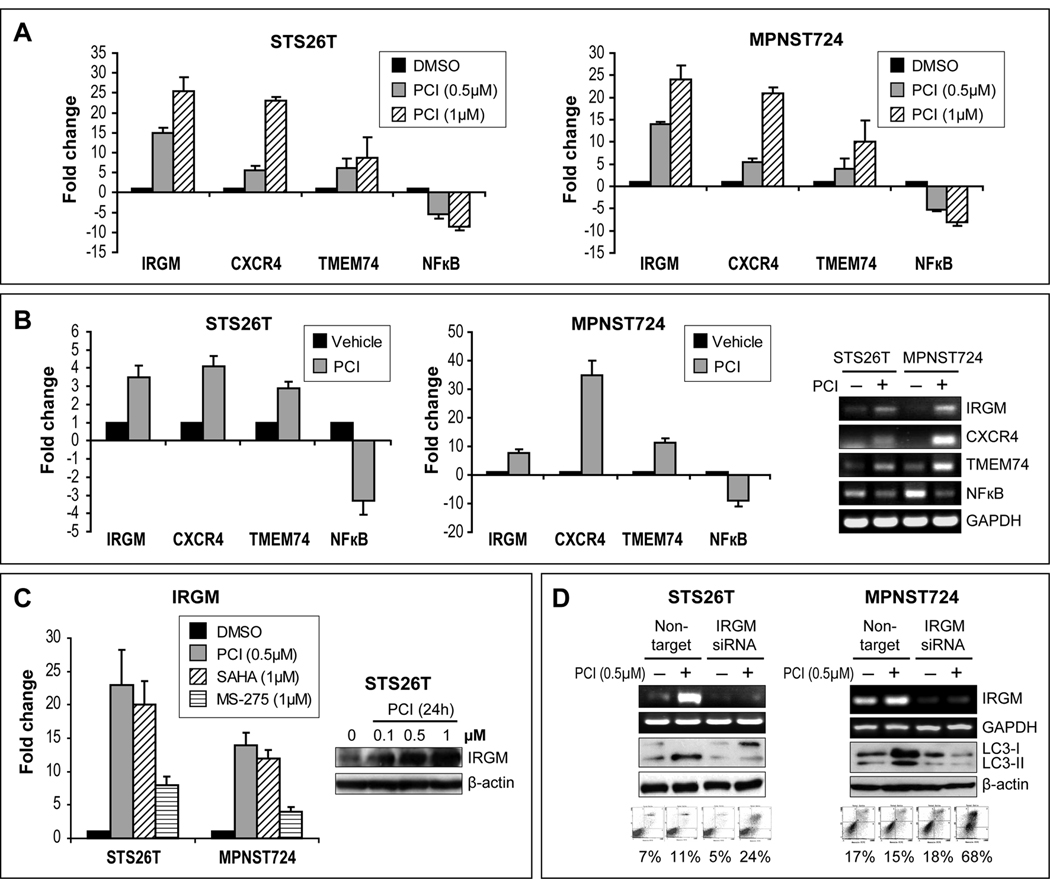
Due to their mechanisms of function, one of the major consequences of HDACi therapy is gene expression modulation. Using a focused autophagy PCR array, we sought to identify potential autophagy-related genes in MPNST cells whose expression is modified secondarily to HDACi treatment. Results revealed four genes that were reproducibly over-expressed in both cell-lines and five that were consistently down-regulated (Table S1). Three over-expressed genes (IRGM, CXCR4, and TMEM74) and one down-regulated gene, NFκB, were selected for validation. A concordant dose-dependent increase/decrease in corresponding RNA expression levels was identified after PCI-24781 treatment (24hr; Fig 6A). Similarly, IRGM, CXCR4, and TMEM74 mRNA expression was increased in PCI-24781 treated xenograft tissues; NFκB mRNA levels were decreased (Fig 6B). Together, these experiments identified several targets modified by HDACi that may play a role in HDACi-induced autophagy and merit further investigation. Accordingly, IRGM, exhibiting the highest fold increased expression, was selected for additional study. To confirm that increased IRGM expression in MPNST is a common consequence of HDACi exposure, the effects of SAHA and MS-275 on IRGM mRNA expression were evaluated (Fig 6C); both agents induced IRGM expression. A PCI-24781-induced increase in IRGM protein was also demonstrated. IRGM siRNA knockdown demonstrated inhibition of PCI-24781-induced autophagy as suggested by decreased LC3B-II expression (Fig 6D). Most importantly, IRGM knockdown resulted in enhanced PCI-24781-induced apoptosis in both cell-lines. Together, our results implicate HDACi-induced IRGM expression and autophagy in therapeutic resistance in sporadic MPNST cells.
Figure 6. HDACis modulate autophagy-related gene expression; a potential role for IRGM in drug-induced autophagy and therapeutic-resistance.
A. A focused autophagy PCR array identified several genes to be reproducibly deregulated in response to PCI-24781 (0.5µM/24h: Table S1). A concordant dose-dependent increase/decrease in corresponding RNA expression levels was identified (qRTPCR) in both cell-lines after treatment with PCI-24781 (24hr); B. Similarly, an increase in IRGM, CXCR4, and TMEM74 mRNA and a decrease in NFκB mRNA levels were identified by qRTPCR and RTPCR in xenograft tissues retrieved from experiments described above; C. An increase in IRGM mRNA was also identified by in response to SAHA and MS-275 (1µM/24h; left panel). Furthermore, a PCI-24781-induced increase in IRGM protein was demonstrated (WB; right panel); D. IRGM siRNA knockdown (pool, 20nM) resulted in PCI-24781-induced autophagy-blockade (decreased LC3B-II expression) and in enhanced PCI-24781-induced apoptosis.
Discussion
There is a crucial need for improved anti-MPNST therapeutic strategies. Studies here demonstrate that a subset of human MPNSTs is highly sensitive to HDACis anti-proliferative, pro-apoptotic effects in vitro and in vivo; importantly, normal human Schwann cells are resistant to these effects. Both broad spectrum hydroxamic acid-based agents (PCI-24781 and SAHA; (17) and the more selective HDAC1/HDAC2 inhibitor, MS-275 (27), result in a similar anti-MPNST response pattern. However, and in support of our previous observations in STS (17), PCI-24781 exhibited growth inhibitory effects at nanomolar versus micromolar doses needed for SAHA/MS-275. Of note, a phase I/II clinical-study examining the effects of PCI-24781 in combination with doxorubicin on advanced sarcomas following failure of anthracycline therapy has recently been initiated (28) results support the inclusion of MPNST patients in this or other HDACi-based clinical investigations.
With the limitations of a small testable MPNST cell-line cohort, it is intriguing and of possible major biological and clinical importance that all NF1-associated cells exhibited marked HDACi sensitivity. The molecular hallmark of NF1-associated MPNSTs is the loss of the GTPase activating protein, Nf1, a RAS negative regulator leading to constitutive activation of the RAS pathway (29, 30). Previous data suggest that HDACis induce cell death selectively in cells exhibiting enhanced RAS signaling (31, 32). Molecular deregulations such as elevated ROS activity and decreased STAT1 expression and/or function, operative in cells exhibiting activated RAS, have been proposed to underlie the increased HDACi susceptibility of these tumor cells (31, 32). HDACis were found to exert some of their pro-apoptotic effects through the induction, acetylation, and/or activation of p53 (22). p53 gene and its protein product are frequently deleted, mutated, and/or inactivated in MPNSTs (23); this molecular deregulation is thought to be one of the major driving forces for NF1-associated neurofibroma transformation and progression into its malignant MPNST counterpart (23). It is thus pertinent that as we have shown previously for other STS cells, no significant HDACi response differences were seen in wt p53 versus p53-mutated cells (17). With the increased interest in HDACi as anti-cancer therapy, our data suggest that the NF1-associated MPNST preclinical model can be used to establish mechanisms of action driving sensitivity, and especially the role of Nf1 loss and RAS activation in this process.
We found sporadic MPNST cell lines to exhibit relative resistance to HDACis. It is important to note that the diagnosis of sporadic MPNSTs, occurring outside the context of NF1, can be difficult (1). Sporadic MPNSTs are generally not associated with pre-existing neurofibromas and diagnosis is mainly one of exclusion. Strict criteria must be applied to ensure diagnostic consistency and at least one of the following conditions must be met: association with a peripheral nerve and ultrastructural, histologic and/or immunohistochemical features characteristic of schwannian differentiation. As described above, germline NF1 deactivating mutations are the hallmark of NF1-associated MPNSTs. Several studies have identified somatic NF1 mutations to occur in a subset of sporadic MPNSTs, although not uniformly (33). The exact prevalence and importance of Nf1 loss in sporadic MPNST tumorigenesis is currently unknown. Both cell lines studied here were shown to retain expression of Nf1 protein (see Fig S1 and S2). Further studies are needed to determine the role of Nf1 loss in HDACi sensitivity. If such a role is confirmed, it is possible that future treatment studies for sporadic MPNST as well as other malignancies where somatic NF1 mutations commonly occur would benefit from the use of NF1 mutation status or protein expression as biomarkers for patients stratification.
A major objective of this study was to identify potential mechanisms of HDACi resistance. Such knowledge will facilitate appropriate patient selection for treatment and enhance development of effective combination strategies to maximize HDACI cytotoxic effects. Enhanced antioxidant expression (34), retinoic acid signaling deregulation (35), and multi-drug resistance (34) are several potential molecular mechanisms previously proposed as contributory to HDACi resistance. The current study highlights the potential role of autophagy as a mechanism of therapeutic resistance.
Described more than 50 years ago, interest in autophagy in tumorigenesis and cancer progression has recently emerged, suggesting a complex molecular and functional interplay (26, 36, 37). Autophagy activation has been reported in response to diverse anticancer therapies (radiation, chemotherapy, targeted therapies; i.e., ER inhibitors and imatinib; (26, 38)). Of note is that a large number of studies demonstrate autophagy-induction based on autophagosome accumulation and LC3 conversion; however such findings can reflect either increased autophagic activity (productive autophagy) or reduced turnover of autophagosomes and autophagy blockade (24). This has major relevance to HDACis due to the recent finding that HDAC6 controls autophagosome maturation and autophagosome-lysosome fusion, and that its inhibition might in turn induce autophagy blockade (39). Taking this into account, here we have utilized a large panel of assays to test both autophagy steady state and flux, and have evaluated the impact of a selective HDACi (MS-275) which does not block HDAC6.
HDACi-induced autophagy has previously been described (40). The impact of drug-induced autophagy is currently being debated. Pro-death effects via a possible (albeit not yet fully substantiated) autophagic death mechanism (programmed cell-death type-II), or apoptosis enhancement have been described (26). In contrast, pro-survival, anti-apoptotic effects have also been reported, consonant with a role for drug-induced autophagy in tumor chemoresistance (37). For example: Shao et al found both apoptosis and autophagy to occur in HELA cells in response to HDACis; apoptosis-blockade did not diminish therapeutic-induced cell death, highlighting the role of autophagy and/or necrosis in this process (41). Similarly, Hrzenjak and colleagues observed that HDACi treatment mediated autophagic, caspase-independent, cytotoxicity in endometrial sarcoma cells (42). In contrast, Carew et al found that autophagy-blockade significantly enhanced HDACi-mediated apoptosis in CML cells (43). These seemingly contradictory data suggest that consequences of drug-induced autophagy may be compound-type, tumor-type, or even molecular context-dependent and depict a complex cross-talk between autophagy and apoptosis (44).
Our observations suggest that in MPNST HDACi-induced autophagy increases cell survival by possibly opposing apoptosis and demonstrate that autophagy-blockade enhances HDACi pro-apoptotic effects. This occurs not only in cells resistant to HDACi but also in MPNSTs demonstrating relative sensitivity where autophagy-blockade further increases HDACi pro-apoptotic effects. Chloroquine is known for its ability to block autophagy and is currently being evaluated in human glioblastoma and lung cancer clinical trials; initial studies already confirm its safety (45). Our studies support the investigation of HDACi/chloroquine combinations for MPNST treatment.
Currently, autophagy-specific inhibitors are lacking; consequently, the majority of studies, including ours, have been conducted with pharmacological agents with known off-target effects beyond autophagy (46). Development of autophagy-specific inhibitors may help determine the role of autophagy in cancer treatment and can form the basis for novel effective therapeutic combinations. It is therefore pertinent to establish an understanding about selective control of autophagy in specific therapeutic contexts. Our studies have identified and validated several molecular targets that potentially contribute to HDACi-induced autophagy in MPNST including IRGM, CXCR4, and TMEM74. A role in autophagy was recently attributed to the protein products of all three genes (see supplementary information (47–49)). However, the mechanisms by which they activate autophagy and possibly contribute to drug-induced autophagy and chemoresistance are yet to be fully elucidated. Accordingly, and as a proof of principle, knockdown of IRGM was found to block HDACi-induced autophagy and enhance apoptosis. Additional studies are currently ongoing and will hopefully lead to findings of clinical relevance. For example, CXCR4 inhibitors are available and are undergoing human cancer clinical trials (50); pending further supportive data HDACi/CXCR4 inhibitor therapeutic combinations could be evaluated as a novel approach for the treatment of MPNST.
Supplementary Material
Acknowledgments
We thank Drs. Joseph J. Buggy and Sriram Balasubramanian (Pharmacyclics, Inc., Sunnyvale, CA) for kindly providing PCI-24781. Dr Juan Fueyo-Margareto is thanked for providing the GFP-LC3 construct, Mr. Kenneth Dunner Jr. for assistance with electron microscopy, and Ms. Kim Vu for her aid in figure preparation. This manuscript was supported in part by a NIH/NCI RO1CA138345 (to DL) and 5T32CA009599-21 (to KT) grants, an NFCR–Hope Fund Seed Grant (to DL), an Amschwand Foundation Seed Grant (to DL), and a Texas NF foundation grant (to IEM). MDACC cell-line characterization and electron microscopy core facilities were further supported by an NCI Cancer Center Support Grant (CA#16672)
Financial Support: This manuscript was supported in part by a NIH/NCI RO1CA138345 (to DL) and 5T32CA009599-21 (to KT) grants, an NFCR–Hope Fund Seed Grant (to DL), an Amschwand Foundation Seed Grant (to DL), and a Texas NF Foundation grant (to IEM).
Footnotes
Potential Conflict of Interest: Studies were partially supported through funds gifted to the Sarcoma Research Laboratory by Pharmacyclics, Inc., Sunnyvale, CA
References
- 1.Zou C, Smith KD, Liu J, et al. Clinical, pathological, and molecular variables predictive of malignant peripheral nerve sheath tumor outcome. Ann Surg. 2009;249:1014–1022. doi: 10.1097/SLA.0b013e3181a77e9a. [DOI] [PubMed] [Google Scholar]
- 2.Zheng H, Chang L, Patel N, et al. Induction of abnormal proliferation by nonmyelinating schwann cells triggers neurofibroma formation. Cancer Cell. 2008;13:117–128. doi: 10.1016/j.ccr.2008.01.002. [DOI] [PubMed] [Google Scholar]
- 3.Pisters PW, Leung DH, Woodruff J, Shi W, Brennan MF. Analysis of prognostic factors in 1,041 patients with localized soft tissue sarcomas of the extremities. J Clin Oncol. 1996;14:1679–1689. doi: 10.1200/JCO.1996.14.5.1679. [DOI] [PubMed] [Google Scholar]
- 4.Stoeckle E, Coindre JM, Bonvalot S, et al. Prognostic factors in retroperitoneal sarcoma. Cancer. 2001;92:359–368. doi: 10.1002/1097-0142(20010715)92:2<359::aid-cncr1331>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 5.Ducatman BS, Scheithauer BW, Piepgras DG, Reiman HM, Ilstrup DM. Malignant peripheral nerve sheath tumors. A clinicopathologic study of 120 cases. Cancer. 1986;57:2006–2021. doi: 10.1002/1097-0142(19860515)57:10<2006::aid-cncr2820571022>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 6.Evans DGR, Baser ME, McGaughran J, Sharif S, Howard E, Moran A. Malignant peripheral nerve sheath tumours in neurofibromatosis. J Med Genet. 2002;39:311–314. doi: 10.1136/jmg.39.5.311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tucker T, Wolkenstein P, Revuz J, Zeller J, Friedman JM. Association between benign and malignant peripheral nerve sheath tumors in NF1. Neurology. 2005;65:205–211. doi: 10.1212/01.wnl.0000168830.79997.13. [DOI] [PubMed] [Google Scholar]
- 8.Anghileri M, Miceli R, Fiore M, et al. Malignant peripheral nerve sheath tumors. Cancer. 2006;107:1065–1074. doi: 10.1002/cncr.22098. [DOI] [PubMed] [Google Scholar]
- 9.Wong WW, Hirose T, Scheithauer BW, Schild SE, Gunderson LL. Malignant peripheral nerve sheath tumor: analysis of treatment outcome. Int J Radiat Oncol Biol Phys. 1998;42:351–360. doi: 10.1016/s0360-3016(98)00223-5. [DOI] [PubMed] [Google Scholar]
- 10.Jones PA, Baylin SB. The fundamental role of epigenetic events in cancer. Nat Rev Genet. 2002;3:415–428. doi: 10.1038/nrg816. [DOI] [PubMed] [Google Scholar]
- 11.Kuo MH, Allis CD. Roles of histone acetyltransferases and deacetylases in gene regulation. BioEssays. 1998;20:615–626. doi: 10.1002/(SICI)1521-1878(199808)20:8<615::AID-BIES4>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 12.Marks PA, Rifkind RA, Richon VM, Breslow R, Miller T, Kelly WK. Histone deacetylases and cancer: causes and therapies. Nat Rev Cancer. 2001;1:194–202. doi: 10.1038/35106079. [DOI] [PubMed] [Google Scholar]
- 13.Marchion D, Munster P. Development of histone deacetylase inhibitors for cancer treatment. Expert Rev Anticancer Ther. 2007;7:583–598. doi: 10.1586/14737140.7.4.583. [DOI] [PubMed] [Google Scholar]
- 14.Bolden JE, Peart MJ, Johnstone RW. Anticancer activities of histone deacetylase inhibitors. Nat Rev Drug Discovery. 2006;5:769–784. doi: 10.1038/nrd2133. [DOI] [PubMed] [Google Scholar]
- 15.Glaser KB. HDAC inhibitors: Clinical update and mechanism-based potential. Biochem Pharmacol. 2007;74:659–671. doi: 10.1016/j.bcp.2007.04.007. [DOI] [PubMed] [Google Scholar]
- 16.Ma X, Ezzeldin HH, Diasio RB. Histone deacetylase inhibitors: Current status and overview of recent clinical trials. Drugs. 2009;69:1911–1934. doi: 10.2165/11315680-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 17.Lopez G, Liu J, Ren W, et al. Combining PCI-24781, a novel histone deacetylase inhibitor, with chemotherapy for the treatment of soft tissue sarcoma. Clin Cancer Res. 2009;15:3472–3483. doi: 10.1158/1078-0432.CCR-08-2714. [DOI] [PubMed] [Google Scholar]
- 18.Miller SJ, Rangwala F, Williams J, et al. Large-scale molecular comparison of human Schwann cells to malignant peripheral nerve sheath tumor cell lines and tissues. Cancer Res. 2006;66:2584–2591. doi: 10.1158/0008-5472.CAN-05-3330. [DOI] [PubMed] [Google Scholar]
- 19.Lahat G, Zhu QS, Huang KL, et al. Vimentin is a novel anti-cancer therapeutic target; insights from in vitro and in vivo mice xenograft studies. PLoS ONE. 2010;5:e10105. doi: 10.1371/journal.pone.0010105. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 20.Zou CY, Smith KD, Zhu QS, et al. Dual targeting of AKT and mammalian target of rapamycin: A potential therapeutic approach for malignant peripheral nerve sheath tumor. Mol Cancer Ther. 2009;8:1157–1168. doi: 10.1158/1535-7163.MCT-08-1008. [DOI] [PubMed] [Google Scholar]
- 21.Lee JH, Choy ML, Ngo L, Foster SS, Marks PA. Histone deacetylase inhibitor induces DNA damage, which normal but not transformed cells can repair. Proc Natl Acad Sci U S A. 2010;107:14639–14644. doi: 10.1073/pnas.1008522107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhao Y, Lu S, Wu L, et al. Acetylation of p53 at lysine 373/382 by the histone deacetylase inhibitor depsipeptide induces expression of p21Waf1/Cip1. Mol Cell Biol. 2006;26:2782–2790. doi: 10.1128/MCB.26.7.2782-2790.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Menon AG, Anderson KM, Riccardi VM, et al. Chromosome 17p deletions and p53 gene mutations associated with the formation of malignant neurofibrosarcomas in von Recklinghausen neurofibromatosis. Proc Natl Acad Sci U S A. 1990;87:5435–5439. doi: 10.1073/pnas.87.14.5435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Klionsky DJ, Abeliovich H, Agostinis P, et al. Guidelines for the use and interpretation of assays for monitoring autophagy in higher eukaryotes. Autophagy. 2008;4:151–175. doi: 10.4161/auto.5338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Levine B, Yuan J. Autophagy in cell death: An innocent convict? J Clin Invest. 2005;115:2679–2688. doi: 10.1172/JCI26390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kondo Y, Kanzawa T, Sawaya R, Kondo S. The role of autophagy in cancer development and response to therapy. Nat Rev Cancer. 2005;5:726–734. doi: 10.1038/nrc1692. [DOI] [PubMed] [Google Scholar]
- 27.Khan N, Jeffers M, Kumar S, et al. Determination of the class and isoform selectivity of small molecule HDAC inhibitors. Biochem J. 2007;409:581–589. doi: 10.1042/BJ20070779. [DOI] [PubMed] [Google Scholar]
- 28.Bethesda (MD): National Institutes of Health (US); ClinicalTrials.gov [homepage on the Internet] :c2009. [updated 2010 Feb 8; cited 2010 September 21]. Available from: http://clinicaltrials.gov/ct2/show/NCT01027910.
- 29.DeClue JE, Papageorge AG, Fletcher JA, et al. Abnormal regulation of mammalian p21(ras) contributes to malignant tumor growth in von Recklinghausen (type 1) neurofibromatosis. Cell. 1992;69:265–273. doi: 10.1016/0092-8674(92)90407-4. [DOI] [PubMed] [Google Scholar]
- 30.Basu TN, Gutmann DH, Fletcher JA, Glover TW, Collins FS, Downward J. Aberrant regulation of ras proteins in malignant tumour cells from type 1 neurofibromatosis patients. Nature. 1992;356:713–715. doi: 10.1038/356713a0. [DOI] [PubMed] [Google Scholar]
- 31.Klampfer L, Huang J, Shirasawa S, Sasazuki T, Augenlicht L. Histone deacetylase inhibitors induce cell death selectively in cells that harbor activated kRasV12: The role of signal transducers and activators of transcription 1 and p21. Cancer Res. 2007;67:8477–8485. doi: 10.1158/0008-5472.CAN-07-0210. [DOI] [PubMed] [Google Scholar]
- 32.Choudhary S, Wang HCR. Role of reactive oxygen species in proapoptotic ability of oncogenic H-Ras to increase human bladder cancer cell susceptibility to histone deacetylase inhibitor for caspase induction. J Cancer Res Clin Oncol. 2009;135:1601–1613. doi: 10.1007/s00432-009-0608-2. [DOI] [PubMed] [Google Scholar]
- 33.Bottillo I, Ahlquist T, Brekke H, et al. Germline and somatic NF1 mutations in sporadic and NF1-associated malignant peripheral nerve sheath tumours. J Pathol. 2009;217:693–701. doi: 10.1002/path.2494. [DOI] [PubMed] [Google Scholar]
- 34.Fantin VR, Richon VM. Mechanisms of resistance to histone deacetylase inhibitors and their therapeutic implications. Clin Cancer Res. 2007;13:7237–7242. doi: 10.1158/1078-0432.CCR-07-2114. [DOI] [PubMed] [Google Scholar]
- 35.Epping MT, Meijer LAT, Bos JL, Bernards R. UNC45A confers resistance to histone deacetylase inhibitors and retinoic acid. Mol Cancer Res. 2009;7:1861–1870. doi: 10.1158/1541-7786.MCR-09-0187. [DOI] [PubMed] [Google Scholar]
- 36.Klionsky DJ. Autophagy: From phenomenology to molecular understanding in less than a decade. Nat Rev Mol Cell Biol. 2007;8:931–937. doi: 10.1038/nrm2245. [DOI] [PubMed] [Google Scholar]
- 37.Hippert MM, O'Toole PS, Thorburn A. Autophagy in cancer: Good, bad, or both? Cancer Res. 2006;66:9349–9351. doi: 10.1158/0008-5472.CAN-06-1597. [DOI] [PubMed] [Google Scholar]
- 38.Ertmer A, Huber V, Gilch S, et al. The anticancer drug imatinib induces cellular autophagy. Leukemia. 2007;21:936–942. doi: 10.1038/sj.leu.2404606. [DOI] [PubMed] [Google Scholar]
- 39.Iwata A, Riley BE, Johnston JA, Kopito RR. HDAC6 and microtubules are required for autophagic degradation of aggregated Huntingtin. J Biol Chem. 2005;280:40282–40292. doi: 10.1074/jbc.M508786200. [DOI] [PubMed] [Google Scholar]
- 40.Carew JS, Giles FJ, Nawrocki ST. Histone deacetylase inhibitors: Mechanisms of cell death and promise in combination cancer therapy. Cancer Lett. 2008;269:7–17. doi: 10.1016/j.canlet.2008.03.037. [DOI] [PubMed] [Google Scholar]
- 41.Shao Y, Gao Z, Marks PA, Jiang X. Apoptotic and autophagic cell death induced by histone deacetylase inhibitors. Pro Natl Acad Sci U S A. 2004;101:18030–18035. doi: 10.1073/pnas.0408345102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hrzenjak A, Kremser ML, Strohmeier B, Moinfar F, Zatloukal K, Denk H. SAHA induces caspase-independent, autophagic cell death of endometrial stromal sarcoma cells by influencing the mTOR pathway. J Pathol. 2008;216:495–504. doi: 10.1002/path.2434. [DOI] [PubMed] [Google Scholar]
- 43.Carew JS, Nawrocki ST, Kahue CN, et al. Targeting autophagy augments the anticancer activity of the histone deacetylase inhibitor SAHA to overcome Bcr-Abl-mediated drug resistance. Blood. 2007;110:313–322. doi: 10.1182/blood-2006-10-050260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Maiuri MC, Zalckvar E, Kimchi A, Kroemer G. Self-eating and self-killing: Crosstalk between autophagy and apoptosis. Nat Rev Mol Cell Biol. 2007;8:741–752. doi: 10.1038/nrm2239. [DOI] [PubMed] [Google Scholar]
- 45.Solomon VR, Lee H. Chloroquine and its analogs: A new promise of an old drug for effective and safe cancer therapies. Eur J Pharmacol. 2009;625:220–233. doi: 10.1016/j.ejphar.2009.06.063. [DOI] [PubMed] [Google Scholar]
- 46.Chen N, Debnath J. Autophagy and tumorigenesis. FEBS Lett. 2010;584:1427–1435. doi: 10.1016/j.febslet.2009.12.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Singh SB, Davis AS, Taylor GA, Deretic V. Human IRGM induces autophagy to eliminate intracellular mycobacteria. Science. 2006;313:1438–1441. doi: 10.1126/science.1129577. [DOI] [PubMed] [Google Scholar]
- 48.Yu C, Wang L, Lv B, et al. TMEM74, a lysosome and autophagosome protein, regulates autophagy. Biochem Biophys Res Commun. 2008;369:622–629. doi: 10.1016/j.bbrc.2008.02.055. [DOI] [PubMed] [Google Scholar]
- 49.Espert L, Denizot M, Grimaldi M, et al. Autophagy is involved in T cell death after binding of HIV-1 envelope proteins to CXCR4. J Clin Invest. 2006;116:2161–2172. doi: 10.1172/JCI26185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Burger JA, Peled A. CXCR4 antagonists: targeting the microenvironment in leukemia and other cancers. Leukemia. 2008;23:43–52. doi: 10.1038/leu.2008.299. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.