Abstract
Artificial selection in rat has yielded high-capacity runners (HCR) and low-capacity runners (LCR) that differ in intrinsic (untrained) aerobic exercise ability and metabolic disease risk. To gain insight into how oxygen metabolism may have been affected by selection, we compared mitochondrial function, oxidative DNA damage (8-dihydroxy-guanosine; 8dOHG), and antioxidant enzyme activities in soleus muscle (Sol) and gastrocnemius muscle (Gas) of adult and aged LCR vs. HCR rats. In Sol of adult HCR rats, maximal ADP-stimulated respiration was 37% greater, whereas in Gas of adult HCR rats, there was a 23% greater complex IV-driven respiratory capacity and 54% greater leak as a fraction of electron transport capacity (suggesting looser mitochondrial coupling) vs. LCR rats. H2O2 emission per gram of muscle was 24–26% greater for both muscles in adult HCR rats vs. LCR, although H2O2 emission in Gas was 17% lower in HCR, after normalizing for citrate synthase activity (marker of mitochondrial content). Despite greater H2O2 emission, 8dOHG levels were 62–78% lower in HCR rats due to 62–96% higher superoxide dismutase activity in both muscles and 47% higher catalase activity in Sol muscle in adult HCR rats, with no evidence for higher 8 oxoguanine glycosylase (OGG1; DNA repair enzyme) protein expression. We conclude that genetic segregation for high running capacity has generated a molecular network of cellular adaptations, facilitating a superior response to oxidative stress.
Keywords: adaptation, mitochondrial respiration, aging, oxidative metabolism, metabolic syndrome
artificial selection for high (HCR) vs. low (LCR) running capacity in rats has generated a unique animal model system for dissection of aerobic endurance capacity and its correlated traits. The major hypothesis is that functional alleles at multiple interacting loci that determine intrinsic aerobic capacity have been enriched or fixed differentially by selection pressure applied across several generations. By using a rotational breeding paradigm that minimizes the coefficient of inbreeding (37), the divergent selected lines maintain genetic complexity (55) and, when bred concurrently, serve as reciprocal controls for unknown environmental changes.
Consistent with the importance of mitochondria to aerobic metabolism during exercise (26), there is mounting evidence that selection for low- and high-running capacity has led to significant divergence in mitochondrial content and function between HCR and LCR rats and that these differences play a crucial role in explaining the differential disease susceptibility between these strains (74). For example, the gastrocnemius (Gas) muscle [primarily mixed fast-twitch fiber type; (6)] of HCR rats has greater citrate synthase enzyme activity (29), and the soleus muscle (Sol) [primarily slow twitch fiber type; (6)] of HCR rats has higher protein expression of cytochrome-c oxidase subunit I and the mitochondrial uncoupling protein, UCP2 (74). In addition, small permeabilized fiber bundles of soleus muscle exhibit a greater sensitivity to creatine-stimulated respiration in HCR rats (73). Because altered mitochondrial function and imbalance in reactive oxygen intermediates are thought to be central to aging (47) and chronic disease (56), we examined mitochondrial function via high-resolution respirometry and fluorometric measurement of hydrogen peroxide (H2O2) emission in small permeabilized muscle bundles from LCR and HCR rats in adult and old age groups. To provide insight into how the expected differences in mitochondrial function between HCR and LCR rats affected oxidative stress responses, we examined oxidative DNA damage via measurement of 8-dihydroxy-guanosine (8dOHG), antioxidant enzyme activities, and the oxidative DNA repair enzyme, 8-oxoguanine glycosylase (OGG1). On the basis of previous reports of elevated markers of muscle mitochondrial enzymes (29, 30, 49) and higher mitochondrial uncoupling protein expression (49, 74) in skeletal muscle of HCR rats, we hypothesized that HCR rats would exhibit alterations in mitochondrial function that would represent a balance between those that favor aerobic metabolism (e.g., high muscle respiratory capacity) and those aimed at minimizing oxidative damage (e.g., low ROS production and/or enhanced antioxidant enzyme activity). We also hypothesized that these adaptations would result in a lower abundance of 8dOHG in skeletal muscle of adult HCR rats and that this benefit would be maintained with aging.
MATERIALS AND METHODS
Animals.
The selection experiment yielding HCR and LCR rats has been described in detail previously (37). In brief, animals were selectively bred for intrinsic (untrained) endurance running capacity from a founder population of 96 male and 96 female heterogeneous rats from N:NIH stock. Intrinsic running capacity was tested in young adult rats (∼3 mo old), with a velocity-ramped treadmill test beginning at a speed of 10 m/min (elevation of 15°) and increasing 1 m/min every 2 min until exhaustion. Thirteen breeding pairs with the highest recorded time to exhaustion were bred, and the 13 breeding pairs with the lowest time to exhaustion were bred to generate contrasting models, which, by generation 11, differed in running distance by 347% (74). For the present experiments, 20 male (10 HCR and 10 LCR) rats from generation 21 were obtained for the adult groups, and 14 male (7 HCR and 7 LCR) rats from generation 20 were obtained for the aged groups. All breeding and tests for intrinsic running capacity were done at the University of Michigan (Ann Arbor, MI), and then the rats were air shipped to the University of Calgary for further study.
The University of Calgary Animal Care Committee approved all experimental methods subsequently described. Upon arrival at the University of Calgary, animals were housed individually in cages with filter bonnets at the biological sciences vivarium (12:12-h light-dark cycle, ambient temperature = 22°C) without access to a running wheel. Animals were given standard chow and water ad libitum. Animals for the adult age group arrived at our institution at 8 mo old and were killed at 13–15 mo of age. Rats for the aged group were 15–17 mo of age upon arrival, and we waited 7 mo before sacrificing these animals at an age of 22–24 mo (approximately the 50% survival rate for LCR rats; S. L. Britton and L. G. Koch, unpublished results). Over time, one LCR rat in the adult group died leaving nine LCR rats for study, and one HCR rat and four LCR rats in the aged group died leaving six HCR and three LCR rats in the old age groups. Because of the low sample size in the aged animals, particularly in LCR rats, all data for aged animals are reported in the supplemental data.
Muscle harvesting and preparation of muscle fiber bundles.
Animals were anesthetized with 50–60 mg/kg ip pentobarbital sodium. Upon establishing deep surgical anesthesia, the right Sol and Gas muscles, which are representative of largely slow-twitch and largely fast-twitch muscles, respectively (6), were harvested and placed in ice-cold buffer X containing 7.23 mM K2EGTA, 2.77 mM CaK2 EGTA, 20 mM imidazole, 0.5 mM taurine, 5.7 mM ATP, 14.3 mM PCr, 6.56 mM Cl2-6H2O, and 50 mM MES (pH 7.1). These are also muscles recruited during locomotor behavior in the rat (41), and thus, likely to exhibit traits favoring high running capacity in HCR animals. Next, the left Sol and Gas muscles were removed and rapidly frozen in liquid nitrogen. The left Gas was pulverized under liquid nitrogen to create a homogenous powder and facilitate representative sampling of all fiber types in this muscle. Muscle samples were stored at −80 C until analyzed for oxidative DNA damage, antioxidant enzyme activities, and OGG1 content (see Measurement of oxidative DNA damage; Antioxidant enzyme activities; and Western blotting for OCG1 content). Following muscle harvest, the rats were promptly euthanized by cardiac removal.
To obtain distinct representation of slow oxidative vs. fast oxidative muscle fibers, (4), small fiber bundles (∼3–5 mg) were cut from the Sol muscle [largely slow twitch in rat (6)] and from the red region of Gas muscle [Gr; ∼50% fast oxidative fibers in rat (6)] of the right leg, placed in ice-cold buffer X and manually teased apart (leaving connections at various points between individual fibers) with sharp ended needles. Samples were then either incubated in buffer X with 100 μl saponin for 30 min on ice with mild stirring, or cryopreserved (see below). Following incubation in saponin, bundles were washed 3 times in ice-cold buffer Z containing 105 mM K-MES, 30 mM KCl, 10 mM KH2PO4, 5 mM MgCl2-6H2O, 1 mM EGTA, and 5 mg/ml BSA (pH 7.4) to remove saponin and metabolites. Fiber bundles were stored (for no more than 60 min) on ice in buffer Z until respirometry measures.
Cryopreservation of muscle fiber bundles.
We cryopreserved 2 or 3 muscle bundles of each fiber type per animal using a cryopreservation method shown previously to have minimal effects on mitochondrial function in small-muscle fiber bundles (38). This method involved placing muscle fiber bundles in polyethylene tubes containing 100 μl buffer X supplemented with 30% DMSO and 10 mg/ml BSA, waiting 5 s, and then rapidly freezing the sample by immersion of the Eppendorff tube in liquid N2 before storing at −80°C until used in H2O2 measurements (see H2O2 production).
Respirometry.
Respiration was measured in 2 ml of buffer Z at 37°C with a polarographic oxygen sensor designed for high-resolution respirometry (Oxygraph-2k; Oroboros, Innsbruck, Austria). All measures were done following the required oxygraph calibration. Two substrate protocols were performed in parallel (one in each chamber), protocol 1: 5 mM glutamate + 2.5 mM malate, 2 mM adenosine diphosphate, 10 mM succinate, 8 μM cytochrome c, 8 μg/ml oligomycin, and 2.5 μM p-trifluoromethoxy carbonyl cyanide phenyl hydrazone (FCCP; a protonophore and uncoupler of respiration); and protocol 2: 5 mM glutamate + 2.5 mM malate (g), 2 mM adenosine diphosphate (ADP), 10 mM succinate (succ), 10 μM antimycin A (aa), 0.6 mM N,N,N′,N′-tetramethyl-p-phenylenediamine (TMPD; an artificial electron donor for complex IV) + 2.4 mM ascorbate (TMPD + Asc), and 2 mM potassium cyanide (KCN). Following respirometry measures, bundles were placed on a cleaning wipe (Kim Wipe, Kimberly-Clark) to remove the buffer, and they were weighed before being frozen in liquid N2 and stored at −80°C for citrate synthase enzyme activity measurement (see Citrate synthase activity).
H2O2 production.
The long duration of the respirometry protocols precluded assessment of H2O2 production on the same day, as tissues were harvested. As such, in preliminary experiments, we determined the impact of bundle cryopreservation on H2O2 production using small-fiber bundles prepared from the soleus muscle of a Sprague-Dawley rat, using a cyropreservation method shown previously to preserve mitochondrial respiratory capacity (38). Eight bundles were immediately prepared for study (as below), whereas five bundles were prepared for cryopreservation as described above. One week after freezing, the cryopreserved bundles were thawed and used in preliminary experiments. All other methods for these preliminary trials were the same as for the LCR and HCR comparisons. As we observed, similar H2O2 production in cryopreserved bundles and freshly prepared bundles for all conditions except state I (basal H2O2 release; see Supplemental Fig. 1 in the online version of this article), the analysis of H2O2 production in muscles from HCR and LCR animals was done using cryopreserved bundles.
On the day of experiment, cryopreserved muscle fiber bundles were thawed in a water bath at 37°C. Once the cryopreservation solution was fully thawed, muscle fiber bundles were washed 3 times in buffer X supplemented with 2 mg/ml BSA to remove DMSO. Measurements of H2O2 production were performed with a Hitachi F-2500 fluorescence spectrophotometer to detect resorufin, a byproduct of the 1:1 reaction of Amplex red and H2O2. Resorufin is detectable at an excitation/emission wavelength of 563 nm/587 nm and is catalyzed by the addition of horseradish peroxidase (HRP). Note that the H2O2 detected by the Amplex Red system largely reflects the stoichiometric conversion of superoxide to H2O2 by endogenous superoxide dismutase (70) and is a widely used system for obtaining insights into ROS generation (4, 45). Washed muscle fiber bundles were incubated in buffer X with 100 μl saponin for 30 min on ice with mild stirring, washed in three changes of ice-cold buffer Z, and then placed in a fluorometer cuvette containing 1 ml of buffer Z supplemented with 5 μM Amplex red and 0.17 U/ml HRP. The cuvette was then placed in the fluorometer, which contained a thermojacketed cuvette holder to maintain a temperature of 37°C. After baseline measures, substrate additions were injected as follows: 5 mM glutamate + 2 mM malate; 3 mM succinate; 10 μM ADP, 100 μM ADP, and 1 mM ADP.
Citrate synthase activity.
Muscle bundles frozen following the respirometry measurements were used in measurement of citrate synthase enzyme activity by the method of Srere (67), following mercaptide ion production in a spectrophotometer at 412 nm. All methodology was carried out, as we have described previously (27), with the exception that we adapted the protocol to permit use with a 96-well plate format. Briefly, previously frozen muscle bundles were thawed on ice, weighed, and homogenized on ice with a hand homogenizer in homogenizing buffer (1:200 ratio) containing 100 mM KPO4 + 5 mM EDTA + 5 mM EGTA (pH 7.4) for ∼40 s. Homogenates were then vortexed and subjected to three freeze thaws in liquid N2. After the third thaw, samples underwent a second dilution of 1:2 with homogenizing buffer for a final dilution of 1:400. Measurements of citrate synthase activity in each sample were done in triplicate and involved loading individual wells on the microplate with 170 μl of reaction buffer (containing 0.3 mM acetyl Co A, 0.1 mM 5.5′-dithiobis[2-nitrobenzoic acid], and 100 mM Tris buffer) and 20 μl of sample homogenate, after which absorbance was read to determine endogenous levels of thiol or deacetylase activity. Following this initial absorbance reading, 10 μl of 10 mM OAA was added to each well to start the enzyme reaction. Citrate synthase activity was calculated by subtracting the endogenous activity from the final reading and expressed as micromoles per minute per gram wet mass.
Measurement of oxidative DNA damage.
Total genomic DNA (nuclear and mitochondrial) was extracted from portions of the Sol and Gas muscles using QIAmp DNA Mini kit (Qiagen, Mississauga, ON, Canada) as per the manufacturer's instructions. The concentration of the isolated DNA was determined using NanoDrop ND-1000 UV-Vis spectrophotometer (ThermoScientific, Ottawa, ON, Canada). The DNA concentrations ranged from 400 to 800 ng/nl, and the average absorbance ratio of A260/A280 was between 1.7 and 1.9. One microgram of total DNA from each sample was dot blotted on a nitrocellulose membrane (Amersham, Piscataway, NJ). 8dOHG immunoblotting was carried out using mouse monoclonal 8-dOHG (N45.1) antibody (Japan Institute for the Control of Aging, Fukuroi, Japan). Membranes were then incubated with anti-mouse HRP-linked secondary antibody (Bio-Rad Laboratories, Burlington, ON, Canada) and were visualized by enhanced chemiluminescence (Amersham). Relative intensities of the circular dots were digitally quantified by using National Institutes of Health (NIH) ImageJ analysis software (version 1.37, Scion Image, NIH, Bethesda, MD).
Antioxidant enzyme activities.
Frozen tissue samples (15–20 mg of Sol and 20–30 mg of powdered Gas) were rapidly weighed and homogenized using a Potter-Elvehjem homogenizer at 4°C, in a buffer containing KH2PO4 (100 mM), DTT (1 mM), and EDTA (2 mM), at a pH of 7.4. After centrifugation (3,000 g for 5 min), the supernatant was used for the enzymatic assays. Superoxide dismutase (SOD) activity was assayed spectrophotometrically (at 550 nm) by monitoring the rate of acetylated cytochrome-c reduction by superoxide radicals generated by the xanthine-xanthine oxidase system at 25°C in reaction buffer (xanthine, 0.5 mM; cytochrome c, 0.2 mM; KH2PO4, 50 mM; and EDTA, 0.1 mM; pH 7.8) (20). One activity unit of SOD is defined as the amount of enzyme that inhibits the rate of acetylated cytochrome-c reduction by 50%. To distinguish manganese-SOD (MnSOD), which is exclusively located in mitochondrial matrix, from copper zinc-SOD (CuZnSOD), which is primarily located in the cytosol and to a lesser degree in the mitochondrial intermembrane space (43, 50), SOD activity was determined after incubation with NaCN (1 mM). At this concentration, cyanide inhibits the CuZnSOD isoform of the enzyme but does not affect the MnSOD isoform (20). SOD activity was expressed as units per gram of total protein.
The total activity of glutathione peroxidase (GPx) activity was assayed with cumene hydroperoxide (1 mg/ml) as a substrate by measuring spectrophotometrically the reduction of NADPH (ε = 6.22 × 103·l·mol−1·cm−1) at 340 nm (68). Measurement was carried out at 37°C in reaction buffer (GSH, 0.25 mM; NADPH, 0.12 mM; GR, 1 U/ml; and NaCN, 10 mM). GPx activity was expressed as micromoles per gram of total protein.
The activity of catalase (CAT) was determined by the method of Aebi (1). Each supernatant (200 μl) was incubated 30 min at 4°C with ethanol (95%, 2 μl). After adding Triton (1%, 2 μl), the sample was centrifuged at 5,000 g for 5 min at 4°C. The supernatant was used for measurement of CAT activity by using the first-order rate constant of the decomposition of H2O2 by tissue CAT at 37°C in buffer (pH 7.4) containing KH2PO4 (40 mM) and HNa2PO4 (60 mM). One unit of catalase activity was calculated by using k = (2.3/dt)(log A1/A2), where k is CAT activity, dt is change in time, A1 is initial absorbance, and A2 is final absorbance. CAT activity was expressed in Kelvins per gram of total protein.
Western blotting for OGG1 content.
Samples (30–50 mg) from each tissue (Sol and Gas muscles) were homogenized in a RIPA buffer (NaCl 150 mM, Tris-HCl 50 mM, NP-40 1%, sodium deoxycholate 0.5%, and SDS 0.1%, pH 8) and incubated 1 h in an orbital shaker (4°C). After a centrifugation at 12,000 rpm (20 min, 4°C), supernatant concentrations were determined by BCA assay (Pierce, Rockford, IL). Equal quantities of protein (20 μg) were loaded into precast 4–15% SDS-PAGE (Bio-Rad, Hercules, CA) and electrophoresed for 1.5 h at 110 V. Proteins were then electrotransferred for 1.5 h at 400 mA onto a PVDF membrane and probed overnight with a polyclonal antibody directed against OGG1 (NOVUS Biologicals, Littleton, CO; dilution 1:750). Equal loading was verified using the Ponceau red stain and by detection of control protein (β-tubulin, dilution 1:1,000; Abcam, Cambridge, MA). Membranes were washed in 0.05% Tween-PBS buffer and incubated with HRP-conjugated secondary antibody (dilution 1:1,000). Signals were detected using the enhanced chemiluminescence (Pierce), and chemiluminescence was digitally captured (Syngene Bio-Imager, Frederick, MD), and densitometry was measured using the Bio-Imager software (Syngene Tools, Frederick, MD).
Statistics.
Within each age group, respirometry and H2O2 emission measures were analyzed with a two-way ANOVA, with selection group (HCR/LCR) and substrate/inhibitor condition as fixed factors, and a Holm-Sidak multiple-comparison test when significant main effects were present. Respiratory control ratio, citrate synthase activity, and 8-dOHG were analyzed by two-way ANOVA, with selection group (HCR/LCR) and age as fixed factors, and a Holm-Sidak multiple-comparison test when significant main effects were present. Values are expressed as means ± SE.
RESULTS
Running capacity and body weight.
Descriptive data for LCR and HCR rats for adult groups are provided in Table 1. Data from aged LCR and HCR rats are provided in Supplemental Table 1 in the online version of this article. LCR rats were significantly heavier than HCR rats. In addition, in another cohort of adult animals we have observed that HCR rats are markedly leaner, exhibiting a body fat of 13.5 ± 1.5% vs. 21.4 ± 2.0% in LCR animals (R. T. Hepple, unpublished data). Running capacity assessed at 11 wk of age was 8- to 9-fold greater in the HCR rats, regardless of the generation in the selection experiment (Table 1 and Supplemental Table 1).
Table 1.
Descriptive data
| n | Selection Group | Generation | Running capacity at 11 wk of age, m | Body mass at 11 wk of age, g | n at time of experiment | Age at time of experiment, mo | Body mass at time of experiment, g |
|---|---|---|---|---|---|---|---|
| 10 | LCR adult | 21 | 214 ± 13 | 353 ± 13 | 9 | 14.3 ± 0.3 | 616 ± 28 |
| 10 | HCR adult | 21 | 1696 ± 61* | 230 ± 12* | 10 | 14.6 ± 0.2 | 451 ± 14* |
Values are expressed as means ± SE.
P < 0.05 vs. LCR.
Citrate synthase activity.
In Sol muscle bundles from adult animals, there was no detectable difference between HCR and LCR groups (Table 2). However, in Gr muscle bundles, HCR rats had significantly greater citrate synthase (CS) activity compared with LCR. In the aged animal groups (see Supplemental Table 2 in the online version of this article), CS activity was higher for both Sol and Gr in the HCR animals.
Table 2.
Citrate synthase activity
| Muscle | LCR, μmol·min−1·g−1 | HCR, μmol·min−1·g−1 |
|---|---|---|
| Soleus | 25.6 ± 1.3 | 22.9 ± 1.2 |
| Red gastrocnemius | 26.4 ± 1.7 | 37.9 ± 1.6* |
Values are expressed as means ± SE.
P < 0.05 vs. LCR.
Respirometry.
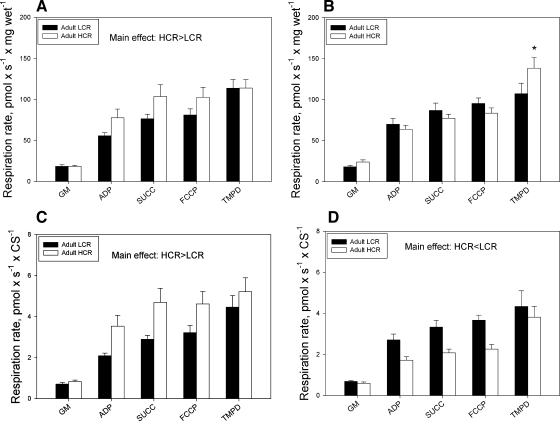
There were no significant increases in oxygen consumption following the addition of exogenous cytochrome c (data not shown), indicating that the permeabilization procedure did not disrupt the mitochondrial outer membrane. For Sol muscle bundles in adult animals, two-way ANOVA showed a significant main effect for selection group, with HCR having higher O2 flux than LCR (Fig. 1A), but no interaction between substrate condition and selection group. In Gr muscle, there were significant interactions between both selection group and substrate condition, with post hoc tests revealing significantly higher flux in HCR animals during TMPD + Asc-stimulated respiration (Fig. 1B). In aged animals, two-way ANOVA revealed no significant interaction between selection group and substrate condition in either Sol or Gr muscle bundles (see Supplemental Fig. 2, A and B, respectively, in the online version of this article). Similarly, there were no significant main effects for selection group in either muscle in the aged animals.
Fig. 1.
Respiration in small bundles of soleus muscle and the red region of gastrocnemius muscle normalized to muscle (wet) mass (A, B) or normalized to citrate synthase actitity (C, D) of adult LCR and HCR rats. *P < 0.05 vs. LCR. GM, gluatamate and malate; ADP, adenosine diphosphate; SUCC, succinate; FCCP, p-trifluoromethoxy carbonyl cyanide phenyl hydrazone; TMPD, N,N,N′,N′-tetramethyl-p-phenylenediamine.
In adult animals, normalization of respirometry measures to CS activity (used as a proxy for mitochondrial content) resulted in no interactions; however, main effects indicated significant differences, with higher respiratory capacity in Sol muscle bundles of HCR rats and lower respiratory capacity in Gr muscle bundles of HCR rats (Fig. 1, C and D, respectively). In aged animals, normalization to CS activity yielded significant main effects for selection group in Sol and Gr muscle bundles, with LCR having significantly higher respiratory capacity than HCR (Supplemental Fig. 2, C and D, respectively).
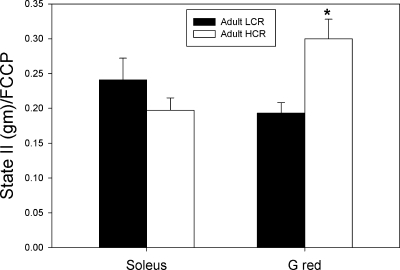
In adult animals, whereas the proportion of electron transport capacity (FCCP) attributable to leak (state II with glutamate and malate as substrates) was not different between HCR and LCR groups in Sol muscle bundles, it was significantly greater in Gr muscle bundles of HCR animals (Fig. 2). In the aged animals (see Supplemental Fig. 3 in the online version of this article), the proportion of electron transport capacity attributable to leak did not differ by selection group in either Sol or Gr muscle bundles.
Fig. 2.
Leak (state II respiration with glutamate and malate as substrates) as a fraction of electron transport capacity (FCCP) in small muscle bundles of soleus muscle and the red region of gastrocnemius (G red) muscle of adult and aged low-capacity runner (LCR) and high-capacity runner (HCR) rats. *P < 0.05 vs. LCR group.
H2O2 production.
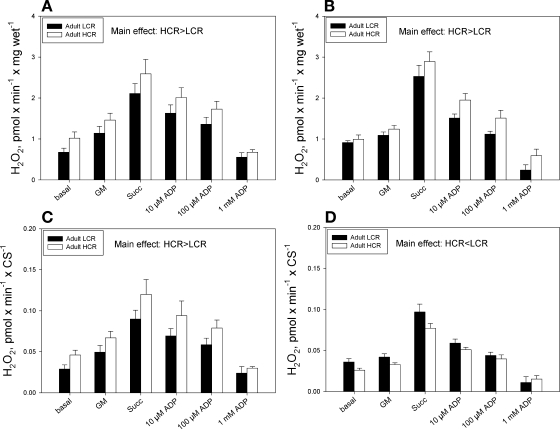
In adult animals, there were no significant interactions between selection group and substrate condition for H2O2 production in either Sol or Gr muscle bundles. Significant main effects were found, however, with higher H2O2 production in both Sol and Gr muscle bundles in HCR vs. LCR rats (Fig. 3, A and B, respectively). Similarly, in aged animals there were no significant interactions between selection group and substrate condition for either muscle, whereas there were significant main effects for selection group in Gr muscle only, with HCR animals having a higher H2O2 emission than LCR animals (see Supplemental Fig. 4B in the online version of this article).
Fig. 3.
H2O2 production in small bundles of soleus muscle and the red region of gastrocnemius muscle in LCR and HCR rats normalized to muscle (wet) mass (A, B) or normalized to citrate synthase activity (C, D).
In adult animals, normalization of H2O2 emission to CS activity did not change the greater H2O2 production in Sol muscle bundles of HCR vs. LCR rats (Fig. 3C); however, because of greater CS activity in Gr muscle in HCR rats, there was a lower H2O2 production per unit of CS activity in Gr muscle of HCR vs. LCR rats (Fig. 3D). In aged animals, although normalization to CS activity had no impact on the lack of differences in H2O2 between selection groups seen in Sol muscle bundles (see Supplemental Fig. 4C in the online version of this article), there was a significant interaction between selection group and substrate condition in Gr muscle bundles, with lower H2O2 production in HCR than LCR rats in the succinate condition (see Supplemental Fig. 4D in the online version of this article).
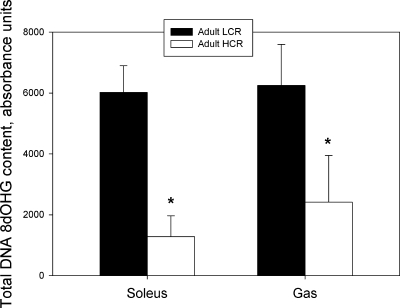
Oxidative DNA damage.
In both adult (Fig. 4) and aged (Supplemental Fig. 5) animals, 8-OHdG content, an indicator of oxidative DNA damage, was significantly lower in HCR animals compared with LCR animals for both the Sol muscle and Gas muscle.
Fig. 4.
Total 8-dOHG content in DNA isolated from soleus muscle and mixed gastrocnemius muscle of adult LCR and HCR rats. *P < 0.05 vs. LCR group.
Antioxidant enzyme activities.
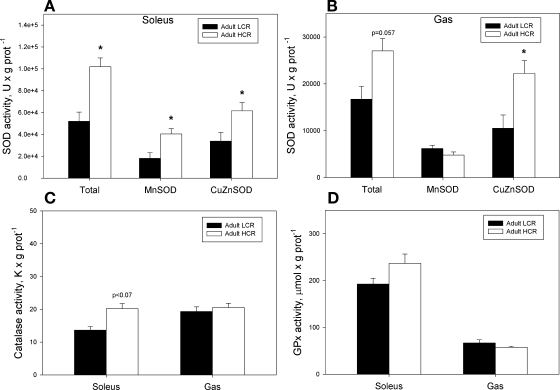
In adult animals, total SOD activity in the Sol muscle was greater in HCR rats due to greater activity of both MnSOD and CuZnSOD (Fig. 5A). In Gas muscle, total SOD activity also tended to be greater in HCR rats (P = 0.057) but only due to greater activity of CuZnSOD (Fig. 5B). In aged animals, there were no differences in SOD activity between selection groups in either muscle (see Supplemental Fig. 6, A and B in the online version of this article).
Fig. 5.
Superoxide dismutase (A, B), catalase (C) and glutathione peroxidase (D) enzyme activities in soleus muscle and gastrocnemius muscle of adult LCR and HCR rats. *P < 0.05 vs. LCR group.
In adult animals, there was a trend (P = 0.07) for a greater CAT activity in the Sol muscle of HCR rats (Fig. 5C), and there was no difference in GPx activity between selection groups in either muscle (Fig. 5D). In aged animals, CAT activity was significantly greater in the Sol muscle of HCR animals (see Supplemental Fig. 6C in the online version of this article), whereas GPx activity was not different between selection groups in either muscle (see Supplemental Fig. 6D in the online version of this article).
OGG1 protein expression.
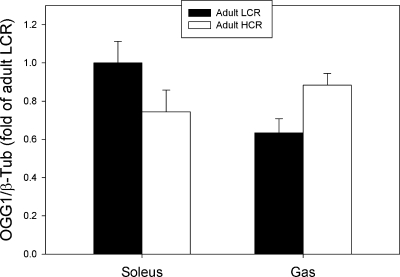
There were no detectable differences in the protein expression of the DNA repair enzyme, OGG1, between selection groups in adult animals in either Sol or Gas muscle using β-tubulin as a loading control (Fig. 6). Similarly, there was no difference in OGG1 expression between selection groups in aged animals (Supplemental Fig. 7 in the online version of this article). Note that these analyses were repeated with sarcomeric actin as a loading control with the same result (data not shown).
Fig. 6.
Protein levels of the DNA repair enzyme, OGG1, in the soleus and gastrocnemius (Gas) adult LCR and HCR rats.
DISCUSSION
Rats selectively bred for high intrinsic running capacity also exhibit markedly lower risk for metabolic disease (11, 49, 69). Given the association between mitochondrial dysfunction and metabolic disease risk (34) and the suspected involvement of reactive oxygen by-products in this association (28, 64), we examined mitochondrial function and markers of oxidative stress responses in a slow-twitch muscle (Sol) and a fast-twitch muscle (Gas) of HCR and LCR rats. Adult HCR rats had greater maximal ADP-stimulated respiration in Sol muscle bundles and greater TMPD-Asc-stimulated respiration in red region of Gr muscle bundles (suggesting greater mitochondrial complex IV activity in the fast oxidative region of this mixed fiber type muscle). Despite greater H2O2 emission per gram of muscle in both Sol and Gr in adult HCR rats, adult and aged HCR rats exhibited lower 8dOHG levels in both Sol and Gas muscles. This lower oxidative damage despite greater ROS production was explained by greater total SOD activity in Sol and Gas of adult HCR rats, as well as a trend to greater CAT activity in Sol of adult HCR rats (P = 0.07); there was no difference in the DNA repair enzyme OGG1. We conclude that HCR rats exhibit superior cellular responses to oxidative stress vs. LCR rats, providing valuable insight into the mechanisms underlying metabolic disease resistance in HCR rats.
Muscle respiratory capacity.
Previous studies show that HCR rats have higher markers of mitochondrial content in their locomotor muscles. Specifically, CS activity (29, 30, 49) and the expression of various mitochondrial proteins (74) are higher in the Gas muscle and Sol muscle, respectively, of adult HCR rats. The only prior study to examine mitochondrial respiratory capacity directly observed a greater mitochondrial sensitivity to ADP in the presence of creatine in fiber bundles of Sol muscle in HCR rats (73). Our results show that Sol fiber bundles exhibited greater maximal ADP-stimulated respiration in adult HCR vs. LCR rats. In contrast, although bundles of the Gr muscle fibers in adult HCR rats had similar maximal ADP-stimulated respiration, they had a greater TMPD-Asc stimulated respiration, indicating a greater capacity of complex IV in the mitochondria of this fast oxidative muscle region in adult HCR rats. In addition, the proportion of electron transport capacity that was attributable to leak, which is one index of mitochondrial coupling (21), was greater in Gr of adult HCR rats. Both the greater relative complex IV capacity and the greater leak as a fraction of electron transport capacity would be expected to reduce mitochondrial ROS generation in Gr of adult HCR rats. Prior studies have noted an increased expression of mitochondrial uncoupling proteins in muscles of HCR rats (49, 74), which may explain the looser coupling that we observed. The looser coupling in Gr of HCR rats is similar to what occurs in muscles of exercise-trained humans (8).
After normalizing the respiration measures to a marker of mitochondrial content (citrate synthase activity), we observed a greater respiratory capacity per mitochondrion in Sol and a lower respiratory capacity per mitochondrion in Gr of adult HCR rats. Interestingly, our results show considerable modulation of the respiratory capacity per mitochondrion through artificial selection for intrinsic running capacity, which is distinct from the adaptation to exercise training where increases in muscle respiratory capacity parallel increases in markers of mitochondrial content (i.e., respiratory capacity per mitochondrion is constant) (12). These results also suggest that the usual maintenance of mitochondrial enzyme stoichiometry seen with increases in mitochondrial content as a result of endurance training (17) can be modulated under the conditions governed within this selection experiment. Furthermore, our results demonstrate that adaptations of mitochondrial function are distinct between a slow oxidative muscle (greater respiratory capacity per mitochondrion in Sol) and a fast oxidative muscle (lower respiratory capacity per mitochondrion and looser coupling in Gr in HCR rats).
Mitochondrial reactive oxygen species generation and oxidative stress.
ROS generated by mitochondria are an important component of normal physiological function (22, 62), but when excessive, ROS can cause cellular damage and contribute to disease (63). Our current results show that H2O2 emission in fiber bundles is elevated by ∼25% in both Sol and Gr muscles of adult HCR over LCR rats. Whereas normalization of H2O2 emission to citrate synthase activity (to obtain an index of ROS generation per mitochondrion) revealed greater H2O2 emission per mitochondrion in Sol muscle of adult HCR rats, H2O2 emission per mitochondrion was lower in the Gr muscle of adult HCR rats. This observation is consistent with the adaptations noted in the previous section, where the Gr muscle of adult HCR rats exhibited a greater relative complex IV activity and looser coupling, both of which facilitate lower mitochondrial ROS production (5, 7, 18, 66). A lower mitochondrial ROS production in the Gr muscle of adult HCR rats may represent an important adaptation to prevent ROS from reaching detrimental levels, while still permitting the benefits of having a higher mitochondrial content for running capacity in these animals (noting that CS activity was 44% greater in Gr of adult HCR rats vs. adult LCR rats). It is also interesting that the lower ROS generation per mitochondrion observed in the Gr of adult HCR rats is similar to the lower mitochondrial ROS generation observed in muscle following adaptation to exercise training in mice (10). On the other hand, because of greater endogenous antioxidant capacity in the Sol, this muscle would have a larger buffer between physiological vs. detrimental levels of ROS, and this may explain why in the Sol muscle, selection for high running capacity permitted the beneficial effect of having a higher respiratory capacity per mitochondrion to dominate over adaptations that would temper ROS generation.
Note that although we used cryopreserved bundles in making the assessment of H2O2 emission in our experiments, as we show in Supplemental Fig. 1, the only condition in which we saw an elevated H2O2 production in cryopreserved bundles vs. fresh bundles was under basal (state I) conditions. Since differences between HCR and LCR rats were observed under all substrate conditions, the use of cryopreserved bundles is unlikely to affect our results. Further to this point, the values for H2O2 emission that we report here in Gr bundles is remarkably similar to what we have observed previously in freshly prepared bundles (54).
What is most striking about our results is that despite the greater ROS generation per gram in both muscles of adult HCR rats, this did not translate to greater 8dOHG damage. In fact, the adult HCR rats had 79% lower 8dOHG levels in Sol and 62% lower 8dOHG levels in Gas, revealing more than simply a proportionally increased ROS buffering capacity, but actually a superior oxidative stress response in HCR rats. Interestingly, these results are consistent with evidence linking mildly elevated oxidative stress to beneficial cellular adaptations (24, 71), including the adaptations seen with exercise training (59), where ROS generated during exercise (9) induce upregulation of endogenous antioxidant defenses via activation of NF-κB and MAPK signaling pathways (32). In this respect, we observed significantly greater SOD activity in both the Sol and Gas, and a trend to greater catalase activity (P = 0.07) in Sol muscle of adult HCR rats, consistent with a ROS-induced upregulation of endogenous antioxidant defenses in adult HCR rats. Although this is similar to what is seen with exercise training (57, 58) and heterozygous knockout of Mclk-1 in mice (39), on the basis of the divergent transcript responses to exercise training in HCR vs. LCR rats (13), HCR rats have a superior cell stress response in general rather than simply upregulated antioxidant defenses secondary to higher ROS exposure.
Since oxidative damage to DNA can be repaired, principally by the DNA glycosylase OGG1 (35), we also determined whether differences in the protein expression of OGG1 might contribute to the lower 8dOHG levels in adult HCR rats. However, as seen in Fig. 6, we found no evidence that this was the case in either fast-twitch or slow-twitch muscle. Although we did not measure OGG1 activity per se in our experiments, it appears that the selection experiment for high running capacity differs from the adaptation to exercise training where OGG1 activity is upregulated in both liver (46) and skeletal muscle (60). These data also suggest that the primary factor responsible for the lower 8dOHG burden in the HCR animals was the upregulation of endogenous antioxidants noted above.
Impact of selecting for high running capacity on aging.
Although our limited sample size in the aged LCR group warrants some caution in our interpretations of the impact of aging on mitochondrial function in this model, the data had very little variability within each group, and we can make some initial observations. First, it is clear that some, but not all, of the mitochondrial function differences observed in adult HCR vs. LCR rats are maintained with aging, and that the Sol muscle maintains fewer of these adaptations as a result of aging than does the Gr region of Gas muscle in HCR rats. Specifically, in aged HCR animals, the Gr region still maintains a greater CS activity than in LCR animals, and this is accompanied by a continued lower respiratory capacity per CS activity, and greater H2O2 emission per gram of muscle. On the other hand, in Sol muscle, the greater respiratory capacity per CS activity and the greater H2O2 emission (per gram and per CS activity) seen in adult HCR animals are both lost with aging. In Gr muscle the lower H2O2 emission per CS activity and greater leak as a fraction of electron transport capacity in adult HCR rats are both lost with aging. Similarly, the higher activity of SOD evident for both muscles of adult HCR animals is not sustained with aging (due to an age-dependent increase in SOD activity in LCR animals). Notwithstanding these alterations, the lower burden of 8dOHG damage in both Sol and Gas muscle of adult HCR animals is maintained in aged animals, demonstrating continued protection against oxidative damage into old age for the HCR animals. Therefore, our results show that despite moderately elevated ROS generation in adult HCR animals, the superior endogenous antioxidant defenses in adulthood translate to reduced oxidative damage accumulation with aging.
Perspectives and Significance
The evolution of our understanding of the positive vs. negative impacts of ROS on organism health is a work in progress, with recent evidence suggesting elevations in ROS and/or oxidative stress can actually be beneficial. Although early studies of the free radical theory of aging were consistent with ROS-induced damage playing an important role in aging (52, 61, 65), subsequent studies have revealed this to be a much more complicated issue. For example, transgenic upregulation of endogenous antioxidants in recent studies is often found to have no benefit for life span (51). In addition, knocking out endogenous antioxidant defenses does not consistently shorten life span (72), and some interventions that exacerbate oxidative stress are found to paradoxically extend life span (40, 71). Further to this emerging evidence that elevated ROS is not harmful to aging, but may also yield beneficial effects, we observed that although rats selected for high intrinsic running capacity exhibit elevated muscle ROS generation, they have a lower level of oxidative DNA damage that persists to old age. Given the well-established metabolic disease resistance of HCR rats (49, 69, 74), our results suggest that a modestly elevated ROS, coupled with a superior transcriptional response to stress (13), may play an important role in resistance to metabolic disease, providing further evidence of the complexity of the responses to elevated ROS in normal physiology. Finally, the fact that this protection from oxidative damage continued into old age in HCR rats may foreshadow superior aging in this model as well.
GRANTS
This work was funded by operating grants from the Canadian Institutes of Health Research (MOP 57808 and IAO 84673 to R. T. Hepple, and BCA 20071 to M. A. Tarnopolsky), Natural Sciences and Engineering Research Council (RPG 238805 to R. T. Hepple), and the National Center for Research Resources of the National Institutes of Health to L. G. Koch and S. L. Britton (R24 RR017718). Expert care of the LCR and HCR rat colony was provided by Lori Gilligan. A. Safdar was supported by a Doctoral Award from the Canadian Institutes of Health Research, R. T. Hepple was supported by a Senior Scholar Award from the Alberta Heritage Foundation for Medical Research, and Y. Burelle was supported by a Chercher Boursier Junior II Award from Fonds de Recherche en Sante du Quebec.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
Supplementary Material
ACKNOWLEDGMENTS
The authors also wish to acknowledge the assistance of Sharon Rowan in the conduct of several of the assay procedures.
REFERENCES
- 1. Aebi H. Catalase in vitro. Methods Enzymol 105: 121–126, 1984 [DOI] [PubMed] [Google Scholar]
- 2. Alcendor RR, Gao S, Zhai P, Zablocki D, Holle E, Yu X, Tian B, Wagner T, Vatner SF, Sadoshima J. Sirt1 regulates aging and resistance to oxidative stress in the heart. Circ Res 100: 1512–1521, 2007 [DOI] [PubMed] [Google Scholar]
- 3. Anastasiou D, Krek W. SIRT1: linking adaptive cellular responses to aging-associated changes in organismal physiology. Physiology 21: 404–410, 2006 [DOI] [PubMed] [Google Scholar]
- 4. Anderson EJ, Neufer PD. Type II skeletal myofibers possess unique properties that potentiate mitochondrial H2O2 generation. Am J Physiol Cell Physiol 290: C844–C851, 2006 [DOI] [PubMed] [Google Scholar]
- 5. Anderson EJ, Yamazaki H, Neufer PD. Induction of endogenous uncoupling protein 3 suppresses mitochondrial oxidant emission during fatty acid-supported respiration. J Biol Chem 282: 31257–31266, 2007 [DOI] [PubMed] [Google Scholar]
- 6. Armstrong RB, Phelps RO. Muscle fiber type composition of the rat hindlimb. Am J Anat 171: 259–272, 1984 [DOI] [PubMed] [Google Scholar]
- 7. Barja G. Mitochondrial oxygen consumption and reactive oxygen species production are independently modulated: implications for aging studies. Rejuvenation Res 10: 215–224, 2007 [DOI] [PubMed] [Google Scholar]
- 8. Befroy DE, Petersen KF, Dufour S, Mason GF, Rothman DL, Shulman GI. Increased substrate oxidation and mitochondrial uncoupling in skeletal muscle of endurance-trained individuals. Proc Natl Acad Sci USA 105: 16701–16706, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bejma J, Ji LL. Aging and acute exercise enhance free radical generation in rat skeletal muscle. J Appl Physiol 87: 465–470, 1999 [DOI] [PubMed] [Google Scholar]
- 10. Brooks SV, Vasilaki A, Larkin LM, McArdle A, Jackson MJ. Repeated bouts of aerobic exercise lead to reductions in skeletal muscle free radical generation and nuclear factor κB activation. J Physiol 586: 3979–3990, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Buck BJ, Kerman IA, Burghardt PR, Koch LG, Britton SL, Akil H, Watson SJ. Upregulation of GAD65 mRNA in the medulla of the rat model of metabolic syndrome. Neurosci Lett 419: 178–183, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Burelle Y, Hochachka PW. Endurance training induces muscle-specific changes in mitochondrial function in skinned muscle fibers. J Appl Physiol 92: 2429–2438, 2002 [DOI] [PubMed] [Google Scholar]
- 13. Bye A, Hoydal MA, Catalucci D, Langaas M, Kemi OJ, Beisvag V, Koch LG, Britton SL, Ellingsen O, Wisloff U. Gene expression profiling of skeletal muscle in exercise-trained and sedentary rats with inborn high and low V̇o2max. Physiol Genomics 35: 213–221, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Bye A, Langaas M, Hoydal MA, Kemi OJ, Heinrich G, Koch LG, Britton SL, Najjar SM, Ellingsen O, Wisloff U. Aerobic capacity-dependent differences in cardiac gene expression. Physiol Genomics 33: 100–109, 2008 [DOI] [PubMed] [Google Scholar]
- 15. Cohen HY, Miller C, Bitterman KJ, Wall NR, Hekking B, Kessler B, Howitz KT, Gorospe M, de Cabo R, Sinclair DA. Calorie restriction promotes mammalian cell survival by inducing the SIRT1 deacetylase. Science 305: 390–392, 2004 [DOI] [PubMed] [Google Scholar]
- 16. Cooke MS, Evans MD, Dizdaroglu M, Lunec J. Oxidative DNA damage: mechanisms, mutation, and disease. FASEB J 17: 1195–1214, 2003 [DOI] [PubMed] [Google Scholar]
- 17. Davies KJ, Packer L, Brooks GA. Biochemical adaptation of mitochondria, muscle, and whole-animal respiration to endurance training. Arch Bioch Biophys 209: 539–554, 1981 [DOI] [PubMed] [Google Scholar]
- 18. Echtay KS. Mitochondrial uncoupling proteins—what is their physiological role? Free Radic Biol Med 43: 1351–1371, 2007 [DOI] [PubMed] [Google Scholar]
- 19. Ferrara N, Rinaldi B, Corbi G, Conti V, Stiuso P, Boccuti S, Rengo G, Rossi F, Filippelli A. Exercise training promotes SIRT1 activity in aged rats. Rejuvenation Res 11: 139–150, 2008 [DOI] [PubMed] [Google Scholar]
- 20. Flohe L, Otting F. Superoxide dismutase assays. Methods Enzymol 105: 93–104, 1984 [DOI] [PubMed] [Google Scholar]
- 21. Gnaiger E. Textbook on Mitochondrial Physiology (electronic ed.), edited by Gnaiger E. Mitochondrial Physiology Society, 2009 [Google Scholar]
- 22. Gomez-Cabrera MC, Borras C, Pallardo FV, Sastre J, Ji LL, Vina J. Decreasing xanthine oxidase-mediated oxidative stress prevents useful cellular adaptations to exercise in rats. J Physiol 567: 113–120, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Gonzalez NC, Kirkton SD, Howlett RA, Britton SL, Koch LG, Wagner HE, Wagner PD. Continued divergence in V̇o2max of rats artificially selected for running endurance is mediated by greater convective blood O2 delivery. J Appl Physiol 101: 1288–1296, 2006 [DOI] [PubMed] [Google Scholar]
- 24. Hartwig K, Heidler T, Moch J, Daniel H, Wenzel U. Feeding a ROS-generator to Caenorhabditis elegans leads to increased expression of small heat shock protein HSP-16.2 and hormesis. Genes Nutr 4: 59–67, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Henderson KK, Wagner H, Favret F, Britton SL, Koch LG, Wagner PD, Gonzalez NC. Determinants of maximal O2 uptake in rats selectively bred for endurance running capacity. J Appl. Physiol. 93: 1265–1274, 2002 [DOI] [PubMed] [Google Scholar]
- 26. Hepple RT. Skeletal muscle: microcirculatory adaptation to metabolic demand. Med Sci Sports Ex 32: 117–123, 2000 [DOI] [PubMed] [Google Scholar]
- 27. Hepple RT, Baker DJ, Kaczor JJ, Krause DJ. Long-term caloric restriction abrogates the age-related decline in skeletal muscle aerobic function. FASEB J 19: 1320–1322, 2005 [DOI] [PubMed] [Google Scholar]
- 28. Hopps E, Noto D, Caimi G, Averna MR. A novel component of the metabolic syndrome: The oxidative stress. Nutr Metab Cardiovasc Dis 20: 72–77, 2009 [DOI] [PubMed] [Google Scholar]
- 29. Howlett RA, Gonzalez NC, Wagner HE, Fu Z, Britton SL, Koch LG, Wagner PD. Genetic models in applied physiology: selected contribution: skeletal muscle capillarity and enzyme activity in rats selectively bred for running endurance. J Appl Physiol 94: 1682–1688, 2003 [DOI] [PubMed] [Google Scholar]
- 30. Howlett RA, Kirkton SD, Gonzalez NC, Wagner HE, Britton SL, Koch LG, Wagner PD. Peripheral oxygen transport and utilization in rats following continued selective breeding for endurance running capacity. J Appl Physiol 106: 1819–1825, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Jeong J, Juhn K, Lee H, Kim SH, Min BH, Lee KM, Cho MH, Park GH, Lee KH. SIRT1 promotes DNA repair activity and deacetylation of Ku70. Exp Mol Med 39: 8–13, 2007 [DOI] [PubMed] [Google Scholar]
- 32. Ji LL, Gomez-Cabrera MC, Vina J. Exercise and hormesis: activation of cellular antioxidant signaling pathway. Ann NY Acad Sci 1067: 425–435, 2006 [DOI] [PubMed] [Google Scholar]
- 33. Kang H, Jung JW, Kim MK, Chung JH. CK2 is the regulator of SIRT1 substrate-binding affinity, deacetylase activity and cellular response to DNA-damage. PLoS ONE 4: e6611, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Kim JA, Wei Y, Sowers JR. Role of mitochondrial dysfunction in insulin resistance. Circ Res 102: 401–414, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Klungland A, Rosewell I, Hollenbach S, Larsen E, Daly G, Epe B, Seeberg E, Lindahl T, Barnes DE. Accumulation of premutagenic DNA lesions in mice defective in removal of oxidative base damage. Proc Natl Acad Sci USA 96: 13300–13305, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Koch LG, Britton SL. Aerobic metabolism underlies complexity and capacity. J Physiol 586: 83–95, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Koch LG, Britton SL. Artificial selection for intrinsic aerobic endurance running capacity in rats. Physiol Genom 5: 45–52, 2001 [DOI] [PubMed] [Google Scholar]
- 38. Kuznetsov AVK, Wolfram S, Saks Valdur, Usson Yves, Mazat J-P, Letellier Thierry, Gellerich Frank N. Margreiter, Raimund Cryopreservation of mitochondria and mitochondrial function in cardiac and skeletal muscle fibers. Analytical Biochemistry 319: 296–303, 2003 [DOI] [PubMed] [Google Scholar]
- 39. Lapointe J, Hekimi S. Early mitochondrial dysfunction in long-lived Mclk1+/− mice. J Biol Chem 283: 26217–26227, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Lapointe J, Stepanyan Z, Bigras E, Hekimi S. Reversal of the mitochondrial phenotype and slow development of oxidative biomarkers of aging in long-lived Mclk1+/− mice. J Biol Chem 284: 20364–20374, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Laughlin MH, Armstrong RB. Muscular blood flow distribution patterns as a function of running speed in rats. Am J Physiol Heart Circ Physiol 243: H296–H306, 1982 [DOI] [PubMed] [Google Scholar]
- 42. Martin-Gronert MS, Tarry-Adkins JL, Cripps RL, Chen JH, Ozanne SE. Maternal protein restriction leads to early life alterations in the expression of key molecules involved in the aging process in rat offspring. Am J Physiol Regul Integr Comp Physiol 294: R494–R500, 2008 [DOI] [PubMed] [Google Scholar]
- 43. Mattiazzi M, D'Aurelio M, Gajewski CD, Martushova K, Kiaei M, Beal MF, Manfredi G. Mutated human SOD1 causes dysfunction of oxidative phosphorylation in mitochondria of transgenic mice. J Biol Chem 277: 29626–29633, 2002 [DOI] [PubMed] [Google Scholar]
- 44. Morris EM, Whaley-Connell AT, Thyfault JP, Britton SL, Koch LG, Wei Y, Ibdah JA, Sowers JR. Low aerobic capacity and high-fat diet contribute to oxidative stress and IRS-1 degradation in the kidney. Am J Nephrol 30: 112–119, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Muller FL, Liu Y, Abdul-Ghani MA, Lustgarten MS, Bhattacharya A, Jang YC, Van Remmen H. High rates of superoxide production in skeletal-muscle mitochondria respiring on both complex I- and complex II-linked substrates. Biochem J 409: 491–499, 2008 [DOI] [PubMed] [Google Scholar]
- 46. Nakamoto H, Kaneko T, Tahara S, Hayashi E, Naito H, Radak Z, Goto S. Regular exercise reduces 8-oxodG in the nuclear and mitochondrial DNA and modulates the DNA repair activity in the liver of old rats. Exp Gerontol 42: 287–295, 2007 [DOI] [PubMed] [Google Scholar]
- 47. Navarro A, Boveris A. The mitochondrial energy transduction system and the aging process. Am J Physiol Cell Physiol 292: C670–C686, 2007 [DOI] [PubMed] [Google Scholar]
- 48. Nemoto S, Fergusson MM, Finkel T. SIRT1 functionally interacts with the metabolic regulator and transcriptional coactivator PGC-1α. J Biol Chem 280: 16456–16460, 2005 [DOI] [PubMed] [Google Scholar]
- 49. Noland RC, Thyfault JP, Henes ST, Whitfield BR, Woodlief TL, Evans JR, Lust JA, Britton SL, Koch LG, Dudek RW, Dohm GL, Cortright RN, Lust RM. Artificial selection for high-capacity endurance running is protective against high-fat diet-induced insulin resistance. Am J Physiol Endocrinol Metab 293: E31–E41, 2007 [DOI] [PubMed] [Google Scholar]
- 50. Okado-Matsumoto A, Fridovich I. Subcellular distribution of superoxide dismutases (SOD) in rat liver: Cu,Zn-SOD in mitochondria. J Biol Chem 276: 38388–38393, 2001 [DOI] [PubMed] [Google Scholar]
- 51. Orr WC, Mockett RJ, Benes JJ, Sohal RS. Effects of overexpression of copper-zinc and manganese superoxide dismutases, catalase, and thioredoxin reductase genes on longevity in Drosophila melanogaster. J Biol Chem 278: 26418–26422-, 2003 [DOI] [PubMed] [Google Scholar]
- 52. Orr WC, Sohal RS. Extension of life-span by overexpression of superoxide dismutase and catalase in Drosophila melanogaster. Science 263: 1128–1130, 1994 [DOI] [PubMed] [Google Scholar]
- 53. Pfluger PT, Herranz D, Velasco-Miguel S, Serrano M, Tschop MH. Sirt1 protects against high-fat diet-induced metabolic damage. Proc Natl Acad Sci USA 105: 9793–9798, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Picard M, Ritchie D, Wright KJ, Thomas MM, Rowan SL, Taivassalo T, Hepple RT. Mitochondrial functional impairment with aging is exaggerated in isolated mitochondria compared to permeabilized myofibers. Aging Cell doi: 9: 1032–1046, 2010 [DOI] [PubMed] [Google Scholar]
- 55. Pickrell JK, Coop G, Novembre J, Kudaravalli S, Li JZ, Absher D, Srinivasan BS, Barsh GS, Myers RM, Feldman MW, Pritchard JK. Signals of recent positive selection in a worldwide sample of human populations. Genome Res 19: 826–837, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Pieczenik SR, Neustadt J. Mitochondrial dysfunction and molecular pathways of disease. Exp Mol Pathol 83: 84–92, 2007 [DOI] [PubMed] [Google Scholar]
- 57. Powers SK, Criswell D, Lawler J, Ji LL, Martin D, Herb RA, Dudley G. Influence of exercise and fiber type on antioxidant enzyme activity in rat skeletal muscle. Am J Physiol Regul Integr Comp Physiol 266: R375–R380, 1994 [DOI] [PubMed] [Google Scholar]
- 58. Radak Z, Chung HY, Goto S. Exercise and hormesis: oxidative stress-related adaptation for successful aging. Biogerontology 6: 71–75, 2005 [DOI] [PubMed] [Google Scholar]
- 59. Radak Z, Chung HY, Goto S. Systemic adaptation to oxidative challenge induced by regular exercise. Free Radic Biol Med 44: 153–159, 2008 [DOI] [PubMed] [Google Scholar]
- 60. Radak Z, Kumagai S, Nakamoto H, Goto S. 8-Oxoguanosine and uracil repair of nuclear and mitochondrial DNA in red and white skeletal muscle of exercise-trained old rats. J Appl Physiol 102: 1696–1701, 2007 [DOI] [PubMed] [Google Scholar]
- 61. Richter C, Park JW, Ames BN. Normal oxidative damage to mitochondrial and nuclear DNA is extensive. Proc Natl Acad Sci USA 85: 6465–6467, 1988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Ristow M, Zarse K, Oberbach A, Kloting N, Birringer M, Kiehntopf M, Stumvoll M, Kahn CR, Bluher M. Antioxidants prevent health-promoting effects of physical exercise in humans. Proc Natl Acad Sci USA 106: 8665–8670, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Roberts CK, Sindhu KK. Oxidative stress and metabolic syndrome. Life Sci 84: 705–712, 2009 [DOI] [PubMed] [Google Scholar]
- 64. Roberts CK, Won D, Pruthi S, Kurtovic S, Sindhu RK, Vaziri ND, Barnard RJ. Effect of a short-term diet and exercise intervention on oxidative stress, inflammation, MMP-9, and monocyte chemotactic activity in men with metabolic syndrome factors. J Appl Physiol 100: 1657–1665, 2006 [DOI] [PubMed] [Google Scholar]
- 65. Sohal RS, Weindruch R. Oxidative stress, caloric restriction, and aging. Science 273: 59–63, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Speakman JR, Talbot DA, Selman C, Snart S, McLaren JS, Redman P, Krol E, Jackson DM, Johnson MS, Brand MD. Uncoupled and surviving: individual mice with high metabolism have greater mitochondrial uncoupling and live longer. Aging Cell 3: 87–95, 2004 [DOI] [PubMed] [Google Scholar]
- 67. Srere PA. Citrate synthase. Methods Enzymol 13: 3–5, 1969 [Google Scholar]
- 68. Tappel AL. Glutathione peroxidase and hydroperoxides. Methods Enzymol 52: 506–513, 1978 [DOI] [PubMed] [Google Scholar]
- 69. Thyfault JP, Rector R, Uptergrove GM, Borengasser SJ, Morris EM, Wei Y, Laye MJ, Burant CF, Qi NR, Ridenour SE, Koch LG, Britton SL, Ibdah JA. Rats selectively bred for low aerobic capacity have reduced hepatic mitochondrial oxidative capacity and susceptibility to hepatic steatosis and injury. J Physiol 587: 1805–1816, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Treberg JR, Quinlan CL, Brand MD. Hydrogen peroxide efflux from muscle mitochondria underestimates matrix superoxide production—a correction using glutathione depletion. FEBS J 277: 2766–2778, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Van Raamsdonk JM, Hekimi S. Deletion of the mitochondrial superoxide dismutase sod-2 extends lifespan in Caenorhabditis elegans. PLoS Genet 5: e1000361, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Van Remmen H, Ikeno Y, Hamilton M, Pahlavani M, Wolf N, Thorpe SR, Alderson NL, Baynes JW, Epstein CJ, Huang TT, Nelson J, Strong R, Richardson A. Life-long reduction in MnSOD activity results in increased DNA damage and higher incidence of cancer but does not accelerate aging. Physiol Genomics 16: 29–37, 2003 [DOI] [PubMed] [Google Scholar]
- 73. Walsh B, Hooks RB, Hornyak JE, Koch LG, Britton SL, Hogan MC. Enhanced mitochondrial sensitivity to creatine in rats bred for high aerobic capacity. J Appl Physiol 100: 1765–1769, 2006 [DOI] [PubMed] [Google Scholar]
- 74. Wisloff U, Najjar SM, Ellingsen O, Haram PM, Swoap S, Al-Share Q, Fernstrom M, Rezaei K, Lee SJ, Koch LG, Britton SL. Cardiovascular risk factors emerge after artificial selection for low aerobic capacity. Science 307: 418–420, 2005 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.