Abstract
Recent evidence suggests that an acute increase in the generation of phagocyte-like NADPH-oxidase (Nox)-mediated reactive oxygen species (ROS) may be necessary for glucose-stimulated insulin secretion. Using rat islets and INS 832/13 cells, we tested the hypothesis that activation of specific G proteins is necessary for nutrient-mediated intracellular generation of ROS. Stimulation of β-cells with glucose or a mixture of mitochondrial fuels (mono-methylsuccinate plus α-ketoisocaproic acid) markedly elevated intracellular accumulation of ROS, which was attenuated by selective inhibitors of Nox (e.g., apocynin or diphenyleneiodonium chloride) or short interfering RNA-mediated knockdown of p47phox, one of the subunits of Nox. Selective inhibitors of protein prenylation (FTI-277 or GGTI-2147) markedly inhibited nutrient-induced ROS generation, suggesting that activation of one (or more) prenylated small G proteins and/or γ-subunits of trimeric G proteins is involved in this signaling axis. Depletion of endogenous GTP levels with mycophenolic acid significantly reduced glucose-induced activation of Rac1 and ROS generation in these cells. Other immunosuppressants, like cyclosporine A or rapamycin, which do not deplete endogenous GTP levels, failed to affect glucose-induced ROS generation, suggesting that endogenous GTP is necessary for glucose-induced Nox activation and ROS generation. Treatment of INS 832/13 cells or rat islets with pertussis toxin (Ptx), which ADP ribosylates and inhibits inhibitory class of trimeric G proteins (i.e., Gi or Go), significantly attenuated glucose-induced ROS generation in these cells, implicating activation of a Ptx-sensitive G protein in these signaling cascade. Together, our findings suggest a prenylated Ptx-sensitive signaling step couples Rac1 activation in the signaling steps necessary for glucose-mediated generation of ROS in the pancreatic β-cells.
Keywords: G protein, pancreatic islets, Rac1 activation, pertussis toxin, inosine monophosphate dehydrogenase
glucose-induced insulin secretion (GSIS) involves a series of metabolic and cationic events, leading to translocation of insulin-laden secretory granules from a distal site toward the plasma membrane for fusion and release of insulin into circulation. It is widely accepted that vesicular transport and fusion involves interplay between signaling proteins, including vesicle-associated membrane proteins on the secretory granule and docking proteins on the plasma membrane (23, 28, 33). Furthermore, interaction between these proteins is widely felt to require cytoskeletal remodeling, which is under the fine control of small molecular mass G proteins belonging to the Rho subfamily (e.g., Cdc42 and Rac1; see Ref. 17 for a recent review). Several effector proteins for these small G proteins have been identified in the islet β-cell, including phospholipases, p21-activated kinase-1 kinase, and ERK1/2 kinases (17, 40, 42).
In the context of G proteins, it is well established that they undergo posttranslational modifications for optimal activation, membrane trafficking, and effector interactions. The majority of small G proteins undergoes a series of modifications at their COOH-terminal cysteine residues, which include prenylation (i.e., farnesylation and geranylgeranylation), carboxylmethylation (CML), and palmitoylation. In addition to small G proteins, the γ-subunits of trimeric G proteins undergo prenylation and CML (2, 13, 16, 17, 40). Indeed, using pharmacological and molecular biological approaches, several recent studies have confirmed the requisite nature of these modifications in GSIS in a variety of insulin-secreting cells, including clonal β-cells, normal rodent islets, and human islets (see Ref. 17 for a recent review).
A growing body of recent evidence implicated roles for reactive oxygen species (ROS) in metabolic dysfunction of the islet β-cell under the duress of glucolipotoxicity, cytokines, and ceramide (26, 38, 39). It has been shown that increased ROS generation seen under the above experimental conditions is derived from the activation of phagocyte-like NADPH oxidase (Nox), since inhibition of this enzyme by selective inhibitors [e.g., diphenyleneiodonium chloride (DPI) or apocynin] or transfection of short interfering RNA (siRNA) against individual subunits of Nox (e.g., p47phox) significantly attenuated deleterious effects of aforementioned noxious stimuli (38, 39).
Despite the negative modulatory role(s) of ROS in cell function, recent evidence appears to indicate that a tonic increase in the ROS generation may be necessary for GSIS and fatty acid-induced insulin secretion (5, 29–32). ROS have also been shown to modulate many physiological processes, including ion transport and protein phosphorylation (1, 4, 9, 21). As reviewed recently by Pi and Collins (32), ROS plays “second messenger” role in modulating islet β-cell function. Along these lines, studies by Pi and coworkers (31) have demonstrated that glucose-mediated generation of H2O2 alters intracellular redox status, leading to augmented GSIS; such effects were attenuated by coprovision of antioxidants. These findings were further strengthened by Leloup and colleagues (20), suggesting that generation of mitochondrial ROS is a requisite stimulus for GSIS to occur. Together, these data implicate an essential role for Nox-derived ROS as a signaling molecule involved in the regulation of β-cell function, specifically at the level of insulin secretion. The present studies are undertaken to determine potential mechanisms underlying nutrient-induced elevation of ROS levels in INS 832/13 cells and normal rat islets. Specifically, we have determined the roles of G proteins in this signaling cascade; this was accomplished by selective inhibitors of protein prenylation (e.g., GGTI-2147 and FTI-277), which have been used to verify the roles for G proteins in GSIS (17). In addition, we have examined permissive roles for endogenous GTP in nutrient-induced ROS generation. Our findings implicate that prenylation-sensitive signaling steps are necessary for glucose- and mitochondrial fuel-induced intracellular generation of ROS in INS 832/13 cells and normal rat islets.
MATERIALS AND METHODS
Materials.
DPI, apocynin, pertussis toxin (Ptx), mycophenolic acid (MPA), cyclosporine A, rapamycin, mono-methylsuccinate, α-keto-isocaproic acid, and 2′,7′-dichlorofluorescein diacetate (DCHFDA) were from Sigma (St. Louis, MO). p47phox siRNA and p47phox antiserum were from Santa Cruz Biotechnology (Santa Cruz, CA). FTI-277 and GGTI-2147 were from Calbiochem (San Diego, CA). Rac1 activation kit was from Cytoskeleton (Denver, CO).
Insulin-secreting cells.
INS 832/13 cells were provided by Dr. Chris Newgard (Duke University Medical Center, Durham, NC) and were cultured in RPMI-1640 medium containing 10% heat-inactivated fetal bovine serum supplemented with 100 IU/ml penicillin and 100 IU/ml streptomycin, 1 mM sodium pyruvate, 50 μM 2-mercaptoethanol, and 10 mM HEPES (pH 7.4). The medium was changed twice, and cells were subcloned weekly. Islets from normal Sprague-Dawley rats were isolated by collagenase digestion method described previously (41). All experiments, including isolation of pancreatic islets from normal Sprague-Dawley rats, were reviewed and approved by the Wayne State University Institutional Animal Care and Use Committee.
Quantitation of ROS.
This was carried out as our laboratory described recently in Ref. 39. In brief, INS 832/13 cells were seeded in six-well plate and treated with various insulin secretagogues and inhibitors (or their respective diluents), as indicated in the text. Following incubation, the medium was removed, and cells were further incubated with DCHFDA (10 μM) at 37°C for 30 min in RPMI. DCHFDA, being a nonpolar compound, diffuses rapidly into the cells and hydrolyzes readily by cellular esterases into polar 2′,7′-dichlorofluorescein. In the presence of ROS, 2′,7′-dichlorofluorescein readily oxidizes to fluorescent dichlorofluorescein. The cells were washed with ice-cold phosphate-buffered saline and harvested, and equal amounts of protein were taken for fluorescence measurements (emission wavelength: 485 nm and excitation wavelength: 535 nm) using luminescence spectrophotometer (PerkinElmer, Waltham, MA).
Inhibition of Nox activity via molecular biological or pharmacological approaches.
INS 832/13 cells were seeded in a 24-well plate and at 50–60% confluence either mock transfected or transfected with antisense p47phox siRNA at a final concentration of 150 nM and cultured for 24 h. Following this, cells were stimulated with low glucose (2.5 mM) or high glucose (20 mM) for 1 h. At the end of stimulation, culture medium was removed; cells were incubated further with DCHFDA (10 μM) at 37°C for 30 min in RPMI, washed with ice cold PBS, and harvested; equal amount of proteins were taken; and fluorescence was measured (excitation wavelength: 485 nm, and emission wavelength: 535 nm) using luminescence spectrophotometer as described above. Alternatively, Nox activity was inhibited via a pharmacological approach by incubating INS 832/13 cells either with apocynin (100 μM; 12 h) or DPI (5 μM; 2 h) in low-serum, low-glucose-containing medium. Following incubation, cells were stimulated with low glucose (2.5 mM) or high glucose (20 mM) for 1 h in the continuous absence or presence of inhibitors, and NADPH activity was measured by DCHFDA assay, as described above. The amount of fluorescence recorded is directly correlated with the amount of superoxide radicals generated due to Nox activity.
Rac1 activation assay.
This was accomplished using a pull-down assay that our laboratory described recently (18). Briefly, INS 832/13 cells were starved overnight in low-serum, low-glucose-containing medium in either the presence or absence of MPA (10 μM). At the end of incubation, cells were stimulated with low glucose (2.5 mM) or high glucose (20 mM) for 30 min in the continuous presence or absence of MPA. Lysates (∼500 μg protein) were clarified by centrifugation for 5 min at 4,800 g, and p21-activated kinase-binding domain beads (20 μl) were added to the supernatant. The mixture was then rotated for 1 h at 4°C and pelleted by centrifugation at 4,000 g for 3 min. The pellet was washed once with lysis buffer followed by a rinse (3×) in wash buffer (25 mM Tris, pH 7.5, 30 mM MgCl2, 40 mM NaCl, and 150 mM EDTA). Proteins in the pellet were resolved by SDS-PAGE and transferred onto a nitrocellulose membrane, and Western blotting method determined the relative abundance of activated Rac1.
Other assays and statistical analysis of data.
Protein concentrations were determined by Bradford's dye-binding method using bovine serum albumin as the standard. Statistical significance of differences between diluent and experimental groups was determined by Student's t-test and ANOVA analysis. P < 0.05 was considered significant.
RESULTS
Pharmacological inhibitors or siRNA-p47phox markedly attenuate glucose-induced ROS generation in insulin-secreting cells.
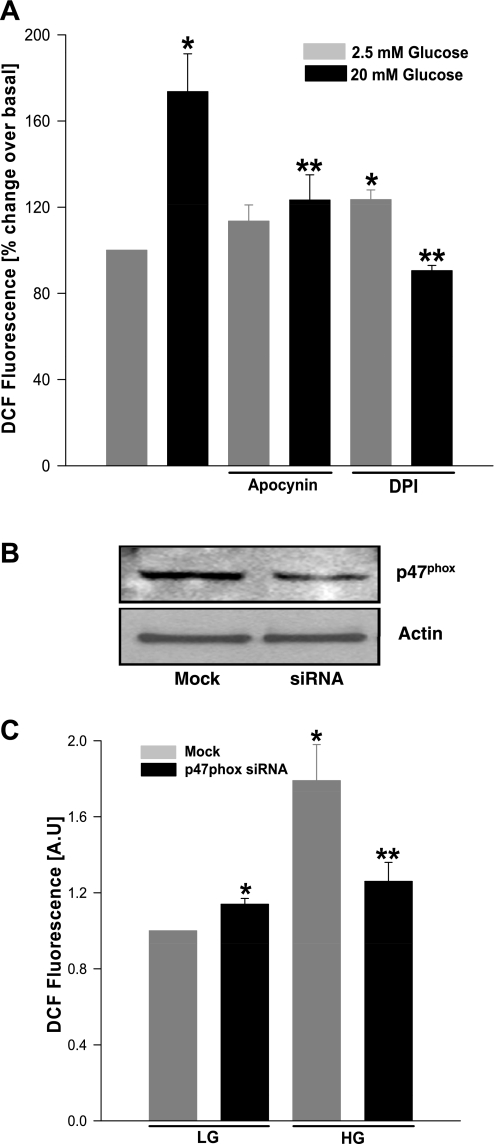
At the outset, we determined whether stimulatory glucose promotes the generation of ROS, and whether selective inhibition of Nox attenuates such an effect in this model system. Data in Fig. 1A demonstrated a significant increase (∼1.7-fold) in glucose-induced ROS generation in INS 832/13 cells, which was markedly attenuated by inhibitors of Nox holoenzyme (e.g., apocynin and DPI). The above observations were further validated by knockdown of p47phox, a cytosolic subunit of Nox. Data in Fig. 1B indicated ∼50% inhibition in the expression of p47phox subunit after siRNA transfection, and under these conditions we noticed a marked attenuation of glucose-induced ROS generation (Fig. 1C).
Fig. 1.
Selective inhibitors of NADPH oxidase or short interfering RNA (siRNA)-p47phox inhibits glucose-stimulated reactive oxygen species (ROS) generation in insulin-secreting cells. INS 832/13 cells were incubated with either diluent or apocynin (100 μM, 12 h; A) or diphenyleneiodonium chloride (DPI; 5 μM, 2 h; A) or transfected with p47phox siRNA (B and C), following which they were stimulated with either low (2.5 mM; LG) or high glucose (20 mM; HG) for 1 h. ROS generated was quantified as dichlorofluorescein (DCF) fluorescence and expressed as arbitrary units (AU). B: transfection efficiency of p47phox siRNA was determined by immunoblotting. Values are means ± SE from three independent experiments done in triplicates in each case. *P < 0.05 vs. LG alone or mock transfected LG. **P < 0.05 vs. HG alone or mock transfected HG.
Selective inhibitors of protein prenylation markedly attenuate glucose-induced ROS generation in INS 832/13 cells and normal rat islets.
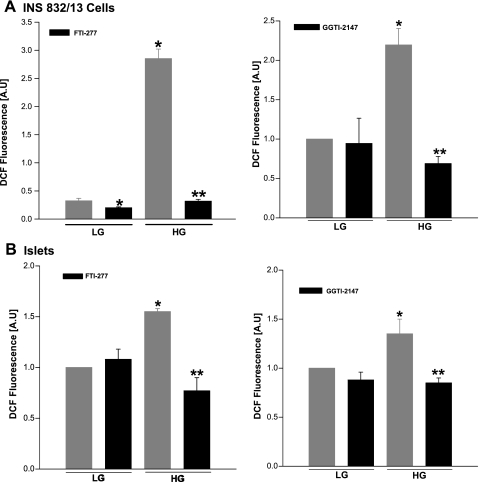
Several earlier studies have demonstrated that posttranslational farnesylation and geranylgeranylation of specific G proteins are necessary for GSIS (17, 42). With this in mind, using a pharmacological approach, we examined whether glucose-induced ROS generation in isolated β-cells is sensitive to inhibition of protein prenylation. Data in Fig. 2 demonstrated a significant reduction in glucose-induced ROS generation by selective inhibitors of farnesylation (e.g., FTI-277) or geranylgeranylation (e.g., GGTI-2147) in INS 832/13 cells (A) or rat islets (B). Together, these findings suggested involvement of farnesylated and geranylgeranylated proteins in the signaling events, leading to glucose-induced ROS generation.
Fig. 2.
Selective inhibitors of protein farnesylation or geranylgeranylation markedly attenuate glucose-induced ROS generation in INS 832/13 cells and normal rodent islets. INS 832/13 cells (A) or normal rat islets (B) were incubated overnight in the absence or presence of FTI-277 (5 μM; left) or GGTI-2147 (10 μM; right), followed by stimulation with either LG (2.5 mM) or HG (20 mM) for 1 h. ROS generated was quantified as DCF fluorescence and expressed as AU. Values are means ± SE from three independent experiments done in triplicates (in INS 832/13 cells) and in duplicates (in islets) in each case. *P < 0.05 vs. LG alone. **P < 0.05 vs. HG alone.
Protein prenylation is also necessary for mitochondrial fuel-, but not KCl-induced ROS generation.
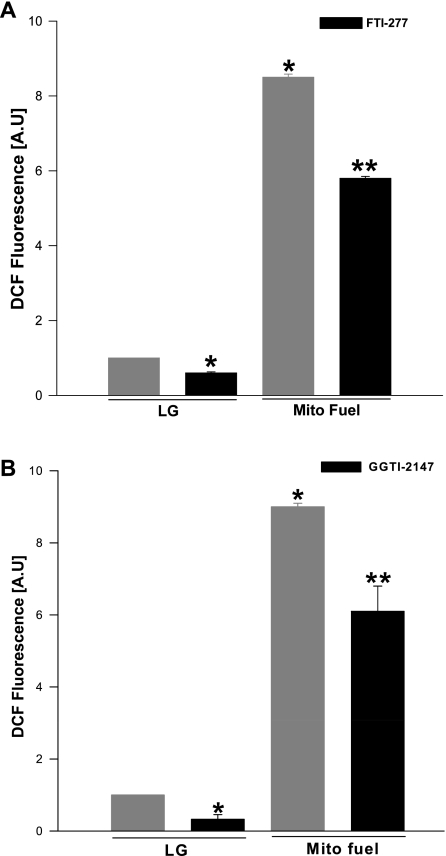
We next examined if a mixture of mitochondrial fuels (e.g., α-keto-isocaproic acid and mono-methylsuccinate), which elicits insulin secretion from INS 832/13 cells (6), also promotes Nox-mediated generation of ROS in these cells. Data in Fig. 3 demonstrated that mitochondrial fuels increased ROS generation in a manner akin to glucose. Furthermore, we observed that such a signaling step was inhibited by FTI-277 and GGTI-2147, albeit to a lesser degree (Fig. 3) compared with glucose-induced ROS generation (Fig. 2). Together, data in Figs. 2 and 3 implicate protein farnesylation and geranylgeranylation in the cascade of events, leading to nutrient-induced generation of ROS in INS 832/13 cells. It should be noted that ROS generation appears to be specific for nutrient secretagogues, since a depolarizing concentration of KCl (40 mM), which facilitates insulin release via membrane depolarization and associated increase in cytosolic calcium, failed to promote ROS generation. (i.e., 109 ± 1.2% of control values; mean ± SE; n = 3; additional data not shown).
Fig. 3.
Selective inhibitors of protein prenylation inhibit ROS generation induced by a mixture of mitochondrial (mito) fuels in INS 832/13 cells. INS 832/13 cells were incubated overnight in the presence or absence of FTI-277 (5 μM; A) and GGTI-2147 (10 μM; B), followed by stimulation with LG (2.5 mM) or a mixture of mito fuels [monomethyl succinate (MMS) = 20 mM and α-ketoisocaproic acid (KIC) = 5 mM] for 1 h in continuous presence or absence of inhibitors. ROS generated was quantified as DCF fluorescence and expressed as AU. Values are means ± SE from three independent experiments done in triplicates in each case. *P < 0.05 vs. glucose alone. **P < 0.05 vs. mito fuels alone.
Depletion of intracellular GTP inhibits glucose-induced Rac1 activation and ROS generation in INS 832/13 cells.
Several previous studies have demonstrated a critical requirement for endogenous GTP in physiological insulin secretion by selectively inhibiting inosine monophosphate dehydrogenase (IMPDH) with MPA (24, 25). Herein, using MPA, we examined if endogenous GTP is required for glucose-induced Nox activation and associated ROS generation in INS 832/13 cells. Cyclosporine A and rapamycin were included as negative controls, which, like MPA, are endowed with immunosuppressive actions, but not GTP-lowering properties. Data in Table 1 suggested a marked attenuation in glucose-induced ROS generation by MPA, but not cyclosporine A or rapamycin. These data indicate a critical requirement for endogenous GTP for glucose to promote ROS generation in these cells. Together, data in Figs. 2 and 3 and Table 1 indicate potential involvement of prenylated G protein, requiring newly synthesized GTP due to the catalytic activation of IMPDH in the signaling events leading to ROS generation.
Table 1.
Depletion of endogenous GTP markedly attenuates glucose-induced ROS generation in INS 832/13 cells
| Condition | Degree of ROS Generation, fold over basal glucose |
|---|---|
| Low glucose | 1.00 |
| High glucose | 1.58 ± 0.06† |
| Low glucose + mycophenolic acid | 1.08 ± 0.02* |
| High glucose + mycophenolic acid | 1.16 ± 0.04‡ |
| Low glucose +cyclosporine A | 1.06 ± 0.09* |
| High glucose + cyclosporine A | 1.46 ± 0.14† |
| Low glucose + rapamycin | 1.05 ± 0.08* |
| High glucose + rapamycin | 1.42 ± 0.15† |
Values are means ± SE from three independent experiments in each case. INS 832/13 cells were incubated with low glucose (2.5 mM) and low serum in the presence or absence of mycophenolic acid (10 μM), cyclosporine-A (5 μM), and rapamycin (100 nM) for 24 h. Following this, cells were stimulated either with low (2.5 mM) or high glucose (20 mM) for 1 h in the continuous presence and or absence of the inhibitors, as indicated. At the end of stimulation, cells were incubated with 2′,7′-dichlorofluorescein diacetate (10 μM) for 30 min and harvested for dichlorofluorescein fluorescence. ROS generated was quantified as dichlorofluorescein fluorescence and expressed as arbitrary units.
No significant difference vs. low glucose alone.
P < 0.05 vs. low glucose alone.
P < 0.05 vs. high glucose alone.
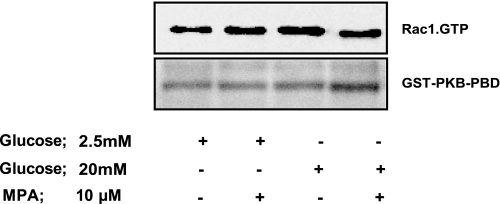
We next examined if GTP depletion impedes glucose-induced activation of specific G proteins involved in GSIS. To test this, we quantitated glucose-induced activation of Rac1 in MPA-treated (i.e., GTP-depleted) INS 832/13 cells. The premise underlying the selection of Rac1 in these studies is based on the evidence that 1) it has been shown to be activated by glucose and involved in GSIS; 2) it undergoes geranylgeranylation, and GGTI-2147 (above) inhibits glucose-induced Rac1 activation and GSIS; and 3) it is a member of the Nox holoenzyme. Data shown in Fig. 4 demonstrated that stimulatory concentration of glucose failed to activate Rac1 in INS 832/13 cells following depletion of endogenous GTP using MPA.
Fig. 4.
Endogenous GTP levels are required for glucose-induced Rac1 activation and subsequent ROS generation in pancreatic β-cells. INS 832/13 cells were incubated overnight with either diluent or mycophenolic acid (MPA; 10 μM), followed by stimulation with either LG (5 mM) or HG (20 mM) for 30 min. The degree of Rac1 activation was determined by p21-activated kinase-binding domain (PAK-PBD) pull-down assay, as described in materials and methods. A representative blot from two pull-down assays yielding similar data is depicted here.
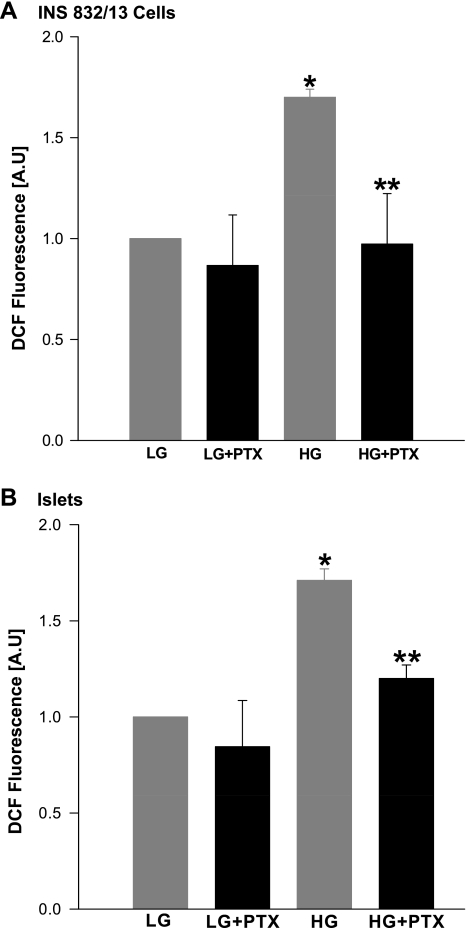
A Ptx-sensitive G protein mediates glucose-induced ROS generation in INS 832/13 cells.
In the last series of studies, we determined the nature of the prenylated protein that might be involved in glucose-induced ROS generation shown in Figs. 2 and 3. In this context, our laboratory recently reported that coprovision of FTI-277 or FTI-2628 or siRNA-mediated knockdown of farnesyltransferase β-subunit resulted in a significant inhibition of glucose-stimulated activation of ERK1/2, Rac1, and insulin secretion, further ruling out the potential involvement of Ras in these signaling steps (18). Based on these findings, we speculated a prenylated protein, most likely the γ-subunit(s) of trimeric G proteins, in the regulation of the above signaling cascade. Herein, we examined if a Ptx-sensitive trimeric G protein is involved in glucose-induced ROS generation. Data shown in Fig. 5 demonstrated marked attenuation of glucose-induced ROS generation in INS 832/13 cells (A) and normal rat islets (B) treated with Ptx.
Fig. 5.
Pertussis toxin (Ptx) pretreatment attenuates glucose-induced ROS generation in INS 832/13 cells or normal rat islets. Untreated or Ptx-treated (100 ng/ml) INS 832/13 cells (A) or normal rat islets (B) were stimulated with either LG (2.5 mM) or HG (20 mM) for 1 h. ROS generated was quantified as DCF fluorescence and expressed as AU. Values are means ± SE from three independent experiments done in triplicates (in INS 832/13 cell) and in duplicates (in islets) in each case. *P < 0.05 vs. LG alone. **P < 0.05 vs. HG alone.
DISCUSSION
The overall objective of the present study was to determine potential mechanisms underlying nutrient-induced generation of ROS in isolated β-cells. Salient features of our studies are as follows: 1) glucose and mitochondrial fuels, but not membrane-depolarizing KCl, increase ROS generation significantly; 2) an increase in ROS seen under these conditions is derived from Nox, since pharmacological or molecular biological inhibition of Nox inhibited ROS generation; 3) such a regulatory effect of glucose requires the activation of farnesylated as well as geranylgeranylated proteins; 4) MPA, but not rapamycin or cyclosporine A, completely inhibits glucose-induced ROS generation, implying that endogenous GTP is necessary for such an effect; and 5) inactivation of Ptx-mediated ADP ribosylation of an inhibitory G protein(s) markedly attenuates glucose-induced ROS generation. Taken together, our findings provide insights into potential G protein-mediated regulation of ROS in the islet β-cells under conditions in which they regulate physiological insulin secretion.
Nox is a highly regulated membrane-associated protein complex that facilitates the one electron reduction of oxygen to superoxide anion involving oxidation of cytosolic NADPH. The Nox holoenzyme is composed of membrane as well as cytosolic components. The membrane-associated catalytic core consists of gp91phox, p22phox, and the small G protein Rap1. The cytosolic regulatory components include p47phox, p67phox, p40phox, and the small G protein Rac. Following stimulation, the cytosolic components of Nox translocate to the membrane fraction for association with the catalytic core for holoenzyme assembly. Available evidence suggests that a protein kinase C-sensitive phosphorylation of p47phox leads to its translocation to the membrane fraction (3). It has also been shown that functional activation of Rac1 (Rac1.GTP) is vital for the holoenzyme assembly and activation of Nox in insulin-secreting cells (38, 39).
Along these lines, Oliveira et al. (30) provided a detailed description of localization, expression, and functional regulation of Nox within the islet. More recent pharmacological and molecular biological observations by Morgan and coworkers (27) have provided compelling evidence for a regulatory role for Nox in glucose-stimulated insulin secretion in rat islets under static incubation and perifusion conditions. Follow-up studies from this group have demonstrated key roles for Nox-derived ROS in palmitate-induced insulin secretion in the presence of submaximal concentration of glucose in islets (5). Under the above conditions, palmitate not only promoted translocation of p47phox to the membrane fraction, but also upregulated the protein content of p47phox and the mRNA levels of p22phox, gp91phox, p47phox, proinsulin, and the G protein-coupled receptor 40. Essential role for Nox in palmitate-induced effects on β-cells was further strengthened by their observations to indicate a marked inhibition of fatty acid stimulation of insulin secretion in the presence of high-glucose concentration by inhibition of Nox activity. Based on these findings, it is evident that Nox plays key roles in islet function, including gene regulation and insulin secretion.
Our present observations also implicate roles for farnesylated and geranylgeranylated proteins in nutrient-induced Nox activation and associated ROS generation; the geranylated protein involved in nutrient-mediated activation of Nox might be Rac1, since it is one of the components of the Nox holoenzyme (39). Pharmacological (i.e., generic as well as more selective inhibitors of geranylgeranylation of Rac1), as well as molecular biological (i.e., dominant-negative mutants of prenyltransferases; Ref. 22) studies from our laboratory have clearly implicated Rac1 in islet function, including insulin secretion (17, 15, 41). The identity of the farnesylated protein, which is required for nutrient-induced ROS generation, remains to be determined. It is likely that it might represent the γ-subunit of a Ptx-sensitive G protein, since our laboratory has demonstrated earlier regulation of Ptx-sensitive G proteins by glucose in clonal β-cells, normal rat islets, and human islets (10–12, 14). Several earlier studies by Seaquist et al. (35), Robertson et al. (34), and Sharp (36) have provided evidence for the expression of inhibitory (e.g., Gi or Go) class of Ptx-sensitive heterotrimeric proteins in the islet β-cell. Furthermore, studies from our laboratory (10) and those of Konrad and coworkers (7) have demonstrated functionally active heterotrimeric G proteins on the insulin granules in isolated β-cells. Lastly, using clonal β-cells, normal rat islets, and human islets, we have been able to demonstrate activation of the CML of γ-subunits by glucose; such effects of glucose were shown to be sensitive to Ptx, GTP, and extracellular calcium (14). Existing experimental evidence also implicates role(s) for trimeric G proteins, specifically the inhibitory Gi class of proteins in the regulation of NADPH-oxidase activity. For example, using human fat cells, Kreuzer and coworker's (19) demonstrate insulin-induced activation of NADPH-dependent H2O2 generation in human adipocyte plasma membranes is mediated by Gαi2, which is regulated via ADP-ribosylation by Ptx. Additional studies are needed to conclusively determine the identity of this protein. However, based on our laboratory's recently published evidence (18), it is unlikely that the farnesylated protein is Ras, since inhibition of Ras (a farnesylated protein) had no effects on glucose-induced ERK1/2 phosphorylation, Rac1 activation, and insulin secretion.
Our findings also suggested that depletion of endogenous GTP by MPA results in a decreased activation of glucose-induced Rac1 and ROS generation. In this context, original studies by Metz and coworkers (24, 25) have documented permissive roles for endogenous GTP in physiological insulin secretion. MPA, which selectively inhibits GTP biosynthesis by inhibiting IMPDH, has been shown to inhibit GSIS and mastoparan-induced insulin secretion (24, 37). Even though inhibition of G protein activation was speculated to be one of the underlying mechanisms in the inhibition of insulin secretion following GTP depletion by MPA, very little information is available to substantiate that speculation. In this context, our laboratory has described earlier the inability of glucose to increase the CML (and activation) of small G proteins in GTP-depleted cells (13). The present studies identify Rac1 as one of the target proteins for glucose-mediated, endogenous GTP-dependent effects in β-cells. Our present findings are also in agreement with observations of Krotz et al. (19a), demonstrating inhibition of endothelial Nox by MPA via a Rac1-dependent mechanism.
Perspectives and Significance
Our studies provide the first evidence to suggest that prenylation-dependent, Nox-mediated generation of ROS is necessary for nutrient-induced insulin secretion in the pancreatic β-cell. Our findings also suggested that IMPDH-derived generation of GTP is necessary for glucose-induced ROS generation and subsequent activation of Rac1 in insulin-secreting cells. Data accrued in studies involving Ptx suggested that glucose-induced Nox activation and ROS generation are under the fine control of a Ptx-sensitive G protein. Potential identity of the prenylated protein whose activation appears to be required for glucose-induced ROS generation (present study) and ERK1/2 phosphorylation, Rac1 activation, and insulin secretion (18) remains to be elucidated.
GRANTS
This research was supported by a merit review award from the Department of Veterans Affairs and the National Institute of Diabetes and Digestive and Kidney Diseases (DK 74921) (to A. Kowluru). A. Kowluru is also the recipient of the Senior Research Career Scientist Award from the Department of Veterans Affairs.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
REFERENCES
- 1. Arrigo AP. Gene expression and the thiol redox state. Free Radic Biol Med 27: 936–944, 1999 [DOI] [PubMed] [Google Scholar]
- 2. Casey PJ, Seabra MC. Protein prenyltransferases. J Biol Chem 271: 5289–5292, 1996 [DOI] [PubMed] [Google Scholar]
- 3. Dang PM, Fontayne A, Hakim J, ElBenna J, Perianin A. Protein kinase C phosphorylates a subset of selective sites of the NADPH oxidase component p47phox and participates in formyl peptide-mediated neutrophil respiratory burst. J Immunol 166: 1206–1213, 2001 [DOI] [PubMed] [Google Scholar]
- 4. Finkel T. Oxygen radicals and signaling. Curr Opin Cell Biol 10: 248–253, 1998 [DOI] [PubMed] [Google Scholar]
- 5. Graciano MF, Santos LR, Curi R, Carpinelli AR. NAD(P)H oxidase participates in the palmitate-induced superoxide production and insulin secretion by rat pancreatic islets. J Cell Physiol. In press [DOI] [PubMed] [Google Scholar]
- 6. Kamath V, Kyathanahalli CN, Jayaram B, Syed I, Olson LK, Ludwig K, Klumpp S, Krieglstein J, Kowluru A. Regulation of glucose- and mitochondrial fuel-induced insulin secretion by a cytosolic protein histidine phosphatase in pancreatic beta-cells. Am J Physiol Endocrinol Metab 299: E276–E286, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Konrad RJ, Young RA, Record RD, Smith RM, Butkerait P, Manning D, Jarett L, Wolf BA. The heterotrimeric G-protein Gi is localized to the insulin secretory granules of beta-cells and is involved in insulin exocytosis. J Biol Chem 270: 12869–12876, 1995 [DOI] [PubMed] [Google Scholar]
- 9. Kourie JI. Interaction of reactive oxygen species with ion transport mechanisms. Am J Physiol Cell Physiol 275: C1–C24, 1998 [DOI] [PubMed] [Google Scholar]
- 10. Kowluru A, Metz SA. Stimulation by prostaglandin E2 of a high-affinity GTPase in the secretory granules of normal rat and human pancreatic islets. Biochem J 297: 399–406, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kowluru A, Rabaglia ME, Muse KE, Metz SA. Subcellular localization and kinetic characterization of guanine nucleotide binding proteins in normal rat and human pancreatic islets and transformed beta cells. Biochim Biophys Acta 1222: 348–359, 1994 [DOI] [PubMed] [Google Scholar]
- 12. Kowluru A, Seavey SE, Rhodes CJ, Metz SA. A novel regulatory mechanism for trimeric GTP-binding proteins in the membrane and secretory granule fractions of human and rodent β cells. Biochem J 313: 97–107, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kowluru A, Seavey SE, Li G, Sorenson RL, Weinhaus AJ, Nesher R, Rabaglia ME, Vadakekalam J, Metz SA. Glucose- and GTP-dependent stimulation of the carboxylmethylation of Cdc42 in rodent and human pancreatic islets and pure β cells: evidence for an essential role for GTP-binding proteins in nutrient-induced insulin secretion. J Clin Invest 98: 540–555, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kowluru A, Li G, Metz SA. Glucose activates the carboxyl methylation of gamma subunits of trimeric GTP-binding proteins in pancreatic beta cells. Modulation in vivo by calcium, GTP, and pertussis toxin. J Clin Invest 100: 1596–610, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kowluru A, Li G, Rabaglia ME, Segu VB, Hofmann F, Aktories K, Metz SA. Evidence for differential roles of the Rho subfamily of GTP-binding proteins in glucose- and calcium-induced insulin secretion from pancreatic β cells. Biochem Pharmacol 54: 1097–1108, 1997 [DOI] [PubMed] [Google Scholar]
- 16. Kowluru A. Protein prenylation in glucose-induced insulin secretion from the pancreatic islet β-cell: a perspective. J Cell Mol Med 12: 164–173, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kowluru A. Small G proteins in islet beta-cell function. Endocr Rev 31: 52–78, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kowluru A, Veluthakal R, Rhodes CJ, Kamath V, Syed I, Koch BJ. Protein farnesylation-dependent Raf/extracellular signal-related kinase signaling links to cytoskeletal remodeling to facilitate glucose-induced insulin secretion in pancreatic beta-cells. Diabetes 59: 967–977, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kreuzer J, Viedt C, Brandes RP, Seeger F, Rosenkranz AS, Sauer H, Babich A, Nürnberg B, Kather H, Krieger-Brauer HI. Platelet-derived growth factor activates production of reactive oxygen species by NAD(P)H oxidase in smooth muscle cells through Gi1,2. FASEB J 17: 38–40, 2003 [DOI] [PubMed] [Google Scholar]
- 19a. Krötz F, Keller M, Derflinger S, Schmid H, Gloe T, Bassermann F, Duyster J, Cohen CD, Schuhmann C, Klauss V, Pohl U, Stempfle HU, Sohn HY. Mycophenolate acid inhibits endothelial NAD(P)H oxidase activity and superoxide formation by a Rac1-dependent mechanism. Hypertension 49: 201–208, 2007 [DOI] [PubMed] [Google Scholar]
- 20. Leloup C, Tourrel-Cuzin C, Magnan C, Karaca M, Castel J, Carneiro L, Colombani AL, Ktorza A, Casteilla L, Pénicaud L. Mitochondrial reactive oxygen species are obligatory signals for glucose-induced insulin secretion. Diabetes 58: 673–681, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Liu H, Colavitti R, Rovira II, Finkel T. Redox-dependent transcriptional regulation. Circ Res 97: 967–974, 2005 [DOI] [PubMed] [Google Scholar]
- 22. Li J, Luo R, Kowluru A, Li G. Novel regulation by Rac1 of glucose- and forskolin-induced insulin secretion in INS-1 beta-cells. Am J Physiol Endocrinol Metab 286: E818–E827, 2004 [DOI] [PubMed] [Google Scholar]
- 23. MacDonald MJ. Elusive proximal signals of β-cells for insulin secretion. Diabetes 39: 1461–1466, 1990 [DOI] [PubMed] [Google Scholar]
- 24. Metz SA, Rabaglia ME, Pintar TJ. Selective inhibitors of GTP synthesis impede exocytotic insulin release from intact rat islets. J Biol Chem 267: 12517–12527, 1992 [PubMed] [Google Scholar]
- 25. Metz SA, Meredith M, Rabaglia ME, Kowluru A. Small elevations of glucose concentration redirect and amplify the synthesis of guanosine 5′-triphosphate in rat islets. J Clin Invest 92: 872–882, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Morgan D, Oliveira-Emilio HR, Keane D, Hirata AE, SantosdaRocha M, Bordin S, Curi R, Newsholme P, Carpinelli AR. Glucose, palmitate and pro-inflammatory cytokines modulate production and activity of a phagocyte-like NADPH oxidase in rat pancreatic islets and a clonal beta cell line. Diabetologia 50: 359–369, 2007 [DOI] [PubMed] [Google Scholar]
- 27. Morgan D, Rebelato E, Abdulkader F, Garciano MFR, Oliveira-Emilio HR, Hirata AE, Rocha MS, Bordin S, Curi R, Carpinelli AR. Association of NAD(P)H oxidase with glucose-induced insulin secretion by pancreatic beta cells. Endocrinology 150: 2197–2201, 2009 [DOI] [PubMed] [Google Scholar]
- 28. Newgard CB, Lu D, Jensen MV, Schissler J, Boucher A, Burgess S, Sherry AD. Stimulus/secretion coupling factors in glucose-stimulated insulin secretion: insights gained from a multidisciplinary approach. Diabetes 51, Suppl 3: S389–S393, 2002 [DOI] [PubMed] [Google Scholar]
- 29. Newsholme P, Morgan D, Rebelato E, Oliveira-Emilio HR, Procopio J, Curi R, Carpinelli AR. Insights into the critical role of NADPH oxidase(s) in the normal and dysregulated pancreatic beta cell. Diabetologia 52: 2489–2498, 2009 [DOI] [PubMed] [Google Scholar]
- 30. Oliveira HR, Verlengia R, Carvalho CR, Britto LR, Curi R, Carpinelli AR. Pancreatic beta cells express phagocyte-like NADPH oxidase. Diabetes 52: 1457–1463, 2003 [DOI] [PubMed] [Google Scholar]
- 31. Pi J, Bai Y, Zhang Q, Wong V, Floering LM, Daniel K, Reece JM, Deeney JT, Andersen ME, Corkey BE, Collins S. Reactive oxygen species as a signal in glucose-stimulated insulin secretion. Diabetes 56: 1783–1791, 2007 [DOI] [PubMed] [Google Scholar]
- 32. Pi J, Collins S. Reactive oxygen species and uncoupling protein 2 in pancreatic beta-cell function. Diabetes Obes Metab 12, Suppl 2: 141–148, 2010 [DOI] [PubMed] [Google Scholar]
- 33. Prentki M, Matschinsky FM. Calcium, cAMP, and phospholipid-derived messengers in coupling mechanisms of insulin secretion. Physiol Rev 67: 1185–1248, 1987 [DOI] [PubMed] [Google Scholar]
- 34. Robertson RP, Seaquist ER, Walseth TF. G proteins and modulation of insulin secretion. Diabetes 40: 1–6, 1991 [DOI] [PubMed] [Google Scholar]
- 35. Seaquist ER, Walseth TF, Nelson DM, Robertson RP. Pertussis toxin-sensitive G-protein mediation of PGE2 inhibition of cAMP metabolism and phasic glucose-induced insulin secretion in HIT cells. Diabetes 38: 1439–1445, 1989 [DOI] [PubMed] [Google Scholar]
- 36. Sharp GW. Mechanisms of inhibition of insulin release. Am J Physiol Cell Physiol 271: C1781–C1799, 1996 [DOI] [PubMed] [Google Scholar]
- 37. Straub SG, James RF, Dunne MJ, Sharp GW. Glucose augmentation of mastoparan-stimulated insulin secretion in rat and human pancreatic islets. Diabetes 47: 1053–1057, 1998 [DOI] [PubMed] [Google Scholar]
- 38. Subasinghe W, Syed I, Kowluru A. Phagocyte-like NADPH oxidase promotes cytokine-induced mitochondrial dysfunction in pancreatic β-cells: evidence for regulation by Rac1. Am J Physiol Regul Integr Comp Physiol 300: R12–R20, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Syed I, Jayaram B, Subasinghe W, Kowluru A. Tiam1/Rac1 signaling pathway mediates palmitate-induced, ceramide-sensitive generation of superoxides and lipid peroxides and the loss of mitochondrial membrane potential in pancreatic beta-cells. Biochem Pharmacol 80: 874–883, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Takai Y, Sasaki T, Matozaki T. Small GTP-binding proteins. Physiol Rev 81: 153–208, 2001 [DOI] [PubMed] [Google Scholar]
- 41. Veluthakal R, Kaur H, Goalstone M, Kowluru A. Dominant negative α-subunit of farnesyl- and geranyl geranyltransferase inhibits glucose-stimulated, but not KCl-stimulated, insulin secretion in INS 832/13 cells. Diabetes 56: 204–210, 2007 [DOI] [PubMed] [Google Scholar]
- 42. Wang Z, Thurmond DC. Mechanisms of biphasic insulin-granule exocytosis–roles of the cytoskeleton, small GTPases and SNARE proteins. J Cell Sci 122: 893–903, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]