Abstract
Heat stress increases limb blood flow and cardiac output (Q̇) in humans, presumably in sole response to an augmented thermoregulatory demand of the skin circulation. Here we tested the hypothesis that local hyperthermia also increases skeletal muscle blood flow at rest and during exercise. Hemodynamics, blood and tissue oxygenation, and muscle, skin, and core temperatures were measured at rest and during exercise in 11 males across four conditions of progressive whole body heat stress and at rest during isolated leg heat stress. During whole body heat stress, leg blood flow (LBF), Q̇, and leg (LVC) and systemic vascular conductance increased gradually with elevations in muscle temperature both at rest and during exercise (r2 = 0.86–0.99; P < 0.05). Enhanced LBF and LVC were accompanied by reductions in leg arteriovenous oxygen (a-vO2) difference and increases in deep femoral venous O2 content and quadriceps tissue oxygenation, reflecting elevations in muscle and skin perfusion. The increase in LVC occurred despite an augmented plasma norepinephrine (P < 0.05) and was associated with elevations in muscle temperature (r2 = 0.85; P = 0.001) and arterial plasma ATP (r2 = 0.87; P < 0.001). Isolated leg heat stress accounted for one-half of the increase in LBF with severe whole body heat stress. Our findings suggest that local hyperthermia also induces vasodilatation of the skeletal muscle microvasculature, thereby contributing to heat stress and exercise hyperemia. The increased limb muscle vasodilatation in these conditions of elevated muscle sympathetic vasoconstrictor activity is closely related to the rise in arterial plasma ATP and local tissue temperature.
Keywords: vasodilatation, hyperthermia, ATP
heat stress augments limb blood flow and cardiac output (Q̇) in resting humans (1, 4, 9, 19, 35, 40, 45). An unresolved question is whether limb muscle vasodilatation contributes to these increases in blood flow and the mechanisms involved. Early investigations into the partition of limb perfusion between skin and skeletal muscle during heat stress produced conflicting results (4–6, 9, 35), with some studies suggesting an elevation in muscle blood flow (4, 5). However, later studies utilizing a variety of experimental techniques found no evidence of an elevation in forearm muscle perfusion during heat stress (2, 8, 19). These observations combined with the estimate of maximal skin blood flow (SkBF) of 6–8 l/min, based on indirect measures of Q̇ and visceral blood flow during whole body heat stress, helped shape the view that heat stress-induced systemic hyperemia and blood flow redistribution are entirely due to an augmented thermoregulatory demand for SkBF (8, 25, 39, 40). Recent evidence, however, shows that calf muscle blood flow increases significantly during isolated leg heating (25). Nevertheless, the mechanisms increasing skeletal muscle perfusion with passive heat stress and the contribution of local vs. systemic elevations in tissue temperature on limb muscle blood flow regulation remain unknown.
The effects of combined heat stress and exercise upon active muscle blood flow also remain equivocal with reports showing that exercising limb and systemic perfusion is either elevated (42, 47), unchanged, or reduced (10, 28, 29, 41). These discrepant findings may be accounted for by differences in the mode and intensity of exercise, the magnitude of heat stress, and the possible influence of dehydration. In this context, limb perfusion is elevated during single limb exercise with exposure to a moderate degree of either local limb or whole body heat stress in conditions where dehydration is negligible (42, 47). On the other end of the spectrum, limb muscle perfusion is reduced in association with the hemoconcentration and the declines in perfusion pressure and Q̇ that accompany severe heat stress or dehydration during prolonged and short moderate- to high-intensity whole body exercise (10, 11). The use of an isolated limb exercise model and the maintenance of the subject's hydration status enable the study of the influence of graded levels of heat stress on exercising limb muscle blood flow without the confounding influences of reflexes underpinning the circulatory limitations to whole body exercise and dehydration (3, 26).
Mechanistically, the elevation in limb perfusion with heat stress occurs in the presence of increased muscle sympathetic nerve activity (30, 33). This indicates that signals responsive to heat stress may directly or indirectly modulate muscle sympathetic vasoconstrictor activity such that vasodilatation prevails over vasoconstriction, allowing limb muscle perfusion and vascular conductance to increase (21). The observation that the plasma concentration of the potent vasodilator and sympatholytic molecule ATP increases during exercise with severe heat stress (12, 36) raises the possibility that ATP is one of the key regulatory signals involved in the prevailing heat stress-induced limb vasodilatation. It is yet to be determined whether increases in local temperature induce elevations in limb muscle perfusion in relation to the rise in circulating ATP.
Accordingly, the main aim of this study was to determine whether skeletal muscle vasodilatation contributes to the generally observed increases in limb and systemic perfusion with heat stress in resting humans and whether heat stress induces similar effects on leg muscle and systemic perfusion during mild exercise in euhydrated individuals. A second aim was to determine the contribution of local tissue temperatures to the increases in leg perfusion with whole body and isolated leg heat stress and gain insight into the role of plasma ATP and muscle temperature into heat stress-mediated limb muscle vasodilatation. To accomplish these aims, we measured leg and systemic hemodynamics, arterial, deep, and common femoral venous blood O2 content, quadriceps muscle oxygenation and temperature, and plasma ATP and catecholamines at rest and during moderate one-legged, knee-extensor exercise. These measurements were performed in healthy male volunteers during control conditions and three graded levels of whole body skin and internal body hyperthermia as well as during isolated leg hyperthermia. The design of the study allowed us to test the overall hypothesis that local hyperthermia induces vasodilatation in resting and exercising human limb muscle and gain an insight into the roles of muscle temperature and intravascular ATP in this response.
METHODS
Subjects.
Eleven healthy, recreationally active males (mean ± SD: ages, 21 ± 2 yr; body wt, 76.3 ± 10.4 kg; and height, 178 ± 6 cm) participated in this study involving two different protocols. This study conformed to the code of Ethics of the World Medical Association (Declaration of Helsinki) and was conducted after ethical approval from the Brunel University Research Ethics Committee. Informed written and verbal consent was obtained from all participants before participation.
Experimental protocols.
In protocol 1 leg and systemic hemodynamics and quadriceps muscle oxygenation were examined at rest and during moderate one-legged, knee-extensor exercise (mean ± SE: 21 ± 1 W at 65 ± 1 rpm for 6 min) in four consecutive whole body, heat-stress conditions. To induce whole body heat stress, a custom-built, water-perfused suit, passively heated participants in the supine position to four progressive levels of heat stress leading to four distinct conditions of skin and internal body hyperthermia: 1) control: normal skin, muscle, and core temperatures (i.e., ∼33, ∼34, and ∼37°C, respectively); 2) Skin Hyperthermia: whole body skin and muscle temperatures were increased, while rectal temperature remained at control (all ∼37°C); 3) Skin and Mild Core Hyperthermia: whole body skin, muscle, and rectal temperatures increased (all ∼38°C); and 4) Skin and Core Hyperthermia: skin temperature remained elevated and core and muscle temperatures increased further (∼38, ∼39, and ∼39°C, respectively) (Fig. 1). The same level of skin and core hyperthermia was maintained during exercise, yet quadriceps muscle temperature increased in all four experimental conditions (∼1–2°C) in response to the ensuing increases in local metabolic heat production and, in the control condition, the additional convective heat transfer from the warmer body core to the cooler leg muscles. The manipulation of body temperatures in this manner allowed the examination of the contribution of muscle, skin, and core temperature on leg hemodynamic responses to heat stress both at rest and during exercise.
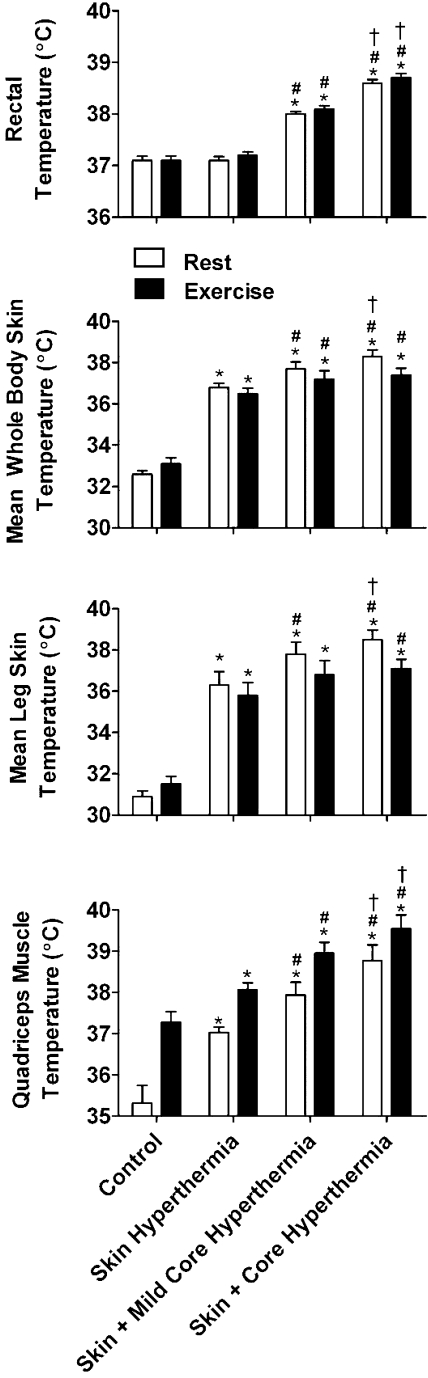
Fig. 1.
Body temperature responses to whole body heat stress. Mean whole body skin temperature increased from control with the skin hyperthermia condition, but core temperature remained unchanged. With further heat stress, core temperature increased in the skin and mild core hyperthermia condition, while mean whole body skin temperature remained elevated. This pattern continued into the skin and core hyperthermia condition where there was a further elevation in core temperature, and skin temperature remained elevated from control. Data are means ± SE for 11 subjects, except for muscle temperature (n = 5). Quadriceps muscle temperature followed the increase in mean skin temperature at rest and increased rapidly during exercise by 1–2°C above corresponding resting values. *Different from control, P < 0.05; #different from skin hyperthermia, P < 0.05; †different from skin and mild core hyperthermia. Significance was accepted at P < 0.05 and refers to differences in the respective conditions, i.e., either rest or exercise.
In protocol 2 leg and systemic hemodynamics were examined using noninvasive methods in the supine position at rest over 60 min of isolated leg heat stress, which created a condition of isolated leg Skin Hyperthermia (i.e., core and experimental leg skin and muscle temperatures of ∼37°C). This protocol allowed the examination of the independent contribution of leg muscle and skin temperatures on the leg hemodynamic response to whole body heat stress. Protocols were separated by at least 2 wk. Subjects ingested a carbohydrate-electrolyte beverage (Gatorade) throughout both protocols to prevent dehydration. The temperature of the beverage was 35–40°C to avoid any decreases in internal temperature caused by its consumption.
Instrumentation of subjects.
In preparation for the whole body heat stress protocol, subjects were familiarized with the one-legged, knee-extensor exercise to minimize the involvement of the gluteal and hamstring muscles and thereby isolating the work load to the knee extensors. Furthermore, all participants reported to the laboratory on two occasions separated by 2 days to undergo heat familiarization by performing cycling exercise at ∼150 W for 60 min in a heat chamber controlled at 37°C and 60% humidity.
On the morning of the protocol, subjects arrived at the laboratory after eating a light breakfast. After insertion of the rectal thermister, participants rested in the supine position, while catheters were placed under local anesthesia into the common femoral vein of the exercising leg (left leg) and in the radial artery (right forearm). The femoral venous catheter was positioned 1–2 cm proximal to the inguinal ligament, running proximally in the anterograde direction. In four out of the eleven participants, an additional catheter was inserted in the femoral vein of the exercising leg that ran in a distal or retrograde direction. This retrograde venous catheter was 12 cm in length and was inserted into the femoral vein ∼1–2 cm from the anterograde common femoral venous catheter. Consequently the catheter tip was inserted past the saphenofemoral junction, resting in the deep portion of the femoral vein. Consequently, the blood drawn from this catheter primarily represented blood-draining leg skeletal muscle tissues and as such was not representative of blood flowing through the great saphenous vein. For the purposes of this publication, the retrograde femoral venous catheter will be referred to as the deep femoral venous catheter. Successful placement of the retrograde catheter in the deep femoral venous portion of the leg was indicated by a lower blood O2 content compared with that drawn from the common femoral venous catheter. Specifically, the absolute venous O2 content values in the deep vs. mixed femoral venous catheter were ∼123 vs. ∼136 ml/l at rest and ∼62 vs. ∼70 ml/l during exercise, respectively. These values from the deep femoral catheter closely agree with previous reports (44) and can be used as an index of deep leg tissue oxygenation and perfusion.
Participants then walked to the experimental room and sat on the knee-extensor ergometer where they were dressed in a custom-built, water-perfused suit that was interwoven with silicone tubing and connected to a water circulator (model F34; Julabo, Seelbach, Germany). The water circulator was fitted with an auxiliary pump and temperature-control unit capable of controlling the temperature of the water in the suit, which covered the subject's entire body except the head, hands, and feet. Whole body heat stress was induced by perfusing water at a temperature of 47°C through the water-perfused suit. Once the specific skin and/or core temperatures were attained at each stage of heat stress, the temperature of the water-perfusing the suit was decreased slightly to limit further increases in skin and/or core temperature during data collection at rest and during exercise. To minimize heat loss during each heat-stress stage, a thermal foil blanket covered the torso and was wrapped around the lower body of the subjects, socks covered both feet, and a thermal hat was also worn. After the participants were dressed in the suit they lay supine on a reclining chair that was part of a knee-extensor ergometer (model LE220; FBJ Engineering, Odense Area, Denmark), while the left foot and ankle were inserted into the boot of the ergometer. Both of the subject's lower legs were supported during resting conditions.
In protocol 2, subjects only wore the left leg of the water-perfused suit. Similarly to protocol 1, foil was wrapped around the heated leg and the water circulator controlled the temperature of the water within the water-perfused leg. Subjects remained supine while a pressure cuff was placed around a finger for the measurement of systemic blood pressure and subsequent determination of systemic hemodynamics.
Temperature measurements.
Skin thermisters were placed on seven sites: forehead, forearm, hand, abdomen, thigh, calf, and foot (Grant Instruments, Cambridge, United Kingdom). Thermisters were securely held in place throughout the protocol by the use of adhesive spray and medical tape. Rectal temperature was measured 10 cm past the sphincter muscle using a commercially available rectal probe (Physitemp, Clifton, NJ). Skin (Squirrel 1000 Series, Grant Instruments) and rectal (Thermalert; Physitemp) temperatures were monitored online. Weighted mean skin temperature was calculated using methods described previously (17). Mean leg skin temperature was calculated using the combined weighted skin temperatures of the thigh and calf.
In the whole body and isolated leg heat stress protocols, quadriceps muscle temperature was measured online (model TC-2000; Sable Systems, Las Vegas, NV) in five and two participants, respectively, with a tissue-implantable thermocouple microprobe (model T-204A; Physitemp) together with core and skin temperatures. Muscle temperature was measured in the vastus lateralis muscle at a depth of 3 cm. Increases in vastus lateralis muscle temperature provide an accurate representation of the mean temperature responses of the different portions of the quadriceps muscles during isolated knee-extensor exercise in conditions where subcutaneous tissue temperature remains unchanged despite external heating (24).
Systemic hemodynamics and muscle oxygenation.
In protocol 1, baseline systemic and leg hemodynamics were measured immediately prior to exercise after a minimum of 10-min supine rest and following the attainment of the desired skin and rectal temperatures. During exercise, these measurements were repeated between minutes 4 and 6. Additionally, arterial and venous blood samples (1 ml for blood gas, metabolite, and electrolyte variables; 2 ml for plasma ATP and plasma hemoglobin; and 2 ml for plasma catecholamines) were obtained at rest and after 5 min of exercise. Arterial and venous catheters were also used to measure arterial and venous blood pressure, respectively. In protocol 2 (n = 7), subjects remained at rest throughout the isolated leg heat stress protocol and temperature and hemodynamic measures were taken every 2 min between 0–10 min and every 10 min thereafter.
In protocol 1, heart rate was obtained from a three-lead electrocardiogram, while arterial and femoral venous pressure waveforms were continuously recorded at the level of the heart via pressure transducers (Pressure Monitoring Kit; Baxter) connected to two amplifiers (BP amp; ADInstruments, Bella Vista, NSW, Australia) and monitored online via a data acquisition system (Powerlab 16/30 ML 880/P; ADInstruments). Q̇ was calculated as the product of heart rate and stroke volume, where stroke volume was estimated using directly measured arterial pressure waveforms via the Modelflow method, incorporating age, gender, height, and weight (BeatScope version 1.1; Finapress Medical Systems, Amsterdam, Netherlands) (46). Q̇ data obtained from estimated stroke volume via the Modelflow method in this study, shares a tight relationship with an independently derived measure of Q̇ data obtained using echocardiography (r2 = 0.831; P = 0.002; data not shown). Systemic vascular conductance was calculated by dividing Q̇ by mean arterial blood pressure (MAP).
Muscle oxygenation of the vastus lateralis muscle was measured using near-infrared spectroscopy (NIRS) (INVOS Cerebral Oximeter; Somanetics, Troy, MI). An adhesive NIRS pad that emitted NIR signals at 730- and 810-nm wavelengths was placed over the vastus lateralis muscle. The NIRS pad contained two optodes at a distance of 3 and 4 cm away from the sensor. Previous research (18) indicates that these NIRS signals penetrated quadriceps tissue to a depth of ∼1.5 and 2 cm, respectively. Therefore, the shallow penetrating NIRS signal enabled an algorithm inherent to the NIRS system to account for any alterations in cutaneous oxygenation. The NIRS pad was securely taped to the skin to ensure that no light interfered with recordings.
In protocol 2, blood pressure waveforms were recorded noninvasively by using photosphygmomanometry (Finapres Medical Systems, Smart Medical, Amsterdam, Netherlands) and heart rate was obtained from a three-lead electrocardiogram, allowing estimates of systemic hemodynamics as described above. In both studies, systemic oxygen uptake was continuously measured and recorded online (Quark b2; Cosmed, Italy).
Leg and skin hemodynamics.
In both studies, leg blood flow (LBF) was measured from the common femoral artery 2–3 cm proximal to the bifurcation point by using an ultrasound Doppler equipped with a 10-MHz linear probe (Vivid 7 Dimension; GE Medical, Horton, Norway). Femoral artery vessel diameter was determined after obtaining three 2D images in the longitudinal view at ∼40 frames/s, depending on artery depth. The diameter was calculated using measurements obtained from the systolic and diastolic phases, which accounted for one-third and two-thirds of the vessel diameter, respectively (32). Mean blood velocity (Vmean) was calculated from an insonation angle that was consistently < 60 degrees (32) and with the sample volume positioned in the center of the femoral artery. Blood velocity was calculated from the average of three measurements each consisting of between 10 and 12 velocity profiles. The contribution of turbulence occurring at the vascular wall to blood flow measurement was reduced by using a low-velocity rejection filter. Finally, LBF (l/min) was calculated using the equation: Vmean × π (vessel diameter/2)2 × 6 × 104.
Our coefficient of variation for measures of LBF during four control rest and exercise conditions is 8.1% at rest and 4.9% during exercise (n = 5), which is small compared with the blood flow increases evoked by heat stress. This control study also showed that resting and exercising LBF is unchanged during four control rest and exercise conditions mimicking the experimental design of the present study.
During whole body and isolated leg heat stress SkBF of the exercising leg was measured via laser-Doppler flowmetry (Periflux Flowmetry System, Jarfalla, Sweden). The probe was secured to the skin of the thigh (i.e., above the vastus lateralis) and was not covered by or in contact with the water-perfused suit.
Perfusion pressure at the level of the leg was calculated as MAP minus femoral venous pressure, where each pressure value was obtained from the integration of the corresponding pressure curves. Leg vascular conductance (LVC) was calculated as LBF divided by perfusion pressure. Leg arteriovenous oxygen (a-vO2) difference was the difference in arterial and femoral venous blood O2 content, while leg O2 delivery was the product of arterial O2 content and LBF. Leg O2 extraction was the ratio between leg a-vO2 difference and arterial O2 content, while leg Q̇ was calculated by multiplying LBF by leg a-vO2 difference. Finally, leg tissue blood flow (i.e., LBF-saphenous BF) was calculated in each experimental condition using the Fick principle: leg tissue blood flow = leg tissue V̇o2/deep femoral-based leg a-vO2 difference, where, leg tissue V̇o2 = leg V̇o2 − estimated leg skin V̇o2, assuming that leg skin V̇o2, i.e., 1.5 ml/min; (1, 43) remained constant with graded heat stress and/or exercise.
Blood and plasma parameters.
Blood gas variables, hemoglobin concentration, metabolites, electrolytes, and osmolality were measured using an automated analyzer (model ABL 825; Radiometer, Copenhagen, Denmark). Plasma ATP was determined with the luciferin-luciferase technique, using a luminometer with three automatic injectors (Orion Microplate Luminometer; Berthold Detection System, Pforzheim, Germany). Blood samples (2.0 ml) were drawn into a stop solution (2.7 ml) containing S-(4-nitrobenzyl)-6-thioinosine (5 nM), IBMX (100 μM), forskolin (10 μM), EDTA (4.15 mM), NaCl (118 mM), KCl (5 mM), and tricine buffer (40 mM) (16). Immediately thereafter, the samples were centrifuged for 3 min at 4,000 g in plastic tubes containing lithium heparin with an inert gel barrier for plasma separation (BD, Franklin Lakes, NJ) and measured in duplicates at room temperature (20–22°C) using an ATP kit (ATP Kit SL; BioTherma, Dalarö, Sweden) with an internal ATP standard procedure. As an indicator of hemolysis, plasma hemoglobin was measured spectrophotometrically (model 3500; Jenway, Essex, UK). Plasma levels of the catecholamines epinephrine and norepinephrine were measured using the catecholamines assay kit Bi-CAT EIA (model 17-EA613–192; Alpco Diagnostics) according to the manufacturer's instructions.
Statistics.
A one-way repeated-measures ANOVA was performed on all dependent variables to test significance among the control and three conditions of heat stress separately at rest and during exercise. When a significant difference (P < 0.05) was found, post hoc analysis of the data was conducted using Tukey's honestly significant difference test and applying a Bonferroni correction where appropriate (P < 0.0125). Where applicable, relationships were determined using Pearson's product moment correlation using the mean data of all participants from each thermal stage (P < 0.05). Stepwise regression analysis was also used to identify the best predictors of the LVC responses to heat stress and exercise.
RESULTS
Hydration and temperature during whole body heat stress.
In protocol 1 body weight, blood electrolytes, osmolality, and hematological variables remained unchanged (Table 1), indicating a maintained intravascular and extravascular fluid status during heat stress. With Skin Hyperthermia, mean skin temperature increased from 32.6 ± 0.2°C to 36.8 ± 0.2°C (P < 0.05), whereas core temperature was unchanged (37.1 ± 0.1°C vs. 37.1 ± 0.1°C; P > 0.05). In the Skin and Mild Core Hyperthermia condition, mean skin temperature was maintained (37.7 ± 0.3°C), while core temperature increased to 38.0 ± 0.1°C (P < 0.05). This pattern was repeated with further whole body heat stress in the Skin and Core Hyperthermia condition: 38.3 ± 0.3°C (mean skin) and 38.6 ± 0.1°C (core) (both P < 0.05). In response to whole body heat stress, mean leg skin temperature, and quadriceps muscle temperature followed the same pattern and magnitude as mean whole body skin temperature (Fig. 1). In five participants, quadriceps muscle temperature increased progressively from 35.3 ± 0.4°C at control rest to 37.0 ± 0.1°C with Skin Hyperthermia, 37.9 ± 0.3°C with Skin and Mild Core Hyperthermia, and finally 38.8 ± 0.4°C with Skin and Core Hyperthermia (all P < 0.05). During exercise, muscle temperature increased (P < 0.05) and was progressively higher with each thermal condition; from 37.3 ± 0.3°C at control, to 38.1 ± 0.2°C, 38.9 ± 0.3°C, and 39.6 ± 0.3°C, respectively (all P < 0.05). With the exception of quadriceps muscle temperature, all reported resting temperatures are representative of exercise conditions as skin or core temperatures were not significantly different between rest and exercise (P > 0.05) (Fig. 1).
Table 1.
Blood variable responses to whole body heat stress at rest and during exercise
| Control |
Skin Hyperthermia |
Skin and Mild Core Hyperthermia |
Skin and Core Hyperthermia |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Rest | Exercise | Rest | Exercise | Rest | Exercise | Rest | Exercise | ||
| Hb, g/l | a | 146 ± 3 | 152 ± 3 | 145 ± 3 | 150 ± 3 | 147 ± 3 | 151 ± 3 | 149 ± 3 | 152 ± 3 |
| v | 147 ± 3 | 156 ± 3 | 148 ± 3 | 152 ± 3 | 150 ± 3 | 153 ± 3 | 151 ± 3 | 153 ± 3 | |
| O2 Sat, % | a | 97.9 ± 0.2 | 97.9 ± 0.3 | 97.8 ± 0.2 | 98.1 ± 0.1 | 98.0 ± 0.3 | 98.0 ± 0.2 | 98.6 ± 0.2 | 98.3 ± 0.2 |
| v | 67.1 ± 3.5 | 32.3 ± 1.5 | 80.5 ± 1.5 | 37.0 ± 1.5 | 83.5 ± 1.6 | 41.8 ± 2 | 83.7 ± 1.7 | 46.1 ± 1.9 | |
| Po2, mmHg | a | 109 ± 4 | 107 ± 4 | 102 ± 3 | 110 ± 4 | 110 ± 5 | 110 ± 5 | 121 ± 5†# | 113 ± 5 |
| v | 38 ± 2 | 25 ± 1 | 48 ± 2 | 26 ± 1 | 50 ± 2* | 28 ± 1* | 48 ± 2* | 29 ± 1* | |
| CtO2, ml/l | a | 199 ± 5 | 209 ± 5 | 197 ± 5 | 204 ± 5 | 200 ± 7 | 206 ± 5 | 204 ± 6# | 207 ± 6 |
| v | 128 ± 8 | 65 ± 4 | 165 ± 3* | 75 ± 4 | 170 ± 5* | 87 ± 6* | 174 ± 7* | 99 ± 5* | |
| Pco2, mmHg | a | 39 ± 2 | 39 ± 2 | 39 ± 1 | 35 ± 1 | 32 ± 2* | 31 ± 3 | 25 ± 3* | 29 ± 3 |
| v | 49 ± 1 | 67 ± 2 | 42 ± 1* | 58 ± 3 | 36 ± 2*# | 50 ± 3 | 29 ± 3*†# | 43 ± 4 | |
| pH | a | 7.41 ± 0.02 | 7.40 ± 0.02 | 7.40 ± 0.01 | 7.43 ± 0.02 | 7.44 ± 0.02 | 7.45 ± 0.02 | 7.52 ± 0.03*# | 7.48 ± 0.03 |
| v | 7.37 ± 0.01 | 7.27 ± 0.01 | 7.40 ± 0.01* | 7.31 ± 0.02* | 7.43 ± 0.01*# | 7.37 ± 0.02*# | 7.50 ± 0.02*†# | 7.40 ± 0.03 | |
| Na+, mmol/l | a | 139 ± 1 | 142 ± 2 | 139 ± 1 | 142 ± 3 | 138 ± 2 | 140 ± 2 | 137 ± 2 | 139 ± 2 |
| v | 139 ± 2 | 143 ± 1 | 139 ± 2 | 142 ± 3 | 138 ± 1 | 141 ± 0 | 137 ± 2 | 139 ± 2 | |
| K+, mmol/l | a | 4.0 ± 0.1 | 4.6 ± 0.1 | 4.0 ± 0.1 | 4.4 ± 0.1 | 3.9 ± 0.1 | 4.1 ± 0.1 | 3.9 ± 0.1 | 4.0 ± 0.1 |
| v | 4.1 ± 0.1 | 4.9 ± 0.1 | 4.0 ± 0.1 | 4.7 ± 0.1 | 3.9 ± 0.1 | 4.3 ± 0.1 | 3.8 ± 0.1 | 4.1 ± 0.1 | |
| Cl−, mmol/l | a | 108 ± 3 | 108 ± 1 | 108 ± 1 | 108 ± 1 | 107 ± 1 | 108 ± 1 | 107 ± 1 | 105 ± 1 |
| v | 105 ± 2 | 105 ± 1 | 106 ± 1 | 104 ± 1 | 106 ± 1 | 104 ± 1 | 105 ± 1 | 104 ± 1 | |
| Glucose, mmol/l | a | 5.9 ± 0.2 | 5.8 ± 0.1 | 7.5 ± 0.3 | 7.2 ± 0.3 | 9.2 ± 0.5 | 8.2 ± 0.4 | 8.7 ± 0.5 | 7.9 ± 0.4 |
| v | 5.5 ± 0.2 | 5.7 ± 0.1 | 7.0 ± 0.3 | 7.0 ± 0.3 | 8.5 ± 0.4 | 7.8 ± 0.4 | 8.1 ± 0.5 | 7.5 ± 0.4 | |
| Lactate, mmol/l | a | 1.0 ± 0.1 | 2.7 ± 0.3 | 1.6 ± 0.2 | 2.6 ± 0.2 | 2.9 ± 0.3 | 3.3 ± 0.3 | 3.0 ± 0.3 | 3.7 ± 0.5 |
| v | 1.0 ± 0.1 | 4.1 ± 0.5 | 1.5 ± 0.1 | 3.4 ± 0.5 | 2.8 ± 0.3 | 3.8 ± 0.5 | 3.0 ± 0.3 | 4.1 ± 0.7 | |
| Osmolality , mOsm·kg−1 | a | 284 ± 2 | 289 ± 3 | 283 ± 2 | 288 ± 3 | 285 ± 2 | 286 ± 2 | 282 ± 3 | 285 ± 3 |
| v | 284 ± 2 | 291 ± 2 | 285 ± 3 | 291 ± 3 | 284 ± 2 | 289 ± 3 | 281 ± 2 | 285 ± 3 | |
| ATP, nmol/l | a | 454 ± 102 | 709 ± 158§ | 639 ± 120*§ | 842 ± 193§ | 704 ± 150§ | 950 ± 227§ | 917 ± 196§ | 1090 ± 200*†§ |
| v | 685 ± 80 | 867 ± 114 | 754 ± 42 | 851 ± 78 | 824 ± 115 | 903 ± 55 | 874 ± 155 | 822 ± 145 | |
| Noradrenaline, nmol/l | a | 0.7 ± 0.3 | 1.3 ± 0.4 | 0.6 ± 0.3 | 1.1 ± 0.3 | 1.5 ± 0.6 | 2.6 ± 0.5 | 2.0 ± 0.7* | 4.6 ± 1.4* |
| v | 0.8 ± 0.2 | 2.1 ± 1.3 | 0.7 ± 0.2 | 1.3 ± 0.3 | 1.3 ± 0.6 | 2.2 ± 1.0 | 2.0 ± 0.9* | 4.9 ± 2.4* | |
| Adrenaline, nmol/l | a | 1.1 ± 0.2 | 1.7 ± 0.3 | 0.8 ± 0.2 | 1.3 ± 0.2 | 0.8 ± 0.2 | 1.0 ± 0.1 | 0.7 ± 0.1 | 0.8 ± 0.2 |
| v | 0.4 ± 0.1 | 1.1 ± 0.2 | 0.7 ± 0.1 | 1.1 ± 0.1 | 0.7 ± 0.1 | 0.7 ± 0.1 | 0.6 ± 0.1 | 0.8 ± 0.1 | |
Values are means ± SE for 11 subjects, except for plasma ATP (a, n = 10 and v, n = 8), noradrenaline (n = 6), and adrenaline (n = 6). a, Arterial; v, femoral venous.
Different from control, P < 0.05;
different from skin hyperthermia, P < 0.05;
different from skin and mild core hyperthermia, P < 0.05. An additional one-way ANOVA with Tukeys honestly significant difference was performed on arterial plasma ATP values to determine any significant differences from control rest conditions (§P < 0.05).
Leg and systemic hemodynamics and oxygenation during whole body heat stress.
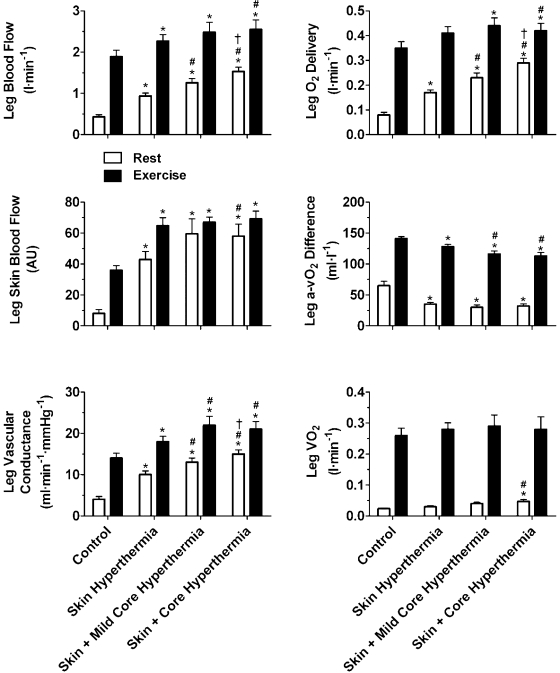
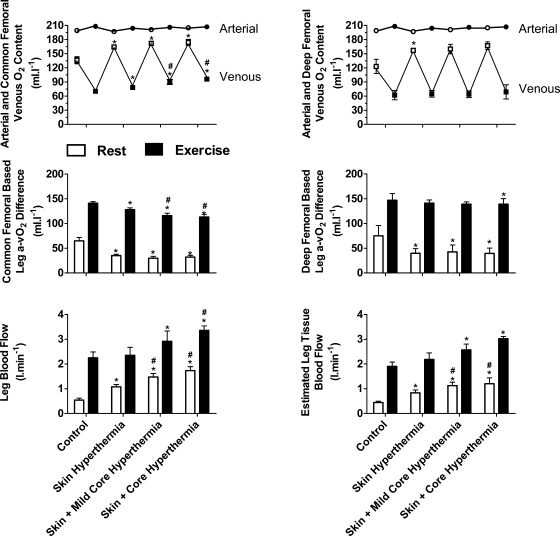
At rest, LBF and SkBF gradually increased with each level of whole body hyperthermia accompanying a decline in leg a-vO2 difference and a significant but small increase in leg and whole body V̇o2 (peak ΔV̇o2 = 0.008 ± 0.005 and 0.15 ± 0.03 l/min; respectively, P < 0.05, Fig. 2). The increase in LBF was due to increases in mean blood velocity as common femoral artery diameter remained unchanged throughout heat stress (overall mean: 0.93 ± 0.02 cm; P = 0.15). Increases in LBF were accompanied by elevations in common femoral venous O2 content and Po2 (136 ± 7 vs. 164 ± 2 ml/l and 38 ± 2 vs. 50 ± 2 mmHg, respectively; P < 0.05, Fig. 3). In four participants, these increases in LBF and common femoral venous O2 content were accompanied by elevations in deep femoral venous O2 content and Po2 (123 ± 15 vs. 157 ± 4 ml/l, P < 0.05, and 36 ± 4 vs. 43 ± 5 mmHg, respectively, Fig. 3).
Fig. 2.
Leg hemodynamics and oxygen consumption during whole body heat stress. Leg blood flow (LBF) increased with each stage of heat stress at rest and was also increased from control levels during exercise, while leg arteriovenous oxygen (a-vO2) difference declined; thus leg V̇o2 was maintained. Leg skin blood flow (SkBF) increased at rest, while leg vascular conductance (LVC) also increased with heat stress at rest and during exercise. Data are means ± SE for 10 subjects, except for LBF and leg SkBF, where n = 11 and n = 8, respectively. *Different from control, P < 0.05; #different from skin hyperthermia, P < 0.05; †different from skin and mild core hyperthermia. Significance refers to differences in the respective conditions, i.e., either rest or exercise.
Fig. 3.
Common and deep femoral venous O2 content and estimated leg tissue blood flow with heat stress. Arterial and common femoral venous O2 content during whole body heat stress. Data are means ± SE for 10 participants. In 4 additional participants, increases in common femoral venous O2 content were accompanied by elevations in deep femoral venous O2 content and estimated leg tissue blood flow, which are suggestive of an increase in blood flow to the muscle tissue within the leg. *Different from control, P < 0.05; #different from skin hyperthermia, P < 0.05; †different from skin and mild core hyperthermia. Significance refers to differences in the respective conditions, i.e., either rest or exercise.
Perfusion pressure declined during whole body heat stress owing to a fall in MAP (P < 0.05), while femoral venous pressure slightly increased (11 ± 1 vs. 14 ± 3 mmHg; P < 0.05) (Table. 2). At the level of the systemic circulation, both Q̇ and systemic vascular conductance increased progressively with each level of whole body hyperthermia (Table 2) accompanying gradual increases in heart rate (P < 0.05).
Table 2.
Systemic hemodynamics during whole-body heat stress at rest and during exercise
| Control |
Skin Hyperthermia |
Skin and Mild Core Hyperthermia |
Skin and Core Hyperthermia |
|||||
|---|---|---|---|---|---|---|---|---|
| Rest | Exercise | Rest | Exercise | Rest | Exercise | Rest | Exercise | |
| Cardiac output, l/min | 5.3 ± 0.2 | 7.8 ± 0.4 | 6.9 ± 0.3* | 9.2 ± 0.4* | 8.5 ± 0.4*# | 10.2 ± 0.4*# | 9.0 ± 0.4*#† | 10.9 ± 0.5*#† |
| Mean arterial pressure, mmHg | 109 ± 3 | 146 ± 2 | 103 ± 0* | 129 ± 2* | 101 ± 2*# | 120 ± 3*# | 105 ± 3† | 119 ± 3*# |
| Femoral venous pressure, mmHg | 11 ± 1 | 20 ± 2 | 11 ± 2 | 20 ± 2 | 14 ± 2# | 19 ± 3 | 14 ± 3† | 18 ± 3 |
| Systemic vascular conductance, ml·min−1·mmHg−1 | 49 ± 2 | 54 ± 3 | 67 ± 3* | 72 ± 4* | 84 ± 4*# | 86 ± 5*# | 86 ± 4*# | 91 ± 4*#† |
| Perfusion pressure, mmHg−1 | 97 ± 3 | 125 ± 2 | 90 ± 2* | 109 ± 2* | 88 ± 3* | 101 ± 4*# | 93 ± 3* | 100 ± 3*# |
Data are means ± SE for 11 subjects. Cardiac output, mean arterial pressure, femoral venous pressure, and systemic vascular conductance during heat stress at rest and one-legged knee extensor exercise.
Different from control, P < 0.05;
different from skin hyperthermia, P < 0.05;
different from skin and mild core hyperthermia. Significance was accepted at P < 0.05 and refers to differences in the respective conditions, i.e., either rest or exercise.
During exercise, LBF increased with Skin Hyperthermia compared with control (2.27 ± 0.16 vs. 1.89 ± 0.15 l/min P < 0.05). Thereafter, LBF remained elevated from control with Skin and Mild Core Hyperthermia (2.48 ± 0.25 l/min P < 0.05 vs. control) and increased further with Skin and Core Hyperthermia (2.55 ± 0.23 l/min P < 0.05 vs. control and Skin and Mild Core Hyperthermia, Fig. 2). The increase in LBF was accompanied by proportional decreases in leg a-vO2 difference (P < 0.05). In line with this, LVC increased from 14 ± 1 to 18 ± 1 and 22 ± 2 ml·min−1·mmHg−1 from control to Skin Hyperthermia and Skin and Mild Core Hyperthermia conditions, respectively (P < 0.05), while no further increase was observed with Skin and Core Hyperthermia. Likewise, Q̇, heart rate, and systemic vascular conductance progressively increased during exercise with heat stress (P < 0.05, Fig. 4). MAP declined from control values of 146 ± 2 to 129 ± 2 mmHg with Skin Hyperthermia and was further reduced to 120 ± 3 mmHg with Skin and Mild Core Hyperthermia (P < 0.05; Table 2). Thereafter, MAP remained stable as did leg and whole body Q̇. Additionally, while vastus lateralis oxygenation decreased with the onset of exercise with control and Skin Hyperthermia conditions, leg SkBF increased rapidly (P < 0.05; Table 2).
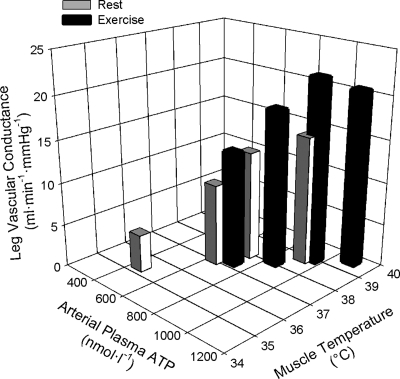
Fig. 4.
Relationship between LVC, muscle temperature, and plasma ATP. With whole body heat stress at rest and during exercise, LVC shares a strong positive relationship with elevations in leg muscle tissue temperature and arterial plasma ATP (r2 = 0.87, P = 0.001). Stepwise regression analysis revealed that the majority of the variance in LVC (87%) can be accounted for by elevations in arterial plasma ATP as opposed to the influence of muscle tissue temperature. However, elevations in muscle tissue temperature shared a strong relationship with elevations in plasma ATP (r2 = 0.85 P = 0.001), which could indicate that ATP is released into the vascular system in response to elevations in local tissue temperature, thereby initiating local vasodilatation and elevations in skeletal muscle perfusion. Data for LVC and arterial plasma ATP are means ± SE for 10 subjects and 5 subjects for leg muscle tissue temperature.
Effect of whole body heat stress at rest vs. exercise.
LBF increased in line with muscle temperature at rest (r2 = 0.99; P < 0.05; 0.34 ± 0.02 l·min−1·°C−1) and to a similar magnitude during exercise (r2 = 0.99; P < 0.05; 0.47 ± 0.04 l·min−1·°C−1). Similarly, increases in Q̇ shared a strong relationship with changes in muscle temperature at rest and during exercise (r2 = 0.93; P < 0.05; 1.09 ± 0.21 l·min−1·°C−1 and r2 = 0.99; P < 0.05; 1.40 ± 0.08 l·min−1·°C−1, respectively). Furthermore, at rest and during exercise, elevations in muscle temperature were accompanied by increases in LVC (r2 = 0.86, P = 0.001). However, when comparing the effect of whole body heat stress at rest vs. during exercise, the increases in LBF and Q̇ (and muscle temperature) with severe heat stress compared with control were significantly attenuated during exercise compared with that at rest (i.e., ΔLBF = 0.66 ± 0.15 vs. 1.10 ± 0.10 l/min and ΔQ̇ = 3.4 ± 0.3 vs. 4.0 ± 0.3 l/min, respectively; both P < 0.05). Correspondingly, the increase in leg and systemic blood flow from rest to exercise (i.e., exercise hyperemia) declined progressively from 1.46 ± 0.14 to 1.02 ± 0.20 l/min for LBF and 2.5 ± 0.3 and 1.9 ± 0.4 l/min for Q̇ during control compared with severe heat stress, respectively.
Circulating plasma ATP and catecholamines during whole body heat stress.
Plasma norepinephrine increased progressively with whole body heat stress and became significantly elevated with Skin and Core Hyperthermia compared with both rest and exercise control conditions (P < 0.05; Table 1). However, plasma epinephrine tended to decline throughout whole body heat stress at rest and during exercise (P > 0.05) in association with elevations in plasma glucose and leg glucose uptake. At rest, venous plasma ATP remained unchanged throughout (P > 0.05; Table 1), while arterial plasma ATP increased at rest and during exercise (P < 0.05; Table 1). This increase in arterial plasma ATP was strongly correlated with increases in LVC (r2 = 0.87; P = 0.001, Fig. 4), muscle tissue temperature (r2 = 0.85; P = 0.001, Fig. 4, n = 5) and arterial plasma norepinephrine (r2 = 0.54; P = 0.03) at rest and during exercise. Stepwise regression analysis revealed that the largest proportion of the variance in LVC during whole body heat stress at rest and during exercise was accounted for by elevations in arterial plasma ATP (∼87%).
Hydration, temperature, and hemodynamics during isolated leg heat stress.
After 60 min of isolated leg Skin Hyperthermia, mean leg skin temperature increased from 31.8 ± 0.1°C at control to 37.4 ± 0.1°C (P < 0.05), while quadriceps muscle temperature also increased from 34.0 ± 0.4 to 36.8 ± 0.2°C (Fig. 1). However, core temperature (37.0 ± 0.1°C) and hydration status, as indicated by body weight, remained unchanged. Correspondingly, LBF and muscle oxygenation increased from 0.47 ± 0.08 to 0.96 ± 0.07 l/min and 75 ± 1% to 86 ± 1%, respectively (P < 0.05). Furthermore, leg SkBF increased from 17 ± 0.5 to 45 ± 2 arbitrary units (AU) (P < 0.05), whereas Q̇, MAP, systemic vascular conductance, heart rate, stroke volume, and whole body V̇o2 all remained unchanged.
Effect of isolated leg and whole body heat stress on resting leg hemodynamics.
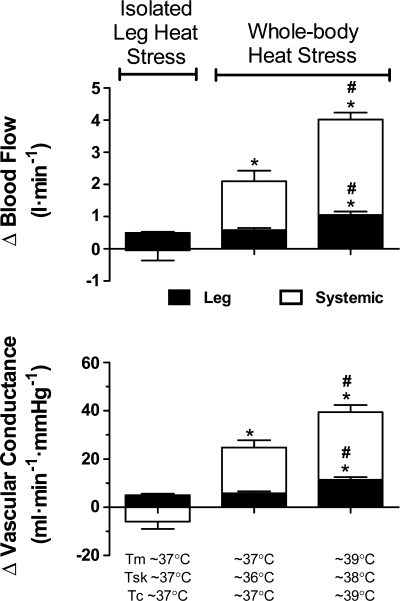
In seven participants where leg and systemic hemodynamics were examined during both protocols 1 and 2, whole body and isolated leg Skin Hyperthermia induced a significant but similar increase in LBF compared with control (ΔLBF = 0.50 ± 0.07 vs. 0.49 ± 0.04 l/min, respectively; P < 0.05, Fig. 5). However, Q̇ only increased during whole body heat stress (i.e., by 2.1 ± 0.3 l/min, P < 0.05). With further whole body heat stress, the increase in LBF and Q̇ doubled (i.e., 1.05 ± 0.11 and 4.0 ± 0.2 l/min, respectively). Thus, the increase in LBF with isolated leg Skin Hyperthermia accounted for up to 52 ± 9% of the LBF increase observed with whole body skin and core hyperthermia. The increases in local and systemic perfusion were matched with an elevated leg and systemic vascular conductance (Fig. 5).
Fig. 5.
Systemic and local responses to whole body and isolated leg heat stress. LVC increased to a similar extent with both isolated leg and whole body skin hyperthermia in association with comparable increases in local tissue temperature [i.e., muscle and skin temperature (Tsk)]. Tm denotes quadriceps muscle temperature. However, systemic vascular conductance only increased with whole body skin hyperthermia. With greater elevations in muscle and core temperature (Tc) with severe whole body heat stress, leg and systemic vascular conductance increased further. Leg and systemic vascular conductance values are representative of LBF and Q̇, respectively. Data are means ± SE for 7 subjects; *different from isolated leg heat stress skin hyperthermia; #different from whole body heat stress skin hyperthermia. Significance was accepted at P < 0.05.
DISCUSSION
This study reveals three key findings that provide further insight into the role of local temperature on limb muscle vasodilatation during heat stress and exercise hyperemia in humans. First, leg and systemic perfusion and vascular conductance increased progressively with elevations in muscle tissue temperature both at rest and during exercise. The increases in leg tissue perfusion paralleled significant reductions in leg a-vO2 differences and O2 extraction due to increases in venous O2 content in the blood circulating through the deep and common femoral veins and the muscle microcirculation. These observations suggest that heat stress not only augments flow and venous O2 content in the skin vasculature but also in the skeletal muscle vasculature. Second, progressive increase in LVC, which occurred in spite of increases in plasma norepinephrine at rest and during exercise, was associated with elevations in muscle temperature (r2 = 0.85; P = 0.001) and arterial plasma ATP (r2 = 0.87; P = 0.001). Third, isolated elevations in leg muscle and skin temperatures to ∼37°C accounted for approximately one-half of the leg hyperemia seen when muscle, skin, and core temperatures increased further to ∼38–39°C during whole body heat stress. These findings suggest that elevations in local tissue temperature also induce vasodilatation in the leg muscle microvasculature, contributing to heat stress and exercise limb hyperemia.
A key question of this study was whether heat stress caused vasodilatation not only of the skin but also of the muscle vasculature. To answer this question we used three different approaches: 1) took blood samples from the deep portion of the femoral vein; 2) measured quadriceps tissue oxygenation with NIRS; and 3) estimated leg tissue blood flow, which excludes the contribution of the skin circulation via the great saphenous vein. In support of the hypothesis of the study, we found that heat stress evoked significant increases in deep femoral venous O2 content, quadriceps tissue oxygenation, and leg tissue blood flow in parallel with significant reciprocal reductions in leg tissue O2 extraction in conditions where arterial O2 content and leg V̇o2 remained essentially unchanged (Fig. 3). Deep femoral venous O2 content and NIRS-determined quadriceps tissue oxygenation can be used as indexes of leg muscle oxygenation and perfusion under normal conditions. This has been shown in experiments where increasing or reducing LBF with intrafemoral artery infusion of vasodilators and/or vascular signal transduction blockers demonstrate that changes in femoral venous O2 content or muscle tissue oxygenation largely reflect variations in leg muscle blood flow in conditions of stable arterial O2 content, muscle metabolism, and environmental temperature (13, 27, 36). A critical question in the present setting is whether they also reflect increases in skeletal muscle perfusion with exposure to heat stress, or according to the general belief, they solely mirror the increases in limb SkBF. In this respect, there are several methodological considerations needed to present the experimental approaches.
First, the possibility exists that skin perfusion of the lower leg contributes significantly to the increases in deep femoral O2 content, thus rendering the rise in venous O2 content with heat stress as merely a cutaneous response. In this regard, some of the leg SkBF can initially be drained by the small saphenous vein and thereafter flow into the deep femoral vein via anastomoses, thereby affecting deep femoral venous O2 content values. Arguing against this possibility, the present findings of an increase in deep venous O2 content and reciprocal reductions in a-vO2 difference are consistent with the observation that human calf blood flow is significantly elevated during passive leg heating (i.e., 56% increase) (21), even when the magnitude of the increase in whole LBF only represented one-third of the 1.1 l/min increase observed in the present study with whole body heat stress. Second, it has been reported that measures of muscle tissue oxygenation using NIRS may be influenced by the rise in SkBF and oxygenation (7) and that the magnitude of this influence can depend on the specific NIRS system. In this regard, the system used here contrasts those used previously, as it is able to account for elevations in shallow tissue oxygenation that occur with increases in SkBF using a two-optode system, which also emits NIRS signals that penetrate to a deeper tissue depth, thereby reaching further into skeletal muscle tissue. Thus, it is quite unlikely that our measures of quadriceps tissue oxygenation only reflected increases in SkBF in the present scenario where deep tissue oxygenation would need to be maintained or decreased (during exercise) for leg SkBF to solely account for the observed 1.1 and 0.7 l/min elevations in resting and exercising LBF with heat stress. Third, the increase in deep femoral O2 content shown in this study was obtained in a small subgroup of subjects (n = 4). Although this is a limitation of the study, the concurrent reductions in deep femoral-based leg a-vO2 difference due only to increases in venous O2 content were so marked that they reached statistical significance both at rest and exercise (Fig. 3). Furthermore, the significant increases in deep femoral venous O2 content at rest paralleled similar increases in common femoral venous O2 content and quadriceps muscle oxygenation in all the subjects, suggesting that this is a general phenomenon in humans. Last, indices of muscle perfusion, deep femoral venous O2 content and NIRS measurements of quadriceps tissue oxygenation increased at rest and during exercise without a meaningful change in leg metabolism (Figs. 2 and 3). Assuming a constant leg skin V̇o2 we estimated that leg tissue blood flow, which excludes the blood flow through the great saphenous vein, increased by 0.8–1.1 l/min in response to the significant reductions in deep femoral-based leg a-vO2 differences evoked by heat stress at rest and during exercise (Fig. 3). Because increases in skin metabolism would have little influence on the flow estimate and muscle is clearly the primary driver of the ∼0.25 l/min elevation in leg V̇o2 in all exercise conditions, it is reasonable to suggest that the rise in deep femoral venous O2 content and the concomitant reductions in a-vO2 difference and O2 extraction across the leg tissues with heat stress and exercise are in part responses to the increase in leg muscle perfusion. We propose that the increased active muscle blood flow during heat stress exercise has an important thermoregulatory role as it removes heat away from the exercising muscles via the circulation (i.e., convective heat transfer within the leg or to the body core) (15).
The examination of the relationships between muscle, skin, and core temperature and leg perfusion during exposure to isolated leg and whole body heat stress provides a novel insight into which temperature elevations contribute to the increases in leg tissue perfusion. A novel observation was that isolated elevations in leg muscle and skin temperatures from 32–35°C to ∼37°C (while maintaining core and other regional skin temperatures) accounted for approximately one-half of the leg hyperemia seen when muscle, skin, and core temperatures increased further to ∼38–39°C during whole body heat stress. Furthermore, of all body temperatures measured, the progressive increases in LBF, LVC, and Q̇ at rest and during exercise with whole body heat stress were only significantly associated with elevations in quadriceps muscle temperature (r2 = 0.93–0.99 all P < 0.05). More importantly, LBF and LVC were equally elevated when leg muscle and skin temperatures were similar between isolated leg and whole body heat stress protocols, i.e., Skin Hyperthermia (Fig. 5), as were muscle oxygenation and leg SkBF. These results therefore support a close relationship between local tissue temperature and limb tissue blood flow and vascular conductance (Fig. 4), which is consistent with the rapid increases in quadriceps muscles and venous blood temperatures during local thigh heating and/or exercise (15).
The mechanisms underpinning heat stress and local hyperthermia induced limb muscle and skin vasodilatation are likely to involve local vasodilator and vasoconstrictor signals and central neural reflexes (20, 38). Here we found that leg tissue perfusion and vascular conductance increased in the presence of an augmented muscle sympathetic vasoconstrictor activity as evidenced by the rise in circulating norepinephrine (Table 1). The observation that plasma epinephrine tended to decline suggests that the increase in plasma norepinephrine was largely the result of spillover of norepinephrine into the circulation from sympathetic nerve terminals. Studies using microneurography support this notion by showing that muscle sympathetic nerve activity is elevated with exposure to heat stress (30, 33). Our data indicates that factors related to heat stress modulate sympathetic vasoconstrictor activity such that vasodilator activity overrides vasoconstrictor activity. This prevailing vasodilatation resembles the functional sympatholysis occurring in skeletal muscle vasculature in conditions of increased sympathetic nerve drive during exercise and hypoxia. (34, 36).
A salient observation of this study was that elevations in LVC with heat stress were closely related to the rise in quadriceps muscle temperature and the increases in arterial plasma ATP at rest and during exercise (r2 = 0.85–0.87, both P < 0.01, Fig. 4). The significant relationship implying a role of local hyperthermia upon limb microcirculatory control is consistent with reports from human forearm (5, 6, 19) and leg studies (24), showing that increases in local tissue temperature are tightly related to forearm and leg vasodilatation. However, the results from studies in isolated vessel preparations are equivocal with some reports showing an altered vascular responsiveness to elevations in temperature (23), while others do not (31). An alternative possibility is that local hyperthermia acted indirectly upon vasodilatation and sympatholysis through increases in intravascular ATP and/or other vascular signals sensitive to temperature. In support of a potential role of intravascular ATP, the stepwise regression analysis identified arterial plasma ATP as the best predictor of the increase in LVC during heat stress at rest and during exercise (r2 = 0.87, P = 0.001). Muscle temperature did not increase the predicting power of ATP because it was significantly correlated with ATP (r2 = 0.95, P = 0.001). This could be interpreted to mean that local temperature might trigger the release of ATP into the vascular lumen of the arterial tree providing the limb tissue vasculature, including that of the skeletal muscle and skin, with an augmented intravascular concentration of ATP. This is an attractive idea in light of recent observations indicating that intravascular ATP can act as a potent vasodilator and sympatholytic molecule in the leg and forearm (13, 14, 22, 36, 37). Interestingly, the observed doubling in the arterial ATP concentration could underlie a major role of ATP in heat stress-induced local and systemic hyperemia, as graded doses of intrafemoral artery infusion of ATP, but not femoral venous infusion, has been shown to progressively increase LBF and Q̇ to 8 and 15 l/min, respectively (13). Therefore, the present findings merit further investigation into the sources and the potential roles of intravascular ATP and local tissue temperature on the control of limb muscle and skin perfusion during heat stress and exercise.
Perspectives and Significance
The concept that elevations in SkBF accounts entirely for the increases in Q̇ and the reductions in blood flow in the visceral organs is a widely held dogma in human thermoregulatory and integrative physiology (25, 39). The present and recent findings (21) challenge this classic dogma by supporting that elevations in skeletal muscle blood flow contribute to the increases in limb tissue and systemic perfusion with heat stress and combined heat stress and mild exercise. The increases in skeletal muscle blood flow in conditions where heat stress and/or exercise markedly increase muscle sympathetic vasocontrictor activity (30, 33) require the involvement of vasodilator and sympatholytic signals capable of overriding the augmented neural vasoconstrictor reflexes on the muscle microvasculature. Our results that heat stress-mediated increases in skeletal muscle blood flow share strong relationships with increases in muscle tissue temperature and the arterial concentration of the vasodilator and sympatholytic molecule ATP open new avenues for future research investigating the signaling mechanisms underlying hyperthermia- and exercise-induced hyperemia. Our knowledge and understanding of the role of muscle blood flow in thermoregulation is very limited compared with the extensive literature in the field of muscle metabolism. Our data are important in this respect because they support the hypothesis that increases in skeletal muscle blood flow during heat stress and exercise might have important thermoregulatory implications for the control of local tissue temperature as they can enhance the removal of heat from the muscles via the circulation (i.e., convective heat exchange) (15).
AUTHORS CONTRIBUTIONS
J. Pearson participated in the conception and design of the study, the analysis and interpretation of data, and writing the article. D. A. Low participated in the data collection and interpretation and drafting of the article. E. Stöhr contributed to the data collection. K. Kalsi participated in the data collection and performed the analysis of the plasma catecholamines and part of the plasma ATP samples. L. Ali and H. Barker participated in the data collection and provided medical support for the study. J. González-Alonso participated in the conception, design of the study, data collection, interpretation of data, and drafting of the article. All authors approved the final version of the manuscript for publication.
GRANTS
This study was funded by the Gatorade Sports Science Institute.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
ACKNOWLEDGMENTS
We thank the participants for dedication and commitment without which this study would not have been possible. We also acknowledge Stephane Dufour, Ioannis Papanikolaou, Doireann McMorrow, and Kelly Street for help and assistance during and in preparation for this study.
Present addresses: J. Pearson. Institute for Exercise and Environmental Medicine, Texas Health Presbyterian Hospital Dallas, 7232 Greenville Ave., Dallas, TX 75231 (e-mail: jamespearson@texashealth.org); D. A. Low, Neurovascular and Autonomic Medicine Unit, St. Mary's Hospital, Clinical Neurosciences, Faculty of Medicine, Imperial College London, London, W2 1NY, UK.
REFERENCES
- 1. Abraham P, Leftheriotis G, Desvaux B, Saumet M, Saumet JL. Venous return in lower limb during heat stress. Am J Physiol Heart Circ Physiol 267: H1337–H1340, 1994 [DOI] [PubMed] [Google Scholar]
- 2. Abramson DI, Kahn A, Tuck S, Jr, Turman GA, Rejal H, Fleischer CJ. Relationship between a range of tissue temperature and local oxygen uptake in the human forearm. I. Changes observed under resting conditions. J Clin Invest 37: 1031–1038, 1958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Andersen P, Adams RP, Sjogaard G, Thorboe A, Saltin B. Dynamic knee extension as model for study of isolated exercising muscle in humans. J Appl Physiol 59: 1647–1653, 1985 [DOI] [PubMed] [Google Scholar]
- 4. Barcroft H, Bonnar WM, Edholm OG. Reflex vasodilatation in human skeletal muscle in response to heating the body. J Physiol 106: 271–278, 1947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Barcroft H, Edholm OG. The effect of temperature on blood flow and deep temperature in the human forearm. J Physiol (Lond) 102: 5–20, 1943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Barcroft H, Edholm OG. Temperature and blood flow in the human forearm. J Physiol 104: 366–376, 1946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Davis SL, Fadel PJ, Cui J, Thomas GD, Crandall CG. Skin blood flow influences near-infrared spectroscopy-derived measurements of tissue oxygenation during heat stress. J Appl Physiol 100: 221–224, 2006 [DOI] [PubMed] [Google Scholar]
- 8. Detry JM, Brengelmann GL, Rowell LB, Wyss C. Skin and muscle components of forearm blood flow in directly heated resting man. J Appl Physiol 32: 506–511, 1972 [DOI] [PubMed] [Google Scholar]
- 9. Edholm OG, Fox RH, Macpherson RK. The effect of body heating on the circulation in skin and muscle. J Physiol 134: 612–619, 1956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. González-Alonso J, Calbet JA. Reductions in systemic and skeletal muscle blood flow and oxygen delivery limit maximal aerobic capacity in humans. Circulation 107: 824–830, 2003 [DOI] [PubMed] [Google Scholar]
- 11. González-Alonso J, Calbet JA, Nielsen B. Muscle blood flow is reduced with dehydration during prolonged exercise in humans. J Physiol 513: 895–905, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. González-Alonso J, Dalsgaard MK, Osada T, Volianitis S, Dawson EA, Yoshiga CC, Secher NH. Brain and central haemodynamics and oxygenation during maximal exercise in humans. J Physiol 557: 331–342, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. González-Alonso J, Mortensen SP, Jeppesen TD, Ali L, Barker H, Damsgaard R, Secher NH, Dawson EA, Dufour SP. Haemodynamic responses to exercise, ATP infusion and thigh compression in humans: insight into the role of muscle mechanisms on cardiovascular function. J Physiol 586: 2405–2417, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. González-Alonso J, Olsen DB, Saltin B. Erythrocyte and the regulation of human skeletal muscle blood flow and oxygen delivery: role of circulating ATP. Circ Res 91: 1046–1055, 2002 [DOI] [PubMed] [Google Scholar]
- 15. González-Alonso J, Quistorff B, Krustrup P, Bangsbo J, Saltin B. Heat production in human skeletal muscle at the onset of intense dynamic exercise. J Physiol 524: 603–615, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Gorman MW, Marble DR, Ogimoto K, Feigl EO. Measurement of adenine nucleotides in plasma. Luminescence 18: 173–181, 2003 [DOI] [PubMed] [Google Scholar]
- 17. Hardy JD, Stolwijk JA. Partitional calorimetric studies of man during exposures to thermal transients. J Appl Physiol 21: 1799–1806, 1966 [DOI] [PubMed] [Google Scholar]
- 18. Homma S, Fukunaga T, Kagaya A. Influence of adipose tissue thickness on near infrared spectroscopic signal in the measurement of human muscle. J Biomed Optics 1: 418–424, 1996 [DOI] [PubMed] [Google Scholar]
- 19. Johnson JM, Brengelmann GL, Rowell LB. Interactions between local and reflex influences on human forearm skin blood flow. J Appl Physiol 53: 744–749, 1976 [DOI] [PubMed] [Google Scholar]
- 20. Johnson JM, Proppe DW. Cardiovascular adjustments to heat stress. In: Handbook of Physiology: Environmental Physiology. Bethesda, MD: Am. Physiol. Soc., 1996, sect. 4, vol. II, chapt. 11, p. 215–243 [Google Scholar]
- 21. Keller DM, Sander M, Stallknecht B, Crandall CG. α-Adrenergic vasoconstrictor responsiveness is preserved in the heated human leg. J Physiol 588: 3799–3808, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kirby BS, Voyles WF, Carlson RE, Dinenno FA. Graded sympatholytic effect of exogenous ATP on postjunctional α-adrenergic vasoconstriction in the human forearm: implications for vascular control in contracting muscle. J Physiol 586: 4305–4316, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kluess HA, Buckwalter JB, Hamann JJ, Clifford PS. Elevated temperature decreases sensitivity of P2X purinergic receptors in skeletal muscle arteries. J Appl Physiol 99: 995–998, 2005 [DOI] [PubMed] [Google Scholar]
- 24. Krustrup P, Soderlund K, Mohr M, González-Alonso J, Bangsbo J. Recruitment of fibre types and quadriceps muscle portions during repeated, intense knee-extensor exercise in humans. Pflügers Arch 449: 56–65, 2004 [DOI] [PubMed] [Google Scholar]
- 25. Minson CT, Wladkowski SL, Cardell AF, Pawelczyk JA, Kenney WL. Age alters the cardiovascular response to direct passive heating. J Appl Physiol 84: 1323–1332, 1998 [DOI] [PubMed] [Google Scholar]
- 26. Mortensen SP, Damsgaard R, Dawson EA, Secher NH, González-Alonso J. Restrictions in systemic and locomotor skeletal muscle perfusion, oxygen supply and V̇o2 during high-intensity whole body exercise in humans. J Physiol 586: 2621–2635, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Mortensen SP, González-Alonso J, Damsgaard R, Saltin B, Hellsten Y. Inhibition of nitric oxide and prostaglandins, but not endothelial-derived hyperpolarizing factors, reduces blood flow and aerobic energy turnover in the exercising human leg. J Physiol 581: 853–861, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Nadel ER, Cafarelli E, Roberts MF, Wenger CB. Circulatory regulation during exercise in different ambient temperatures. J Appl Physiol 46: 430–437, 1979 [DOI] [PubMed] [Google Scholar]
- 29. Nielsen B, Savard G, Richter EA, Hargreaves M, Saltin B. Muscle blood flow and muscle metabolism during exercise and heat stress. J Appl Physiol 69: 1040–1046, 1990 [DOI] [PubMed] [Google Scholar]
- 30. Niimi Y, Matsukawa T, Sugiyama Y, Shamsuzzaman AS, Ito H, Sobue G, Mano T. Effect of heat stress on muscle sympathetic nerve activity in humans. J Auton Nerv Syst 63: 61–67, 1997 [DOI] [PubMed] [Google Scholar]
- 31. Padilla J, Garcia-Villalon AL, Fernandez N, Monge L, Gomez B, Dieguez G. Effects of hyperthermia on contraction and dilatation of rabbit femoral arteries. J Appl Physiol 85: 2205–2212, 1998 [DOI] [PubMed] [Google Scholar]
- 32. Rådegran G. Ultrasound Doppler estimates of femoral artery blood flow during dynamic knee extensor exercise in humans. J Appl Physiol 83: 1383–1388, 1997 [DOI] [PubMed] [Google Scholar]
- 33. Ray CA, Gracey KH. Augmentation of exercise-induced muscle sympathetic nerve activity during muscle heating. J Appl Physiol 82: 1719–1725, 1997 [DOI] [PubMed] [Google Scholar]
- 34. Remensnyder JP, Mitchell JH, Sarnoff SJ. Functional sympatholysis during muscular activity. Observations on influence of carotid sinus on oxygen uptake. Circ Res 11: 370–380, 1962 [DOI] [PubMed] [Google Scholar]
- 35. Roddie IC, Shepherd JT, Whelan RF. Evidence from venous oxygen saturation measurements that the increase in forearm blood flow during body heating is confined to the skin. J Physiol 134: 444–450, 1956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Rosenmeier JB, Hansen J, González-Alonso J. Circulating ATP-induced vasodilatation overrides sympathetic vasoconstrictor activity in human skeletal muscle. J Physiol 558: 351–365, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Rosenmeier JB, Yegutkin GG, González-Alonso J. Activation of ATP/UTP selective receptors increase blood flow and blunt sympathetic vasoconstriction in human skeletal muscle. J Physiol 586: 4993–5002, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Rowell LB. Cardiovascular aspects of human thermoregulation. Circ Res 52: 367–379, 1983 [DOI] [PubMed] [Google Scholar]
- 39. Rowell LB. Human cardiovascular adjustments to exercise and thermal stress. Physiol Rev 54: 75–159, 1974 [DOI] [PubMed] [Google Scholar]
- 40. Rowell LB, Brengelmann GL, Murray JA. Cardiovascular responses to sustained high skin temperature in resting man. J Appl Physiol 27: 673–680, 1969 [DOI] [PubMed] [Google Scholar]
- 41. Savard GK, Nielsen B, Laszczynska J, Larsen BE, Saltin B. Muscle blood flow is not reduced in humans during moderate exercise and heat stress. J Appl Physiol 64: 649–657, 1988 [DOI] [PubMed] [Google Scholar]
- 42. Smolander J, Louhevaara V. Effect of heat stress on muscle blood flow during dynamic handgrip exercise. Eur J Appl Physiol Occup Physiol 65: 215–220, 1992 [DOI] [PubMed] [Google Scholar]
- 43. Tayefeh F, Kurz A, Sessler DI, Lawson CA, Ikeda T, Marder D. Thermoregulatory vasodilation increases the venous partial pressure of oxygen. Anesth Analg 85: 657–662, 1997 [DOI] [PubMed] [Google Scholar]
- 44. van Hall G, González-Alonso J, Sacchetti M, Saltin B. Skeletal muscle substrate metabolism during exercise: methodological considerations. Proc Nutr Soc 58: 899–912, 1999 [DOI] [PubMed] [Google Scholar]
- 45. Wenger CB, Bailey RB, Roberts MF, Nadel ER. Interaction of local and reflex thermal effects in control of forearm blood flow. J Appl Physiol 58: 251–257, 1985 [DOI] [PubMed] [Google Scholar]
- 46. Wesseling KH, Jansen JR, Settels JJ, Schreuder JJ. Computation of aortic flow from pressure in humans using a nonlinear, three-element model. J Appl Physiol 74: 2566–2573, 1993 [DOI] [PubMed] [Google Scholar]
- 47. Williams CA, Lind AR. Measurement of forearm blood flow by venous occlusion plethysmography: influence of hand blood flow during sustained and intermittent isometric exercise. Eur J Appl Physiol Occup Physiol 42: 141–149, 1979 [DOI] [PubMed] [Google Scholar]