Abstract
Estrogens are known to cause hyperprolactinemia, most probably by acting on the tuberoinfundibular dopaminergic (TIDA) system of the hypothalamus. Dopamine (DA) produced by TIDA neurons directly inhibits prolactin secretion and, therefore, to stimulate prolactin secretion, estrogens inhibit TIDA neurons to decrease DA production. However, the mechanism by which estrogen produces this effect is not clear. In the present study, we used a paradigm involving chronic exposure to low levels of estradiol-17β (E2) to mimic prolonged exposures to environmental and endogenous estrogens. We hypothesized that chronic exposure to low levels of E2 induces oxidative stress in the arcuate nucleus (AN) of the hypothalamus that contains TIDA neurons and causes nitration of tyrosine hydroxylase (TH), the rate-limiting enzyme in the synthesis of DA. This results in a significant decrease in DA and consequently, hyperprolactinemia. To investigate this, adult, intact female cycling rats were implanted with slow-release E2 pellets (20 ng/day) for 30, 60, or 90 days and were compared with old (16–18 mo old) constant estrous (OCE) rats. Chronic E2 exposure significantly increased the expression of glial fibrillary acidic protein and the concentrations of interleukin-1β (IL-1β) and nitrate in the AN that contains perikarya of TIDA neurons and increased nitration of TH in the median eminence (ME) that contains the terminals. These levels were comparable to those seen in OCE rats. We observed a significant decrease in DA concentrations in the ME and hyperprolactinemia in an exposure-dependent manner similar to that seen in OCE rats. It was concluded that chronic exposure to low levels of E2 evokes oxidative stress in the AN to inhibit TIDA neuronal function, most probably leading to hyperprolactinemia.
Keywords: prolactin, nitration, tyrosine hydroxylase
estrogens are pleiotropic hormones and have been shown to have several beneficial effects: Estrogen therapy can prevent bone loss (27) and decrease the risk of coronary disease (45). Estrogens can also provide neuroprotection during ischemic brain injury and Alzheimer's disease and are believed to improve memory in postmenopausal women (13, 40, 43). In rats, both acute (5) and subchronic treatment (16, 23) with moderate to high doses of estradiol are known to increase prolactin (PRL) levels. PRL levels also increase during aging, specifically after rats have been in the constant estrous state for 4 or 5 mo (10). This increase in PRL levels is known to promote mammary and pituitary tumor formation (15, 17). Estrogen is known to act directly on the pituitary gland and also inhibit hypothalamic dopamine (DA) to stimulate PRL release (10). While several studies have examined the mechanism by which estrogen acts on the pituitary gland (9, 38), the possible molecular mechanism by which estrogen decreases hypothalamic DA to inhibit PRL levels is not clear.
The purpose of this study was to understand the mechanisms by which hypothalamic DA levels are reduced after chronic exposure to estradiol at levels comparable to that seen during proestrus. The tuberoinfundibular dopaminergic (TIDA) system is one of the most estrogen-sensitive neuronal systems in the hypothalamus (37), and estrogen's effects are known to be mediated through estrogen receptor-α (ER-α) (41). The cell bodies of TIDA neurons are located in the arcuate nucleus (AN), and their terminals reach the median eminence (ME). DA released from these terminals acts on the lactotrophs in the anterior pituitary to inhibit PRL secretion (3). A reduction in TIDA neuronal function results in decreased DA synthesis and causes hyperprolactinemia (29). This has been implicated in the development of mammary and pituitary tumors in aging animals (28, 29) and more recently in humans (11).
Although estrogens are known to impact DA levels in the hypothalamus (33), the mechanism by which chronic estradiol exposure reduces TIDA neuronal activity is unclear. In this study, we propose a novel hypothesis that involves the induction of the cytokine, interleukin-1β (IL-1β) in the AN after chronic estradiol exposure. We chose this cytokine over others because IL-1β levels are known to increase in the brain in response to stressors (12). We propose that IL-1β causes the generation of nitric oxide-related free radicals that promote nitration of tyrosine hydroxylase (TH), a key enzyme involved in DA synthesis. This would result in decreased DA production and elevated PRL levels. To test this, we used female Sprague-Dawley rats and treated them for 30, 60, or 90 days with low doses of estradiol-17β (E2) that mimic endogenous levels of estrogen. Old rats that were in constant estrus were used for comparison purposes to determine whether a similar phenomenon is operating in aging animals.
MATERIALS AND METHODS
Animals
Female Sprague-Dawley rats (4–5 mo) were purchased from Harlan Sprague Dawley (Indianapolis, IN) and housed in air-conditioned (23 ± 1°C), and light-controlled (lights on from 0500 to 1900) animal quarters. Rats were provided food and water ad libitum. Estrous cyclicity was determined in young (3–4 mo old) and old (16–18 mo old) rats by vaginal cytology (24). Young animals showing 4- to 5-day regular estrous cycles and old animals that exhibited estrous smears for more than 10 consecutive days (old constant estrous, OCE) were used in the experiments. All of the animal care and treatment protocols were approved by, and were followed in accordance with, the Michigan State University Institutional Animal Care and Use Committee.
Treatment
A large cohort (n = 30–40) of regularly cycling adult female rats (4–5 mo old) were either sham-implanted (control) or implanted with estradiol-17β (E2; 20 ng/day) slow-release pellets (Innovative Research America, Sarasota, FL) for a period of 30 (E-30), 60 (E-60), or 90 (E-90) days. Control animals were age-matched to the E-90 group. A group of OCE rats (n = 8–12) were used for comparison. Animals were checked for the presence of mammary tumors by periodic physical examination. Treatment with E2 for 30, 60, or 90 days did not induce mammary tumors. About 20% of the older animals had palpable mammary tumors and were excluded from the study. Vaginal cytology was performed for the large cohort. Most animals in the E-30 (80%) and control groups (95%) cycled regularly; they were euthanized when they were in estrus at 1200. Most of the animals in E-60 and E-90 groups were in the state of constant estrus and were also euthanized at 1200. OCE rats were killed at 1200 for comparison. Upon death, the brains were quickly removed, frozen on dry ice, and stored at −70°C until the time of sectioning. Serum was separated from trunk blood and stored at −70°C until they were used for hormone assays. From the large cohort, tissues and serum from a subset (n = 7) were used for measuring the following parameters.
Radioimmunoassay for Hormones
E2 and PRL levels in the serum were measured in duplicate by double antibody radioimmunoassay (RIA). The RIA kit for E2 was obtained from Diagnostic Products (Los Angeles, CA). The assays were performed according to the manufacturer's instructions. The standards and antibody for rat PRL were obtained from Dr. A. F. Parlow, National Hormone and Peptide Program, National Institute of Diabetes and Digestive and Kidney Diseases. The PRL tracer was obtained from Amersham Pharmacia Biotech (Waukesha, WI). The assay was performed as described previously (24, 30).
Palkovits' Microdissection
The brains were mounted and serial sections of 300-μm thickness were obtained using a cryostat (Slee, London, UK) maintained at −10°C as described before (31, 32). The sections were transferred to glass slides placed on a cold stage set at −10°C. The AN and ME were located with the help of a rat brain stereotaxic atlas (35) and microdissected using the Palkovits' microdissection technique (32, 34). Tissue samples of the ME were removed first, and the AN samples were obtained bilaterally using a 250-μm punch. They were stored at −70°C until they were analyzed for various factors.
Measurement of Glial Fibrillary Acidic Protein and IL-1β in Brain Homogenates
Glial fibrillary acidic protein (GFAP) levels in the AN were measured using a sandwich ELISA based on an assay developed by O'Callaghan (1991). Briefly, Immulon-2 flat-bottomed microtiter plates were coated with anti-GFAP N-18 (Santa Cruz, cat. no. sc-6171, goat polyclonal; 1:100 diluted in I Ab dilution buffer) for 1 h at 37°C and overnight at 4°C. After washing with PBS, the next day, the wells were blocked for an hour at room temperature and washed. Standards and samples were added in duplicate and incubated for 1 h at 37°C. Premixed anti-GFAP antibody (DAKO, Carpinteria, CA) was used as the second antibody and incubated for 1 h at 37°C. After washing, an anti-rabbit IgG horseradish peroxidase conjugate (cat. no. A1949; Sigma) was added at 1:3,000 dilution and incubated for 1 h at 37°C. Premixed TMB solution was used as the developing reagent. The reaction was stopped with 2N HCl, and the plate was read at 450 nm using an ELISA reader. The rat IL-1β ELISA kit was purchased from Biosource International (Camarillo, CA). The protocol supplied by the manufacturer was used to perform this assay.
Measurement of Nitric Oxide Generation in the AN
A commercially available kit (total nitric oxide assay kit; Assay Designs, Ann Arbor, MI) that involved the Griess reaction was used to measure total nitrate levels in the AN. Nitric oxide (NO), by nature, is transient and volatile and cannot be measured easily. However, its two stable breakdown products, nitrate and nitrite, can be measured colorimetrically. Tissue samples contain both nitrate and nitrite. To measure the total NO generated, nitrate is enzymatically converted to nitrite by nitrate reductase, and this is measured as a colored azo dye product. The assay quantifies the total NO produced and has been used to measure NO generation by microglial cells in mesencephalic cultures (44).
Immunoprecipitation of Tyrosine Hydroxylase and Detection of Nitrotyrosine Residues by Western Blot Analysis
Immunoprecipitation of TH was carried out as described by Ara et al. (2). Microdissected ME tissue samples were homogenized in 50-μl cell lysis buffer (composition: 20 mM Tris·HCl, 150 mM NaCl, 4 mM EGTA, 10% glycerol, 1% Triton X-100, 1 mM PMSF, 0.2 mM sodium orthovanadate, pH 7.4) and incubated with 0.5 μg of anti-TH antibody (Chemicon, Temecula, CA) for 12 h at 4°C. Protein-A agarose slurry (100 μl; KPL, Gaithersburg, MD) was added to the mixture and incubated at 4°C for 1.5 h. The mixture containing the antigen:antibody complex was centrifuged at 14,000 rpm for 10 min, and 30 μl of the elution buffer (0.2 M glycine, pH 3) was added to the pellet, mixed, and left on ice for 5 min. The mixture was centrifuged at high speed for 10 min, and 10 μl of the supernatant containing TH was loaded onto two separate 20% SDS-PAGE and electrophoresed at 70 V for 1.5 h. Gels were electroblotted onto nitrocellulose membranes for 30 min at 22 V. Membranes were immersed in blocking solution, probed with primary antibody [anti-TH, 1:1,000 dilution (Chemicon) or anti-nitrotyrosine, 1:1,000 dilution (Sigma, Saint Louis, MO)] overnight, and exposed to anti-rabbit- IgG tagged with horseradish peroxidase (Sigma), for 4 h. Bands were visualized using 4-chloro-1-naphthol (Bio-Rad, Hercules, CA). Pixel intensities were determined by densitometric scanning using a Kodak Digital Science Image analysis system (Kodak, Rochester, NY).
Measurement of DA Concentrations
ME homogenates were analyzed for DA concentrations using HPLC-EC as described before (31, 32). Briefly, the HPLC-EC system consisted of the following: a phase II, 5-μm ODS reverse-phase C-18 column (Phenomenex, Torrance, CA), a glassy carbon electrode, a CTO-10 AT/VP column oven, a LC-10 AT/VP pump (Shimadzu, Columbia, MD), and a LC-4C amperometric detector (Bioanalytical Systems, West Lafayette, IN). The mobile phase was filtered and degassed through a Milli-Q purification system (Millipore, Bedford, MA) and pumped at a flow rate of 1.8 ml/min. The sensitivity of the detector was 1 nA full scale, and the potential of the working electrode was 0.65 V. The column was maintained at a temperature of 37°C. At the time of analysis, ME punches were homogenized in 150 μl of 0.1 M perchloric acid. After separating the homogenates for protein analysis, they were centrifuged at 14,000 g for 5 min. One hundred microliters of the supernatant along with the 30 μl of 0.05 M dihydroxy benzylamine (internal standard) was injected into the HPLC system using an autoinjector. DA concentrations were determined using the Class-VP software ver. 7.2. The sensitivity of the system was <1 pg.
Protein Assay
Protein concentrations in the AN and ME homogenates were determined using a micro bicinchoninic acid assay (Pierce, Rockford, IL). Concentrations of DA, GFAP, IL-1β, and NO were expressed in terms of protein concentrations.
Statistical Analysis
Differences in serum E2 and PRL; DA concentrations in the ME, GFAP, IL-1β, NO concentrations in the AN; and the ratio of TH to nitrated TH in the ME were analyzed using one-way ANOVA followed by Fisher's least significant difference test.
RESULTS
Effects of Chronic E2 Exposure on Estrous Cyclicity and Serum E2
Estrous cyclicity.
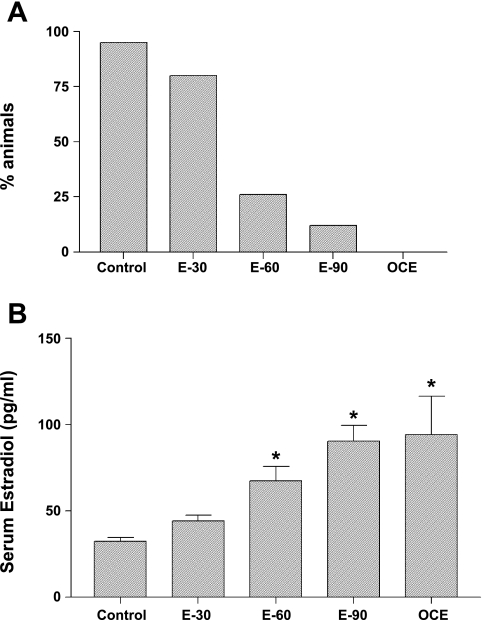
Effects of E2 exposure on estrous cyclicity is shown in Fig. 1A. All the sham-implanted (control) animals had regular 4- to 5-day estrous cycles. Exposure to 30 days of E2 did not affect estrous cyclicity, and 80% of the animals exhibited regular estrous cycles. In contrast, treatment with E2 for 60 or 90 days resulted in 74% and 88% of the animals becoming acyclic, and these animals were in the state of constant estrus. All of the OCE animals were in the state of constant estrus.
Fig. 1.
A: effects of chronic E2 exposure on estrous cyclicity indicated as % of animals that showed regular estrous cycles. Adult young (4- to 5-mo old) sham implanted (control) and 30 (E-30), 60 (E-60), or 90 (E-90)-day slow release E2 pellets implanted rats and old constant estrous (OCE) rats that are 15- to 19-mo old were used. B: serum E2 levels (means ± SE; pg/ml) in young (4- to 5-mo old) sham implanted (control) and 30 (E-30), 60 (E-60), or 90 (E-90) day slow-release E2 pellet-implanted rats and OCE rats that are 16- to 18-mo old. *Significant difference from control animals (P < 0.05).
Serum estradiol.
Effects of chronic E2 exposure on serum E2 levels are shown in Fig. 1B. Serum E2 levels (pg/ml; means ± SE) in control animals were 32.3 ± 2.3, and exposure to E2 for 30 days did not alter these levels. However, E2 exposure for 60 or 90 days resulted in a significant increase in serum E2 levels (67.2 ± 8.4 and 90.2 ± 9.2 in E-60 and E-90 groups, respectively) (P < 0.05). This is most likely due to the presence of cystic follicles in the ovaries of these animals, as previously reported (24). Similarly, serum E2 levels in OCE rats (94.2 ± 22) were significantly higher compared with control and E-30 rats (P < 0.05).
GFAP and IL-1β Levels in the AN
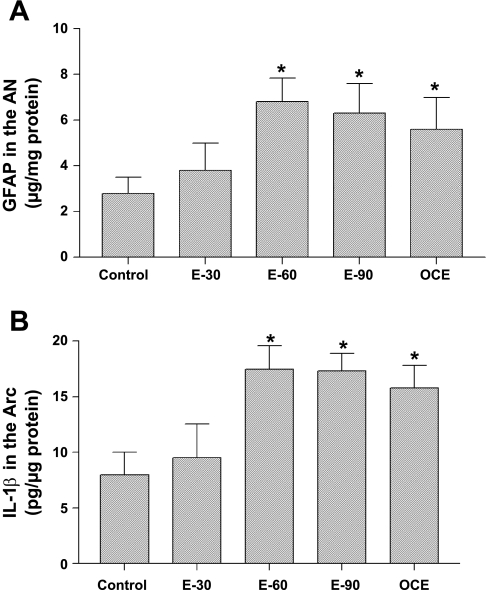
In control animals, GFAP concentrations in the AN (μg/mg protein; Fig. 2A) remained low at 3 ± 0.7. Treatment with E2 for 30 days did not alter GFAP levels. In contrast, treatment with 60 or 90 days of E2 increased GFAP levels to 6.8 ± 1 and 6.3 ± 1, respectively (P < 0.05). GFAP levels in OCE animals were also significantly high (5.6 ± 1.4) compared with the control group (P < 0.05).
Fig. 2.
A: glial fibrillary acidic protein (GFAP) concentrations (means ± SE; μg/mg protein) in the arcuate nucleus (AN) of sham-implanted (control) and 30, 60, or 90 day E2 pellet-implanted (E-30, E-60, and E-90) and OCE rats. *Significant difference from control animals (P < 0.05). B: interleukin-1β (IL-1β) concentrations (means ± SE; pg/μg protein) in the AN of sham-implanted (control) and 30 (E-30), 60 (E-60), or 90 (E-90) day slow-release E2 pellet-implanted rats and OCE rats that are 16- to 18-mo old. *Significant difference from control animals (P < 0.05).
Chronic exposure to E2 produced a similar effect on IL-1β levels in the AN (Fig. 2B). IL-1β concentrations in 30-day E2-treated rats (9.5 ± 3.0) were similar to that seen in control animals (8.0 ± 2). In contrast, exposure to E2 for 60 or 90 days increased IL-1β concentrations to 17.5 ± 2.1 and 17.3 ± 1.6, respectively (P < 0.05), that was similar to that seen in OCE rats (15.8 ± 2; P < 0.05).
Total NO Levels in the AN and TH and Nitrated TH Levels in the ME
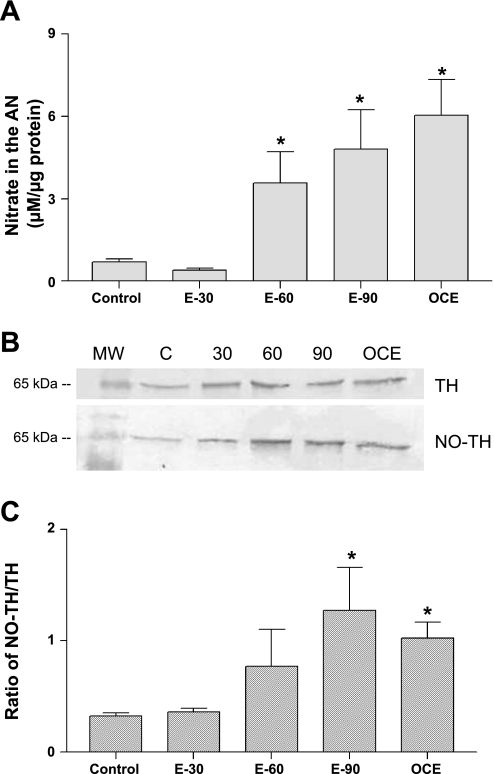
Total NO levels in the AN measured in terms of nitrite concentrations (means ± SE; μM/μg protein) of E2-treated and OCE animals are given in Fig. 3A. Exposure to E2 for 30 days did not alter NO levels in the AN compared with control rats. In contrast, E2 treatment for 60 or 90 days increased NO levels in the AN to 3.6 ± 1.1 and 4.8 ± 1.5, respectively, compared with control rats (0.7 ± 0.1; P < 0.05). NO levels in the AN of OCE rats was also significantly higher compared with control rats (P < 0.05).
Fig. 3.
A: total nitrate concentrations (means ± SE; μM/μg protein) in the median eminence (ME) of sham (control) and 30 (E-30), 60 (E-60), or 90 (E-90) day E2 pellet implanted and OCE rats. *Significant difference from control animals (P < 0.05). B: representative Western blots of tyrosine hydroxylase (TH) and nitrated TH in the ME of sham-implanted (control) and 30 (E-30), 60 (E-60), or 90 (E-90)-day slow-release E2 pellet-implanted rats and OCE rats that are 16- to 18-mo old. C: ratio of nitrated TH to TH in the ME of sham implanted (control) and 30 (E-30), 60 (E-60), or 90 (E-90) day E2 pellet-implanted and OCE rats. The intensities of the bands were calculated by measuring the pixel intensities that were determined using densitometric scanning with a Kodak Digital Science Image analysis system. *Significantly different from control animals (P < 0.05).
Fig. 3B shows representative Western blots of TH and nitrated TH in control, E2-treated, and OCE rats in the ME. The ratio of densities of nitrated TH to TH is shown in Fig. 3C. As shown in the figure, E2 increases the ratio of nitrated TH to TH in a duration-dependent manner. The ratio of nitrated TH to TH also increased significantly in OCE rats compared with control rats.
DA Concentrations in the ME and Serum PRL
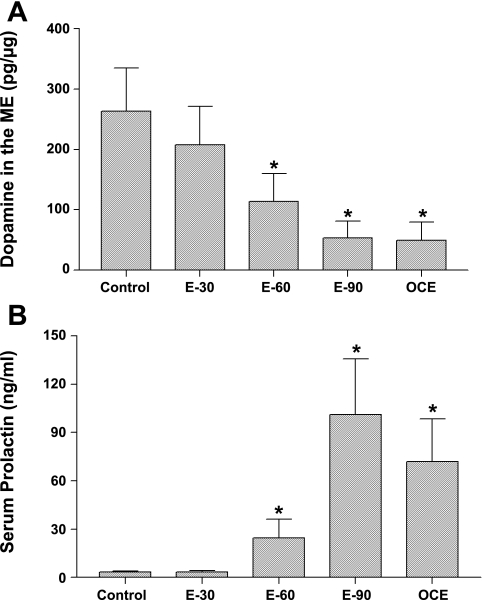
DA concentrations in the ME (means ± SE; pg/μg protein) in control, E2-treated and OCE animals are shown in Fig. 4A. DA concentrations in the ME were 263.5 ± 72.0 in control animals and treatment with E2 for 30 days did not alter DA levels in the ME. In contrast, exposure to E2 for 60 or 90 days decreased DA concentrations significantly to 114.2 ± 46 and 53.2 ± 28, respectively (P < 0.05). DA concentrations in the ME of OCE rats were also significantly lower (49.7 ± 30) compared with control rats (P < 0.05).
Fig. 4.
A: dopamine (DA) concentrations (means ± SE; pg/μg protein) in the ME of sham-implanted (control) and 30, 60, or 90 day E2 pellet implanted (E-30, E-60, and E-90) and OCE rats. *Significant difference from control animals (P < 0.05). B: serum prolactin (PRL) concentrations (means ± SE; ng/ml) in sham-implanted (control) and 30 (E-30), 60 (E-60), or 90 (E-90) day slow-release E2 pellet-implanted rats and OCE rats that are 16- to 18-mo old. *Significant difference from control animals (P < 0.05).
Serum PRL concentrations (means ± SE; ng/ml; Fig. 4B) in the control group was 3.5 ± 0.4. Exposure to E2 for 30 days did not alter PRL levels. In contrast, exposure to E2 for 60 days produced a modest increase in PRL levels (24.5 ± 11.7) and exposure for 90 days further increased PRL levels to 101.1 ± 34.7 (P < 0.05). Similarly, serum PRL levels were elevated in OCE rats (71.9 ± 26.6) compared with animals in the control, E-30, and E-60 groups (P < 0.05).
DISCUSSION
Estrogens are one of the major factors that are known to promote hyperprolactinemia (6–8). Several estrogenic preparations, including estradiol benzoate (22), estradiol valerate (5), and estradiol 17β (25) are known to stimulate PRL secretion. This is known to be brought about by either a direct stimulatory action on the pituitary gland (1) and through a reduction in hypothalamic DA, especially DA that is produced by TIDA neurons (37). The mechanism by which estradiol-17β affects TIDA neurons to increase serum PRL levels has not been studied in detail. Results from this study demonstrate a novel possibility that estrogens could increase proinflammatory cytokines to stimulate the production of NO-related free radical production in the AN that would, in turn, cause nitration of TH and decrease DA synthesis in the ME. In the following paragraphs, we will explain how this cascade of events could take place.
DA synthesized by TIDA neurons is the primary regulator of PRL secretion. As described earlier, cell bodies of TIDA neurons are located in the AN, and their terminals extend to the ME. DA released from these terminals passes through the portal vessels and acts on the lactotrophs in the pituitary to inhibit PRL secretion (3). Several studies indicate that TIDA neurons are sensitive to estrogen. E2 has been shown to bind to TIDA neurons (26), most probably through ERα (41). Moreover, acute estrogen treatment decreases TH mRNA levels in TIDA neurons, suggesting that it can act directly on these neurons to decrease DA synthesis (36). However, this does not explain the reduction in DA levels observed with chronic exposures or aging. The results from this study indicate that E2 produces a duration-dependent reduction in DA synthesis and hyperprolactinemia that is very comparable to changes observed in aging and that these changes are accompanied by gradual alterations in glial-neuron interactions as described below.
We hypothesized that chronic exposure to low levels of E2 causes gliosis in the AN that up-regulates the production of proinflammatory cytokines and NO. This is, in fact, supported by a few studies that have described degenerative changes in the AN after animal exposure to various forms of estrogen (5–7). These changes have been marked by the presence of reactive microglial cells and astrocytes (4). In the present study, chronic exposure to E2 increases the levels of GFAP, a marker for astrocytes, in a duration-dependent manner, indicating that E2 exposure does activate astrocytes in the AN. The changes, observed in E2-treated young animals, are very similar to what is observed in OCE rats. Aging has been shown to increase the number of microglia and astrocytic granules in the AN of female rats that could be prevented by ovariectomy (39), suggesting that ovarian steroids, most probably estrogen, play an important role in this phenomenon. Moreover, estrogen receptors are expressed in different types of glial cells (34), suggesting the possibility that estrogen can act on glial cells. Young rats supplemented with estrogen are known to have similar changes in glia in the AN mimicking the glial hyperactivity observed in aging rats (5). The reactivity of astrocytes to estrogen exposure could translate into increased cytokine production as explained below.
Astrocytes and glial cells are capable of producing many cytokines, including IL-1β (8). In the present study, activation of astrocytes by E2 exposure was accompanied by an increase in the production of IL-1β. This increase was duration dependent, suggesting that astrocytes continue to react to E2 exposure for extended periods of time. Cytokines, in turn, are known to cause elevation of inducible nitric oxide synthase, nitrite, and associated free radicals in the hypothalamus (18, 42). Our results demonstrate that chronic exposure to E2 increases the concentrations of nitrate, a stable product of the nitric oxide metabolism, in the AN in a duration-dependent manner. We hypothesize that the increases in IL-1β and nitrate probably play a critical role in decreasing TIDA neuronal activity. A recent study demonstrated that intracerebroventricular injections of LPS increased IL-1β production by microglial cells in the AN. This led to a reduction in TH activity and TH mRNA by 6 h and increased PRL levels by 12 h (14). A similar phenomenon could be in operation with E2 exposure.
There is evidence to indicate that an increase in NO-related free radicals can increase nitration of tyrosine residues on TH, the rate-limiting enzyme in DA biosynthesis (19, 21). Peroxynitrite, one of the products of NO metabolism, facilitates the formation of nitrotyrosine, which results in nitration of tyrosine residues in proteins (19, 21). Nitration of tyrosine residues is one of the important hallmarks of NO-induced pathology and can lead to inactivation of protein function, particularly of proteins like TH that contain several tyrosine residues in their structure (2). The tyrosine moieties in TH are clustered around the active site of the enzyme (20). Nitration of the tyrosine residues in TH causes steric hindrance and inhibits the activity of this enzyme (20). In the present study, the ratio of nitrated TH to TH increased significantly after chronic E2 exposure. Thus, it is possible that E2-induced increase in NO metabolism probably caused nitration of tyrosine residues in TH. This most likely contributed to the reduction in DA levels in the ME and elevations and the increase in serum PRL levels that were observed in this study.
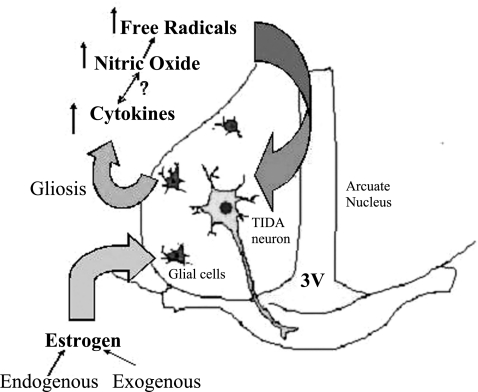
In conclusion, results from this study provide evidence that chronic exposure to E2 activates astrocytes in the AN, resulting in elevated levels of IL-1β and NO-related free radical production. This increase in oxidative stress is probably responsible for the nitration of tyrosine residues of TH observed in these animals. Nitration of TH, as mentioned before, is believed to be one of the hallmarks of NO-induced pathology. Thus, it is possible that estrogen-induced increase in NO production could be responsible for reduction in TIDA neuronal function (fig. 5). The changes observed in young animals after prolonged E2 exposure was similar to what was observed in OCE rats. Hyperprolactinemia and the development of spontaneous mammary and pituitary tumors are commonly observed in aging rats, especially after they have been in constant estrus for 4–6 mo (37). Therefore, the results from this study provide important clues about how prolonged exposure to endogenous estrogens could decrease DA levels to produce hyperprolactinemia during aging.
Fig. 5.
Figure depicting the possible mechanism by which chronic E2 exposure decreases tuberoinfundibular dopaminergic (TIDA) activity to cause hyperprolactinemia. Chronic estradiol exposure activates glial cells in the AN and increases the production of cytokines. This stimulates NO-related free radical production that causes nitration of TH. This leads to a reduction in DA synthesis and an increase in PRL.
Although this study appears to link independent molecular and cellular changes that occur in the AN after estrogen exposure, it nevertheless suggests a possible mechanism for E2 effects and aging-induced decreases in TIDA neuronal activity and hyperprolactinemia. Mechanistic studies are in progress to provide a cause-and-effect relationship between these different molecular and cellular components of this cascade.
Perspectives and Significance
Estrogen exposure is known to inhibit TIDA neuronal function but the mechanisms are not clear. Results from this study suggest that chronic exposure to low levels of estradiol can increase IL-1β and nitric oxide-related free radicals in the hypothalamus. There was an associated increase in the nitration of tyrosine hydroxylase, and a reduction in dopamine levels. This could be a possible mechanism by which chronic exposures to low levels of estrogenic compounds can impact the TIDA system.
GRANTS
This work was supported by National Institutes of Health AG027697, National Science Foundation IBN0236385, and the Michigan Agricultural Experiment Station.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
ACKNOWLEDGMENT
The authors would like to thank Ms. Katrina Linning for editorial assistance.
REFERENCES
- 1. Aguilar R, Bellido C, Garrido-Gracia JC, Alonso R, Sanchez-Criado JE. Estradiol and its membrane-impermeable conjugate estradiol-BSA inhibit tamoxifen-stimulated prolactin secretion in incubated rat pituitaries. Reproduction 131: 763–769, 2006 [DOI] [PubMed] [Google Scholar]
- 2. Ara J, Przedborski S, Naini AB, Jackson-Lewis V, Trifiletti RR, Horwitz J, Ischiropoulos H. Inactivation of tyrosine hydroxylase by nitration following exposure to peroxynitrite and 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP). Proc Natl Acad Sci USA 95: 7659–7663, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ben-Jonathan N, Arbogast LA, Hyde JF. Neuroendocrine [corrected] regulation of prolactin release. Prog Neurobiol 33: 399–447, 1989 [DOI] [PubMed] [Google Scholar]
- 4. Brawer JR, Beaudet A, Desjardins GC, Schipper HM. Pathologic effect of estradiol on the hypothalamus. Biol Reprod 49: 647–652, 1993 [DOI] [PubMed] [Google Scholar]
- 5. Brawer JR, Naftolin F, Martin J, Sonnenschein C. Effects of a single injection of estradiol valerate on the hypothalamic arcuate nucleus and on reproductive function in the female rat. Endocrinology 103: 501–512, 1978 [DOI] [PubMed] [Google Scholar]
- 6. Brawer JR, Ruf KB, Naftolin F. Effects of estradiol-induced lesions of the arcuate nucleus on gonadotropin release in response to preoptic stimulation in the rat. Neuroendocrinology 30: 144–149, 1980 [DOI] [PubMed] [Google Scholar]
- 7. Brawer JR, Schipper H, Naftolin F. Ovary-dependent degeneration in the hypothalamic arcuate nucleus. Endocrinology 107: 274–279, 1980 [DOI] [PubMed] [Google Scholar]
- 8. Chao CC, Hu S, Ehrlich L, Peterson PK. Interleukin-1 and tumor necrosis factor-alpha synergistically mediate neurotoxicity: involvement of nitric oxide and of N-methyl-d-aspartate receptors. Brain Behav Immun 9: 355–365, 1995 [DOI] [PubMed] [Google Scholar]
- 9. Chaturvedi K, Sarkar DK. Alteration in G proteins and prolactin levels in pituitary after ethanol and estrogen treatment. Alcoholism Clin Exp Res 32: 806–813, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Clemens JA, Meites J. Neuroendocrine status of old constant-estrous rats. Neuroendocrinology 7: 249–256, 1971 [DOI] [PubMed] [Google Scholar]
- 11. Clevenger CV, Furth PA, Hankinson SE, Schuler LA. The role of prolactin in mammary carcinoma. Endocr Rev 24: 1–27, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Deak T, Bordner KA, McElderry NK, Barnum CJ, Blandino P, Jr, Deak MM, Tammariello SP. Stress-induced increases in hypothalamic IL-1: a systematic analysis of multiple stressor paradigms. Brain Res Bull 64: 541–556, 2005 [DOI] [PubMed] [Google Scholar]
- 13. Dubal DB, Kashon ML, Pettigrew LC, Ren JM, Finklestein SP, Rau SW, Wise PM. Estradiol protects against ischemic injury. J Cereb Blood Flow Metab 18: 1253–1258, 1998 [DOI] [PubMed] [Google Scholar]
- 14. Gonzalez MC, Abreu P, Barroso-Chinea P, Cruz-Muros I, Gonzalez-Hernandez T. Effect of intracerebroventricular injection of lipopolysaccharide on the tuberoinfundibular dopaminergic system of the rat. Neuroscience 127: 251–259, 2004 [DOI] [PubMed] [Google Scholar]
- 15. Gottschall PE, Meites J. Evidence for a permanent decline in tuberoinfundibular dopaminergic neuronal function after chronic estrogen treatment is terminated in Fischer 344 rats. Neuroendocrinology 44: 211–216, 1986 [DOI] [PubMed] [Google Scholar]
- 16. Gottschall PE, Sarkar DK, Meites J. Persistence of low hypothalamic dopaminergic activity after removal of chronic estrogen treatment. Proc Soc Exp Biol Med 181: 78–86, 1986 [DOI] [PubMed] [Google Scholar]
- 17. Goya RG, Lu JK, Meites J. Gonadal function in aging rats and its relation to pituitary and mammary pathology. Mech Ageing Dev 56: 77–88, 1990 [DOI] [PubMed] [Google Scholar]
- 18. Hu S, Peterson PK, Chao CC. Cytokine-mediated neuronal apoptosis. Neurochem Int 30: 427–431, 1997 [DOI] [PubMed] [Google Scholar]
- 19. Huie RE, Padmaja S. The reaction of no with superoxide. Free Radic Res Commun 18: 195–199, 1993 [DOI] [PubMed] [Google Scholar]
- 20. Imam SZ, el-Yazal J, Newport GD, Itzhak Y, Cadet JL, Slikker W, Jr, Ali SF. Methamphetamine-induced dopaminergic neurotoxicity: role of peroxynitrite and neuroprotective role of antioxidants and peroxynitrite decomposition catalysts. Annals N Y Acad Sci 939: 366–380, 2001 [DOI] [PubMed] [Google Scholar]
- 21. Ischiropoulos H, Duran D, Horwitz J. Peroxynitrite-mediated inhibition of DOPA synthesis in PC12 cells. J Neurochem 65: 2366–2372, 1995 [DOI] [PubMed] [Google Scholar]
- 22. Jaques S, Jr, Gala RR. The influence of oestrogen administration in vivo on in vitro prolactin release Interaction between dopamine and thyrotrophin releasing hormone. Acta Endocrinol 92: 437–447, 1979 [DOI] [PubMed] [Google Scholar]
- 23. Jeng YJ, Kochukov M, Nauduri D, Kaphalia BS, Watson CS. Subchronic exposure to phytoestrogens alone and in combination with diethylstilbestrol - pituitary tumor induction in Fischer 344 rats. Nutr Metab 7: 40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kasturi BS, MohanKumar SM, Sirivelu MP, MohanKumar PS. Chronic exposure to low levels of oestradiol-17beta affects oestrous cyclicity, hypothalamic norepinephrine and serum luteinising hormone in young intact rats. J Neuroendocrinol 21: 568–577, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Lawson DM. Evidence for a rapid in vivo effect on estradiol-17 beta on prolactin secretion in ovariectomized rats. Endocr Res Commun 6: 135–148, 1979 [DOI] [PubMed] [Google Scholar]
- 26. Maeda T, Ikegami H, Sakata M, Yamaguchi M, Wada K, Koike K, Adachi K, Kurachi H, Hirota K, Miyake A. Intraventricular administration of estradiol modulates rat prolactin secretion and synthesis. J Endocrinol Invest 19: 586–592, 1996 [DOI] [PubMed] [Google Scholar]
- 27. Meema S, Bunker ML, Meema HE. Preventive effect of estrogen on postmenopausal bone loss. Arch Intern Med 135: 1436–1440, 1975 [PubMed] [Google Scholar]
- 28. Meites J. Aging: hypothalamic catecholamines, neuroendocrine-immune interactions, and dietary restriction. Proc Soc Exp Biol Med 195: 304–311, 1990 [DOI] [PubMed] [Google Scholar]
- 29. Meites J. Role of hypothalamic catecholamines in aging processes. Acta Endocrinol (Copenh) 125 Suppl 1: 98–103, 1991 [PubMed] [Google Scholar]
- 30. Mohankumar PS, Thyagarajan S, Quadri SK. Correlations of catecholamine release in the medial preoptic area with proestrous surges of luteinizing hormone and prolactin: effects of aging. Endocrinology 135: 119–126, 1994 [DOI] [PubMed] [Google Scholar]
- 31. Mohankumar PS, Thyagarajan S, Quadri SK. Cyclic and age-related changes in norepinephrine concentrations in the medial preoptic area and arcuate nucleus. Brain Res Bull 38: 561–564, 1995 [DOI] [PubMed] [Google Scholar]
- 32. MohanKumar SM, Smith CL, MohanKumar PS. Central adaptation to chronic administration of interleukin-1beta (IL-1beta) in rats. Brain Res Bull 62: 71–76, 2003 [DOI] [PubMed] [Google Scholar]
- 33. Morel GR, Caron RW, Console GM, Soaje M, Sosa YE, Rodriguez SS, Jahn GA, Goya RG. Estrogen inhibits tuberoinfundibular dopaminergic neurons but does not cause irreversible damage. Brain Res Bull 80: 347–352, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33a. O'Callaghan JP. Quantification of glial fibrillary acidic protein: comparision of slot-immuno-binding assays with a novel sandwich ELISA. Neurotoxicol Teratol 13: 275–281, 1991 [DOI] [PubMed] [Google Scholar]
- 34. Palkovits M, Zaborszky L, Brownstein MJ, Fekete MI, Herman JP, Kanyicska B. Distribution of norepinephrine and dopamine in cerebral cortical areas of the rat. Brain Res Bull 4: 593–601, 1979 [DOI] [PubMed] [Google Scholar]
- 35. Paxinos GW, Watson C. The Rat Brain in Stereotaxic Coordinates. San Diego, CA: Academic Press, 1998 [Google Scholar]
- 36. Piroli GG, Grillo CA, Ferrini MG, Lux-Lantos V, De Nicola AF. Antagonism by progesterone of diethylstilbestrol-induced pituitary tumorigenesis in Fischer 344 rats: effects on sex steroid receptors and tyrosine hydroxylase mRNA. Neuroendocrinology 63: 530–539, 1996 [DOI] [PubMed] [Google Scholar]
- 37. Reymond MJ, Porter JC. Involvement of hypothalamic dopamine in the regulation of prolactin secretion. Horm Res 22: 142–152, 1985 [DOI] [PubMed] [Google Scholar]
- 38. Saitoh Y, Hikake T, Hayashi S, Iguchi T, Sato T. Involvement of insulin-like growth factor-I for the regulation of prolactin synthesis by estrogen and postnatal proliferation of lactotrophs in the mouse anterior pituitary. Cell Tiss Res 340: 147–158 [DOI] [PubMed] [Google Scholar]
- 39. Schipper H, Brawer JR, Nelson JF, Felicio LS, Finch CE. Role of the gonads in the histologic aging of the hypothalamic arcuate nucleus. Biol Reprod 25: 413–419, 1981 [DOI] [PubMed] [Google Scholar]
- 40. Simpkins JW, Green PS, Gridley KE, Singh M, de Fiebre NC, Rajakumar G. Role of estrogen replacement therapy in memory enhancement and the prevention of neuronal loss associated with Alzheimer's disease. Am J Med 103: 19S–25S, 1997 [DOI] [PubMed] [Google Scholar]
- 41. Steyn FJ, Anderson GM, Grattan DR. Expression of ovarian steroid hormone receptors in tuberoinfundibular dopaminergic neurones during pregnancy and lactation. J Neuroendocrinol 19: 788–793, 2007 [DOI] [PubMed] [Google Scholar]
- 42. Wakita T, Shintani F, Yagi G, Asai M, Nozawa S. Combination of inflammatory cytokines increases nitrite and nitrate levels in the paraventricular nucleus of conscious rats. Brain Res 905: 12–20, 2001 [DOI] [PubMed] [Google Scholar]
- 43. Waring SC, Rocca WA, Petersen RC, O'Brien PC, Tangalos EG, Kokmen E. Postmenopausal estrogen replacement therapy and risk of AD: a population-based study. Neurology 52: 965–970, 1999 [DOI] [PubMed] [Google Scholar]
- 44. Xing B, Xin T, Hunter RL, Bing G. Pioglitazone inhibition of lipopolysaccharide-induced nitric oxide synthase is associated with altered activity of p38 MAP kinase and PI3K/Akt. J Neuroinflammation 5: 4, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Yusuf S, Anand S. Hormone replacement therapy: a time for pause. Can Med Assoc J 167: 357–359, 2002 [PMC free article] [PubMed] [Google Scholar]