Abstract
Our previous studies showed that stimulation of adenosine A1 receptors located in the nucleus of the solitary tract (NTS) exerts counteracting effects on the iliac vascular bed: activation of the adrenal medulla and β-adrenergic vasodilation vs. sympathetic and vasopressinergic vasoconstriction. Because NTS A1 adenosine receptors inhibit baroreflex transmission in the NTS and contribute to the pressor component of the HDR, we hypothesized that these receptors also contribute to the redistribution of blood from the visceral to the muscle vasculature via prevailing sympathetic and vasopressinergic vasoconstriction in the visceral (renal and mesenteric) vascular beds and prevailing β-adrenergic vasodilation in the somatic (iliac) vasculature. To test this hypothesis, we compared the A1 adenosine-receptor-mediated effects of each vasoactive factor triggered by NTS A1 adenosine receptor stimulation [N6-cyclopentyladenosine (CPA), 330 pmol in 50 nl] on the regional vascular responses in urethane/chloralose-anesthetized rats. The single-factor effects were separated using adrenalectomy, β-adrenergic blockade, V1 vasopressin receptor blockade, and sinoaortic denervation. In intact animals, initial vasodilation was followed by large, sustained vasoconstriction with smaller responses observed in renal vs. mesenteric and iliac vascular beds. The initial β-adrenergic vasodilation prevailed in the iliac vs. mesenteric and renal vasculature. The large and sustained vasopressinergic vasoconstriction was similar in all vascular beds. Small sympathetic vasoconstriction was observed only in the iliac vasculature in this setting. We conclude that, although A1 adenosine-receptor-mediated β-adrenergic vasodilation may contribute to the redistribution of blood from the visceral to the muscle vasculature, this effect is overridden by sympathetic and vasopressinergic vasoconstriction.
Keywords: purinergic receptors, V1 receptor blockade, β-adrenergic blockade, adrenalectomy, sinoaortic denervation, iliac vascular conductance, mesenteric vascular conductance, renal vascular conductance
recent studies have firmly established that adenosine operating as a neuromodulator in the nucleus of the solitary tract (NTS) modifies cardiovascular control (14, 20, 24, 25, 34, 40–44). The NTS is a major integrative center for visceral and autonomic reflexes and contains the greatest density of adenosine uptake sites within the central nervous system (4). Adenosine operates in the NTS in both physiological and pathological situations. Under normal, physiological conditions, a natural source of adenosine is ATP released from neurons and glial cells (6). This occurs, for example, during the stress or hypothalamic defense response (HDR) (42–44). Extracellular ATP is catabolized via ectonucleotidases to adenosine, which acts globally on pre- or postsynaptic A1 or A2a adenosine receptors located in the NTS (6, 49). Adenosine is also released under pathological conditions such as ischemia, hypoxia, and severe hemorrhage via the breakdown of intracellular ATP (28, 46, 47). Therefore, adenosine is an important neuromodulator helping to regain homeostasis in these life-threatening situations via specific modulation of central mechanisms of cardiovascular control. Although adenosine is released globally, it may, however, differentially modulate autonomic reflexes integrated in the NTS (20, 34). The differential action of adenosine is most likely due to differential localization of adenosine receptor subtypes on NTS terminals/interneurons involved in different reflexes that finally target different sympathetic outputs and vascular beds as we suggested previously (35, 40).
Adenosine may either inhibit or facilitate neurotransmission via A1 or A2a receptors, respectively. Selective stimulation of A1 vs. A2a receptors often, but not always, exerts counteracting hemodynamic effects. Stimulation of A1 adenosine receptors in the NTS yields predominately pressor and vasoconstrictor effects in the hindlimb accompanied with differential regional sympathoactivation (adrenal > renal ≥ lumbar) (24, 39). In contrast, NTS A2a adenosine receptor stimulation yields depressor responses, decreases in renal sympathetic nerve activity (RSNA), no changes in lumbar sympathetic nerve activity (LSNA), and activation of preganglionic adrenal sympathetic nerve activity (pre-ASNA) (36, 37). Interestingly, both receptor subtypes preferentially activate the adrenal medulla. We have previously shown that both A1 and A2a adenosine receptors evoke β-adrenergic vasodilation in the iliac vascular bed, since β-adrenergic receptors are predominantly located in the skeletal muscle vasculature (45). However, stimulation of A1 adenosine receptors alone in the NTS often yields variable responses, although predominantly pressor and iliac vasoconstrictor responses prevail (24, 39). Recent studies have attributed this variability to the activation of the adrenal medulla, the subsequent release of epinephrine eliciting activation of β-adrenergic vasodilation that is simultaneously opposed by sympathetic and vasopressinergic vasoconstriction (24, 25). The increased vasoconstrictor drive and simultaneously triggered β-adrenergic vasodilation were most likely mediated via inhibition of NTS baroreflex mechanisms (32, 34, 39).
Stimulation of NTS A1 adenosine receptors differentially affects baroreflex control and baseline levels of regional sympathetic outputs, and baroreflex control of vascular beds is regionally different (11, 30, 34). Furthermore, there is differential regional distribution of α1- and β2-adrenergic receptors (19, 45) as well as differential reactivity of regional vascular beds to vasopressin and α1-adrenergic activation (12, 13, 17, 23). Therefore, it was likely that the contribution of vasoconstrictor and vasodilatory effects may be different between somatic (iliac) vs. visceral (mesenteric and renal) vascular beds. In support of this hypothesis, it has been shown that adenosine, operating mostly via A1 receptors located in the NTS and rostral ventrolateral medulla, participates in the pressor component of the HDR (42–44) and may possibly contribute to the redistribution of blood flow from the viscera to skeletal muscle, which is a key element of this response (48). We hypothesized that NTS A1 adenosine receptor-induced sympathetic and vasopressinergic vasoconstriction would prevail over β-adrenergic vasodilation to a greater extent in the visceral vascular beds compared with muscle. To address this hypothesis, we compared the simultaneously recorded vascular responses of iliac, mesenteric, and renal vascular beds evoked by selective activation of NTS A1 adenosine receptors in intact animals and after selective elimination of each major vasodilating and vasoconstricting factor.
MATERIALS AND METHODS
All protocols and surgical procedures employed in this study were reviewed and approved by the institutional Animal Care and Use Committee and were performed in accordance with the Guiding Principles in the Care and Use of Animals endorsed by the American Physiological Society and published by the National Institutes of Health.
Instrumentation and measurements.
All procedures were described in detail previously (2, 21, 24, 25, 32, 37–39). Briefly, male Sprague-Dawley rats (350–400 g; Charles River) were anesthetized with a mixture of α-chloralose (80 mg/kg) and urethane (500 mg/kg) intraperitoneally, tracheotomized, connected to a small animal respirator (SAR-830; CWE, Ardmore, PA), and artificially ventilated with a 40% oxygen-60% nitrogen mixture. Catheterizations of the right femoral artery and vein were performed to monitor arterial blood pressure and infuse drugs. Arterial blood gases were tested occasionally for appropriate experimental values (ABL500, OSM3; Radiometer). Averaged values measured at the end of each experiment were the following: pH =7.37 ± 0.01, Po2 = 144.2 ± 5.0 mmHg, and Pco2 = 36.2 ± 0.9 mmHg.
From a midabdominal incision, the left iliac artery, superior mesenteric artery, and the left renal artery were exposed. Pulsed Doppler blood flow velocity transducers (Baylor Electronics) were placed around the arteries and connected to the flowmeter. From the same midabdominal incision, in some animals, bilateral adrenalectomy was performed at the time of inserting the flow probes, i.e., 1–2 h before starting respective experimental protocols.
Arterial blood pressure and iliac, mesenteric, and renal flow signals were digitized and recorded with an analog-digital converter (Modular Instruments) interfaced to a laboratory computer. The signals were recorded continuously using Biowindows software (Modular Instruments), averaged over 5 s intervals, and stored on hard disk for subsequent analysis.
Microinjections into the NTS.
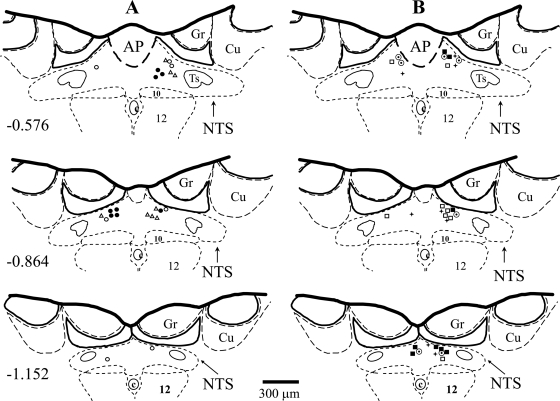
After the exposure of the brain stem via dissection of the atlantooccipital membrane, the animals were allowed to stabilize for at least 30 min before microinjections. Unilateral microinjections of N6-cyclopentyladenosine [CPA, 330 pmol in 50 nl of artificial cerebrospinal fluid (ACF)] were made through multibarrel glass micropipettes in the medial region of the caudal subpostremal NTS as described previously (2, 32, 37–39). Briefly, with the rat skull adjusted to a 45° angle from the horizontal plane of the stereotaxic apparatus and the micropipette barrel held at a 22° angle from the vertical plane, the surface coordinates used for insertion of the micropipette relative to the caudal tip of the area postrema were as follows: anteroposterior = −0.1 mm; mediolateral = 0.3 mm; dorsoventral = 0.3 mm from the dorsal surface of the brain stem. This dose of CPA produced most consistent, predominantly pressor responses in our previous study (39). The CPA was dissolved in ACF, and the pH was adjusted to 7.2. In several previous studies, we have shown that microinjections of the same amount of vehicle (ACF) in the same site of the NTS did not markedly affect mean arterial pressure (MAP), heart rate (HR), RSNA, LSNA, pre-ASNA, and regional vascular blood flows (2, 31, 34, 36–39). The changes in all these variables were either not different from zero or smaller than natural, random fluctuations of these variables over the time of measurements. To avoid the effect of desensitization of A1 adenosine receptors, in all experiments, only one dose of the agonist was microinjected in the left or right side of the NTS. All microinjection sites were marked by microinjections of DiI fluorescent dye (Molecular Probes, Eugene, OR) and verified histologically (Fig. 1).
Fig. 1.
Microinjection sites in the caudal subpostremal nucleus tractus solitarii (NTS) for all experiments. Schematic diagrams of transverse sections of the medulla oblongata from a rat brain. AP, area postrema; c, central canal; 10, dorsal motor nucleus of the vagus nerve; 12, nucleus of the hypoglossal nerve; Ts, tractus solitarius; Gr, gracile nucleus; Cu, cuneate nucleus. Scale is shown at the bottom; the no. on the left of the schematic diagram denotes the rostrocaudal position in mm of the section relative to the obex according to the atlas of the rat subpostremal NTS by Barraco et al. (1). Microinjection sites were marked with fluorescent dye and are denoted with the following symbols: A, microinjections of N6-cyclopentyladenosine (CPA) in intact animals (filled circles), after bilateral adrenalectomy (open triangles), and following β-adrenergic blockade (open circles); B, microinjections of CPA after V1 vasopressin receptor blockade (open square), following bilateral sinoaortic denervation (open circle with dot), after combined adrenalectomy and V1 vasopressin receptor blockade (filled square) and following combined sinoaortic denervation and adrenalectomy (plus symbol).
Experimental protocols.
The relative contribution of three neural and humoral vasoactive factors (sympathetic and vasopressinergic vasoconstriction opposed by β-adrenergic vasodilation) to the regional vascular responses evoked by selective stimulation of NTS A1 adenosine receptors was studied in 53 male Sprague-Dawley rats. Our previous studies showed that only these three vasoactive factors significantly contributed to iliac vascular responses evoked by stimulation of NTS A1 adenosine receptors (25). The relative changes in iliac (IVC), mesenteric (MVC), and renal (RVC) vascular conductance evoked by microinjections into the NTS by the selective A1 adenosine receptor agonist CPA (Tocris) observed in intact animals (n = 8) were compared with the responses evoked after the following six experimental procedures: protocol 1, bilateral adrenalectomy (ADX, n = 8); protocol 2, blockade of β-adrenergic receptors (n = 7); protocol 3, selective vasopressin V1 receptor blockade (VX, n = 7); protocol 4, sinoaortic denervation (SAD, n = 7); protocol 5, bilateral adrenalectomy combined with selective vasopressin V1 receptor blockade (ADX + VX, n = 8); and protocol 6, sinoaortic denervation combined with bilateral adrenalectomy (SAD + ADX, n = 8).
Blockade of β-adrenergic receptors was performed as in our previous studies via injection of propranolol (2 mg/kg iv) (21, 24). The β-adrenergic blockade (protocol 2) was used to confirm that bilateral adrenalectomy (protocol 1) removed mostly β-adrenergic vasodilation, whereas simultaneous elimination of adrenal secretion of norepinephrine (as well as dopamine, endorphins, and other potential vasoactive neuropeptides) had no significant vascular effects. In protocols 5 and 6, bilateral adrenalectomy instead of β-adrenergic blockade was used to avoid potential α1-adrenergic vasoconstriction that may be mediated by epinephrine when β2-receptors are blocked as we observed previously (21). Vasopressin V1 receptors, mediating vascular constriction, were blocked by intravenous injections of [β-mercapto-β,β-cyclopentylmethylenepropionyl,1-O-Me-Tyr,2Arg8]vasopressin, a selective V1 antagonist (20 μg/kg; Sigma) in doses that completely blocked vasoconstriction evoked by intavenous injection of vasopressin (50 mU/kg; Sigma). The blockades were applied ∼10 min before the microinjection in the NTS (24, 25). Sinoaortic denervation was accomplished at the beginning of surgery, as described previously (33, 39). The completeness of the denervation was tested ∼2 h later before the start of the protocol. The procedure was considered complete if intravenous phenylephrine-induced increases in MAP >30 mmHg did not decrease HR >5 beats/min.
Basic experimental protocols allowed for comparisons of how elimination of one vasoactive factor affects the responses observed in intact animals. However, selective denervation of each vascular bed, which would allow for selective removal of the sympathetic component of the responses, was extremely difficult, and in a few successful experiments arterial pressure was markedly lower than in other experimental groups (<70 mmHg). Therefore, we evaluated the contribution of sympathetic vasoconstriction alone to the regional vascular responses indirectly by removing the adrenal and vasopressinergic components of the responses via adrenalectomy combined with systemic V1 vasopressin receptor blockade (protocol 5). Adrenalectomy and β-adrenergic blockade (protocols 1 and 2, respectively) removed β-adrenergic vasodilation; therefore, these protocols showed how sympathetic and vasopressinergic vasoconstriction triggered by activation of NTS A1 adenosine receptors affected regional vascular beds. Blockade of V1 vasopressin receptors (protocol 3) removed the vascular action of vasopressin and, therefore, showed how regional vascular beds respond to β-adrenergic vasodilation opposed by sympathetic vasoconstriction. The contribution of the vasopressinergic component alone to the regional vascular responses was evaluated by subtracting sympathetic vasoconstriction (evaluated in protocol 5) from combined vasopressinergic and sympathetic vasoconstriction (protocol 1). The contribution of β-adrenergic vasodilation alone to the regional vascular bed responses was evaluated by subtracting sympathetic vasoconstriction (protocol 5) from combined sympathetic neural and adrenergic responses (evaluated in protocol 3). To evaluate separate effects of each humoral factor, we subtracted the sympathetic component of the responses (averaged values obtained in protocol 5) from each single animal response in protocols 1 and 3 and then averaged the data in each experimental group.
In addition, sinoaortic denervation (protocol 4), which removed baroreflex vasopressinergic and sympathetic vasoconstriction and the baroreflex-dependent part of the activation of the adrenal medulla, evaluated the relative regional vasodilation mediated via the nonbaroreflex component of the activation of the adrenal medulla (39). Finally, sinoaortic denervation combined with bilateral adrenalectomy (protocol 6) removed all three major vasoactive factors triggered by NTS A1 adenosine receptor stimulation: SAD removed the effects of A1-adenosine-receptor-mediated inhibition of baroreflex mechanisms (i.e., increases in sympathetic nerve activity, vasopressin, and epinephrine), whereas adrenalectomy removed the remaining, nonbaroreflex activation of the adrenal medulla by NTS A1 adenosine receptors (34, 39). This protocol tested if the three vasoactive factors (sympathetic and vasopressinergic vasoconstriction opposed by β-adrenergic vasodilation), known to affect the somatic (iliac) vascular bed (24, 25), are the only major factors affecting visceral vascular beds (mesenteric and/or renal) in response to stimulation of NTS A1 adenosine receptors.
Data analysis.
The regional vascular conductance was calculated similarly as in our previous studies by dividing the regional blood flows, expressed as Doppler shift (in Hz), by MAP (in mmHg) (24, 25). The absolute values of vascular conductance depend to some extent on positioning of the probe around the arteries; therefore, comparisons between the relative changes were more reliable. In all experimental groups, the hemodynamic data were averaged in 1-min intervals over a 20-min period of the response. Because the responses were biphasic, with an initial decrease in MAP and vasodilation (first ∼5 min of the response) followed by the pressor and vasoconstrictor phase of the response (last ∼15 min), we calculated the overall responses for each vascular bed by the integral of the response for the first 5 min, when vasodilation prevailed, and the last 15 min when vasoconstriction prevailed. To make the integral values comparable between the phases of the responses, we normalized the integral values to 1 min by dividing the total integral for the first and second phase of the response by 5 and 15, respectively.
Two-way ANOVA was used to evaluate the relative contribution of the three vasoactive factors (sympathetic and vasopressinergic vasoconstriction opposed by β-adrenergic vasodilation) to the responses of the three vascular beds (iliac, mesenteric, and renal) in respect to the integral responses. Two-way ANOVA was also used for comparing the dynamics of the response in each vascular bed observed under each experimental condition, and for each single vasoactive factor (20 time points of the responses vs. 3 vascular beds). A t-test with Bonferroni adjustments was used for the calculation of specific differences between the groups for those comparisons where interactions of the vasoactive factors or the time vs. the vascular beds were significant.
RESULTS
Resting values of MAP, HR, iliac, mesenteric, and renal blood flow, and conductance for each experimental group, measured just before stimulation of NTS A1 adenosine receptors, are presented in Table 1. In most cases, vascular conductance observed in specific beds in all experimental groups was not different from that observed in intact animals; the only exemptions were the increase in iliac vascular conductance following bilateral adrenalectomy combined with V1 vasopressin receptor blockade and a smaller increase in renal conductance following adrenalectomy alone and vasopressin receptor blockade alone. However, there were no significant differences between renal conductance measured before and ∼10 min after β-adrenergic and V1 vasopressin receptor blockades (Table 2). Therefore, changes in the relative vascular responses evoked by activation of NTS A1 adenosine receptors were comparable across the experimental groups with only small limitations. HR was significantly higher in the two groups where sinoaortic denervation was performed, as expected. MAP was lower compared with intact animals only in two experimental groups: following bilateral adrenalectomy combined with V1 vasopressin receptor blockade or adrenalectomy combined with sinoaortic denervation.
Table 1.
Resting values of hemodynamic parameters in each experimental group
| Blood Flow, Hz |
Vascular Conductance, Hz/mmHg |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Experimental Procedure | n | Mean Arterial Pressure, mmHg | Heart Rate, beats/min | Iliac | Mesenteric | Renal | Iliac | Mesenteric | Renal |
| Intact | 8 | 91.4 ± 4.0 | 323.5 ± 9.4 | 826.6 ± 63.1†‡ | 1,228.4 ± 72.3 | 1,771.9 ± 213.7 | 9.3 ± 1.0†‡ | 13.7 ± 1.2 | 19.3 ± 2.2 |
| ADX | 8 | 82.3 ± 1.8 | 336.1 ± 16.3 | 834.4 ± 68.7†‡ | 1,344.0 ± 169.7§ | 2,283.6 ± 215.2 | 10.3 ± 0.9†‡ | 16.4 ± 2.0§ | 27.8 ± 2.5* |
| ßX | 7 | 92.1 ± 3.8 | 300.0 ± 7.5 | 697.0 ± 71.3‡ | 1,424.8 ± 138.8 | 1,761.4 ± 307.3 | 7.6 ± 0.7‡ | 15.5 ± 1.3 | 19.3 ± 3.6 |
| VX | 7 | 81.2 ± 2.6 | 351.7 ± 22.3 | 997.0 ± 194.4‡ | 1,184.6 ± 129.8§ | 2,685.2 ± 123.5* | 12.5 ± 2.6‡ | 14.6 ± 1.5§ | 33.1 ± 1.3* |
| SAD | 7 | 86.1 ± 5.7 | 386.7 ± 16.3* | 732.4 ± 86.8‡ | 1,150.7 ± 163.7 | 1,609.9 ± 200.2 | 8.7 ± 1.2†‡ | 13.6 ± 1.9 | 18.7 ± 2.1 |
| ADX + VX | 8 | 76.5 ± 2.7* | 341.8 ± 10.1 | 1,554.6 ± 149.1* | 1,384.8 ± 154.4 | 1,550.9 ± 153.3 | 20.6 ± 2.2* | 18.6 ± 2.4 | 20.3 ± 1.9 |
| SAD + ADX | 8 | 78.8 ± 2.3* | 414.4 ± 21.9* | 1,076.3 ± 192.2‡ | 969.1 ± 71.7*§ | 2,115.6 ± 231.5 | 13.7 ± 2.4‡ | 12.4 ± 1.0§ | 27.1 ± 3.2 |
Data are means ± SE; n, no. of rats. Resting values for intact (INT) animals and following: bilateral adrenalectomy (ADX), β-adrenergic blockade (βX), vasopressin V1 receptor blockade (VX), adrenalectomy +V1 receptor blockade (ADX + VX), sinoaortic denervation (SAD), and sinaortic denervation + adrenalectomy (SAD + ADX).
P < 0.05 vs. intact.
P < 0.05, iliac vs. mesenteric (†), iliac vs. renal (‡), and mesenteric vs. renal (§).
Table 2.
Average maximum and steady-state hemodynamic responses evoked by β-adrenergic and V1 vasopressin receptor blockades
| Response | Experimental Procedure | n | ΔMAP, % | ΔHR, % | Δ Iliac, % | Δ Mesenteric Vascular Conductance, % | ΔRenal, % |
|---|---|---|---|---|---|---|---|
| Maximal | βX | 7 | 19.2 ± 4.0 | −8.6 ± 1.6 | −30.3 ± 4.2†‡ | −16.2 ± 4.0 | −13.0 ± 3.3 |
| VX | 8 | −16.6 ± 4.7 | 6.1 ± 4.0 | 38.4 ± 9.9 | 26.7 ± 6.3 | 25.9 ± 7.4 | |
| Steady state | βX | 7 | 5.9 ± 5.7 | −4.2 ± 1.8 | −11.7 ± 7.8†‡ | 6.4 ± 5.8 | 11.0 ± 6.7 |
| VX | 8 | −3.2 ± 3.6 | −2.9 ± 2.5 | 13.6 ± 8.9 | 7.6 ± 5.6 | 15.2 ± 10.3 |
Data are means ± SE; n, no. of rats. Δ, Change; MAP, mean arterial pressure; HR, heart rate. P < 0.05, iliac vs. mesenteric (†) and iliac vs. renal (‡). No significant differences between mesenteric vs. renal vascular beds were observed. All maximal responses, except for the increase in HR following VX, were significantly different from 0, whereas no steady-state responses were different from 0, except for the decrease in HR following βX.
The maximal and steady-state effects evoked by β-adrenergic and V1 vasopressin receptor blockades in the regional vascular beds, MAP, and HR are presented in Table 2. Note that the maximal iliac vasoconstriction in response to β-adrenergic blockade was approximately two times as large as that observed in the mesenteric and renal vascular beds. In contrast, no significant differences between the regional vascular beds were observed in maximal responses to V1 vasopressin receptor blockade. This suggests larger tonic β-adrenergic vasodilation in the iliac vs. both visceral vascular beds. The maximal responses were observed immediately after the blockades, and then all variables gradually returned toward the preblockade levels. Approximately 10 min after the blockades, the residual changes in all variables, except for the decreases in HR following β-adrenergic blockade, became not different from zero (Table 2). Nevertheless, the steady-state decrease in iliac conductance following β-adrenergic blockade was still significantly different compared with small, insignificant increases in mesenteric and renal conductance, which is consistent with preferential tonic β-adrenergic vasodilation of the iliac vasculature.
Comparison of hemodynamic responses observed in intact vs. experimental groups.
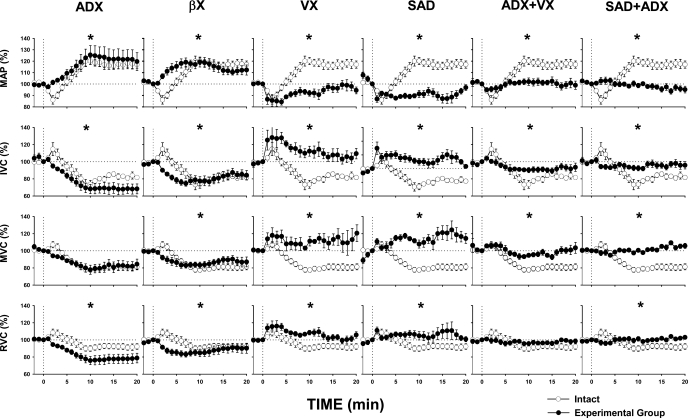
Figure 2 presents comparisons of averaged responses evoked by selective stimulation of NTS A1 adenosine receptors in intact animals and following specific experimental procedures. Two-way ANOVA showed significant differences between all experimental vs. intact conditions except for MVC in adrenalectomized animals and RVC following combined adrenalectomy plus vasopressin receptor blockade, which did not reach statistical significance (P = 0.195 and P = 0.112, respectively) (Fig. 2). In intact animals, the typical variability of the responses to stimulation of NTS A1 adenosine receptors was observed, similar to that reported in previous studies from our laboratory (24, 25, 39). In the initial phase of the responses (approximately first 5 min), depressor and vasodilatory responses prevailed. In the subsequent 15 min (or more) of the responses, pressor and vasoconstrictor responses prevailed. Overall, the increases in MAP and vasoconstriction in all vascular beds dominated over 20 min of the responses in the intact group. Adrenalectomy and β-adrenergic blockade eliminated initial vasodilation, thus sympathetic and vasopressinergic vasoconstriction dominated in these groups. Blockade of V1 vasopressin receptors virtually eliminated the vasoconstrictor component of the responses; adrenergic vasodilation prevailed over sympathetic vasoconstriction in this setting in all vascular beds. Sinoaortic denervation eliminated reflex sympathetic vasoconstriction and vasopressin release (25, 39). In this situation, nonbaroreflex activation of the adrenal medulla (39) was smaller than the activation mediated via combined baro- and nonbaroreflex mechanisms; however, in this setting, the vasodilatory factor was not opposed by any vasoconstricting factors (sympathetic nerves and vasopressin). Therefore, vasodilation observed in this group did not differ substantially compared with that observed after blockade of V1 vasopressin receptors alone. Combined adrenalectomy and V1 vasopressin receptor blockade eliminated both major humoral factors, revealing that the contribution of sympathetic vasoconstriction to the responses was very small, especially in the mesenteric and renal vascular beds. Finally, combined sinoaortic denervation and bilateral adrenalectomy eliminated all three primary vasoactive factors triggered by stimulation of NTS A1 adenosine receptors, and these residual responses were not different from those where only sympathetic nerves were active. Because in most of the experimental groups more than one vasoactive factor was at play, the relative comparisons of the effect of the vasoactive factors on regional vascular beds were rather complex, especially that these factors may differentially interact with each other in a specific vascular bed. To simplify the picture, we further compared regional vascular responses due to the separate effects of sympathetic nerves, vasopressin, and epinephrine; these single-factor effects were calculated from basic experimental groups as described in Experimental protocols.
Fig. 2.
Mean arterial pressure (MAP) and iliac (IVC), mesenteric (MVC), and renal (RVC) vascular conductance responses to microinjection of adenosine A1 receptor agonist (CPA, 330 pmol/50 nl) in the subpostremal NTS in intact rats (open circles) compared with the responses evoked following different experimental procedures (filled circles). Data are means ± SE. *P < 0.05 vs. intact animals. ADX, bilateral adrenalectomy; βX, β-adrenergic blockade; VX, vasopressin V1 receptor blockade; SAD, bilateral sinoaortic denervation; ADX + VX, bilateral adrenalectomy + vasopressin V1 receptor blockade; SAD + ADX, bilateral sinoaortic denervation + bilateral adrenalectomy. The unstable resting level of MAP and MVC in the SAD group is the result of baroreceptor denervation; in time periods longer than 3 min, these values oscillated around the 100% level measured at time 0.
Differential regional vascular responses to single vasoactive factors triggered by stimulation of NTS A1 adenosine receptors.
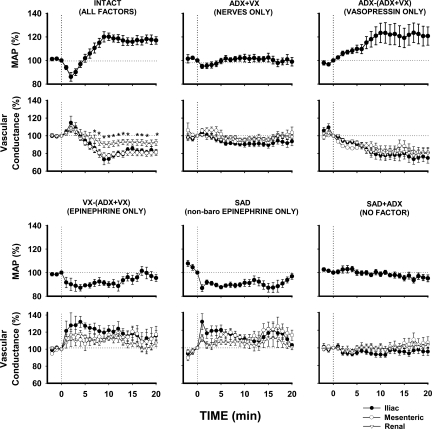
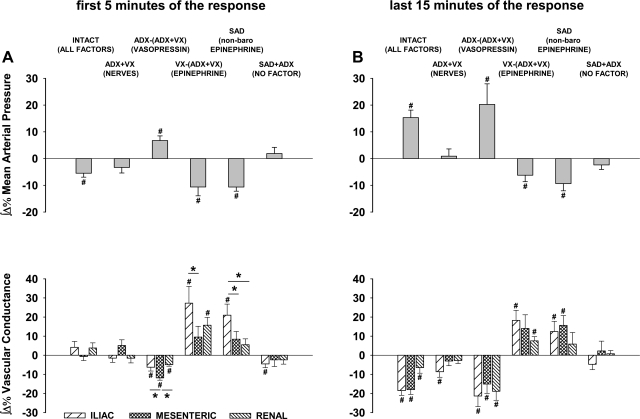
Figure 3 compares the averaged responses of three vascular beds (iliac vs. mesenteric vs. renal) mediated via the three major vasoactive factors (sympathetic nerves, vasopressin, and epinephrine) activated by the selective stimulation of A1 adenosine receptors located in the NTS. The impact of each factor on each vascular bed was compared with the combined impact of all these factors in intact animals and with a “negative control” group where activation of all these factors was prevented via combined bilateral sinoaortic denervation and adrenalectomy (SAD + ADX). In intact animals, where β-adrenergic vasodilation acted together with vasopressinergic and sympathetic vasoconstriction, no significant differences were observed between initial vasodilation across regional vascular beds. However, in the later phase of the responses, significantly smaller vasoconstriction was observed in renal compared with iliac and mesenteric vascular beds (Fig. 3, first panel on top). Two-way ANOVA did not show significant differences between time courses of regional vascular responses evoked by each vasoactive factor alone (P > 0.05 for vascular bed × time interactions); however, it confirmed significant differences across regional vascular beds in the intact group (P = 0.024 for vascular beds × time interaction). The lack of differences between time courses of whole vascular responses to single vasoactive factors was not surprising taking into consideration the biphasic dynamics of the responses: β-adrenergic vasodilation prevailed in the first 5 min of the responses, whereas neural and vasopressinergic vasoconstriction prevailed in the remaining portion of responses. Two-way ANOVA performed separately on the first 5 and last 15 min of the responses also did not show significant interactions between vascular beds vs. time, probably because the changes in regional vascular conductance were rather parallel, although different in amplitude, especially so for the effects of total and nonbaroreflex epinephrine (Fig. 3, two first panels on bottom). The differences between regional vascular responses became significant when both phases of the responses were analyzed as integrals (reflecting both amplitude and duration of the responses) separately for the first 5 min (mostly vasodilation) and the last 15 min (mostly vasoconstriction) of the responses (Fig. 4, A and B). In the initial phase of the responses (first 5 min), two-way ANOVA showed significant vascular bed vs. experimental condition interaction (P = 0.026), whereas in the later phase the regional vascular responses were more uniform (P = 0.441 and P = 0.205 for vascular beds and vascular bed × experimental conditions interaction, respectively).
Fig. 3.
Comparison of regional hemodynamic responses (iliac, mesenteric, and renal) mediated via three major vasoactive factors (sympathetic nerves, vasopressin, and epinephrine) triggered by selective stimulation of A1 adenosine receptors located in the NTS (microinjections of CPA, 330 pmol/50 nl). Data are means ± SE. *P < 0.05, renal vs. iliac and mesenteric vascular beds. Abbreviations are as in Fig. 2. Single-factor effects were calculated from basic experimental groups {sympathetic nerve effect (ADX + VX), vasopressin [ADX − (ADX + VX)], epinephrine [VX − (ADX + VX)]} and epinephrine released via the nonbaroreflex mechanism following SAD. These effects are compared with the combined effect of all vasoactive factors operating in intact animals and after elimination of all major vasoactive factors in the SAD + ADX group.
Fig. 4.
Integral hemodynamic responses (iliac, mesenteric, and renal) evoked by selective stimulation of A1 adenosine receptors located in the NTS. Data were calculated from traces presented in Fig. 3. A: integrals measured over the first 5 min of the responses, when decreases in MAP and vasodilation prevailed. B: integrals measured over the last 15 min of the responses, when increases in MAP and vasoconstriction prevailed. Data are means ± SE. *P < 0.05 between vascular beds linked with the horizontal lines. #P < 0.05 vs. 0. Abbreviations are as in Fig. 2.
Interestingly, in the initial phase of the responses, virtually no neural vasoconstriction was observed; the mesenteric vascular bed even tended to dilate (P = 0.114 vs. zero). In the later phase of the response, iliac vasoconstriction was significant (P = 0.010 vs. 0), whereas renal and mesenteric vasoconstrictions remained very small and not different from zero. Vasopressin initially evoked relatively small responses with significantly greater vasoconstriction in the mesenteric vs. the iliac and renal vascular beds (Fig. 4A); however, in the later phase of the responses, vasopressinergic vasoconstriction increased systematically, and large, sustained vasoconstriction with no differences between the vascular beds was observed (Fig. 3, top left). In the initial phase of the responses, iliac β-adrenergic vasodilation was significantly greater than that observed in the mesenteric vascular bed (P = 0.020) and tended to be greater than that observed in the renal vasculature (P = 0.113) (Fig. 4A). In the later phase of the responses, these regional differences persisted to some degree (Fig. 4B). The regional effects of epinephrine released via nonbaroreflex mechanisms (in sinoaortic-denervated animals) were similar to those observed when the adrenal medulla was activated by both baro- and nonbaroreflex mechanisms (Figs. 3 and 4, A and B). Finally, the elimination of all three vasoactive factors by means of combined bilateral sinoaortic denervation and adrenalectomy virtually abolished the responses in all vascular beds.
DISCUSSION
Activation of A1 adenosine receptors in the NTS contributes to the cardiovascular component of the HDR; specifically, it contributes to the inhibition of baroreflex transmission in the NTS and to the pressor component of HDR (34, 39, 42–44). The present study tested the hypothesis that activation of NTS A1 adenosine receptors may also contribute to the crucial component of HDR, which is the redistribution of blood from the viscera to the muscle (48). Therefore, in the present study for the first time, regional vascular responses evoked by selective stimulation of NTS A1 adenosine receptors were compared. Furthermore, we investigated the mechanisms mediating the complex hemodynamic responses and their regional variability. The major finding of the present study is that three major vasoactive factors naturally triggered in response to the stimulation, i.e., sympathetic and vasopressinergic vasoconstriction counteracted by β-adrenergic vasodilation (24, 25), had differential effects on somatic (iliac) vs. visceral (mesenteric and renal) vasculatures. Sympathetic vasoconstriction was observed in the iliac vasculature only. The greatest vasodilation mediated via released epinephrine and a β-adrenergic mechanism was observed in the iliac compared with the two other vascular beds. The released vasopressin initially evoked small preferential mesenteric vasoconstriction, and then large, steady vasoconstriction that was similar in all vascular beds. Taken together, these results suggest that A1 adenosine receptors operating in the NTS may contribute to the redistribution of blood from the visceral to the somatic (muscle) vasculature via preferential iliac vasodilation; however, two vasoconstricting factors simultaneously triggered by the stimulation diminished this effect by limiting skeletal muscle vasodilation. When all vascular factors act simultaneously (intact group), vasoconstriction prevailed in the iliac and mesenteric vs. the renal vascular bed. These data combined with previous studies from our laboratory and by others show that, although the A1 adenosine receptors located in the NTS contribute to the pressor component of the stress/HDR, the activation of these receptors may have a small, if any, effect on the redistribution of blood from the visceral to the muscle vasculature (34, 39, 42–44).
NTS adenosine receptors and differential control of regional vascular beds by neural and humoral factors: sympathetic nerves.
It has been reported that stimulation of the superior laryngeal nerve, which also conducts the aortic depressor nerve fibers in rats, evokes much greater iliac than renal and mesenteric vasodilation (11). Also, stimulation of the aortic depressor nerve evoked preferential iliac vasodilation in rats (30). The activation of α1-adrenergic receptors (a major mechanism mediating sympathetic baroreflex responses) with metoxamine in mongrel dogs led to a greater vasoconstriction in iliac compared with renal and mesenteric vascular beds (17). Similarly, iliac capacity for baroreflex vasoconstriction and vasodilation in greyhounds was greater in iliac than mesenteric and renal vascular beds (8). These reports suggested that baroreflex sympathetic responses may be greater in iliac than in renal and mesenteric vascular beds. Therefore, we expected that A1 adenosine receptor-mediated inhibition of NTS baroreflex mechanisms (34, 39) will result in a greater sympathetic iliac vasoconstriction compared with that observed in mesenteric and renal vascular beds. The present study generally confirmed this hypothesis. However, the lack of neural vasoconstrictor responses in renal and mesenteric vascular beds was unexpected (Fig. 4, A and B). The neural vasoconstrictor component of the responses was relatively small in all vascular beds compared with the relatively large effects evoked by the increases in circulating epinephrine and vasopressin. No significant vasoconstriction was observed in the first 5 min of the responses, whereas in the later phase of the response (the last 15 min) only iliac vasoconstriction was significantly different from zero as well as from both other vascular beds, consistent with our previous reports (24, 25).
Interestingly, the mesenteric vascular bed tended to vasodilate in the initial phase of the response, although the difference did not reach statistical significance vs. zero (P = 0.114). This may suggest that, in the early phase of the responses, sympathetic vasoconstriction was counteracted by neurogenic vasodilation and this vasodilation weakened with time. The most likely vasodilator factor responsible for this counteraction could be preabsorbed epinephrine released from sympathetic terminals, as suggested previously by Berecek and Brody (3). The action of preabsorbed epinephrine would decrease with time over the response in adrenalectomized animals. Consistently sympathetic (α1-adrenergic) vasoconstriction would increase with time as was observed. This is also consistent with an initially smaller and then greater neural vasoconstriction in the iliac vascular bed (P = 0.018) (Fig. 4, A and B). Among other vasodilatory neurotransmitters potentially released from sympathetic nerve terminals in the mesenteric and iliac vasculature, for example, nitric oxide (NO), calcitonin gene-related peptide (CGRP), and adenosine as a catabolite of neuronally released ATP should be considered (6, 10, 22, 29).
Vasopressin.
In the present study, we found that vasopressin triggered by selective activation of NTS A1 adenosine receptors initially constricts the mesenteric vascular bed to a greater extent than the renal and iliac vasculature, whereas in the later phase of the response no regional differences between vasopressinergic vasoconstriction were observed. Our data are consistent with regional vascular responses observed following intravenous infusion of exogenous vasopressin in rats where small doses of vasopressin evoked preferential vasoconstriction in the mesenteric compared with the renal and iliac vasculature, whereas with larger doses the regional differences diminished or disappeared (12, 13). It should be mentioned that regional effects of vasopressin in rats are different from those observed in dogs where vasopressinergic vasoconstriction dominates in the iliac vasculature (17, 23). Most likely, in the present study, the initial release of vasopressin was small, and it increased with the time of the response (Figs. 3 and 4). In our previous study, we found that the level of circulating vasopressin measured ∼8 min after microinjection of A1 receptor agonist (CPA) in the NTS increased over fourfold compared with the resting level. Note that, in the present study, vasopressinergic vasoconstriction increased systematically up to ∼8 min of the response and then was maintained at this high, steady level throughout the observed response (Fig. 3, last panel on top). Circulating vasopressin is quickly catabolized at normal body temperature (7); therefore, the large, sustained vasopressinergic vasoconstriction observed in the present study suggests that vasopressin was continuously released in the circulation as long as the baroreflex mechanism was inhibited by stimulation of A1 adenosine receptors, which can last over an hour at this dose of A1 agonist (CPA, 330 pmol) (34).
In contrast to activation of NTS A1 adenosine receptors, the activation of A2a adenosine receptors does not affect vasopressin release, since A2a receptors do not inhibit baroreflex transmission in the NTS (20, 37). In addition, previous studies from our laboratory showed that preferential iliac vasodilation observed after selective activation of NTS A2a adenosine receptors was completely abolished following bilateral adrenalectomy and lumbar sympathectomy, and no vasoconstrictor component of the response persisted following these procedures (2, 21).
Epinephrine.
The preferential iliac vs. mesenteric and renal vasodilation dominated in the initial phase of the responses (Figs. 3 and 4). This vasodilation was mediated via activation of the adrenal medulla, release of epinephrine, and β-adrenergic vasodilation. The initial vasodilation was abolished in all vascular beds after bilateral adrenalectomy or β-adrenergic blockade (Fig. 2). The greatest β-adrenergic vasodilation was observed in the iliac vascular bed, which supplies mostly skeletal muscles, compared with the vasodilation of both visceral vascular beds. This is consistent with preferential expression of β-adrenergic receptors in the skeletal muscle vasculature (45) as well as with the greater tonic β-adrenergic vasodilation in iliac compared with mesenteric and renal vascular beds as observed in the present study (Table 2). The preferential iliac vasodilation was observed in the group of animals where the whole epinephrine effect was calculated from basic experimental groups [VX − (ADX + VX)] as well as in the group where sinoaortic denervation was performed, and nonbaroreflex epinephrine effects were directly measured (Figs. 3 and 4). The consistent results obtained using the two different experimental approaches attested that the arithmetical separation of single vasoactive factors in the present study was accurate.
According to our previous reports, sinoaortic denervation or blockade of glutamatergic transmission in the NTS abolishes pressor and regional sympathoexcitatory responses (39) mediated via A1 adenosine receptors. However, A1 receptor-mediated activation of the adrenal medulla is only attenuated but not abolished in this setting. Therefore, following sinoaortic denervation, the adrenal medulla was still activated by stimulation of NTS A1 adenosine receptors, although to a lesser extent than in intact animals where both baro- and nonbaroreflex components of the activation of the adrenal medulla were present. In fact, in the present study, regional vasodilatory responses evoked by combined baro- and nonbaroreflex mechanisms tended to be greater compared with those where only nonbaroreflex mechanism was active (Fig. 4), although these differences did not reach statistical significance.
Preferential β-adrenergic iliac vasodilation, triggered by selective stimulation of NTS A1 adenosine receptors, was masked by simultaneous neural and vasopressinergic vasoconstriction. However, combined activation of both A1 and A2a adenosine receptor subtypes in the NTS may significantly contribute to the preferential iliac vasodilation and to the redistribution of blood from visceral to somatic vascular beds. Previous studies from our laboratory showed that selective stimulation of NTS A2a adenosine receptors evoked much greater iliac vs. renal and mesenteric vasodilation (2); the β-adrenergic vasodilation contributed to ∼80% of this preferential iliac vasodilation (21).
Potential mechanisms.
The present study showed that selective activation of NTS A1 adenosine receptors preferentially disinhibited vasopressin release and activated sympathetic outputs to the adrenal medulla, whereas other sympathetic outputs were activated to a much lesser extent. What possible mechanism(s) may be responsible for this difference? Our previous studies strongly suggested that A1 adenosine receptors act mainly via inhibition of baroreflex transmission in the NTS (34, 39). This inhibition is responsible for all pressor and sympathoexcitatory responses, including vasopressin release (25). Because the vasopressinergic vasoconstriction observed in the present study was severalfold greater than the regional sympathetic vasoconstriction, it seems that A1 adenosine receptors may be located preferentially on those NTS baroreflex terminals/interneurons that are responsible for the tonic baroreflex restraint of vasopressin release and to a much lesser extent on NTS neurons responsible for tonic baroreflex restraint of regional sympathetic outputs. In fact, the increases in LSNA and RSNA, observed in a previous study from our laboratory (39), were two- to threefold smaller than that observed in the adrenal nerve. Pre- and postsynaptic A1 adenosine receptors, coupled to inhibitory Gi/o proteins, may be located on both glutamatergic and γ-aminobutyric acid (GABA)-ergic terminals and neurons, respectively (6). Therefore, total effects of activation of these receptors may depend on the ratio between inhibition of activatory (glutamatergic) vs. inhibitory (GABA-ergic) NTS neurons and/or terminals. This ratio may be higher for NTS neurons controlling vasopressin release compared with the neurons controlling sympathetic outputs. Nevertheless, A1 adenosine receptor-mediated inhibition of glutamatergic transmission in the baroreflex pathway dominates over potential inhibition of GABA-ergic neurons, since stimulation of these receptors results in inhibition of baroreflex mechanisms and baroreflex-dependent regional sympathoexcitation (34, 39).
One other reason for much smaller neural than humoral vasoconstriction observed in the present study may be the nonhomogenous character of efferent sympathetic fibers selectively disinhibited by A1 adenosine receptors in the NTS. Efferent sympathetic terminals, in addition to the major neurotransmitter norepinephrine, may also secrete several vasodilatory neurotransmitters, including NO, CGRP, and ATP, which, after degradation to adenosine via ectonucleotidases, may cause vasodilation via both A1 and A2a adenosine receptor subtypes (6, 9, 10, 22, 29, 49). A1 adenosine receptors may disinhibit both vasoconstrictor and vasodilatory efferent fibers, and these effects may cancel each other. The net effect may be relatively small vasoconstriction (as observed in the iliac vasculature), no significant response (as observed in renal vasculature), or even a tendency to vasodilation (as observed in the mesenteric vascular bed). In a previous study from our laboratory, we observed a similar phenomenon revealed by selective stimulation of NTS A2a adenosine receptors. This stimulation triggered preferential iliac vasodilation compared with the mesenteric and renal vasculatures, whereas sympathetic activity directed to the iliac vascular bed did not change in this setting (2, 36). Taken together, these observations could suggest that LSNA may not contribute to the response. However, although the total LSNA did not alter, the lumbar sympathectomy did contribute to >20% of the preferential iliac vasodilation, suggesting that both vasoconstricting and vasodilatory fibers (most likely releasing NO) might have been simultaneously activated (10, 21, 29). Which specific neurotransmitters are released from these regional sympathetic nerves in response to stimulation of NTS adenosine receptor subtypes awaits further investigation.
The responses observed in intact animals were biphasic; therefore, unloading of arterial baroreceptors during the initial, depressor phase of the responses could contribute to the total pressor and vasoconstrictor effects observed in the later phase of the responses. This initial, depressor phase of the responses was a result of baroreflex- and nonbaroreflex-dependent activation of the adrenal medulla, release of epinephrine, and β-adrenergic vasodilation, which prevailed over vasopressinergic and sympathetic vasoconstriction in approximately the first 5 min of the responses. However, when bilateral adrenalectomy or β-adrenergic blockade eliminated this initial decrease in MAP (protocols 1 and 2), the vasoconstrictor and pressor effects observed in the later phase of the responses were not smaller than those observed in intact animals (Fig. 2). This strongly suggests that the potential unloading of arterial baroreceptors in the early phase of the response had a negligible (if any) contribution to the overall vasoconstrictor effects evoked by direct inhibition of baroreflex mechanisms following stimulation of NTS A1 adenosine receptors.
Elevated levels of circulating vasopressin due to activation of NTS A1 adenosine receptors may facilitate baroreflex mechanisms at the level of the NTS via activation of the area postrema neurons (16, 25), thus competing with A1 adenosine-mediated inhibition of the baroreflex mechanisms (34, 39). However, the effective inhibition of baroreflex mechanisms lasted over 20 min of the responses, as shown by large, sustained vasopressinergic vasoconstriction (Figs. 3 and 4B). This indicates that the A1 adenosoinergic inhibition prevailed over V1 vasopressinergic facilitation of the baroreflex mechanisms in this setting.
In absolute terms, the regional β-adrenergic vasodilatory responses were much greater than the sympathetic vasoconstrictor responses, since blockade of V1 vasopressin receptors reversed vasoconstriction (which dominated in intact animals) into marked vasodilation in all three vascular beds (Fig. 2). This indicated that β-adrenergic vasodilation markedly prevailed over sympathetic vasoconstriction in all vascular beds. It should be stressed that NTS A1 adenosine receptors activate the adrenal medulla via both baroreflex and nonbaroreflex mechanisms, which is consistent with the greater overall activation of ASNA compared with other sympathetic outputs (39). The baroreflex component of this response was relatively weak (similarly as it was observed for baroreflex-mediated regional vasoconstriction discussed above), since there were no significant differences between total vs. nonbaroreflex β-adrenergic vasoconstriction observed in each vascular bed in these two situations (Fig. 4). The nonbaroreflex component of the activation of the adrenal medulla is most likely mediated via descending pathways from hypothalamic nuclei that use nonglutamatergic neurotransmitters as we proposed previously (40). However, specific pathways and neurotransmitters responsible for nonbaroreflex activation of the adrenal medulla remain unknown. All of the above hypotheses should be addressed in future studies.
Limitations of the method.
Anesthesia and recent surgical stress most likely elevated resting levels of sympathetic activity, circulating epinephrine, and vasopressin. This could attenuate further increases of these vasoactive factors due to stimulation of NTS A1 adenosine receptors. In fact, in our previous study, which was performed under similar experimental conditions, the resting vasopressin levels were moderately elevated compared with the levels measured in conscious animals (5, 15, 18, 25–27). Nevertheless, the activation of NTS A1 adenosine receptors evoked a large (>4-fold) increase of circulating vasopressin compared with the elevated baseline (25). Also, regional sympathetic outputs, especially that directed to the adrenal medulla, were significantly activated in response to stimulation of NTS A1 adenosine receptors (39). This attests that, despite the increased resting levels of humoral and neural factors, the stimulation of NTS A1 receptors is potent to evoke marked responses in all of these factors. In addition, the potential effects of anesthesia and recent surgery presumably affected all vascular beds similarly; therefore, we believe that the relative regional differences in responsiveness to the vasoactive factors were preserved under these experimental conditions.
We measured changes in regional vascular conductance using acutely inserted Doppler flow probes placed around the large arteries (common iliac, mesenteric, and renal) in proximity of the aorta. Blood flow was calculated from Doppler frequency shift, which is proportional to changes in velocity of circulating blood. This method was routinely used in many previous studies from our laboratory and by others (2, 3, 10, 24, 25). Although blood flow in specific vascular bed is controlled mainly at the level of the arterioles, whereas the contractility of large arteries is rather minimal, it was possible that these large arteries participated to some extent in vasoconstriction and vasodilation occurring downstream of the flow probes. If the diameter of the artery on which the flow probe was placed decreased (vasoconstriction) and increased (vasodilation), measured velocity of blood flow could increase and decrease, respectively. If this was the case, our measurements of both decreases and increases of regional vascular conductance could be underestimated because local, mechanical changes in blood velocity occurring under the flow probe could oppose experimental changes in blood flow occurring mostly downstream of the flow probe at the level of the arterioles. However, even if this underestimation occurred, it should have a proportional effect in all regional vascular beds; therefore, the relative changes in regional vascular conductance, compared in the present study, should not be affected. Despite this potential limitation (decreased sensitivity of the method), we were able to detect very small and very large increases and decreases in regional vascular conductance as well as the dynamic properties on biphasic vascular responses. It should be stressed that, in our previous study, we showed that marked iliac vasoconstriction, abolished by systemic V1 vasopressin receptor blockade, was indeed accompanied with large (>4-fold) increases in circulating vasopressin levels, which directly confirmed the conclusions based on this type of recordings (25). Therefore, we believe that theoretically possible underestimation of both vasoconstrictor and vasodilator effects in the present study did not have significant effects on the conclusions.
In this and our previous study, MAP decreased following V1 vasopressin receptor blockade and was allowed to return spontaneously toward resting levels. We did not compensate for the decrease of MAP with phenylephrine infusion to avoid potentially different α1-adrenergic vasoconstriction of different vascular beds due to differential regional expression of these receptors (17, 19); such a compensation could distort the responses to the experimental factors. The baroreflex compensation of the depressor response evoked by V1 vasopresinergic blockade probably elevated baseline sympathetic activity, which may have attenuated the sympathoactivation in response to stimulation of NTS A1 adenosine receptors. Therefore, the neural sympathetic component of the responses could be underestimated in this study. Nevertheless, we believe that regional differences in sympathetic responses were preserved, since vasoconstriction in the iliac vascular bed significantly increased, whereas the responses of the mesenteric and renal vascular beds were not different from zero.
In contrast, removing β-adrenergic vasodilation could differentially exaggerate regional vascular responses to vasoconstrictor factors (sympathetic vasoconstriction and vasopressin release). For example, β-adrenergic vasodilation was significantly greater in iliac compared with mesenteric and renal vascular beds; therefore, elimination of this factor could allow for relatively greater vasoconstriction in iliac than in the two other vascular beds. This effect could partially contribute to the greater iliac than mesenteric and renal sympathetic vasoconstriction (Fig. 4B). However, it did not significantly affect the powerful vasopressinergic vasoconstriction in the later phase of the responses, whereas, in the initial phase of the responses, vasopressinergic vasoconstriction was significantly greater in mesenteric compared with iliac and renal vascular beds (Fig. 4).
Adrenalectomy and β-adrenergic blockade most likely diminished cardiac output regulatory capacity. Therefore, following combined adrenalectomy and V1 vasopressin receptor blockade (protocol 5), MAP and IVC did not fully recover, whereas, following V1 vasopressin receptor blockade alone (protocol 3), full recovery of these parameters was observed (Table 1).
Renal autoregulation was most likely responsible for much smaller responses evoked in this vascular bed in intact animals and under most of the experimental conditions (Fig. 4). Nevertheless, vasopressin had a relatively large effect on the renal vasculature, not different from that observed in the iliac and mesenteric vasculature beds during the whole time course of the responses (Fig. 3). Consistently, following V1 vasopressin receptor blockade, significantly increased RVC remained significantly elevated compared with that observed in intact animals, whereas MAP, IVC, and MVC completely recovered following the blockade (Table 1).
Although we used a standard method of recording regional vascular conductance, which is well established in our laboratory, the relatively small neural vs. humoral regional vascular responses could raise a question if sympathetic nerves were potentially damaged during the procedure. This was rather unlikely, because electrical stimulation of the aortic depressor nerve (8 volts, 0.1 ms, 8–64 Hz) and activation of cardiopulmonary receptors with intravenous phenylbiguanide (1–8 μg/kg) applied in some intact animals evoked large sympathetic vasodilation in all vascular beds with patterns similar to that described previously by Faber and Brody (11).
In conclusion, three major vasoactive factors triggered by stimulation of NTS A1 adenosine receptors in the NTS (sympathetic and vasopressinergic vasoconstriction opposed by β-adrenergic vasodilation) differentially affect regional vascular beds. The β-adrenergic vasodilation, which dominates in the initial phase of the response, was significantly greater in the iliac than the mesenteric and renal vasculatures. Significant sympathetic vasoconstriction was observed in the iliac but not in the mesenteric and renal vascular beds. In contrast, vasopressin exerted similar, marked, sustained vasoconstriction in all vascular beds, except for a small, initial preferential mesenteric vasoconstriction. This pattern of regional vascular responses suggests that activation of A1 adenosine receptors in the NTS has minor, if any, effect on redistribution of blood from the visceral to somatic (muscle) vasculature.
Perspectives and Significance
Previous studies from our laboratory and by others strongly suggested that adenosine operating via A1 adenosine receptors in the NTS may contribute to the pressor component of HDR, most likely via the inhibition of baroreflex transmission in the NTS, which disinhibits efferent sympathetic vasoconstriction and vasopressin release (25, 34, 39, 42–44). Adenosine operating in the NTS via both A1 and A2a receptor subtypes preferentially activates the adrenal medulla leading to preferential iliac vasodilation (2, 21), which is the major mechanism mediating increases in blood supply to skeletal muscles during stress/HDR in the rat (48). However, the present study showed that, although activation of NTS A1 adenosine receptors preferentially increases iliac vascular conductance in the early phase of the response, this effect is overridden by the powerful V1 vasopressinergic vasoconstriction in later phase of the response, which diminished the contribution of NTS A1 adenosine receptors to the redistribution of blood. To what extent do these potential mechanisms, triggered by stimulation of NTS adenosine receptor subtypes, contribute to the pattern of autonomic responses evoked by the stress or HDR requires further investigation. Interestingly, some components of the autonomic responses evoked by selective activation of adenosine receptor subtypes in the NTS remain inconsistent with the pattern of HDR. For example, the decreases in HR due to A1 and A2a adenosine receptor stimulation, the depressor responses evoked by A2a receptor stimulation, and the lack of contribution of NTS A2a receptors to the baroreflex inhibition are most likely components of other autonomic mechanisms, not related to the HDR. Alternatively, the responses opposite to the HDR pattern may contribute to fine tuning of the HDR autonomic pattern. Further studies are necessary to specify the autonomic mechanisms to which these non-HDR effects mediated by NTS adenosine receptor subtypes may contribute.
GRANTS
This study was supported by National Heart, Lung, and Blood Institute Grant HL-67814.
DISCLOSURES
No conflicts of interest are declared by the authors.
REFERENCES
- 1. Barraco R, el Ridi M, Ergene E, Parizon M, Bradley D. An atlas of the rat subpostremal nucleus tractus solitarius. Brain Res Bull 29: 703–765, 1992 [DOI] [PubMed] [Google Scholar]
- 2. Barraco RA, O'Leary DS, Ergene E, Scislo TJ. Activation of purinergic receptor subtypes in the nucleus tractus solitarius elicits specific regional vascular response patterns. J Auton Nerv Syst 59: 113–124, 1996 [DOI] [PubMed] [Google Scholar]
- 3. Berecek KH, Brody MJ. Evidence for a neurotransmitter role for epinephrine derived from the adrenal medulla. Am J Physiol Heart Circ Physiol 242: H593–H601, 1982 [DOI] [PubMed] [Google Scholar]
- 4. Bisserbe JC, Patel J, Marangos PJ. Autoradiographic localization of adenosine uptake sites in rat brain using [3H]nitrobenzylthioinosine. J Neurosci 5: 544–550, 1985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bonjour JP, Malvin RL. Plasma concentrations of ADH in conscious and anesthetized dogs. Am J Physiol 218: 1128–1132, 1970 [DOI] [PubMed] [Google Scholar]
- 6. Burnstock G. Physiology and pathophysiology of purinergic neurotransmission. Physiol Rev 87: 659–797, 2007 [DOI] [PubMed] [Google Scholar]
- 7. Cowley AW., Jr Vasopressin and cardiovascular regulation. Int Rev Physiol 26: 189–242, 1982 [PubMed] [Google Scholar]
- 8. Cox RH, Bagshaw RJ, Detweiler DK. Baroreceptor reflex cardiovascular control in mongrel dogs and racing greyhounds. Am J Physiol Heart Circ Physiol 249: H655–H662, 1985 [DOI] [PubMed] [Google Scholar]
- 9. Dampney RA. Functional organization of central pathways regulating the cardiovascular system. Physiol Rev 74: 323–364, 1994 [DOI] [PubMed] [Google Scholar]
- 10. Davisson RL, Possas OS, Murphy SP, Lewis SJ. Neurogenically derived nitrosyl factors mediate sympathetic vasodilation in the hindlimb of the rat. Am J Physiol Heart Circ Physiol 272: H2369–H2376, 1997 [DOI] [PubMed] [Google Scholar]
- 11. Faber JE, Brody MJ. Reflex hemodynamic response to superior laryngeal nerve stimulation in the rat. J Auton Nerv Syst 9: 607–622, 1983 [DOI] [PubMed] [Google Scholar]
- 12. Gardiner SM, Compton AM, Bennett T. Regional haemodynamic effect of vasopressin infusion in conscious, unrestrained, Brattleboro rats. Br J Pharmacol 97: 147–152, 1989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Gardiner SM, Compton AM, Kemp PA, Bennett T. Effects of NG-nitro-l-arginine methyl ester or indomethacin on differential regional and cardiac haemodynamic actions of arginine vasopressin and lysine vasopressin in conscious rats. Br J Pharmacol 102: 65–72, 1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Gourine AV, Wood JD, Burnstock G. Purinergic signalling in autonomic control. Trends Neurosci 32: 241–248, 2009 [DOI] [PubMed] [Google Scholar]
- 15. Hammond RL, Augustyniak RA, Rossi NF, Lapanowski K, Dunbar JC, O'Leary DS. Alteration of humoral and peripheral vascular responses during graded exercise in heart failure. J Appl Physiol 90: 55–61, 2001 [DOI] [PubMed] [Google Scholar]
- 16. Hasser EM, Bishop VS, Hay M. Interactions between vasopressin and baroreflex control of the sympathetic nervous system. Clin Exp Pharmacol Physiol 24: 102–108, 1997 [DOI] [PubMed] [Google Scholar]
- 17. Heyndrickx GR, Boettcher DH, Vatner SF. Effects of angiotensin, vasopressin, and methoxamine on cardiac function and blood flow distribution in conscious dogs. Am J Physiol 231: 1579–1587, 1976 [DOI] [PubMed] [Google Scholar]
- 18. Howard RL, Summer S, Rossi N, Kim JK, Schrier RW. Short-term hypothyroidism and vasopressin gene expression in the rat. Am J Kidney Dis 19: 573–577, 1992 [DOI] [PubMed] [Google Scholar]
- 19. Hrometz SL, Edelmann SE, McCune DF, Olges JR, Hadley RW, Perez DM, Piascik MT. Expression of multiple alpha1-adrenoceptors on vascular smooth muscle: correlation with the regulation of contraction. J Pharmacol Exp Ther 290: 452–463, 1999 [PubMed] [Google Scholar]
- 20. Ichinose TK, O'Leary DS, Scislo TJ. Activation of NTS A2a adenosine receptors differentially resets baroreflex control of renal vs. adrenal sympathetic nerve activity. Am J Physiol Heart Circ Physiol 296: H1058–H1068, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kitchen AM, Scislo TJ, O'Leary DS. NTS A2a purinoceptor activation elicits hindlimb vasodilation primarily via a beta-adrenergic mechanism. Am J Physiol Heart Circ Physiol 278: H1775–H1782, 2000 [DOI] [PubMed] [Google Scholar]
- 22. Lappe RW, Todt JA, Wendt RL. Regional vasodilator actions of calcitonin gene-related peptide in conscious SHR. Peptides 8: 747–749, 1987 [DOI] [PubMed] [Google Scholar]
- 23. Liard JF, Deriaz O, Schelling P, Thibonnier M. Cardiac output distribution during vasopressin infusion or dehydration in conscious dogs. Am J Physiol Heart Circ Physiol 243: H663–H669, 1982 [DOI] [PubMed] [Google Scholar]
- 24. McClure JM, O'Leary DS, Scislo TJ. Stimulation of NTS A1 adenosine receptors evokes counteracting effects on hindlimb vasculature. Am J Physiol Heart Circ Physiol 289: H2536–H2542, 2005 [DOI] [PubMed] [Google Scholar]
- 25. McClure JM, Rossi NF, Chen H, O'Leary DS, Scislo TJ. Vasopressin is a major vasoconstrictor involved in hindlimb vascular responses to stimulation of adenosine A1 receptors in the nucleus of the solitary tract. Am J Physiol Heart Circ Physiol 297: H1661–H1672, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Mueller PJ, Sullivan MJ, Grindstaff RR, Cunningham JT, Hasser EM. Regulation of plasma vasopressin and renin activity in conscious hindlimb-unloaded rats. Am J Physiol Regul Integr Comp Physiol 291: R46–R52, 2006 [DOI] [PubMed] [Google Scholar]
- 27. O'Leary DS, Rossi NF, Churchill PC. Muscle metaboreflex control of vasopressin and renin release. Am J Physiol Heart Circ Physiol 264: H1422–H1427, 1993 [DOI] [PubMed] [Google Scholar]
- 28. Phillis JW, Walter GA, O'Regan MH, Stair RE. Increases in cerebral cortical perfusate adenosine and inosine concentrations during hypoxia and ischemia. J Cereb Blood Flow Metab 7: 679–686, 1987 [DOI] [PubMed] [Google Scholar]
- 29. Possas OS, Lewis SJ. NO-containing factors mediate hindlimb vasodilation produced by superior laryngeal nerve stimulation. Am J Physiol Heart Circ Physiol 273: H234–H243, 1997 [DOI] [PubMed] [Google Scholar]
- 30. Salgado HC, Barale AR, Castania JA, Machado BH, Chapleau MW, Fazan R., Jr Baroreflex responses to electrical stimulation of aortic depressor nerve in conscious SHR. Am J Physiol Heart Circ Physiol 292: H593–H600, 2007 [DOI] [PubMed] [Google Scholar]
- 31. Scislo TJ, Augustyniak RA, Barraco RA, Woodbury DJ, O'Leary DS. Activation of P2x-purinoceptors in the nucleus tractus solitarius elicits differential inhibition of lumbar and renal sympathetic nerve activity. J Auton Nerv Syst 62: 103–110, 1997 [DOI] [PubMed] [Google Scholar]
- 32. Scislo TJ, Augustyniak RA, O'Leary DS. Differential arterial baroreflex regulation of renal, lumbar, and adrenal sympathetic nerve activity in the rat. Am J Physiol Regul Integr Comp Physiol 275: R995–R1002, 1998 [DOI] [PubMed] [Google Scholar]
- 33. Scislo TJ, DiCarlo SE. Gender difference in cardiopulmonary reflex inhibition of sympathetic nerve activity. Am J Physiol Heart Circ Physiol 267: H1537–H1543, 1994 [DOI] [PubMed] [Google Scholar]
- 34. Scislo TJ, Ichinose TK, O'Leary DS. Stimulation of NTS A1 adenosine receptors differentially resets baroreflex control of regional sympathetic outputs. Am J Physiol Heart Circ Physiol 294: H172–H182, 2008 [DOI] [PubMed] [Google Scholar]
- 35. Scislo TJ, Kitchen AM, Augustyniak RA, O'Leary DS. Differential patterns of sympathetic responses to selective stimulation of nucleus tractus solitarius purinergic receptor subtypes. Clin Exp Pharmacol Physiol 28: 120–124, 2001 [DOI] [PubMed] [Google Scholar]
- 36. Scislo TJ, O'Leary DS. Activation of A2a adenosine receptors in the nucleus tractus solitarius inhibits renal but not lumbar sympathetic nerve activity. J Auton Nerv Syst 68: 145–152, 1998 [DOI] [PubMed] [Google Scholar]
- 37. Scislo TJ, O'Leary DS. Differential control of renal vs. adrenal sympathetic nerve activity by NTS A2a and P2x purinoceptors. Am J Physiol Heart Circ Physiol 275: H2130–H2139, 1998 [DOI] [PubMed] [Google Scholar]
- 38. Scislo TJ, O'Leary DS. Differential role of ionotropic glutamatergic mechanisms in responses to NTS P2x and A2a receptor stimulation. Am J Physiol Heart Circ Physiol 278: H2057–H2068, 2000 [DOI] [PubMed] [Google Scholar]
- 39. Scislo TJ, O'Leary DS. Mechanisms mediating regional sympathoactivatory responses to stimulation of NTS A1 adenosine receptors. Am J Physiol Heart Circ Physiol 283: H1588–H1599, 2002 [DOI] [PubMed] [Google Scholar]
- 40. Scislo TJ, O'Leary DS. Purinergic mechanisms of the nucleus of the solitary tract and neural cardiovascular control. Neurol Res 27: 182–194, 2005 [DOI] [PubMed] [Google Scholar]
- 41. Scislo TJ, O'Leary DS. Adenosine receptors located in the NTS contribute to renal sympathoinhibition during hypotensive phase of severe hemorrhage in anesthetized rats. Am J Physiol Heart Circ Physiol 291: H2453–H2461, 2006 [DOI] [PubMed] [Google Scholar]
- 42. St. Lambert JH, Dashwood MR, Spyer KM. Role of brainstem adenosine A1 receptors in the cardiovascular response to hypothalamic defence area stimulation in the anaesthetized rat. Br J Pharmacol 117: 277–282, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. St. Lambert JH, Dawid-Milner MS, Silva-Carvalho L, Spyer KM. Action of adenosine receptor antagonists on the cardiovascular response to defence area stimulation in the rat. Br J Pharmacol 113: 159–164, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. St. Lambert JH, Thomas T, Burnstock G, Spyer KM. A source of adenosine involved in cardiovascular responses to defense area stimulation. Am J Physiol Regul Integr Comp Physiol 272: R195–R200, 1997 [DOI] [PubMed] [Google Scholar]
- 45. Vanhoutte B. Heterogeneity in vascular smooth muscle . In: Microcirculation, edited by Kaley G, Altura BM. Baltimore, MD: University Park, 1978, p. 181–310 [Google Scholar]
- 46. Van Wylen DG, Park TS, Rubio R, Berne RM. Cerebral blood flow and interstitial fluid adenosine during hemorrhagic hypotension. Am J Physiol Heart Circ Physiol 255: H1211–H1218, 1988 [DOI] [PubMed] [Google Scholar]
- 47. Yan S, Laferriere A, Zhang C, Moss IR. Microdialyzed adenosine in nucleus tractus solitarii and ventilatory response to hypoxia in piglets. J Appl Physiol 79: 405–410, 1995 [DOI] [PubMed] [Google Scholar]
- 48. Yardley CP, Hilton SM. Vasodilatation in hind-limb skeletal muscle evoked as part of the defence reaction in the rat. J Auton Nerv Syst 19: 127–136, 1987 [DOI] [PubMed] [Google Scholar]
- 49. Zimmermann H. Biochemistry, localization and functional roles of ecto-nucleotidases in the nervous system. Prog Neurobiol 49: 589–618, 1996 [DOI] [PubMed] [Google Scholar]