Abstract
Evidence suggests that consumption of over-the-counter cyclooxygenase (COX) inhibitors may interfere with the positive effects that resistance exercise training has on reversing sarcopenia in older adults. This study examined the influence of acetaminophen or ibuprofen consumption on muscle mass and strength during 12 wk of knee extensor progressive resistance exercise training in older adults. Thirty-six individuals were randomly assigned to one of three groups and consumed the COX-inhibiting drugs in double-blind placebo-controlled fashion: placebo (67 ± 2 yr; n = 12), acetaminophen (64 ± 1 yr; n = 11; 4 g/day), and ibuprofen (64 ± 1 yr; n = 13; 1.2 g/day). Compliance with the resistance training program (100%) and drug consumption (via digital video observation, 94%), and resistance training intensity were similar (P > 0.05) for all three groups. Drug consumption unexpectedly increased muscle volume (acetaminophen: 109 ± 14 cm3, 12.5%; ibuprofen: 84 ± 10 cm3, 10.9%) and muscle strength (acetaminophen: 19 ± 2 kg; ibuprofen: 19 ± 2 kg) to a greater extent (P < 0.05) than placebo (muscle volume: 69 ± 12 cm3, 8.6%; muscle strength: 15 ± 2 kg), when controlling for initial muscle size and strength. Follow-up analysis of muscle biopsies taken from the vastus lateralis before and after training showed muscle protein content, muscle water content, and myosin heavy chain distribution were not influenced (P > 0.05) by drug consumption. Similarly, muscle content of the two known enzymes potentially targeted by the drugs, COX-1 and -2, was not influenced (P > 0.05) by drug consumption, although resistance training did result in a drug-independent increase in COX-1 (32 ± 8%; P < 0.05). Drug consumption did not influence the size of the nonresistance-trained hamstring muscles (P > 0.05). Over-the-counter doses of acetaminophen or ibuprofen, when consumed in combination with resistance training, do not inhibit and appear to enhance muscle hypertrophy and strength gains in older adults. The present findings coupled with previous short-term exercise studies provide convincing evidence that the COX pathway(s) are involved in the regulation of muscle protein turnover and muscle mass in humans.
Keywords: cyclooxygenase, prostaglandin, sarcopenia
sarcopenia, defined as the loss of skeletal muscle mass, is a debilitating clinical condition associated with old age (13, 40). This age-related loss of muscle mass is associated with a loss of muscle strength and function, a reduced functional independence, and a financial burden to the US healthcare system estimated at $20 billion per year (19, 23, 24, 51). One of the most proven treatments for sarcopenia is resistance exercise training, which increases muscle protein synthesis, muscle mass, and muscle function in men and women from 60 to > 90 yr of age (12, 14, 17, 53).
Short-term studies in humans using stable isotopic tracers to examine the rate of muscle protein synthesis following a single session of resistance exercise show that over-the-counter doses of acetaminophen (4,000 mg/day) and ibuprofen (1,200 mg/day) block the normal increase in muscle protein synthesis (50). This removal of the normal muscle protein synthesis response corresponded with the blockade of an increase in the cyclooxygenase (COX) product prostaglandin F2α (48), a known regulator of muscle protein synthesis (32, 39, 45, 52). Studies in animals also show that chronic COX-inhibitor consumption attenuates muscle hypertrophy and regrowth from atrophy (4, 29, 43).
We hypothesized that daily consumption of acetaminophen or ibuprofen during 12 wk of resistance exercise training would reduce the normal muscle mass and strength benefits typically observed in older adults.
MATERIALS AND METHODS
Overall Study Design
This study was a randomized, placebo-controlled, double-blind, 12-wk investigation. During the 12 wk, subjects completed a progressive resistance training program of the lower extremities three times per week and consumed a placebo, acetaminophen, or ibuprofen. The study was conducted at the Human Performance Laboratory at Ball State University and Ball Memorial Hospital and was approved by the Institutional Review Boards of both institutions. All study procedures, risks, and benefits were explained to the subjects before giving written consent to participate.
Subjects
Men and women were recruited from the Muncie, IN area. Subjects completed a medical screening exam, which included routine blood and urine clinical chemistries, a resting and exercise electrocardiogram, and a detailed health and exercise history questionnaire. Subjects were excluded if they had any cardiac, orthopedic, or neuromuscular conditions that would preclude them from participating in a resistance exercise training program, abnormal blood or urine chemistries, arthritis, diabetes, uncontrolled hypertension, or any condition that would be a contraindication to taking acetaminophen or ibuprofen for 3 mo, if they were chronically consuming any prescription or nonprescription COX-inhibiting drugs, if they were involved in any formal aerobic or resistance exercise training program, if they smoked, or if they were < 60 or > 85 yr of age.
Of the 61 individuals that consented to be screened for participation in the study, 17 were excluded due to medical reasons, seven decided not to participate for personal reasons, and one individual sustained a back injury unrelated to the study after starting the resistance training. Subject characteristics of the 36 men and women included in the study are presented in Table 1.
Table 1.
Subject characteristics
| Placebo | Acetaminophen | Ibuprofen | |
|---|---|---|---|
| Number in group | 12 | 11 | 13 |
| Males/females | 8/4 | 7/4 | 9/4 |
| Age, yr† | 67 ± 2 | 64 ± 1 | 64 ± 1 |
| Range | 60–78 | 60–71 | 60–77 |
| Height, cm† | 170 ± 3 | 172 ± 5 | 175 ± 2 |
| Weight, kg | 77.3 ± 4.4 | 93.1 ± 5.6* | 82.6 ± 2.8 |
Data are means ± SE.
Significant difference from placebo, P < 0.05;
no significant difference among values for all 3 groups, P > 0.05.
Interventions
Resistance exercise training protocol.
All subjects completed a progressive resistance exercise training program of bilateral knee extension that was designed to hypertrophy and strengthen the m. quadriceps femoris (14, 17, 42), using a protocol employed for several previous investigations in our laboratory (42). Each subject was scheduled for resistance training three times per week over the 12 wk for a total of 36 sessions on an isotonic knee extension device (Cybex Eagle, Medway, MA). All sessions were supervised by a member of the research team. Each session was separated by at least 1 day and consisted of 5 min of light cycling (model 828E; Monark Exercise, Vansbro, Sweden), two sets of five knee extensions at a light weight, followed by three sets of 10 repetitions with 2 min of rest between sets. Training intensity was based on each individual's one repetition maximum (1 RM) and was adjusted during the training based on each individual's training session performance and biweekly 1 RM.
COX-inhibitor consumption.
Drugs were administered in double-blind, placebo-controlled fashion as we have previously described (6, 50). Each drug was administered in 3 doses/day (∼8 AM, ∼2 PM, ∼8 PM) corresponding to the maximal over-the-counter daily dose (acetaminophen: 1,500 mg, 1,500 mg, 1,000 mg, 4,000 mg total; ibuprofen: 400 mg/dose, 1,200 mg total). The placebo group was given an identical number of pills/dose (3 pills), which were indistinguishable from the drug doses. Each subject was given the doses in weekly batches (21 doses) in pillboxes labeled with the date and consumption time. At the end of each week subjects were asked to return all of the pillboxes. Subjects were instructed to not consume any other COX-inhibiting drugs outside of the study.
Compliance with the requested drug consumption was completed in two ways: direct and indirect. Direct compliance was determined by a member of the research team watching the subject consume their dose in person while at their scheduled training session (3 doses/wk) or by personal digital video (18 doses/wk), as previously described (6). Each subject was provided a small camera that allowed them to video record, by virtue of a rotating lens feature, the consumption of each dose. Each video was automatically time and date stamped, downloaded to a laboratory computer, and watched by a research team member to confirm dose consumption. Indirect compliance was monitored by counting the number of pills remaining in the pillboxes returned by the subjects each week.
Potential side effects of drug consumption were monitored via monthly blood draws for renal (creatinine), hepatic (alanine aminotransferase), and hematologic (hematocrit) measures.
Muscle Volume
Knee extensor (m. quadriceps femoris) muscle volume was measured with MRI before and at the end of the 12-wk period as we have previously described in detail for sarcopenia studies of aging and chronic bed rest (47, 49). The hamstrings muscles (mm. semimembranosus, semitendinosus, and biceps femoris) were also measured to determine the influence of drug consumption on nonexercised muscle.
Subjects rested in the supine horizontal position for 1 h prior to scanning to prevent the influence of fluid shifts on muscle volume (1). No exercise or strenuous activity was allowed within 24 h of scanning, which was completed at the same time of day for each subject using a nonmetallic foot restraint to control joint angle (muscle length) and leg compression. Imaging was completed in a 1.5T scanner (Genesis Signa, GE Medical Systems, Milwaukee, WI) by using serial interleaved images 8-mm thick (TR: 2,000 ms, TE: 9.0 ms, 512 × 512 matrix, field of view: 480 × 480 mm). MR images were transferred electronically from the scanner to a personal computer (iMac G5) at the Human Performance Laboratory and analyzed with NIH Image software (Image J, version 1.34 h) using manual planimetry. A detailed description of the manual planimetry measurements and associated variability has been presented previously (47, 49). The cross-sectional area (cm2) of the muscle(s) of interest in a given slice was determined, and the muscle volume (cm3) was calculated by multiplying the cross-sectional area by the slice thickness. The right limb of each subject was used for all measurements. Measurements were completed by the same investigator in blinded fashion.
Muscle Strength
Muscle strength was measured three times prior to training and twice during the final week of the 12-wk training period by determining the maximum amount of weight each subject could lift through a full range of motion one time (i.e., 1 RM) on the resistance exercise training device (42). The highest weight lifted before and at the end of the 12 wk was considered the pre- and posttraining 1 RM.
Muscle Biopsy
Subjects underwent a muscle biopsy (2) of the m. vastus lateralis before and at the end of the resistance training program for the measurement of muscle protein content, muscle water content, myosin heavy chain (MHC) distribution, and COX protein and mRNA levels. Biopsies were obtained in the early morning (∼7 AM) after at least 30 min of supine rest and after an overnight fast of ∼12 h. The evening meals prior to the biopsy were supplied in liquid form (Ensure Plus, 57% carbohydrate, 15% protein, and 28% fat), and provided 50% of the estimated daily caloric need (1.5× predicted resting metabolic rate) to standardize the composition, amount, and timing (i.e., duration of the fast) of the final meal consumed prior to the biopsy. In addition, no testing or training was completed for 3 days prior to each biopsy.
Following the biopsy, excess blood, visible fat, and connective tissue were removed, and portions of the muscle to be used for all of the aforementioned analyses except COX mRNA were immediately frozen and stored in liquid nitrogen (−190°C) until analysis. A separate portion of the muscle was immediately stored in 0.5 ml RNAlater (Ambion, Austin, TX) at 4°C for a 24-h incubation period and then placed at −20°C until analysis of COX mRNA.
Biopsies were obtained from and analyses were completed on 34 individuals (minus one subject from each drug group) for muscle protein and water content, MHC distribution, and COX proteins, and 33 individuals (minus one subject from each group) for COX mRNA.
Muscle Protein and Water Quantification
For each biopsy sample, a piece of muscle (14.95 ± 0.46 mg) was weighed on a precision microbalance (Cahn 35; Orion Research, Beverly, MA) at −35°C, placed in a vented cryovial, freeze-dried for ∼72 h (Flexi-Dry; FTS Industries, New York, NY), and reweighed at −35°C. Following freeze drying, each sample was homogenized in 40 volumes of cold homogenizing buffer (250 mM sucrose, 100 mM potassium chloride, 20 mM imidazole, and 5 mM EDTA; pH 6.8) in a ground-glass homogenizer (Radnoti Glass Technology, Monrovia, CA) (10). An aliquot of each homogenate was measured for protein concentration using the bicinchoninic acid assay (Sigma) with bovine serum albumin used as the protein standard (20, 44). The amount of protein and water in each sample was normalized to muscle wet weight.
Muscle MHC Isoform Distribution
Each muscle protein homogenate sample was centrifuged at 20,000 g for 30 min at 4°C, the supernatant removed, and the pellet resuspended in 40 volumes of cold homogenizing buffer. MHC isoform composition of each resuspended protein sample was determined in triplicate from an SDS buffer-diluted aliquot heated to 100°C for 5 min. MHC isoforms were separated with SDS-PAGE with a 3.5% stacking gel and 5% separating gel. Electrophoresis was performed at 150 V for ∼15 h with a Tris-glycine electrode buffer at 4°C (Hoeffer SE 600; Amersham Pharmacia Biotech, Piscataway, NJ). The separating gels were silver stained (18), and digitally photographed (ChemImager 5500; Alpha Innotech, San Leandro, CA), and densitometry was completed to determine the percent contribution of each isoform of the total (100%). The average density of each isoform from the three lanes loaded was taken as the MHC distribution for that sample (20, 44).
Muscle COX mRNA Measurements
RT-quantitative PCR (RT-qPCR) was completed to determine the mRNA levels of the known COX isoforms and variants: COX-1 [both variant 1: COX-1v1 and variant 2: COX-1v2 (11, 41)], COX-2, and the intron-retaining COX-1 [also referred to as COX-3 (7)] variants (COX-1b1, -1b2, and -1b3), as we have previously described (5, 52).
Total RNA extraction and RNA quality check.
Each muscle sample was removed from the RNAlater and placed in a mixture of 0.8 ml of TRI Reagent and 4 μl of PolyAcryl Carrier (Molecular Research Center, Cincinnati, OH). The tissue was homogenized and total RNA was extracted according to the manufacturer's protocol. The RNA pellet was dissolved in 30 μl of nuclease-free water and then stored at −80°C. The quality and integrity (RIN of 8.12 ± 0.03) of extracted total RNA (113.28 ± 4.51 ng/μl) was evaluated using a RNA 6000 Nano LabChip kit on an Agilent 2100 Bioanalyzer (Agilent Technologies, Palo Alto, CA).
Reverse transcription.
Oligo(dT) primed first-strand cDNA was synthesized (150 ng of total RNA) using SuperScript II RT (Invitrogen, Carlsbad, CA) according to the manufacturer's protocol. All thermal incubations and chilling were done in a Peltier Thermal Cycler with dual-block DNA engine (MJ Research, Waltham, MA) to provide temperature homogeneity and identical temperature ramping for all samples.
RT-qPCR.
Quantification of mRNA levels (in duplicate) was performed in a 72-well Rotor-Gene 3000 centrifugal real-time cycler (Corbett Research, Mortlake, NSW, Australia). Housekeeping gene GAPDH was used as a reference gene. The validation of GAPDH was performed to insure that its expression was unaffected by the experimental treatments, as we have previously described (25, 36, 52). All primers used in this study were mRNA specific and designed for qPCR (Vector NTI Advance 9 software, Invitrogen) using SYBR Green chemistry. Details about primer characteristics and sequences, as well as the qPCR parameters and amplicon melting curve analysis have been reported previously (5, 52).
Relative qPCR data analysis.
The COX gene expression before and after the 12 wk of resistance exercise training was compared using the 2−ΔCT (arbitrary units) quantification method (28, 36, 52).
A serial dilution curve was generated for each qPCR run to evaluate reaction efficiencies. To make the dilution curve for GAPDH and COX isoforms and variants, the cDNA from total RNA of human skeletal muscle (500 ng; Ambion, Austin, TX) was used. The amplification calculated by the Rotor-Gene software was specific and highly efficient (efficiency = 1.16 ± 0.03; R2 = 0.99 ± 0.00; slope = 3.02 ± 0.06).
Muscle COX Protein Measurements
COX-1 and COX-2 protein measurements were completed as we have previously described (5). For each biopsy sample, a piece of muscle (15.82 ± 0.45 mg) previously frozen in liquid nitrogen was weighed on a precision microbalance (Cahn 35, Orion Research, Beverly, MA) at −35°C. Each sample was homogenized (PowerGen 700; Fisher Scientific, Pittsburg, PA) in 30 volumes of cold RIPA buffer (25 mM Tris·HCl pH 7.6, 150 mM NaCl, 1% NP-40, 1% sodium deoxycholate, 0.1% SDS) (Pierce, Rockford, IL) with Halt Protease Inhibitor Cocktail and 5 mM EDTA (Pierce). Total protein concentration was determined (macroBCA Assay, Pierce), and aliquots of the homogenate were diluted in SDS sample buffer and heated to 95°C for 5 min. Proteins (80 μg) were separated with a 4–20% gradient gel (Pierce) using SDS-PAGE for 2 h at 75 V (Mini Protean 3 system; Bio-Rad Laboratories, Hercules, CA) and then transferred to a polyvinylidene fluoride membrane (Immobilon-P; Millipore, Bedford, MA) for 2 h at 200 mA at 4°C. The membrane was blocked with 5% milk for 60 min and then incubated with a monoclonal COX-1 antibody diluted 1:200 (cat. no. 160110; Cayman Chemical, Ann Arbor, MI) in 1× Tris buffered saline, 0.1% Tween-20 (TBS-T) with 0.5% milk or a monoclonal COX-2 antibody diluted 1:500 (cat. no. 160112; Cayman Chemical) in 1× TBS-T at 4°C overnight. Blots were identified by incubation with horseradish peroxidase-conjugated goat anti-mouse IgG diluted 1:7100 (cat. no. 10004302; Cayman Chemical) in 1× TBS-T with 5% milk and then exposed to an enhanced chemiluminescent substrate (ChemiGlow West; Alpha Innotech, San Leandro, CA). Digital images were captured using a chemiluminescent imaging system (FluorChem; Alpha Innotech). Equal protein loading was verified by Ponceau S staining, and sizes of the immunodetected proteins were confirmed by molecular weight markers (Invitrogen) and a positive control COX-1 protein (cat. no. 60100; Cayman Chemical) or COX-2 protein (cat. no. 10009624; Cayman Chemical). To control for intra-assay variability, subject's pre- and posttraining samples were analyzed on the same blot, and each blot contained subjects from all three experimental groups.
COX-1 and COX-2 considerations.
We have previously discussed the issues of COX-1 and COX-2 protein quantification by Western blot analysis in detail (5). Briefly, no attempt was made to determine whether our COX-1 measurements reflect the protein product from COX-1v1 or COX-1v2 (41). Interestingly, COX-1v2 continues to show the highest mRNA expression levels of the COX-1 variants and is responsive to acute and chronic exercise [data reported here and previously (5, 52)]. For the COX-2 measurements, we did not detect any COX-2 band that migrated with the aforementioned recombinant human COX-2 protein (cat. no. 10009624; Cayman Chemical) or an ovine COX-2 electrophoresis standard protein (cat. no. 360120; Cayman Chemical) in any of the pre- or posttraining samples. All of the muscle samples also had two detectable bands below the 70 kDa region (5), which may be a glycosylated form of COX-2 (8, 22, 27, 30, 31, 33), but these bands were also unchanged over the 12-wk interventions.
Statistical Analysis
Data were analyzed using commercially available software (Statistica; Statsoft, Tulsa, OK). A one-way ANOVA was used to compare the subject characteristics, intervention compliance, and resistance training parameters. A two-way (group and time) ANOVA with repeated measures was used to compare all other parameters. Due to an apparent enhancement of muscle size and strength with drug consumption and the effects of both of these COX-inhibitors on suppressing muscle prostaglandin production and muscle protein synthesis following resistance exercise (48, 50), a one-way ANCOVA, considering initial muscle size and strength, was used to compare the change in quadriceps muscle size, strength, strength normalized to muscle size, and the muscle biopsy parameters between the drug groups and the placebo group. Post hoc comparisons were made with Tukey's test. Significance was accepted at P < 0.05. Data are presented as means ± SE.
RESULTS
Intervention compliance with the drug consumption was high and similar (P > 0.05) among all three groups (Table 2). The 6–7% noncompliance with the observed compliance was primarily due to technical issues with the personal digital video cameras (6). Reported compliance from the return of the pillboxes was nearly 100% for all three groups (Table 2). There were no measured changes (P > 0.05) in the renal, hepatic, or hematologic markers during the 12 wk (Table 3). Resistance training compliance was also excellent, with all individuals from each group completing 100% of their scheduled training sessions (Table 2). All of the resistance training parameters (Table 2) were similar (P > 0.05) among the three groups, including training intensity and the actual training load.
Table 2.
Intervention compliance and resistance training parameters
| Placebo | Acetaminophen | Ibuprofen | |
|---|---|---|---|
| Intervention compliance | |||
| Drug consumption | |||
| Observed, %† | 93 ± 2 | 93 ± 2 | 94 ± 2 |
| Reported, %† | 100 ± 0.1 | 99 ± 1 | 99 ± 0.3 |
| Exercise training, %† | 100 | 100 | 100 |
| Resistance training | |||
| Mean training, %1 RM† | 74 ± 1 | 71 ± 1 | 73 ± 1 |
| Mean training load, kg† | 58 ± 7 | 55 ± 6 | 56 ± 4 |
| Total lifted over 36 training sessions, kg† | 62,843 ± 7076 | 59,562 ± 6311 | 60,491 ± 4636 |
Data are means ± SE. Drug consumption is the % of 252 scheduled doses consumed by the subjects; Observed means % consumed as directly viewed by the research staff in person or on personal digital video; Reported is % consumed based on the number of doses remaining in the returned pillboxes; Exercise training is the % of 36 scheduled exercise training sessions completed by the subjects. RM, repetition maximum.
No significant difference among values for all 3 groups, P > 0.05.
Table 3.
Blood clinical chemistries
| Placebo | Acetaminophen | Ibuprofen | |
|---|---|---|---|
| Creatinine, mg/dl | |||
| Pretraining | 0.9 ± 0.1 | 1.0 ± 0.1 | 0.9 ± 0.1 |
| 4 wk | 1.0 ± 0.1 | 1.0 ± 0.1 | 0.9 ± 0.1 |
| 8 wk | 1.0 ± 0.1 | 0.9 ± 0.1 | 0.9 ± 0.1 |
| 12 wk | 0.9 ± 0.1 | 0.9 ± 0.04 | 0.9 ± 0.1 |
| ALT, U/l | |||
| Pretraining | 25 ± 2 | 27 ± 4 | 20 ± 3 |
| 4 wk | 27 ± 3 | 35 ± 4 | 23 ± 3 |
| 8 wk | 25 ± 2 | 34 ± 6 | 24 ± 3 |
| 12 wk | 24 ± 2 | 26 ± 3 | 29 ± 5 |
| Hematocrit, % | |||
| Pretraining | 43 ± 1 | 45 ± 1 | 44 ± 1 |
| 4 wk | 44 ± 1 | 43 ± 1 | 43 ± 1 |
| 8 wk | 44 ± 1 | 43 ± 1 | 43 ± 1 |
| 12 wk | 43 ± 1 | 43 ± 1 | 42 ± 1 |
ALT, alanine aminotransferase. There were no significant differences from pretraining for each of the 3 groups in any of the listed renal, hepatic, or hematologic markers, P > 0.05.
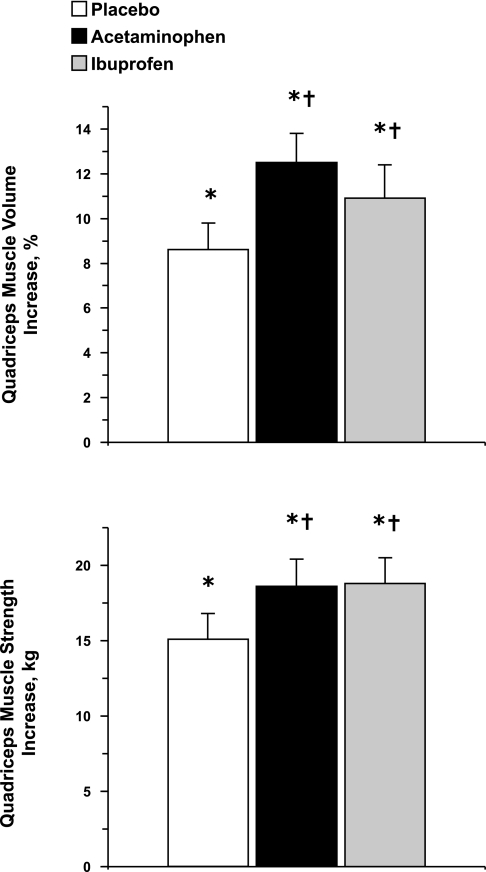
Quadriceps muscle volume increased (P < 0.05) substantially over the 12 wk from pretraining values (placebo: 848 ± 103; acetaminophen: 878 ± 76; ibuprofen: 809 ± 62 cm3), but increased by a greater (P < 0.05) amount in the drug groups compared with the placebo group (placebo: 69 ± 12; acetaminophen: 109 ± 14; ibuprofen: 84 ± 10 cm3) (Fig. 1). Similarly, quadriceps muscle strength increased (P < 0.05) substantially over the 12 wk from pretraining values (placebo: 71 ± 8; acetaminophen: 70 ± 8; ibuprofen: 68 ± 6 kg), and increased by a greater (P < 0.05) amount in the drug groups compared with the placebo group (Fig. 1). Drug consumption had no influence on the increase (15 ± 1%; P < 0.05) in quadriceps' strength normalized to muscle size (placebo: pretraining, 1.22 ± 0.06; posttraining, 1.38 ± 0.06; acetaminophen: pretraining, 1.18 ± 0.05; posttraining, 1.34 ± 0.05; ibuprofen: pretraining, 1.20 ± 0.04; posttraining, 1.39 ± 0.04 kg/cm2). There were no differences (P > 0.05) from pre- to posttraining in hamstrings muscle size (placebo: pretraining, 28 ± 2; posttraining, 27 ± 2; acetaminophen: pretraining, 33 ± 3; posttraining, 33 ± 3; ibuprofen: pretraining, 28 ± 2; posttraining 28 ± 2 cm2).
Fig. 1.
Change in quadriceps muscle volume and muscle strength from the beginning to the end of the 12-wk resistance exercise training and drug intervention. *Significant increase from pretraining across all three groups, P < 0.05; †ANCOVA revealed a significant increase compared with placebo, P < 0.05.
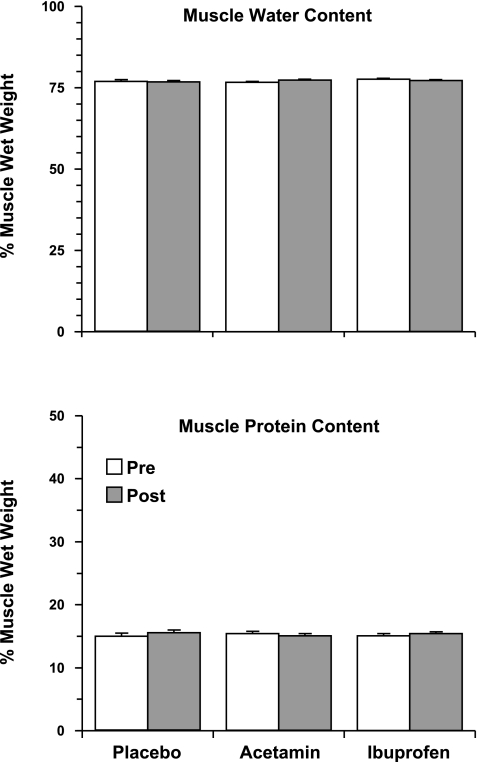
There were no differences (P > 0.05) from pre- to posttraining in muscle protein or water content (Fig. 2). MHC distribution was not influenced by drug consumption, but the amount of MHC IIa and IIx in the muscle was altered by resistance training across all three groups (MHC I: pretraining, 38 ± 1; posttraining, 39 ± 1%, P > 0.05; MHC IIa: pretraining, 30 ± 1; posttraining, 33 ± 1%, P < 0.05; MHC IIx: pretraining, 32 ± 1; posttraining, 28 ± 1%, P < 0.05).
Fig. 2.
Skeletal muscle (vastus lateralis) water content and protein content at the beginning and the end of the 12-wk resistance exercise training and drug intervention. There was no influence of drug consumption or resistance training on either parameter. Acetamin, acetaminophen.
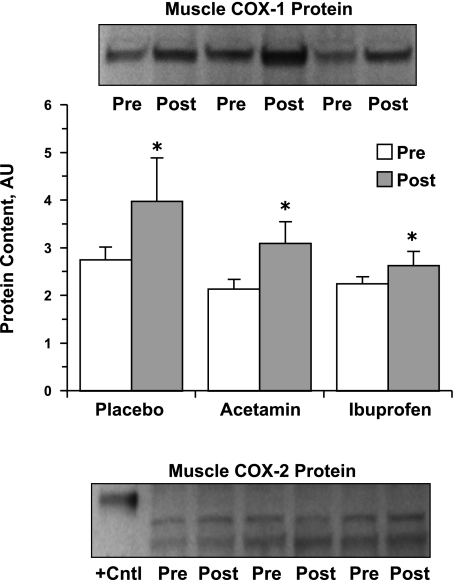
COX-1 protein content was not influenced by drug consumption but did increase (P < 0.05) by 32 ± 8% across all three groups with resistance training (Fig. 3). Correspondingly, the COX-1 mRNA associated variants -v1 and -v2 were not influenced by drug consumption, but did increase (P < 0.05) by 43 ± 20% and 63 ± 24%, respectively, from pre- to posttraining (Table 4). COX-2 protein content was not influenced by drug consumption or resistance training (Fig. 3). COX-2 mRNA was present in the muscle at relatively low levels and was not influenced by drug consumption, but did increase (P < 0.05) pre- to posttraining across all three groups (Table 4). The mRNA levels of the COX-1b variants [also referred to as COX-3 (7)] showed low-level expression in only a few of the subjects from each group before and after resistance training (data not shown), which is consistent with our previous findings (5, 52).
Fig. 3.
Skeletal muscle (vastus lateralis) cyclooxygenase (COX)-1 and COX-2 protein content at the beginning and the end of the 12-wk resistance exercise training and drug intervention. *Significant increase from pretraining across all 3 groups, P < 0.05. There was no influence of drug consumption on COX-1 protein. The image for the COX-1 protein reflects a representative Western blot showing an increase from pre- to posttraining in each of the 3 groups. The image for the COX-2 protein reflects a representative Western blot showing a lack of detectable COX-2 protein migrating with the human recombinant COX-2 positive control protein pre- or posttraining in any of the 3 groups. See materials and methods and Burd et al. (5) for further description of the analytical considerations and the two lower migrating bands, which also did not change over the 12 wk, AU, arbitrary units.
Table 4.
Muscle cyclooxygenase (COX) mRNA with 12 wk of resistance training
| Placebo | Acetaminophen | Ibuprofen | |
|---|---|---|---|
| COX-1v1 | |||
| Pretraining | 2.99 ± 0.28 | 3.44 ± 0.33 | 4.17 ± 0.61 |
| Posttraining | 4.42 ± 0.75* | 5.80 ± 1.59* | 4.04 ± 0.60* |
| COX-1v2 | |||
| Pretraining | 5.26 ± 0.60 | 6.06 ± 0.36 | 7.08 ± 0.78 |
| Posttraining | 8.69 ± 1.36* | 11.64 ± 3.65* | 7.70 ± 1.29* |
| COX-2 | |||
| Pretraining | 0.19 ± 0.03 | 0.14 ± 0.03 | 0.15 ± 0.03 |
| Posttraining | 0.31 ± 0.09* | 0.44 ± 0.14* | 0.27 ± 0.03* |
Data are means ± SE. mRNA expression of COX normalized to GAPDH in arbitrary units (2−ΔCT × 105).
Significant increase from pretraining across all 3 groups, P < 0.05.
DISCUSSION
The results of this study were unexpected and contrary to our original hypothesis, which was rooted in two parts. First, it is generally believed that the repeated increase in muscle protein synthesis following each session of resistance exercise is, at least in part, responsible for the training-induced increase in muscle mass (34, 37, 53). Second, we had previously shown that both acetaminophen and ibuprofen eliminated this typical increase in muscle protein synthesis following a single session of resistance exercise (50). The main findings from the present study were: 1) chronic exposure of skeletal muscle to acetaminophen or ibuprofen during resistance training does not inhibit muscle mass gain; 2) skeletal muscle appears to adapt to these COX-inhibiting drugs during resistance training in a way that ultimately promotes additional muscle hypertrophy and strength gains in older individuals; and 3) this effect of the drugs only occurs when the muscle is exercised, as the hamstrings were not trained and did not hypertrophy but were exposed to the drugs for the entire 12-wk period.
The amount of hypertrophy induced in the placebo group by the resistance exercise program used in the present study (90 contractions/wk; ∼5 min of contraction time/wk) was substantial and similar to what has been reported for resistance training of similar aged individuals (12). To increase muscle mass by up to an additional 4% (i.e., up to ∼50% more than the placebo group) with over-the-counter COX inhibitors is remarkable and clinically relevant (14, 15, 23, 24). Considering the yearly rate of quadriceps muscle mass loss in individuals of this age (12, 16, 19), the placebo group reversed the equivalent of 6 to 7 yr of sarcopenia with the resistance training. The response of the drug groups reversed nearly 10 yr of sarcopenia. In addition, the similar normalized muscle strength increase in all three groups coupled with the additional ∼4 kg increase in strength in the drug groups suggests the additional quadriceps mass added by the drug groups was functional muscle and contained appropriate proportions of contractile proteins (44). This notion is supported by the muscle biopsy findings showing a lack of drug influence on muscle protein content, muscle water content, and MHC distribution after the 12 wk of resistance training.
We considered the possibility that the COX enzymes in the muscle may have been upregulated to overcome the constant inhibition by acetaminophen or ibuprofen over the 12 wk, and in doing so provided for an enhanced PGF2α and protein synthesis response to each exercise session. From the present findings it does not appear this adaptation occurred with either COX-1 or COX-2, as the protein content of both enzymes was not influenced by drug consumption. However, COX-1 protein and mRNA levels were increased as a result of the resistance training, suggesting this enzyme is part of the normal adaptive response to resistance exercise training. These findings are consistent with the recent findings that COX-2 inhibition does not block the acute muscle protein synthesis response to resistance exercise, suggesting the COX-1 enzyme is likely the main isoform responsible for the COX-mediated increase in muscle protein synthesis following resistance exercise in humans (5).
Although speculative, it is possible the COX inhibition provided by the drugs may have had a relatively stronger inhibitory effect on muscle protein breakdown compared with protein synthesis. Support for this hypothesis comes from the original investigation of prostaglandins and protein turnover in skeletal muscle by Rodemann and Goldberg (39). They showed COX inhibition suppressed protein degradation by reducing the intramuscular production of PGE2 in addition to suppressing PGF2α and protein synthesis. However, COX inhibition suppressed protein degradation to a greater extent than protein synthesis, resulting in a significantly higher (+39%) net protein balance. Our original investigation (48, 50), which provided the basis for the present study hypothesis, focused on the COX regulation of PGF2α production and protein synthesis after exercise primarily due to methodological limitations in studying skeletal muscle protein breakdown in humans (3, 46). However, that study did show COX inhibition eliminated the normal increase in PGE2 following resistance exercise (48). Thus, in the present study it is possible that after each resistance exercise bout protein breakdown was inhibited to a greater extent than protein synthesis, resulting in a more positive net protein balance, more protein accretion, and ultimately more muscle mass over time.
Interestingly, Rieu et al. (38) recently showed in old rodents that daily ibuprofen consumption (approximately double the dose per kilogram body weight used in the present study) over 5 mo increased muscle mass. This response occurred by reducing the low-grade inflammation that occurs with aging, which restored the stimulation of muscle protein synthesis and suppression of muscle protein breakdown associated with feeding (38). These results do not directly explain the present results (since the hamstring muscles did not hypertrophy in the drug groups), but provide a potential avenue of investigation that may be related to the present findings. Regardless of the underlying basis of the present findings, it is likely the daily effect on muscle mass was subtle, and the cumulative effects of the drug consumption and resistance training manifested only in the latter portion of the study. This suggestion is consistent with the similar training intensity and loads of the drug and placebo groups, which confirms an analgesic component of the drug consumption did not allow the drug groups to train harder (Table 2). It would be interesting to know the impact of an even longer drug and exercise intervention.
The results of the present study are in contrast to animal studies that show an inhibitory effect on muscle hypertrophy with chronic COX-inhibitor consumption (4, 29, 43). For similar reasons to what we have previously detailed (5), this discrepancy is likely related to different mechanisms regulating the extreme rates of muscle hypertrophy in the animal studies (25–40%/wk) compared with the ∼0.75 to 1%/wk in the present study. In addition, a 6-wk resistance training study in young men utilizing a within-subjects design and intermittent drug consumption showed no effect of 400 mg/day of ibuprofen on muscle mass (26). The short-duration training program and low COX inhibitor dose likely explain the discrepancy between that study and the present findings.
Perspectives and Significance
The present findings coupled with previous short-term exercise studies (5, 45, 48, 50, 52) provide convincing evidence that the COX pathway(s) are involved in the regulation of muscle protein turnover and muscle mass in humans. Daily COX inhibitor consumption during resistance training resulted in an ∼25–50% greater increase in muscle mass and strength compared with a placebo consuming group of older men and women who completed the same 12-wk resistance training program. Although over-the-counter doses of acetaminophen and ibuprofen were used in the present study, the risks associated with COX inhibitors (9, 21, 35) need to be considered before the use of these drugs in any population. Given the millions of individuals that consume acetaminophen, ibuprofen, and other nonprescription or prescription COX inhibitors on a regular basis, the role of this class of drugs in skeletal muscle metabolism needs to be further defined. Further understanding of the COX-associated mechanisms that regulate skeletal muscle mass may provide insight into the mechanisms that regulate sarcopenia, and other conditions of cachexia and skeletal muscle atrophy.
GRANTS
This investigation was funded by National Institute on Aging grant R01-AG-020532 (to T. Trappe).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
ACKNOWLEDGMENTS
The authors thank the faculty, staff, and students at the Human Performance Lab, and the staffs in the Radiology Department and Medical Consultants at Ball Memorial Hospital for their efforts with this investigation. We also extend a special thank you to all the volunteers who dedicated themselves to this project.
REFERENCES
- 1. Berg HE, Tedner B, Tesch PA. Changes in lower limb muscle cross-sectional area and tissue fluid volume after transition from standing to supine. Acta Physiol Scand 148: 379–385, 1993 [DOI] [PubMed] [Google Scholar]
- 2. Bergstrom J. Muscle electrolytes in man. Scand J Clin Lab Invest 14: 7–110, 1962. 13862378 [Google Scholar]
- 3. Biolo G, Fleming RY, Maggi SP, Wolfe RR. Transmembrane transport and intracellular kinetics of amino acids in human skeletal muscle. Am J Physiol Endocrinol Metab 268: E75–E84, 1995 [DOI] [PubMed] [Google Scholar]
- 4. Bondesen BA, Mills ST, Pavlath GK. The COX-2 pathway regulates growth of atrophied muscle via multiple mechanisms. Am J Physiol Cell Physiol 290: C1651–C1659, 2006 [DOI] [PubMed] [Google Scholar]
- 5. Burd NA, Dickinson JM, Lemoine JK, Carroll CC, Sullivan BE, Haus JM, Jemiolo B, Trappe SW, Hughes GM, Sanders CE, Jr, Trappe TA. Effect of a cyclooxygenase-2 inhibitor on postexercise muscle protein synthesis in humans. Am J Physiol Endocrinol Metab 298: E354–E361, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Carroll CC, Trappe TA. Personal digital video: a method to monitor drug regimen adherence during human clinical investigations. Clin Exp Pharmacol Physiol 33: 1125–1127, 2006 [DOI] [PubMed] [Google Scholar]
- 7. Chandrasekharan NV, Dai H, Roos KL, Evanson NK, Tomsik J, Elton TS, Simmons DL. COX-3, a cyclooxygenase-1 variant inhibited by acetaminophen and other analgesic/antipyretic drugs: cloning, structure, and expression. Proc Natl Acad Sci USA 99: 13926–13931, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Chandrasekharan NV, Simmons DL. The cyclooxygenases. Genome Biol 5: 241, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Chen YF, Jobanputra P, Barton P, Bryan S, Fry-Smith A, Harris G, Taylor RS. Cyclooxygenase-2 selective non-steroidal anti-inflammatory drugs (etodolac, meloxicam, celecoxib, rofecoxib, etoricoxib, valdecoxib and lumiracoxib) for osteoarthritis and rheumatoid arthritis: a systematic review and economic evaluation. Health Technol Assess 12: 1–178, 2008 [DOI] [PubMed] [Google Scholar]
- 10. Chi MY, Hintz CS, Coyle EF, Martin WH, Ivy JL, Nemeth PH, Holloszy JO, Lowry OH. Effects of detraining on enzymes of energy metabolism in individual human muscle fibers. Am J Physiol Cell Physiol 244: C276–C287, 1983 [DOI] [PubMed] [Google Scholar]
- 11. Diaz A, Reginato AM, Jimenez SA. Alternative splicing of human prostaglandin G/H synthase mRNA and evidence of differential regulation of the resulting transcripts by transforming growth factor-β1, interleukin-1β, and tumor necrosis factor-α. J Biol Chem 267: 10816–10822, 1992 [PubMed] [Google Scholar]
- 12. Doherty TJ. Aging and sarcopenia. J Appl Physiol 95: 1717–1727, 2003 [DOI] [PubMed] [Google Scholar]
- 13. Dutta C, Hadley EC, Lexell J. Sarcopenia and physical performance in old age: overview. Muscle Nerve Suppl 5: S5–S9, 1997 [PubMed] [Google Scholar]
- 14. Fiatarone MA, Marks EC, Ryan ND, Meredith CN, Lipsitz LA, Evans WJ. High-intensity strength training in nonagenarians. Effects on skeletal muscle. JAMA 263: 3029–3034, 1990 [PubMed] [Google Scholar]
- 15. Fiatarone MA, O'Neill EF, Ryan ND, Clements KM, Solares GR, Nelson ME, Roberts SB, Kehayias JJ, Lipsitz LA, Evans WJ. Exercise training and nutritional supplementation for physical frailty in very elderly people. N Engl J Med 330: 1769–1775, 1994 [DOI] [PubMed] [Google Scholar]
- 16. Frontera WR, Hughes VA, Fielding RA, Fiatarone MA, Evans WJ, Roubenoff R. Aging of skeletal muscle: a 12-yr longitudinal study. J Appl Physiol 88: 1321–1326, 2000 [DOI] [PubMed] [Google Scholar]
- 17. Frontera WR, Meredith CN, O'Reilly KP, Knuttgen HG, Evans WJ. Strength conditioning in older men: skeletal muscle hypertrophy and improved function. J Appl Physiol 64: 1038–1044, 1988 [DOI] [PubMed] [Google Scholar]
- 18. Giulian G, Moss RL, Greaser M. Improved methodology for analysis and quantification of proteins on one-dimensional silver-stained slab gels. Anal Biochem 129: 277–287, 1983 [DOI] [PubMed] [Google Scholar]
- 19. Goodpaster BH, Park SW, Harris TB, Kritchevsky SB, Nevitt M, Schwartz AV, Simonsick EM, Tylavsky FA, Visser M, Newman AB. The loss of skeletal muscle strength, mass, and quality in older adults: the health, aging and body composition study. J Gerontol A Biol Sci Med Sci 61: 1059–1064, 2006 [DOI] [PubMed] [Google Scholar]
- 20. Haus JM, Carrithers JA, Trappe SW, Trappe TA. Collagen, cross-linking, and advanced glycation end products in aging human skeletal muscle. J Appl Physiol 103: 2068–2076, 2007 [DOI] [PubMed] [Google Scholar]
- 21. Hersh EV, Moore PA, Ross GL. Over-the-counter analgesics and antipyretics: a critical assessment. Clin Ther 22: 500–548, 2000 [DOI] [PubMed] [Google Scholar]
- 22. Hla T, Neilson K. Human cyclooxygenase-2 cDNA. Proc Natl Acad Sci USA 89: 7384–7388, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Janssen I, Heymsfield SB, Ross R. Low relative skeletal muscle mass (sarcopenia) in older persons is associated with functional impairment and physical disability. J Am Geriatr Soc 50: 889–896, 2002 [DOI] [PubMed] [Google Scholar]
- 24. Janssen I, Shepard DS, Katzmarzyk PT, Roubenoff R. The healthcare costs of sarcopenia in the United States. J Am Geriatr Soc 52: 80–85, 2004 [DOI] [PubMed] [Google Scholar]
- 25. Jemiolo B, Trappe S. Single muscle fiber gene expression in human skeletal muscle: validation of internal control with exercise. Biochem Biophys Res Commun 320: 1043–1050, 2004 [DOI] [PubMed] [Google Scholar]
- 26. Krentz JR, Quest B, Farthing JP, Quest DW, Chilibeck PD. The effects of ibuprofen on muscle hypertrophy, strength, and soreness during resistance training. Appl Physiol Nutr Metab 33: 470–475, 2008 [DOI] [PubMed] [Google Scholar]
- 27. Liu YT, Kardosh A, Cooc J, Schonthal AH. Potential misidentification of cyclooxygenase-2 by Western blot analysis and prevention through the inclusion of appropriate controls. Mol Biotechnol 34: 329–335, 2006 [DOI] [PubMed] [Google Scholar]
- 28. Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT Method. Methods 25: 402–408, 2001 [DOI] [PubMed] [Google Scholar]
- 29. Novak ML, Billich W, Smith SM, Sukhija KB, McLoughlin TJ, Hornberger TA, Koh TJ. COX-2 inhibitor reduces skeletal muscle hypertrophy in mice. Am J Physiol Regul Integr Comp Physiol 296: R1132–R1139, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. O'Neill GP, Mancini JA, Kargman S, Yergey J, Kwan MY, Falgueyret JP, Abramovitz M, Kennedy BP, Ouellet M, Cromlish W, Culp S, Evans JF, Ford-Hutchinson AW, Vickers PJ. Overexpression of human prostaglandin G/H synthase-1 and -2 by recombinant vaccinia virus: inhibition by nonsteroidal anti-inflammatory drugs and biosynthesis of 15-hydroxyeicosatetraenoic acid. Mol Pharmacol 45: 245–254, 1994 [PubMed] [Google Scholar]
- 31. Otto JC, DeWitt DL, Smith WL. N-glycosylation of prostaglandin endoperoxide synthases-1 and -2 and their orientations in the endoplasmic reticulum. J Biol Chem 268: 18234–18242, 1993 [PubMed] [Google Scholar]
- 32. Palmer RM. Prostaglandins and the control of muscle protein synthesis and degradation. Prostaglandins Leukot Essent Fatty Acids 39: 95–104, 1990 [DOI] [PubMed] [Google Scholar]
- 33. Percival MD, Ouellet M, Vincent CJ, Yergey JA, Kennedy BP, O'Neill GP. Purification and characterization of recombinant human cyclooxygenase-2. Arch Biochem Biophys 315: 111–118, 1994 [DOI] [PubMed] [Google Scholar]
- 34. Phillips SM, Tipton KD, Aarsland A, Wolf SE, Wolfe RR. Mixed muscle protein synthesis and breakdown after resistance exercise in humans. Am J Physiol Endocrinol Metab 273: E99–E107, 1997 [DOI] [PubMed] [Google Scholar]
- 35. Rainsford KD, Roberts SC, Brown S. Ibuprofen and paracetamol: relative safety in non-prescription dosages. J Pharm Pharmacol 49: 345–376, 1997 [DOI] [PubMed] [Google Scholar]
- 36. Raue U, Slivka D, Jemiolo B, Hollon C, Trappe S. Proteolytic gene expression differs at rest and after resistance exercise between young and old women. J Gerontol A Biol Sci Med Sci 62: 1407–1412, 2007 [DOI] [PubMed] [Google Scholar]
- 37. Rennie MJ, Wackerhage H, Spangenburg EE, Booth FW. Control of the size of the human muscle mass. Annu Rev Physiol 66: 799–828, 2004 [DOI] [PubMed] [Google Scholar]
- 38. Rieu I, Magne H, Savary-Auzeloux I, Averous J, Bos C, Peyron MA, Combaret L, Dardevet D. Reduction of low grade inflammation restores blunting of postprandial muscle anabolism and limits sarcopenia in old rats. J Physiol 587: 5483–5492, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Rodemann HP, Goldberg AL. Arachidonic acid, prostaglandin E2 and F2α influence rates of protein turnover in skeletal and cardiac muscle. J Biol Chem 257: 1632–1638, 1982 [PubMed] [Google Scholar]
- 40. Roubenoff R, Castaneda C. Sarcopenia-understanding the dynamics of aging muscle. JAMA 286: 1230–1231, 2001 [DOI] [PubMed] [Google Scholar]
- 41. Schneider C, Boeglin WE, Brash AR. Human cyclo-oxygenase-1 and an alternative splice variant: contrasts in expression of mRNA, protein and catalytic activities. Biochem J 385: 57–64, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Slivka D, Raue U, Hollon C, Minchev K, Trappe S. Single muscle fiber adaptations to resistance training in old (>80 yr) men: evidence for limited skeletal muscle plasticity. Am J Physiol Regul Integr Comp Physiol 295: R273–R280, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Soltow QA, Betters JL, Sellman JE, Lira VA, Long JH, Criswell DS. Ibuprofen inhibits skeletal muscle hypertrophy in rats. Med Sci Sports Exerc 38: 840–846, 2006 [DOI] [PubMed] [Google Scholar]
- 44. Trappe S, Gallagher P, Harber M, Carrithers J, Fluckey J, Trappe T. Single muscle fibre contractile properties in young and old men and women. J Physiol 552: 47–58, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Trappe T, Raue U, Williams R, Carrithers J, Hickner R. Effects of age and resistance exercise on skeletal muscle interstitial prostaglandin F2α. Prostaglandins Leukot Essent Fatty Acids 74: 175–181, 2006 [DOI] [PubMed] [Google Scholar]
- 46. Trappe T, Williams R, Carrithers J, Raue U, Esmarck B, Kjaer M, Hickner R. Influence of age and resistance exercise on human skeletal muscle proteolysis: a microdialysis approach. J Physiol 554: 803–813, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Trappe TA, Burd NA, Louis ES, Lee GA, Trappe SW. Influence of concurrent exercise or nutrition countermeasures on thigh and calf muscle size and function during 60 days of bed rest in women. Acta Physiol (Oxf) 191: 147–159, 2007 [DOI] [PubMed] [Google Scholar]
- 48. Trappe TA, Fluckey JD, White F, Lambert CP, Evans WJ. Skeletal muscle PGF2α and PGE2 in response to eccentric resistance exercise: influence of ibuprofen and acetaminophen. J Clin Endocrinol Metab 86: 5067–5070, 2001 [DOI] [PubMed] [Google Scholar]
- 49. Trappe TA, Lindquist DM, Carrithers JA. Muscle-specific atrophy of the quadriceps femoris with aging. J Appl Physiol 90: 2070–2074, 2001 [DOI] [PubMed] [Google Scholar]
- 50. Trappe TA, White F, Lambert CP, Cesar D, Hellerstein M, Evans WJ. Effect of ibuprofen and acetaminophen on postexercise muscle protein synthesis. Am J Physiol Endocrinol Metab 282: E551–E556, 2002 [DOI] [PubMed] [Google Scholar]
- 51. Visser M, Kritchevsky SB, Goodpaster BH, Newman AB, Nevitt M, Stamm E, Harris TB. Leg muscle mass and composition in relation to lower extremity performance in men and women aged 70 to 79: the health, aging and body composition study. J Am Geriatr Soc 50: 897–904, 2002 [DOI] [PubMed] [Google Scholar]
- 52. Weinheimer EM, Jemiolo B, Carroll CC, Harber MP, Haus JM, Burd NA, Lemoine JK, Trappe SW, Trappe TA. Resistance exercise and cyclooxygenase (COX) expression in human skeletal muscle: implications for COX-inhibiting drugs and protein synthesis. Am J Physiol Regul Integr Comp Physiol 292: R2241–R2248, 2007 [DOI] [PubMed] [Google Scholar]
- 53. Yarasheski KE, Pak-Loduca J, Hasten DL, Obert KA, Brown MB, Sinacore DR. Resistance exercise training increases mixed muscle protein synthesis rate in frail women and men ≥ 76 yr old. Am J Physiol Endocrinol Metab 277: E118–E125, 1999 [DOI] [PubMed] [Google Scholar]