Abstract
When capsaicin is applied repeatedly to dorsal root ganglion (DRG) neurons for brief periods (10–15 s) at short intervals (5–10 min), the evoked responses rapidly decline, a phenomenon termed tachyphylaxis. In addition to this phenomenon, the present study using Ca2+ imaging revealed that repeated application of capsaicin to rat dissociated DRG neurons at longer intervals (20–40 min) or during multiple applications at short intervals elicited an enhancement of the responses, termed potentiation. The potentiation occurred in 50–60% of the capsaicin-responsive cells, on average representing a 20- to 30% increase in the peak amplitude of the Ca2+ signal, and was maximal at a 40-min application interval. An analysis of the mechanisms underlying potentiation revealed that it was suppressed by block of Ca2+/calmodulin-dependent protein kinase II (CaMKII) with 5 μM KN-93 or block of the activation of extracellular signal-regulated kinase (ERK) 1/2 with 2 μM U-0126. Lowering the extracellular Ca2+ concentration from 2 to 1 mM or pretreatment with deltamethrin (1 μM), which blocks calcineurin and tachyphylaxis, enhanced potentiation. Potentiation was not affected by: 1) inhibition of protein kinase C or protein kinase A, 2) block of the three subtypes of neurokinin receptors, or 3) block of the trafficking of transient receptor potential V1 channel to the membrane. These results indicate that the potentiation is a slowly developing Ca2+-modulated process that is mediated by a complex intracellular signaling pathway involving activation of CaMKII and ERK1/2. Potentiation may be an important peripheral autosensitization mechanism that occurs independently of the pronociceptive effects of inflammatory mediators and neurotrophic factors.
Keywords: nociceptor, capsaicin, sensitization, rat, calcium/calmodulin-dependent protein kinase II, extracellular signal-regulated kinase, dorsal root ganglion
intradermal injection of capsaicin evokes an immediate painful sensation followed by the development of primary or secondary hyperalgesia or allodynia (27, 47). Thus, capsaicin-evoked pain has been used widely as a neurogenic inflammatory pain model for the study of peripheral or central pain mechanisms (48). It is generally thought that primary hyperalgesia results from the sensitization of primary afferent nociceptors (4, 27, 39) while secondary hyperalgesia and allodynia are caused by central sensitization in the spinal dorsal horn or in the brain (47).
Several mechanisms could contribute to capsaicin or other noxious stimuli-induced peripheral sensitization of nociceptors: 1) release of substance P (SP) or calcitonin gene-related peptide (CGRP) from activated nociceptors (28); 2) release of inflammatory mediators such as nerve growth factor (NGF), bradykinin, or a protease-activated receptor-2 agonist from nonneuronal cells (24, 45, 49); 3) dorsal root reflex (29); 4) hyperexcitability induced by changes in expression of voltage-gated ion channels (5, 21, 44); and 5) activation-induced sensitization of nociceptors (50). The first four mechanisms have been widely studied; however, the last mechanism has received less attention. Direct sensitization of nociceptors after noxious stimulation is potentially an important contributor to peripheral sensitization because capsaicin or other noxious stimuli induce large increases in Ca2+ influx and activate Ca2+-dependent intracellular signaling pathways involving protein kinase C (PKC), protein kinase A (PKA), Ca2+/calmodulin-dependent protein kinase II (CaMKII), phosphatidylinositol 3-kinase (PI3-kinase), and extracellular signal-regulated kinases (ERK) (1, 12, 35, 53), which have been reported to enhance the activity of transducer molecules and ion channels located at the peripheral endings of nociceptors.
Capsaicin increases intracellular Ca2+ in primary afferent nociceptors by activating TRPV1 channels that belong to the transient receptor potential (TRP) channel superfamily (11). The TRPV1 channel is a nonselective cation channel mainly permeable to Ca2+ and Na+. Influx of Ca2+ can induce desensitization of TRPV1 channel, a process that is evident as a reduced response of nociceptors to prolonged (desensitization) or repeated (tachyphylaxis) application of capsaicin (10, 26). Influx of Ca2+ can also induce release of proinflammatory mediators such as SP, which can enhance TRPV1 activity by activating neurokinin-1 (NK-1) or neurokinin 2 (NK-2) receptors located on dorsal root ganglion (DRG) neurons (40, 52). Activation of CaMKII, PI3-kinase, and ERK1/2 by Ca2+ influx has also been reported to potentiate TRPV1 channel activity in vivo and in vitro (1, 25, 52, 53). Therefore, we hypothesized that responses evoked by capsaicin stimulation of TRPV1 channels might be enhanced after previous capsaicin exposure. Because many functional characteristics of nociceptors, such as capsaicin desensitization or tachyphylaxis and sensitization by NGF, are exhibited by dissociated DRG in culture where the complexity of the in vivo condition involving sensitization by inflammatory mediators can be eliminated (17, 21), dissociated DRG neurons were selected to test our hypothesis.
Ca2+ imaging and the perforated patch-clamp methods were employed to record capsaicin-induced intracellular Ca2+ increases and inward currents, respectively. We observed a time-dependent potentiation of the responses when capsaicin was administered repeatedly at long intervals (20–60 min). The potentiation was mediated by multiple mechanisms, including activation of CaMKII and ERK1/2.
MATERIALS AND METHODS
Animal
Adult male Sprague-Dawley rats (200–250 g; Harlan, Indianapolis, IN) were used in this study. All experimental protocols were approved by the University of Pittsburgh Institutional Animal Care and Use Committee and were consistent with the guidelines of the National Institutes of Health and the International Association for the Study of Pain.
DRG Neuron Culture
L5, L6, and S1 DRGs were removed bilaterally after laminectomy under isoflurane anesthesia. DRGs were enzymatically (collagenase type 4 and trypsin; Worthington Biochemical, Lakewood, NJ) treated and mechanically dissociated as described elsewhere (17). The cells were plated on poly-l-lysine-coated (Sigma, St. Louis, MO) glass cover slips and incubated at 37°C in 5% CO2 and 90% humidity for at least 2–3 h to allow recovery from the dissociation procedure before Ca2+ imaging or electrophysiological studies. Cells were studied within 3–10 h after dissociation.
Ca2+ Imaging
DRG cells were loaded with fura 2-AM (2 μm; Molecular Probes, Eugene, OR) for 30 min at 37°C in an atmosphere of 5% CO2. Fura 2-AM was dissolved in the bath solution [Hank's balanced salt solution (HBSS)] containing (in mM): 138 NaCl, 5 KCl, 0.3 KH2PO4, 4 NaHCO3, 2 CaCl2, 1 MgCl2, 10 HEPES, and 5.6 glucose, pH 7.4, 320 mosm/l, to which BSA was added (5 mg/ml; Sigma) to promote loading. Fura 2 Ca2+ imaging was performed as previously described (40). Briefly, cover slips were placed on an inverted epifluorescence microscope (Olympus IX70) and continuously superfused (3–4 ml/min) with HBSS. Fura 2 was excited alternately with ultraviolet light at 340 and 380 nm, and the fluorescence emission was detected at 510 nm using a computer-controlled monochromator. Image pairs were acquired every 1–30 s using illumination periods between 20 and 50 ms. Wavelength selection, timing of excitation, and the acquisition of images were controlled using the program C-Imaging (Compix, Cranberry Township, PA) running on a personal computer. Digital images were stored on hard disk for off-line analysis.
Perforated Patch Clamp
Voltage-clamp recording was performed using an Axopatch 200B (Molecular Devices, Sunnyvale, CA) controlled with pClamp (version 8.2). Data were low-pass filtered at 5–10 kHz with a four-pole Bessel filter and digitally sampled at 25–100 Hz. HBSS was used as bath solution. Electrode solution contained (in mM): 140 KCl, 1 MgCl2, 0.1 CaCl2, 10 HEPES, and 1 EGTA, pH was adjusted to 7.2 with Tris base, and osmolality adjusted to 310 mosmol/l with sucrose. Gramicidin (Sigma) was used to obtain whole cell access, and stock solution was prepared in DMSO (60 mg/ml) then diluted to a final concentration (90 μg/ml) in electrode solution immediately before use. All salts used for electrophysiology were obtained from Sigma. Small- to medium-sized (20–30 μM) DRG neurons were chosen for recording. After formation of a tight seal (>1 GΩ) and compensation of pipette capacitance with amplifier circuitry, whole cell access (<10 MΩ) was obtained within 10–30 min. Cell capacitance was determined with five hyperpolarizing pulses (10 ms) from −60 to −80 mV. Whole cell capacitance and series resistance were compensated with the amplifier circuitry to obtain series resistance compensation >80%. Holding potential was −60 mV. Capsaicin was applied for 20 s at a 40-min interval from a glass pipette connected to a gravity-driven perfusion system (4–5 ml/min). The access resistance was measured just before each recording. If the access resistance changed >5% just before the second capsaicin application then data were not included in the study.
Data Analysis
In Ca2+ imaging studies, data were analyzed using program C-Imaging (Compix). Background was subtracted to minimize camera dark noise and tissue autofluorescence. An area of interest was drawn around each cell, and the average value of all pixels included in this area was taken as one measurement. The ratio of fluorescence signal measured at 340 nm divided by the fluorescence signal measured at 380 nm was used to measure the increase of intracellular Ca2+. Baseline intracellular Ca2+ concentration was determined from the average of five to eight measurements obtained before drug application. Amplitudes of peak Ca2+ responses were computed as the difference between the peak value and the baseline value. Each treatment was tested on three to four cover slips from one rat on one day. One cover slip usually contained 20–40 DRG neurons/microscopic field. To test one treatment, experiments were conducted over the course of 3–4 days, and each day had its own control. Statistical significance was tested using unpaired t-test, Chi square test, and ANOVA followed by Dunnett's post hoc test. All data are expressed as means ± SE.
Drugs
Drugs were dissolved in HBSS from concentrated stock solutions and delivered via bath application using a gravity-driven application system. Capsaicin (Sigma) was prepared as a 1 mm stock solution in 100% ethanol and then administered in HBSS where the final concentration of ethanol was 1 or 0.5%, which had no influence on the capsaicin response. PKC inhibitor bisindolylmaleimide (BIM) was obtained from Calbiochem; NK-1 antagonist aprepitant was from Helsinn Healthcare (Switzerland); NK-3 antagonist SB-235375 was a gift from SmithKline Beecham; NK-2 antagonist MEN-10376, PKA inhibitor adenosine 3′,5′-cyclic monophosphothioate (RP-cAMPS), brefeldin A (BFA), CaMKII inhibitor N-[2-({[3-(4-chlorophenyl)-2-propenyl]methylamino}methyl)phenyl]-N-(2-hydroxyethyl)-4-methoxybenzenesulfonamide (KN-93), and mitogen/extracellular signal-regulated kinase (MEK) inhibitor 1,4-diamino-2,3 dicyano-1,4-bis(o-aminophenylmercapto)butadiene monoethanolate (U-0126) were all obtained from Sigma.
RESULTS
Changes in Response to Capsaicin During Repeated Applications
Ca2+ imaging study.
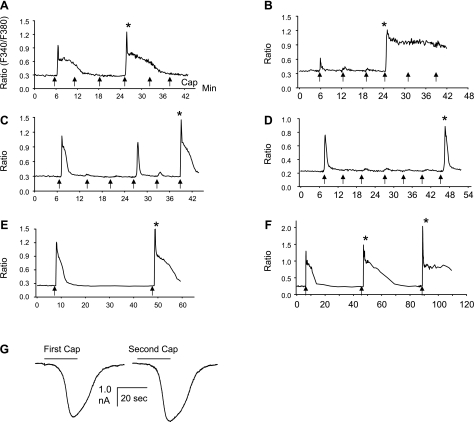
In the initial Ca2+ imaging experiments, 0.5 μM capsaicin, which is approximately two to three times the EC50 concentration, was applied for 15 s because this concentration and period of application has been shown to induce some degree of tachyphylaxis but also allow recovery after 30–40 min (14). As reported by other investigators (14), the time course of 0.5 μM capsaicin-induced Ca2+ response was generally composed of a rapid transient phase followed by a sustained phase that varied in different cells but could last many minutes (2–15 min) (Fig. 1). When capsaicin was applied every 6 min, the second to fourth applications elicited responses markedly reduced in peak amplitude; however, in some cells, responses were increased in peak amplitude above control levels, an effect that will be termed potentiation in this paper (indicated by stars in Fig. 1). Potentiation occurred at 18 min (Fig. 1, A and B), 30 min (Fig. 1C), and 36 min (Fig. 1D) after the first application and varied between cells, but was detected more frequently at 30–40 min after the first application. Another period of tachyphylaxis occurred following the potentiation (e.g., after the 5th capsaicin application in Fig. 1, A and B). Higher concentrations (1 and 2 μM) and lower concentrations (100 or 300 nM) of capsaicin also elicited potentiation (data not shown); however, 0.5 μM was used in the subsequent experiment.
Fig. 1.
A–F: potentiation of intracellular Ca2+ responses elicited by repeated application of capsaicin in 6 small-size dorsal root ganglion (DRG) neurons. Capsaicin-induced Ca2+ increase is expressed as the ratio of fluorescence at 340 and 380 nm (F340/F380). Capsaicin (0.5 μM) was applied for 15 s (indicated by arrows) 6–7 times at 6-min intervals in A–D and 2 times at a 40-min interval in E and F. Repeated capsaicin application induces an initial decrease in responses followed by a late-occurring enhancement of the response (indicated by stars). Potentiation occurred at an 18-min interval in A and B, at a 30-min interval in C, and at a 36-min interval in D. Note, in B, even though the first capsaicin response is very small, it still induced tachyphylaxis followed by prominent potentiation. A second period of tachyphylaxis occurred following the potentiation (after 5th capsaicin application in A and B). F: both the second and third application induced potentiation, and the third response is larger than the second. G: inward currents induced by two capsaicin applications (0.5 μM for 20 s) at a 40-min interval in a small DRG neuron (25 μm, membrane capacitance = 26 pF). Currents were recorded using the gramicidin perforated patch-clamp method. The input resistance was 8.52 MΩ just before the first and 8.16 MΩ just before the second capsaicin application. The second application induced a 10% increase of inward current compared with the first application.
Because the potentiation elicited by 0.5 μM capsaicin was larger at the 30- to 40-min application interval in our preliminary experiments and other studies showed that recovery from tachyphylaxis usually occurred at 30–40 min (14), subsequent experiments examined the effects of capsaicin administered at a 40-min interval, which consistently showed potentiation during the second and third application (Fig. 1, E and F). In addition, the third application usually elicited a larger response than the second application. Capsaicin was applied in two vehicles (in 100% ethanol and in 10% Tween 80, 10% ethanol, and 80% saline), which yielded potentiation of comparable magnitudes (P > 0.05).
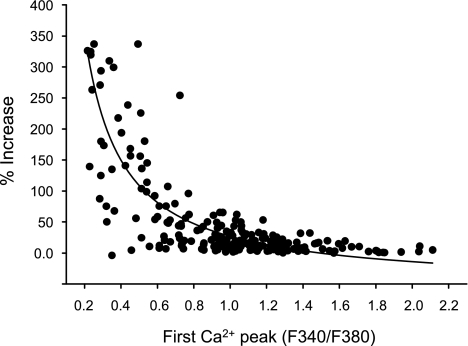
Potentiation may reflect increased TRPV1 channel activity, increased plasma membrane expression of TRPV1 channels, or changes in intracellular Ca2+ handling. These possibilities were explored in a series of experiments to characterize potentiation and to determine the underlying mechanisms. The dual application protocol at a 40-min interval (Fig. 1E) was employed unless otherwise stated. In 25 cultures from 25 rats, 58% of DRG neurons (1,200/2,070) were responsive to capsaicin, a percentage comparable to that reported in other studies (31). Two parameters were calculated to evaluate potentiation: 1) amplitude of potentiation (calculated as %increase of second response relative to the first response) and 2) percentage of cells exhibiting potentiation (cell number exhibiting potentiation/total number of capsaicin-responsive cells × 100%). The magnitude of potentiation ranged from a few percent increase in peak amplitude of the intracellular Ca2+ signal up to a 350% increase in the signal (Fig. 2). Cells exhibiting at least a 4% increase in peak amplitude of the second response, which comprised 90.6% of the cells exhibiting potentiation, were included in the analysis. Using this threshold criterion, potentiation was present in 59% of capsaicin-responsive neurons (414/703). Cells exhibiting 4–35% potentiation account for 81% (335/414) of this population, whereas cells with potentiation exceeding 35% represent 19% of the population. Among the latter cells, the Ca2+ response induced by first capsaicin application was usually small (Figs. 1B and 2).
Fig. 2.
Relationship between the amplitude of the first capsaicin-induced Ca2+ responses and the magnitude of potentiation of the second capsaicin-induced Ca2+ responses. The protocol to test for potentiation is the same as in Fig. 1E. Capsaicin (0.5 μM) was applied for 15 s every 40 min. Data were obtained from 214 cells from 5 rats. Curve is fitted with single exponential with the equation, y = y0+ae−bx in which the three parameters are y0 = 0.13; a = 6.0; and b = 3.7 with correlation coefficient r = 0.68.
Patch-clamp recording.
To establish that the potentiation of capsaicin-induced Ca2+ responses was due at least in part to enhanced TRPV1 channel current, we performed perforated patch-clamp recording in DRG neurons. Capsaicin (0.5 μM) was applied two times at a 40-min interval to induce inward currents, as shown in Fig. 1G. Analysis of DRGs from six rats yielded successful recording from 10 cells (diameter: 20–30 μM) in which the access resistance did not change between the first and second capsaicin application. The inward current induced by the first capsaicin application was 1,495 ± 371 pA (n = 10), and the current density was 56 ± 18 pA/pF. In 6 of 10 cells, the second application induced a 15.5 ± 5% (range: 5–30%) increase of the inward current relative to the first application, suggesting that the potentiation observed in the Ca2+ imaging study was at least in part mediated by an enhancement of TRPV1 channel activity or increased number of activated TRPV1 channels. Because Ca2+ imaging is a more efficient method for studying large numbers of cells, it was used in the remainder of the experiments for evaluating the properties and mechanisms underlying potentiation.
Properties of Potentiation
Time dependence.
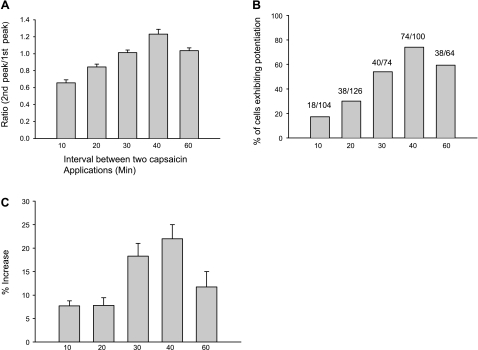
To assess the time course of potentiation, we varied the interval between two capsaicin applications from 10 to 60 min. For each time point, we tested three cover slips a day on two different days (n = 2 rats; Fig. 3). For all capsaicin-responsive neurons (Fig. 3A) in which 74% of the cells exhibited potentiation (Fig. 3B), the average ratio of the second peak vs. the first peak was greater than one at the 40-min interval. The average amplitude of potentiation in this population of cells was ∼23% (Fig. 3C). At 30- and 60-min intervals, smaller percentages (54 and 59%, respectively) of cells showed potentiation (Fig. 3B), and the average peak response for all capsaicin-responsive cells was not significantly different from the control level (ratio approximately equal to 1; Fig. 3A). At 10- and 20-min intervals, the average peak Ca2+ response was below control (Fig. 3A) although a small percentage (17–30%) of the cells exhibited potentiation (Fig. 3B).
Fig. 3.
Time dependence of capsaicin-induced potentiation. Capsaicin (0.5 μM) was applied for 15 s two times at 10-, 20-, 30-, 40-, and 60-min intervals. Data for each interval are averages of responses from 6 cover slips from 2 days of experiments (n = 2 rats). A: all capsaicin-responsive cells were included in the analysis, and the ratio of the second intracellular Ca2+ peak to the first peak was calculated. At 10- and 20-min intervals, the second peak is less than the first (ratio <1). At the 30-min interval, the second Ca2+ peak is almost the same as the first (ratio = 1.01). However, at the 40-min interval, the second Ca2+ peak is 23% larger on average than the first (ratio = 1.23). The ratio returned to 1.03 at the 60-min interval. One-way ANOVA followed by Dunnett's analysis reveals a significant difference (P < 0.001) among different intervals (40 vs. 10, 20, and 30 min). B: %cells exhibiting potentiation. C: amplitude of potentiation, calculated as %increase of second peak relative to the first peak but only includes cells that exhibit potentiation. Potentiation was defined as ≥4%. Cells with <4% increase are not included in the graph. One-way ANOVA followed by Dunnett's analysis indicates a significant difference (P < 0.001) among different intervals (40 vs. 10, 20, and 30 min). All data are expressed as means ± SE.
Cell types exhibiting potentiation.
Capsaicin-responsive DRG neurons are small to medium size. To determine whether there was a differential distribution of potentiation in different-sized capsaicin-responsive neurons, a size analysis was performed on data from 20 cover slips studied on four separate days. These data revealed that potentiation occurred more frequently (63.4%, 350/522 cells) in small-size DRG neurons (diameter <30 μM) than in medium-size neurons (diameter >30 μM) (45%, 28/62 cells) (P < 0.01, Chi square test).
Ca2+ modulation.
Lowering the extracellular Ca2+ concentration or decreasing the intracellular Ca2+ by the Ca2+ chelator BAPTA can reduce tachyphylaxis (26). To determine whether potentiation is modulated by Ca2+, in two days of experiments (n = 2 rats), the extracellular Ca2+ concentration was lowered from 2 mM (normal HBSS) to 1 mM. This change increased the percentage of cells exhibiting potentiation from 60% (40/68) to 90% (38/42) (P < 0.001, Chi square test) and enhanced the average potentiation from 18 ± 2 to 44 ± 6% (P < 0.001, unpaired t-test). The first capsaicin-induced Ca2+ peak in HBSS with 1 mM Ca2+ was much smaller (ratio of fluorescence at 340 to 380 nm = 0.68 ± 0.06, n = 83) than that in normal HBSS (1.21 ± 0.12, n = 95, P < 0.001). In addition, a cross-correlation analysis of the relationship between the amplitude of the first capsaicin-induced intracellular Ca2+ peak and the magnitude of potentiation measured in 214 cells (obtained from 5 rats) in normal extracellular Ca2+ revealed that the magnitude of potentiation was negatively correlated with the amplitude of the intracellular Ca2+ signal evoked by the first application of capsaicin (Fig. 2). Therefore, the enhanced potentiation in 1 mM Ca2+ HBSS may result from the smaller Ca2+ increase induced by first application.
Mechanisms of Potentiation
Release of SP.
Capsaicin can induce the release of SP and CGRP from dissociated DRG neurons (20, 36, 42), and SP can enhance TRPV1 activity by activating NK-1 or NK-2 receptors located on DRG neurons (40, 52). Therefore, we tested the possibility that potentiation is mediated by release of SP and activation of neurokinin receptors. A combination of NK-1 (1 μM aprepitant), NK-2 (1 μM MEN-10376), and NK-3 (1 μM SB-235375) receptor antagonists was applied starting 2 min before the first capsaicin application and extending to the end of the second application. These three antagonists at the concentrations applied in our experiments have been shown to effectively block the effects of the corresponding receptors in DRG neurons (40). As shown in Table 1, experiments on cells from three rats revealed that the magnitude of potentiation and percentage of cells exhibiting potentiation were comparable to the results in control experiments (P > 0.05, unpaired t-test), indicating that the release of SP did not contribute to potentiation under conditions of our experiments.
Table 1.
Failure of various treatments to alter capsaicin-induced potentiation
| Amplitude of Potentiation, %increase |
Cells Exhibiting Potentiation, % |
|||
|---|---|---|---|---|
| Treatments | Control | Treatment | Control | Treatment |
| NK-1, NK-2, and NK-3 antagonists | 21 ± 2 | 23 ± 4 | 58 (142) | 57 (122) |
| BIM (1 μM) | 24 ± 3 | 24 ± 3 | 61 (97) | 54 (125) |
| RP-cAMPS (10 μM) | 18 ± 2 | 19 ± 2 | 58 (114) | 52 (146) |
| BFA (20 μM) | 20 ± 2 | 25 ± 3 | 51 (119) | 55 (143) |
| Wortmannin (1 μM) | 18 ± 2 | 22 ± 6 | 61 (115) | 61 (114) |
Values for amplitude of potentiation are means ± SE. Nos. in parentheses represent the no. of cells tested. Each treatment was tested on 3 rats.
NK, neurokinin; BIM, bisindolylmaleimide; RP-cAMPS, adenosine 3′,5′-cyclic monophosphothioate; BFA, brefeldin A.
The protocol to test the effect of treatments on potentiation is same as in Fig. 1E. Capsaicin (0.5 μM) was applied for 15 s every 40 min. All treatments were applied 2–5 min before the first capsaicin application and during the period between the two capsaicin applications. A combination of NK-1 (1 μM aprepitant), NK-2 (1 μM MEN-10376), and NK-3 (1 μM SB-235375) antagonists was used. Data from control and experimental groups were not significantly different (P > 0.05, unpaired t-test).
Activation of PKC.
Because activation of PKC can sensitize TRPV1 in primary sensory neurons (6, 32) and PKCδ and PKCγ can be directly activated by the influx of Ca2+ (12), it is possible that activation of PKC by the first capsaicin application contributes to the potentiation. To test this possibility, on cells from three rats, 1 μM BIM, an inhibitor of all isoforms of PKCs, was applied 2 min before and during the period of the two capsaicin administrations in a concentration sufficient to block the phorbol 12,13-dibutyrate enhancement of TRPV1 in DRG neurons (40). BIM did not alter the amplitude of potentiation or the percentage of cells exhibiting potentiation (P > 0.05, unpaired t-test; Table 1), suggesting that the PKC pathway does not contribute to potentiation.
Activation of PKA.
Because phosphorylation of the TRPV1 channel by PKA can increase TRPV1 activity and reduce desensitization (7) and the influx of Ca2+ can indirectly activate PKA (35), we tested the possibility on cells from three rats that PKA activation by the first capsaicin application contributes to potentiation. Rp-cAMPS was administered throughout the experiments starting 2 min before the first capsaicin application in a concentration (10 μM) commonly used to inhibit PKA (19). As shown in Table 1, this treatment did not significantly change the amplitude of potentiation or the percentage of cells exhibiting potentiation (P > 0.05, unpaired t-test), indicating that activation of the PKA pathway did not contribute to potentiation.
Activation of CaMKII.
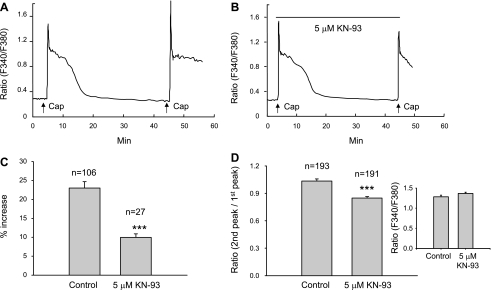
CaMKII, which is expressed in small- and medium-diameter DRG neurons (8), can be directly activated by the influx of Ca2+ (25). CaMKII is coexpressed with TRPV1 channels in small- to medium-diameter DRG (9) or trigeminal ganglion neurons (22). Phosphorylation of TRPV1 channels by CaMKII has been reported to increase the binding with capsaicin (25). To test the contribution of CaMKII, KN-93 (5 μM), a membrane-permeable competitive CaMKII inhibitor, was applied continuously starting 2 min before the first capsaicin application. KN-93 at 5 to 10 μM concentrations directly binds to the CaM-binding site of CaMKII to prevent the activation of CaMKII (41). In nine cover slips tested on 3 days (n = 3 rats), KN-93 significantly reduced both the amplitude of potentiation and percentage of cells exhibiting potentiation. As summarized in Fig. 4, 55% (106/193) of cells in control cover slips exhibited potentiation, whereas, in cover slips treated with KN-93, only 14% (27/191) of cells showed potentiation (P < 0.001, Chi square test). Similarly, the magnitude of potentiation expressed as the average percentage increase (P < 0.001, unpaired t-test; Fig. 4C), and the ratio of the second peak vs. the first peak (P < 0.001, unpaired t-test; Fig. 4D) were significantly decreased. KN-93 (5 μM) did not induce any change in basal intracellular Ca2+ or have any influence on the Ca2+ response induced by the first capsaicin application when compared with responses in control experiments (P > 0.05, unpaired t-test; Fig. 4D, inset).
Fig. 4.
Block of Ca2+- and calmodulin-dependent protein kinase II (CaMKII) activity reduces capsaicin-induced potentiation of the intracellular Ca2+ responses. The protocol to test for potentiation is the same as in Fig. 1E. Capsaicin (0.5 μM) was applied for 15 s every 40 min. A: a cell from a control cover slip where the second application elicited a 25% increase in the response. B: a cell from a KN-93-treated cover slip that did not exhibit potentiation. KN-93 (5 μM) was applied continuously starting 2 min before the first capsaicin application (indicated by long black bar). C: summarized data from all cells (n = 3 rats) exhibiting potentiation. KN-93 treatment significantly attenuated the amplitude of potentiation (***P < 0.001, unpaired t-test). D: summarized data (n = 3 rats) from all capsaicin-responsive cells. KN-93 treatment also significantly reduced the ratio of the second peak to the first peak (***P < 0.001, unpaired t-test). However, preincubation of DRG neurons with 5 μM KN-93 for 10 min did not affect the Ca2+ responses induced by the first application of capsaicin (D, inset).
Activation of ERK1/2.
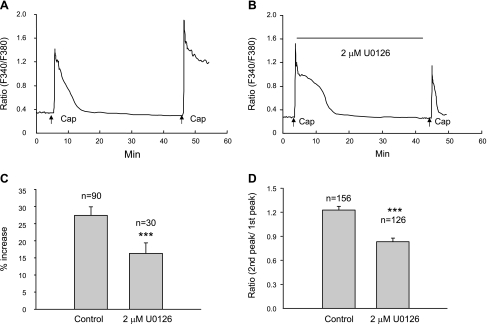
ERKs, which are members of the mitogen-activated protein kinase family are expressed in nociceptive DRG neurons (3) and can be activated by membrane depolarization and Ca2+ influx (38). Because ERKs in primary sensory neurons are activated in vitro or in vivo following capsaicin or other noxious stimuli and contribute to the sensitization of primary sensory neurons by enhancing TRPV1 (16), we determined if activation of ERKs contributes to the potentiation. U-0126 (2 μM), a highly selective MEK1 and MEK2 inhibitor that blocks the activation of ERKs at 1 to 10 μM concentrations (18), was applied continuously starting 10 min before the first capsaicin application. In nine cover slips on 3 days of experiments (n = 3 rats), both amplitude of potentiation and percentage of cells exhibiting potentiation were reduced by the treatment. As shown in Fig. 5, 58% (90/156) of the cells in control cover slips exhibited potentiation, whereas, in treated cover slips, potentiation was present in only 24% (30/126) of cells (P < 0.001, Chi square test). Amplitude of potentiation in control cover slips was 27.4 ± 2.4%, whereas, in the presence of U-0126, it was 16.3 ± 3% (P < 0.001, unpaired t-test; Fig. 5C). Treatment with U-0126 also significantly reduced the ratio of the second peak vs. the first peak in all capsaicin-responsive cells (P < 0.001, unpaired t-test; Fig. 5D). However, 2 μM U-0126 did not change the basal intracellular Ca2+ levels or the peak Ca2+ response induced by the first capsaicin application (P > 0.05, unpaired t-test).
Fig. 5.
Extracellular signal-regulated kinase (ERK) 1/2 inhibition suppresses potentiation. The same protocol as in Fig. 4A was employed. Capsaicin (0.5 μM) was applied for 15 s every 40 min. A: one cell from a control cover slip showing the second capsaicin application induced a potentiated response. B: one cell from a cover slip treated with 2 μM U-0126 showing no potentiation. U-0126 was applied continuously starting 10 min before the first capsaicin application (indicated by the long black bar). C: summarized data (from 3 rats) from all cells showing potentiation demonstrating that U-0126 significantly attenuated the amplitude of potentiation compared with control (***P < 0.001, unpaired t-test). D: summarized data (n = 3 rats) from all capsaicin-responsive cells showing that 2 μM U-0126 significantly reduced the ratio of the second vs. the first Ca2+ peak (***P < 0.001, unpaired t-test). All data were expressed as means ± SE.
Effect of blocking trafficking of TRPV1.
The potentiation of TRPV1 may result either from enhanced channel activity or from increased expression of the channels in the plasma membrane. To test the second possibility, BFA (20 μM), which has been shown to block the insertion of newly synthesized TRPV5 channels in the plasma membrane (43), was applied continuously to cells from 3 rats starting 5 min before the first application of capsaicin. This treatment did not significantly change the amplitude of potentiation or the percentage of cells exhibiting potentiation (P > 0.05, unpaired t-test; Table 1). BFA (20 μM) did not change basal intracellular Ca2+ or influence the Ca2+ responses induced by the first capsaicin application (P > 0.05, unpaired t-test). Incubation of DRG neurons with BFA (10 μg/ml) for a longer period (1 h) also did not significantly reduce the potentiation (data not shown).
Relationship Between Tachyphylaxis and Potentiation
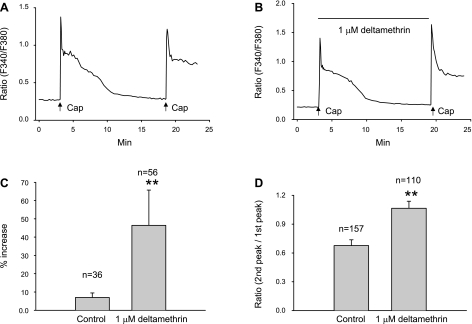
Tachyphylaxis is a time- and Ca2+-dependent process (26). Activation of Ca2+- and calmodulin-dependent phosphatase 2B (calcineurin) is thought to contribute to tachyphylaxis (25, 34). Our studies indicated that potentiation is also a time- and Ca2+-modulated process. To determine the relationship between potentiation and tachyphylaxis, we blocked tachyphylaxis by applying deltamethrin (1 μM), a potent calcineurin inhibitor, during the interval between the two capsaicin applications. Deltamethrin (0.5 or 1 μM) has been reported to reduce capsaicin desensitization or tachyphylaxis (40). In these experiments, capsaicin was administered at a short interval (15 min) during which only 23% (36/157) of the cells in control experiments (9 cover slips from 4 rats) exhibited potentiation. However, in nine cover slips (n = 3 rats) treated with deltamethrin, 51% (56/110) of cells exhibited potentiation (P < 0.01, Chi square test). In addition, both amplitude of potentiation for cells exhibiting potentiation (Fig. 6C) and the ratio of the second peak vs. the first peak in all capsaicin-responsive cells (Fig. 6D) were increased in deltamethrin-treated cover slips (P < 0.01 for potentiation amplitude; P < 0.01 for ratio). Deltamethrin (1 μM) had no influence on the Ca2+ responses when applied 5 min before the first capsaicin application (P > 0.05, unpaired t-test). These results suggest that block of tachyphylaxis enhanced the magnitude of potentiation.
Fig. 6.
Suppression of tachyphylaxis by deltamethrin (1 μM) facilitated the capsaicin-induced potentiation. In this experiment, the interval between two capsaicin applications (0.5 μM for 15 s) was reduced to 15 min to increase the probability of overlap between tachyphylaxis and potentiation. A: a cell from a control cover slip showed no potentiation. B: a cell from a deltamethrin-treated cover slip showing an ∼20% increase of the second response. Deltamethrin (1 μM) was applied during the 15-min interval to inhibit tachyphylaxis. C: summarized data from cells showing potentiation demonstrating that the amplitude of potentiation is significantly enhanced after deltamethrin treatment (**P < 0.01, unpaired t-test). D: summarized data from all capsaicin-responsive cells showing that deltamethrin treatment significantly increased the ratio of the second vs. the first Ca2+ responses (**P < 0.01, unpaired t-test). All data are expressed as means ± SE.
Relationship Between Potentiation and Recovery From Desensitization
After the first capsaicin exposure, at least three processes occur: 1) desensitization, 2) recovery from desensitization, and 3) potentiation. The recovery from desensitization has been reported to be completely blocked by 1 μM wortmannin, a phosphatidylinositol 4-kinase and PI3-kinase inhibitor (30). To determine whether potentiation is a process that is independent of recovery mechanisms, 1 μM wortmannin was applied during the 40-min interval between the two capsaicin applications to block the recovery from desensitization. In nine cover slips tested on 3 days (n = 3 rats), after block of recovery from desensitization, both the amplitude of potentiation and percentage of cells exhibiting potentiation were comparable to values in the control experiment (P > 0.05; Table 1). Wortmannin (1 μM) did not induce any change in basal intracellular Ca2+. These results indicate that there is no interaction between potentiation and recovery from desensitization. These results also indicate that activation of PI3-kinase does not contribute to potentiation.
DISCUSSION
The present results provide the first evidence in acutely dissociated nociceptive DRG neurons that capsaicin can enhance the response to the subsequent application of capsaicin, a phenomena that contrasts with the commonly observed tachyphylaxis or desensitization to repeated application of capsaicin. This sensitization or potentiation is delayed, occurring after partial or full recovery from tachyphylaxis, is influenced by extracellular Ca2+ concentration, and is mediated by the activation of CaMKII and ERK1/2 but not by activation of PKC or PKA or intracellular trafficking of the TRPV1 channels. Potentiation of TRPV1 responses in nociceptors in response to noxious stimulation may contribute to peripheral sensitization.
Clearly, the magnitude of tachyphylaxis induced by capsaicin is greater than the magnitude of potentiation. This feature coupled with the more rapid onset of tachyphylaxis compared with the delayed onset of potentiation probably accounts, in part, for the scarcity of reports in the literature of potentiation following capsaicin application. Furthermore, the temporal overlap between the two phenomena at long application intervals, which contributes to the masking of weaker potentiation by greater tachyphylaxis, is another factor. However, in studies where tachyphylaxis is suppressed by drugs, one might expect that potentiation would be more obvious. This was observed in the present study; and a few studies in other laboratories have also identified potentiation of capsaicin responses under special recording conditions. For example, it was reported that TRPV1 channel activity recovered to >100% following desensitization when high concentrations of ATP were contained in intracellular solution (30, 51). Another study described potentiation of TRPV1 currents when BAPTA was added to the intracellular solution to chelate Ca2+ and when barium was included in the extracellular solution (26). The latter treatments should reduce activation of calcineurin and in turn reduce dephosphorylation and desensitization of TRPV1.
Ca2+ Influence on Potentiation
Lowering the extracellular Ca2+ concentration enhanced potentiation, which seems to be at odds with our other findings indicating that potentiation is mediated by the activation of Ca2+-sensitive signaling pathways (CaMKII or ERK). Lowering extracellular Ca2+ will result in less Ca2+ influx through the TRPV1 channel and should lead to weaker activation of CaMKII or ERK. However, there should also be a simultaneous decrease in Ca2+-dependent tachyphylaxis, which is the stronger and more immediate modulator of capsaicin responses. The Ca2+ dependence of the two processes might be different with potentiation, requiring lower levels of Ca2+. Furthermore, recent studies have indicated certain Ca2+ actions can be confined to small intracellular compartments within neurons and not be closely linked with the total intracellular Ca2+ concentration (2). Thus Ca2+ influx could activate tachyphylaxis and potentiation signaling pathways at the same time but in different compartments.
Mechanisms of Potentiation
Most of our studies were conducted with the Ca2+ imaging method, which has the disadvantage that it is more difficult to identify the mechanisms underlying the potentiation. The enhanced Ca2+ response may result from increased Ca2+ influx through the TRPV1 channel or more Ca2+ release from intracellular Ca2+ stores. However, our patch-clamp data suggested that the enhanced Ca2+ increase is mediated at least in part by increased influx through the TRPV1 channel (Fig. 1G). Our data showing that a CaMKII inhibitor (KN-93) reduced the potentiation also indirectly suggests a contribution of increased TRPV1 channel activity to potentiation, since CaMKII was shown to increase single TRPV1 channel activity (25). Our BFA data indicated that this TRPV1 channel sensitization resulted from enhanced channel activity rather than from more surface expression of the channel protein. Further experiments are needed to determine if the enhanced channel activity is due to prolonged opening of TRPV1 channels, an increase in the binding affinity for capsaicin, or increased permeability to Ca2+.
The role of CaMKII.
CaMKII is coexpressed with TRPV1 channels in small- to medium-diameter DRG or trigeminal ganglion neurons (13, 22, 37) and can be activated by influx of Ca2+. Phosphorylation of TRPV1 channel by CaMKII can increase TRPV1 binding to capsaicin (25). Exposure of trigeminal ganglion neurons to capsaicin elicits a 50% increase of phospho-CaMKII (37). Our results indicate that capsaicin-induced potentiation of the TRPV1 channel is mediated by the activation of CaMKII. Both CaMKII and phosphatase 2B (calcineurin) can be activated by Ca2+-calmodulin but have opposite effects on TRPV1 channel activity. Two hypotheses have been proposed to explain the interactions between these opposing mechanisms (25). First, CaMKII and calcineurin have different kinetics for activation. Following Ca2+ influx through TRPV1, calcineurin is activated first, leading to dephosphorylation of the channel and tachyphylaxis, whereas CaMKII is activated more slowly, leading to rephosphorylation and delayed recovery of channel activity. Second, the two mechanisms have different sensitivities to Ca2+ or calmodulin. Our finding that potentiation occurs later than tachyphylaxis fits well with the above explanations.
The role of ERK activation.
Membrane depolarization and Ca2+ influx can activate ERK, via the Ras-Raf pathway (38) or via activation of PI3-kinase (53). ERK appears to have a role in regulation of excitability and synaptic plasticity in neurons (33) in addition to its well-known involvement in the regulation of the cell cycle, gene expression, proliferation, and cell death. Recently, it was reported that ERK activation in rat DRG neurons occurs within 2 min after capsaicin application, reaches a peak level at 10 min, and is maintained at a high level for 90 min (16). Even though there was no evidence to indicate a direct relationship between activation of ERK and the sensitization of TRPV1, Zhuang et al. (53) proposed that TRPV1 is one of the molecular targets of the ERK-activated pathway. Our results that ERK activation contributes to the capsaicin-induced TRPV1 potentiation are consistent with this proposal. In our studies, block of either CaMKII or ERK activation separately suppressed potentiation, indicating that TRPV1 is a molecular target and point of convergence of these two signaling pathways. Interactions between CaMKII and ERK pathways have been reported in other cells as well as neurons (13, 15, 23).
The role of SP.
It was well documented that capsaicin stimulation can induce the release of SP from cultured DRG neurons (20, 42), and SP can enhance capsaicin-induced currents by activating neurokinin receptors (40, 52); thus, capsaicin-induced SP release could contribute to potentiation. However, under the conditions of our experiments, this did not occur, presumably because we used a rapid superfusion system that washed out the released neuropeptides. The use of the rapid superfusion system also reduces the likelihood that other substances (e.g., other tachykinins, CGRP, or brain-derived neurotrophic factor) released from DRG cells by capsaicin stimulation contribute to potentiation. Nevertheless, a possible role of these substances should be examined in the future.
Relationship Between Potentiation and Tachyphylaxis
Capsaicin has three well-studied effects on nociceptive primary sensory neurons: excitation, desensitization/tachyphylaxis, and neurotoxic effects. After capsaicin application, the first response is the activation of TRPV1 channels and a large Ca2+ and Na+ influx in the cell, leading to depolarization and generation of action potentials and painful sensations (10, 11). Influx of Ca2+ can induce the desensitization or tachyphylaxis by activating phosphatase 2B (calcineurin) (10, 25). A high concentration or a longer period of application can result in neurotoxic effects, including degeneration of afferent nerve terminals. Our results identified a fourth effect of capsaicin, delayed sensitization of the TRPV1 channel leading to potentiation of capsaicin-evoked responses. Potentiation and tachyphylaxis are closely related in the following four ways: 1) potentiation occurs after tachyphylaxis and is followed by another cycle of tachyphylaxis; 2) both are activated by Ca2+ influx; 3) based on the signaling pathways, both potentiation and tachyphylaxis seem to be linked with the level of phosphorylation of the TRPV1 channel, i.e., influx of Ca2+ can activate phosphatase 2B (calcineurin) to dephosphorylate TRPV1 channel to induce tachyphylaxis, and influx of Ca2+ can activate protein kinases (CaMKII or ERK1/2) to phosphorylate TRPV1 channel to induce potentiation; and 4) at certain times after capsaicin administration, potentiation and tachyphylaxis exert opposing effects on TRPV1 activity because blocking tachyphylaxis enhances potentiation.
Perspectives and Significance
The capsaicin-induced inflammatory pain model, which is widely studied using behavioral techniques, is characterized by immediate spontaneous pain behavior, such as lifting and licking the affected paw after intradermal injection of capsaicin, followed in 15–20 min by hypoalgesia at the injection site, and then the appearance of heat hyperalgesia. Heat hyperalgesia by capsaicin is detectable within 15 min, reaches a peak at 30–45 min, and lasts <3 h (46). Even though the time course of these behaviors is a reflection of many peripheral and central sensitization mechanisms, it has been suggested that primary heat hyperalgesia results from sensitization of primary afferent nociceptors (27). The onset of potentiation observed in our study (Fig. 3) fits well with the time course of heat hyperalgesia in behavioral studies. Because dissociated DRG neurons exhibit many of the properties of nociceptive afferent terminals, it is possible that capsaicin-induced nociceptor autosensitization may play a role in this response.
Neuroplasticity in nociceptive terminals is an important peripheral mechanism for the generation of hyperalgesia after inflammation and nerve injury (50). However, most studies of the mechanisms underlying this phenomenon focus on the role of inflammatory mediators and/or neurotrophic factors as modulators of nociceptive neurons. Because the capsaicin-induced autosensitization of TRPV1 channel revealed in our study is an early response to capsaicin stimulation and may further amplify other sensitization mechanisms mediated by inflammatory mediators in vivo, it could have a large impact on the initiation of hyperalgesia even though the magnitude of potentiation is modest (average 20% enhancement). In this regard, it will be important to determine if other noxious stimuli, such as high K+, noxious heat, or ATP, etc., which also can induce an increase in intracellular Ca2+ concentration, have a sensitizing effect on TRPV1 channels.
GRANTS
This research is supported by National Institute of Diabetes and Digestive and Kidney Diseases Grant DK-149430.
DISCLOSURES
The authors have no conflict of interest to disclosure.
REFERENCES
- 1. Aley KO, Martin A, McMahon T, Mok J, Levine JD, Messing RO. Nociceptor sensitization by extracellular signal-regulated kinases. J Neurosci 21: 6933–6939, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Augustine GJ, Santamaria F, Tanaka K. Local calcium signaling in neurons. Neuron 40: 331–346, 2003 [DOI] [PubMed] [Google Scholar]
- 3. Averill S, Delcroix JD, Michael GJ, Tomlinson DR, Fernyhough P, Priestley JV. Nerve growth factor modulates the activation status and fast axonal transport of ERK 1/2 in adult nociceptive neurones. Mol Cell Neurosci 18: 183–196, 2001 [DOI] [PubMed] [Google Scholar]
- 4. Baumann TK, Simone DA, Shain CN, LaMotte RH. Neurogenic hyperalgesia: the search for the primary cutaneous afferent fibers that contribute to capsaicin-induced pain and hyperalgesia. J Neurophysiol 66: 212–227, 1991 [DOI] [PubMed] [Google Scholar]
- 5. Bhave G, Gereau RW., IV Posttranslational mechanisms of peripheral sensitization. J Neurobiol 61: 88–106, 2004 [DOI] [PubMed] [Google Scholar]
- 6. Bhave G, Hu HJ, Glauner KS, Zhu W, Wang H, Brasier DJ, Oxford GS, Gereau RW IV. Protein kinase C phosphorylation sensitizes but does not activate the capsaicin receptor transient receptor potential vanilloid 1 (TRPV1). Proc Natl Acad Sci USA 100: 12480–12485, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bhave G, Zhu W, Wang H, Brasier DJ, Oxford GS, Gereau RW., IV cAMP-dependent protein kinase regulates desensitization of the capsaicin receptor (VR1) by direct phosphorylation. Neuron 35: 721–731, 2002 [DOI] [PubMed] [Google Scholar]
- 8. Carlton SM. Localization of CaMKIIalpha in rat primary sensory neurons: increase in inflammation. Brain Res 947: 252–259, 2002 [DOI] [PubMed] [Google Scholar]
- 9. Carlton SM, Hargett GL. Stereological analysis of Ca(2+)/calmodulin-dependent protein kinase II alpha -containing dorsal root ganglion neurons in the rat: colocalization with isolectin Griffonia simplicifolia, calcitonin gene-related peptide, or vanilloid receptor 1. J Comp Neurol 448: 102–110, 2002 [DOI] [PubMed] [Google Scholar]
- 10. Caterina MJ, Julius D. The vanilloid receptor: a molecular gateway to the pain pathway. Ann Rev Neurosci 24: 487–517, 2001 [DOI] [PubMed] [Google Scholar]
- 11. Caterina MJ, Schumacher MA, Tominaga M, Rosen TA, Levine JD, Julius D. The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature 389: 816–824, 1997 [DOI] [PubMed] [Google Scholar]
- 12. Cesare P, McNaughton P. A novel heat-activated current in nociceptive neurons and its sensitization by bradykinin. Proc Natl Acad Sci USA 93: 15435–15439, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Choe ES, Wang JQ. CaMKII regulates amphetamine-induced ERK1/2 phosphorylation in striatal neurons. Neuroreport 13: 1013–1016, 2002 [DOI] [PubMed] [Google Scholar]
- 14. Cholewinski A, Burgess GM, Bevan S. The role of calcium in capsaicin-induced desensitization in rat cultured dorsal root ganglion neurons. Neuroscience 55: 1015–1023, 1993 [DOI] [PubMed] [Google Scholar]
- 15. Cipolletta E, Monaco S, Maione AS, Vitiello L, Campiglia P, Pastore L, Franchini C, Novellino E, Limongelli V, Bayer KU, Means AR, Rossi G, Trimarco B, Iaccarino G, Illario M. Calmodulin-dependent kinase II mediates vascular smooth muscle cell proliferation and is potentiated by extracellular signal regulated kinase. Endocrinology 151: 2747–2759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Dai Y, Iwata K, Fukuoka T, Kondo E, Tokunaga A, Yamanaka H, Tachibana T, Liu Y, Noguchi K. Phosphorylation of extracellular signal-regulated kinase in primary afferent neurons by noxious stimuli and its involvement in peripheral sensitization. J Neurosci 22: 7737–7745, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Dang K, Bielefeldt K, Gebhart GF. Differential responses of bladder lumbosacral and thoracolumbar dorsal root ganglion neurons to purinergic agonists, protons, and capsaicin. J Neurosci 25: 3973–3984, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Firner M, Greffrath W, Treede RD. Phosphorylation of extracellular signal-related protein kinase is required for rapid facilitation of heat-induced currents in rat dorsal root ganglion neurons. Neuroscience 143: 253–263, 2006 [DOI] [PubMed] [Google Scholar]
- 19. Fu Y, Han J, Ishola T, Scerbo M, Adwanikar H, Ramsey C, Neugebauer V. PKA and ERK, but not PKC, in the amygdala contribute to pain-related synaptic plasticity and behavior (Abstract). Mol Pain 4: 26, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Garland A, Necheles J, White SR, Neeley SP, Leff AR, Carson SS, Alger LE, McAllister K, Solway J. Activated eosinophils elicit substance P release from cultured dorsal root ganglion neurons. Am J Physiol Lung Cell Mol Physiol 273: L1096–L1102, 1997 [DOI] [PubMed] [Google Scholar]
- 21. Gold MS, Reichling DB, Shuster MJ, Levine JD. Hyperalgesic agents increase a tetrodotoxin-resistant Na+ current in nociceptors. Proc Natl Acad Sci USA 93: 1108–1112, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ichikawa H, Gouty S, Regalia J, Helke CJ, Sugimoto T. Ca2+/calmodulin-dependent protein kinase II in the rat cranial sensory ganglia. Brain Res 1005: 36–43, 2004 [DOI] [PubMed] [Google Scholar]
- 23. Ji RR, Woolf CJ. Neuronal plasticity and signal transduction in nociceptive neurons: implications for the initiation and maintenance of pathological pain. Neurobiol Dis 8: 1–10, 2001 [DOI] [PubMed] [Google Scholar]
- 24. Julius D, Basbaum AI. Molecular mechanisms of nociception. Nature 413: 203–210, 2001 [DOI] [PubMed] [Google Scholar]
- 25. Jung J, Shin JS, Lee SY, Hwang SW, Koo J, Cho H, Oh U. Phosphorylation of vanilloid receptor 1 by Ca2+/calmodulin-dependent kinase II regulates its vanilloid binding. J Biol Chem 279: 7048–7054, 2004 [DOI] [PubMed] [Google Scholar]
- 26. Koplas PA, Rosenberg RL, Oxford GS. The role of calcium in the desensitization of capsaicin responses in rat dorsal root ganglion neurons. J Neurosci 17: 3525–3537, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. LaMotte RH, Lundberg LE, Torebjork HE. Pain, hyperalgesia and activity in nociceptive C units in humans after intradermal injection of capsaicin. J Physiol 448: 749–764, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Li D, Ren Y, Xu X, Zou X, Fang L, Lin Q. Sensitization of primary afferent nociceptors induced by intradermal capsaicin involves the peripheral release of calcitonin gene-related Peptide driven by dorsal root reflexes. J Pain 9: 1155–1168, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Lin Q, Li D, Xu X, Zou X, Fang L. Roles of TRPV1 and neuropeptidergic receptors in dorsal root reflex-mediated neurogenic inflammation induced by intradermal injection of capsaicin (Abstract). Mol Pain 3: 30, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Liu B, Zhang C, Qin F. Functional recovery from desensitization of vanilloid receptor TRPV1 requires resynthesis of phosphatidylinositol 4,5-bisphosphate. J Neurosci 25: 4835–4843, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Lu SG, Zhang X, Gold MS. Intracellular calcium regulation among subpopulations of rat dorsal root ganglion neurons. J Physiol 577: 169–190, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Mandadi S, Numazaki M, Tominaga M, Bhat MB, Armati PJ, Roufogalis BD. Activation of protein kinase C reverses capsaicin-induced calcium-dependent desensitization of TRPV1 ion channels. Cell Calcium 35: 471–478, 2004 [DOI] [PubMed] [Google Scholar]
- 33. Martin KC, Michael D, Rose JC, Barad M, Casadio A, Zhu H, Kandel ER. MAP kinase translocates into the nucleus of the presynaptic cell and is required for long-term facilitation in Aplysia. Neuron 18: 899–912, 1997 [DOI] [PubMed] [Google Scholar]
- 34. Mohapatra DP, Nau C. Regulation of Ca2+-dependent desensitization in the vanilloid receptor TRPV1 by calcineurin and cAMP-dependent protein kinase. J Biol Chem 280: 13424–13432, 2005 [DOI] [PubMed] [Google Scholar]
- 35. Mons N, Cooper DM. Adenylate cyclases: critical foci in neuronal signaling. Trends Neurosci 18: 536–542, 1995 [DOI] [PubMed] [Google Scholar]
- 36. Planells-Cases R, Garcia-Sanz N, Morenilla-Palao C, Ferrer-Montiel A. Functional aspects and mechanisms of TRPV1 involvement in neurogenic inflammation that leads to thermal hyperalgesia. Pflugers Arch 451: 151–159, 2005 [DOI] [PubMed] [Google Scholar]
- 37. Price TJ, Jeske NA, Flores CM, Hargreaves KM. Pharmacological interactions between calcium/calmodulin-dependent kinase II alpha and TRPV1 receptors in rat trigeminal sensory neurons. Neurosci Lett 389: 94–98, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Rosen LB, Ginty DD, Weber MJ, Greenberg ME. Membrane depolarization and calcium influx stimulate MEK and MAP kinase via activation of Ras. Neuron 12: 1207–1221, 1994 [DOI] [PubMed] [Google Scholar]
- 39. Schmelz M, Schmidt R, Ringkamp M, Handwerker HO, Torebjork HE. Sensitization of insensitive branches of C nociceptors in human skin. J Physiol 480: 389–394, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Sculptoreanu A, Aura Kullmann F, de Groat WC. Neurokinin 2 receptor-mediated activation of protein kinase C modulates capsaicin responses in DRG neurons from adult rats. Eur J Neurosci 27: 3171–3181, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Sumi M, Kiuchi K, Ishikawa T, Ishii A, Hagiwara M, Nagatsu T, Hidaka H. The newly synthesized selective Ca2+/calmodulin dependent protein kinase II inhibitor KN-93 reduces dopamine contents in PC12h cells. Biochem Biophys Res Commun 181: 968–975, 1991 [DOI] [PubMed] [Google Scholar]
- 42. Tang HB, Li YS, Arihiro K, Nakata Y. Activation of the neurokinin-1 receptor by substance P triggers the release of substance P from cultured adult rat dorsal root ganglion neurons (Abstract). Mol Pain 3: 42, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. van de Graaf SF, Rescher U, Hoenderop JG, Verkaart S, Bindels RJ, Gerke V. TRPV5 is internalized via clathrin-dependent endocytosis to enter a Ca2+-controlled recycling pathway. J Biol Chem 283: 4077–4086, 2008 [DOI] [PubMed] [Google Scholar]
- 44. Wang JG, Strong JA, Xie W, Zhang JM. Local inflammation in rat dorsal root ganglion alters excitability and ion currents in small-diameter sensory neurons. Anesthesiology 107: 322–332, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Williams M, Kowaluk EA, Arneric SP. Emerging molecular approaches to pain therapy. J Med Chem 42: 1481–1500, 1999 [DOI] [PubMed] [Google Scholar]
- 46. Willis WD. Long-term potentiation in spinothalamic neurons. Brain Res Brain Res Rev 40: 202–214, 2002 [DOI] [PubMed] [Google Scholar]
- 47. Willis WD. Role of neurotransmitters in sensitization of pain responses. Ann NY Acad Sci 933: 142–156, 2001 [DOI] [PubMed] [Google Scholar]
- 48. Willis WD., Jr The role of TRPV1 receptors in pain evoked by noxious thermal and chemical stimuli. Exp Brain Res 196: 5–11, 2009 [DOI] [PubMed] [Google Scholar]
- 49. Wood JN, Docherty R. Chemical activators of sensory neurons. Annu Rev Physiol 59: 457–482, 1997 [DOI] [PubMed] [Google Scholar]
- 50. Woolf CJ, Salter MW. Neuronal plasticity: increasing the gain in pain. Science 288: 1765–1769, 2000 [DOI] [PubMed] [Google Scholar]
- 51. Yao J, Qin F. Interaction with phosphoinositides confers adaptation onto the TRPV1 pain receptor. PLoS Biol 7: e46, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Zhang H, Cang CL, Kawasaki Y, Liang LL, Zhang YQ, Ji RR, Zhao ZQ. Neurokinin-1 receptor enhances TRPV1 activity in primary sensory neurons via PKCepsilon: a novel pathway for heat hyperalgesia. J Neurosci 27: 12067–12077, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Zhuang ZY, Xu H, Clapham DE, Ji RR. Phosphatidylinositol 3-kinase activates ERK in primary sensory neurons and mediates inflammatory heat hyperalgesia through TRPV1 sensitization. J Neurosci 24: 8300–8309, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]